Abstract
The bioproduction of 2-phenylethanol (2-PE), a high-value aromatic compound widely used in the fragrance, cosmetic, food and beverage, and pharmaceutical industries, through yeast fermentation offers a sustainable alternative to chemical synthesis and rose extraction. This study explores the fermentation of Yarrowia lipolytica strains using mixed agro-industrial by-products as substrates to produce 2-PE via de novo synthesis, without supplementation with the costly precursor L-phenylalanine. Y. lipolytica strains were genetically engineered to enhance flux through the shikimate pathway and enable the hydrolysis of a broader range of substrates. The culture media consisted solely of a mixture of agro-industrial by-products: sugar beet molasses (SBM), brewer’s spent grain (BSG) pressing extract, and chicory root (CR) pressing extract, serving as the primary carbon and nitrogen sources without the addition of nutrients, minerals, synthetic, complex ingredients, or costly additives. The co-culture approach enhanced substrate utilization, leading to an increase in 2-PE titers, reaching approximately 2.5 g/L 2-PE production after 240 h of fermentation. This study demonstrates the feasibility of integrating co-culture fermentation and agro-industrial waste valorization for sustainable 2-PE production, offering a scalable bioprocess for industrial applications.
1. Introduction
Y. lipolytica is a non-conventional yeast belonging to the phylum Ascomycota [1]. It was previously known as Candida lipolytica, then Endomycopsis lipolytica, and later Saccharomycopsis lipolytica, before finally being assigned the genus name “Yarrowia” in 1980 [2]. Y. lipolytica has been granted GRAS status by the FDA, producing various metabolites. It has also received the Qualified Presumption of Safety status from the European Food Safety Authority and the International Dairy Federation, further supporting its safe use in food and feed industries [3,4].
Y. lipolytica is one of the most extensively studied non-conventional yeasts due to its unique metabolic versatility, robustness, and industrial potential. It is known for its ability to metabolize a wide range of carbon sources, including both hydrophilic substrates (e.g., glucose, fructose, glycerol, ethanol, and organic acids) and hydrophobic substrates (e.g., fatty acids, triacylglycerols, and alkanes) [5]. Wild-type Y. lipolytica can efficiently consume some low-cost and renewable substrates, such as crude glycerol (CG), oils, and industrial by-products, making it a promising candidate for sustainable biomanufacturing processes [6]. While it can utilize multiple hexoses (e.g., glucose, fructose, galactose, and mannose), it cannot metabolize sucrose, lactose, or other complex substrates like lignocellulosic materials and polysaccharides. Recent metabolic engineering efforts have expanded its substrate range to include lignocellulose hydrolysates, xylose, cellulose, starch, and molasses, enhancing its industrial applicability [4].
Beyond its metabolic flexibility, Y. lipolytica exhibits exceptional tolerance to environmental stressors, including high salt concentrations, osmotic pressure, and a broad pH range (4–8). It thrives in both acidic and alkaline conditions and grows well at low pH, which helps prevent bacterial contamination during fermentation. Furthermore, it tolerates a wide temperature range (18–32 °C) and high concentrations of metal ions and organic compounds, simplifying industrial process optimization [7]. Unlike S. cerevisiae, Y. lipolytica lacks the Crabtree effect. The Crabtree effect in yeast refers to the phenomenon where, in the presence of high glucose concentrations, yeast cells preferentially undergo fermentation to produce ethanol even under aerobic conditions [8]. As a result, Y. lipolytica shows no overflow metabolism toward ethanol under high-glucose conditions. This Crabtree-negative trait, characterized by the absence of ethanol production, is particularly advantageous for 2-phenylethanol (2-PE) synthesis, as it eliminates the risk of synergistic toxicity between ethanol and 2-PE, which could otherwise amplify cell toxicity [9]. On the other hand, it is noteworthy that Y. lipolytica exhibits an overflow metabolism toward citric acid under excess carbon conditions. This behavior can lead to medium acidification and reduced production efficiency, representing an important physiological limitation that must be considered in process optimization [10,11]. Another key advantage of Y. lipolytica is its low nutritional requirements and high growth potential, allowing it to be cultivated in various conditions. Combined with its adaptability to various environments and ability to metabolize industrial by-products, these traits make Y. lipolytica a valuable yeast for biotechnology, the bioproduction of value-added compounds, and cost-effective biomanufacturing [12].
Due to its desirable industrial traits, Y. lipolytica has been a prominent biotechnological workhorse for producing commercially valuable biochemicals for over 70 years [13]. Y. lipolytica is an exceptionally versatile yeast, widely recognized for its ability to produce a diverse range of bio-based molecules, making it a key player in biotechnological applications. Its amenability to genetic and metabolic engineering and the availability of advanced synthetic biology tools further enhance its potential as a promising industrial host [14].
2-PE is an aromatic alcohol with a rose-like odor widely used as a fragrance and flavor ingredient in foods, beverages, perfumery, cosmetics and pharmaceuticals [15]. Although present naturally in the essential oils of roses and other plants, extraction from these sources is costly and yields are relatively low; so, plant-derived supply cannot meet market demand. Consequently, most commercial 2-PE is currently produced by chemical synthesis from petrochemical precursors such as benzene or styrene, a process that is environmentally unfriendly and increases cost through the generation of unwanted byproducts [16]. Fermentation has therefore emerged as a promising, more sustainable alternative, since many yeasts can biosynthesize 2-PE either from L-phenylalanine (L-Phe) via the Ehrlich pathway or de novo from sugars through the shikimate pathway together with the final steps of the Ehrlich pathway. The Ehrlich pathway is a microbial catabolic route that converts amino acids, namely L-Phe, into higher alcohols via three steps, transamination to an α-keto acid, decarboxylation to an aldehyde, and reduction to the corresponding alcohol; however, reliance on the expensive precursor L-Phe can limit overall cost-effectiveness [17]. On the other hand, de novo production of 2-PE from sugars is typically economically attractive because it avoids supplementation with costly L-Phe and can use abundant, low-cost feedstocks such as lignocellulosic biomass, starch, molasses, cassava wastewater, and whey [15]. A variety of microorganisms including Yarrowia lipolytica, Saccharomyces cerevisiae, Pichia fermentans, and Meyerozyma guilliermondii have been reported to produce 2-PE from L-Phe via the Ehrlich pathway [16]. The high cost and limited availability of L-Phe constrain the commercial viability of L-Phe-based processes and have motivated efforts to develop de novo biosynthetic routes from sugars. Recently, de novo biosynthesis of 2-PE from glucose was demonstrated in engineered Escherichia coli, underscoring a sustainable route that could enable production from renewable sugars [18]. Agro-industrial by-products encompass a wide range of residual materials generated from agricultural and industrial activities. Industrial sectors like brewing, food production, sugar refining, and cereal processing contribute significantly to waste generation, producing by-products such as BSG, molasses, bagasse, and rice and wheat bran. These by-products represent a valuable source of biomass, yet they are often underutilized or discarded. If not properly managed, their accumulation could lead to environmental challenges [19,20]. What makes this biomass particularly valuable is its rich nutritional composition, which includes a high content of carbohydrates, proteins, lipids, and essential minerals. It also serves as an excellent source of natural polysaccharides such as cellulose, chitin, hyaluronic acid, inulin, and pectin [19,21]. Linking waste streams from agro-industrial sectors to the biotechnological field for effective valorization and the production of value-added compounds can significantly reduce waste accumulation while promoting a circular economy [22]. This strategy aligns with the European Union’s directives on ‘Bioeconomy’ and ‘Biorefinery,’ which emphasize the transformation of industrial by-products into valuable resources [20].
Sugar beets (Beta vulgaris L.), first recognized as a viable sugar source in the mid-18th century, have since become a key alternative to sugarcane [23]. During the vacuum-pan crystallization of sucrose, molasses is formed as a by-product, remaining as a viscous dark liquid after the separation of sugar crystals [24]. Sugar beet molasses (SBM) is rich in carbohydrates, containing between 40 and 60% total sugars, primarily composed of sucrose and some reducing sugars. Additionally, it contains nitrogen, vitamins, minerals, trace elements (e.g., calcium, sodium, sulfate, magnesium, copper, and iron), organic acids (e.g., lactic acid and pyrocarbonic acid), proteins, and ash [25,26].
Beer is one of the world’s oldest and most widely consumed alcoholic beverages, ranking third in popularity after water and tea [27]. Like other manufacturing processes, industrial beer production generates significant waste and by-products. After mashing the barley, the insoluble solid fraction of the grains, brewers’ spent grains (BSG), is separated from the wort, the sugary liquor destined for fermentation [28]. BSG accounts for roughly 85% of the total solid residues produced during brewing [29], and about 20 kg of wet BSG are generated for every 100 L of beer produced [30]. Chemically, BSG is a lignocellulosic material composed mainly of cellulose, hemicellulose, lignin and residual starch, and it also contains appreciable amounts of protein and fermentable monosaccharides, together with smaller quantities of lipids, minerals and vitamins [31].
Chicory (Cichorium intybus L.) is a globally cultivated perennial herb originating in Europe’s Mediterranean region. It grows well in temperate zones and semi-arid areas such as Central Asia, northern Africa, the eastern United States, and Australia [32]. Chicory offers diverse culinary and industrial applications. The roots are widely known for their use as coffee substitutes, particularly when roasted, and are often blended with coffee to enhance flavor. Additionally, chicory root (CR) serves as a source of inulin, a valuable ingredient in food production [33], and are also used as a livestock feedstuff and pet food [34]. Multiple studies have reported that the moisture content of the roots is around 75%. Similarly, various studies have found differing percentages of inulin in CR, ranging from 40% to 68% of their dry weight, which could depend on factors such as the plant’s cultivation method and weather conditions [33,34,35]. CRs are the most carbohydrate-rich part of the plant, primarily consisting of fibers and sugars [32].
The high sugar content and valuable nutrients of agro-industrial by-products make them suitable and cost-effective carbon sources for microbial cultivation, supporting the growth of many microorganisms [36].
2-PE was previously produced through de novo synthesis using Y. lipolytica fermentation on SBM- and BSG-based media supplemented with yeast extract (YE) as the nitrogen source. A production of 0.71 g/L 2-PE was obtained after 146 h of fermentation in a medium containing 44.14 g/L SBM and 3.2 g/L YE using the JMY9398 strain [37]. In contrast, fermentation in 45.9 g/L CG diluted in BSG and supplemented with 2.87 g/L YE resulted in a production of 1.52 g/L 2-PE after 168 h using the JMY9385 strain [38].
There is an increasing need for sustainable, innovative, cost-effective, and environmentally friendly methods to produce 2-PE, in order to replace the environmentally harmful chemical synthesis and costly rose extraction methods currently used. Yeast fermentation represents a promising approach for the natural production of 2-PE, especially when renewable substrates are used, reducing the production cost while promoting a circular economy. Consequently, establishing greener industrial processes through biotechnological innovations and the valorization of agro-industrial residues can further advance the field of fragrance synthesis.
The objective of this study is to bioproduce 2-PE through the fermentation of the yeast Y. lipolytica using three agro-industrial by-products: SBM, BSG, and CR. Fermentations is carried out at the flask scale in culture media composed of solely mixed agro-industrial by-products, without supplementation with any costly or synthetic ingredients, nor with the expensive precursor L-Phe. The media is inoculated with a co-culture of two Y. lipolytica strains, each carrying the necessary genetic information to hydrolyze the complex sugars present in one by-product into simple sugars, which are subsequently metabolized through the shikimate pathway to produce the target molecule.
2. Materials and Methods
2.1. Raw Materials and Pre-Treatments
Three main substrates were used as ingredients for the culture media, particularly SBM, BSG, and CR.
First, the SBMs used have an initial sucrose concentration of 800 g/L, as previously determined by high performance liquid chromatography (HPLC) [26].
Second, the BSG, consisting of 90% barley and 10% wheat, was supplied by a local brewery in Compiègne. BSG was immediately cold-pressed using a pneumatic press (Hydro Press Grifo PEW20, Grifo Marchetti, Piadena, Italy) at 2 bar for approximately 5 min. The liquid fraction from all pressed grains was combined in a single container, mixed thoroughly, and filtered to remove any remaining large particles [38]. The BSG pressing extract was used in the following fermentations.
Third, French CRs were washed with water and cut into cubes of 0.7 × 0.7 × 0.7 cm3 dimensions using a vegetable cutter robot CL60 (S.N.C, France). Then, 1 kg of the cut CR was placed in a filter bag and transferred to the PEF treatment chamber. In addition, 1 L of ultrapure water was added to the chamber prior to the 100 ms PEF treatment. After treatment, the filter bag containing the roots was taken out of the PEF chamber and set in a funnel for 5 min to allow excess water to drain. The pre-treated roots were then pressed for 15 min at 10 bar in a hydraulic press (Creusot-Loire, France). This process generated the CR pressing extract used in the fermentations.
2.2. Plasmid and Strain Construction
Three main Y. lipolytica strains were used in this study (Table 1). All were derived from the chassis strain JMY8032, which was previously engineered by Larroude et al. to overexpress key enzymes in the aromatic amino acid (AAA) pathway, including YlARO1, YlARO2, ScARO4K229L, ScARO7T226I, ARO8, ARO10, and TKL1, thereby driving the production of 2-PE [39]. The strains were further engineered to hydrolyze the different agro-industrial by-products used as substrates in the fermentations.

Table 1.
Main strains used in this study.
The JMY9398 strain, which was described in detail in [37], overexpresses the invertase (SUC2) gene, the amyloglucosidase (AMG) gene, the YlARO3 gene, and the α-amylase gene. It was mainly used for fermentations in SBM-based media, as the expressed SUC2 gene enabled the hydrolysis of sucrose in SBM into glucose and fructose.
The JMY9385 strain, described in detail in [38], overexpresses the AMG gene to hydrolyze the starch in BSG into glucose monomers, which are readily consumed by Y. lipolytica.
During the construction of the Yl119 strain, two additional strains, JMY8131 and Yl117, were also generated. One approach to enhancing the pool of Ehlrich metabolites is to knock out the gene encoding the 4-hydroxyphenylpyruvate dioxygenase (4HPPD), an enzyme responsible for converting tyrosine or phenylalanine to homogentisic acid. Using a replicative Leu2 CRISPR vector [40], the 4HPPD gene was deleted from the JMY8032 strain, generating the JMY8131 strain.
To enable growth on inulin, the Kluyveromyces marxianus inulinase gene (KmINU1) was overexpressed in Y. lipolytica. The KmINU1 coding sequence, previously shown to be expressible in Y. lipolytica [41], was cloned into a JMP62-based vector carrying a hygromycin-resistance marker. Briefly, plasmid JME2730 (containing KmINU1) and the JMP62hygroEx recipient vector were digested with BamHI and AvrII, and the KmINU1 fragment was ligated into JMP62hygroEx so that expression is driven by the pTEF promoter. The final construct was confirmed by whole-plasmid sequencing. Plasmid DNA was then purified, linearized with NotI, and transformed into JMY8131 strain using the lithium acetate method, yielding Yl117 strain. Transformants were selected on YPD medium supplemented with hygromycin. To further improve Yl117 strain, the PHA2 gene was overexpressed to enhance phenylalanine production, the precursor of 2-PE. The YlPHA2 coding sequence (YALI1_B22549g) was codon-optimized by Gencust for Golden Gate cloning. Assembly was performed following Larroude et al. (2019) using the Y. lipolytica Golden Gate toolkit [42], with the 4UASptef promoter and Leu2ex as the selection marker. The plasmid was linearized with NotI and introduced into Yl117 by the lithium-acetate/DTT transformation method, yielding strain Yl119. Integration of KmINU1 and YlPHA2 was confirmed by colony PCR using Phire Plant Direct PCR Master Mix [43].
2.3. Synthetic Media and Culture Conditions
E. coli strains were cultivated in Luria Bertani medium (LB), composed of 10 g/L tryptone, 5 g/L YE and 10 g/L NaCl, with constant shaking at 180 rpm and 37 °C in a Stuart SI500 orbital incubator (Cole Parmer, Villepinte, France). For plasmid selection, LB was supplemented with kanamycin at 50 μg/mL or ampicillin at 100 μg/mL when required.
Y. lipolytica strains were grown, at 28 °C with constant shaking (180 rpm), either in rich yeast extract peptone dextrose medium (YPD) for inoculum preparation or in a minimal yeast nitrogen base medium (YNB) for selection and experimental cultures. YPD consisted of 10 g/L glucose, 10 g/L peptone and 10 g/L YE and was supplemented with hygromycin at 0.25 g/L when necessary. The YNB medium comprised 1.7 g/L YNB without amino acids and ammonium sulfate (Difco, BD, Le Pont-de-Claix, France), 5 g/L NH4Cl, 50 mM phosphate buffer at pH 6.8 and the indicated carbon source, for example 10 g/L soluble starch or 20 g/L glucose or sucrose. For selection of auxotrophic transformants after marker excision, transformants were plated on YNB medium supplemented with 0.1 g/L leucine or 0.1 g/L uracil as appropriate.
Solid media for both E. coli and Y. lipolytica were prepared by adding 15 g/L agar (Invitrogen, Saint Aubin, France) to the corresponding liquid formulations.
For long term preservation of Y. lipolytica strains, stock cultures were prepared in 500 mL baffled Erlenmeyer flasks. A single colony from a YPD agar plate was used to inoculate 100 mL of sterile rich YPD broth. The culture was incubated at 28 °C with shaking at 170 rpm for 24 h. After incubation, sterile glycerol was added to a final concentration of 25%. The culture was aliquoted into 2 mL cryotubes, which were then stored at −80 °C.
2.4. Fermentation Conditions and Growth Media Composition
The aim of these fermentations was to evaluate the production of 2-PE in media composed solely of agro-industrial by-products, without the addition of any nutrients, minerals, or complex ingredients, such as YE. Various combinations of SBM, BSG, and CR pressing extract were tested using the JMY9398, JMY9385, and Yl119 strains, respectively. The buffering agents (0.05 M Na2HPO4 and 0.05 M KH2PO4) added to each preculture were prepared and dissolved in the same medium composition as the preculture. Each preculture was inoculated with two cryotubes of the appropriate strain containing 2 mL volume each. Table 2 provides a summary of all the precultures and co-cultures prepared.

Table 2.
Summary of the precultures and co-cultures tested.
All synthetic and non-synthetic culture media and glassware were sterilized at 121 °C for 20 min using an HMC 110 V autoclave (HMC Europe GmbH, Tusling, Germany) before use. Fermentations were carried out in 500 mL Erlenmeyer flasks.
The first co-culture combined SBM and BSG. Two 100 mL precultures, each containing 44.14 g/L SBM diluted in liquid BSG, were inoculated, one with JMY9398 and the other with JMY9385.
The second co-culture combined BSG and CR pressing extracts. One 100 mL preculture of BSG pressing extract was inoculated with JMY9385, while another preculture, containing 100 mL of CR pressing extract, was inoculated with Yl119.
The third co-culture combined SBM and CR pressing extract. A preculture containing 44.14 g/L SBM was diluted in CR pressing extract and inoculated with the JMY9398 strain, while a separate preculture containing 100 mL of CR pressing extract was inoculated with Yl119.
The fourth co-culture combined the three by-products SBM, CR pressing extract, and BSG pressing extract. Three 100 mL precultures were prepared: one with BSG pressing extract inoculated with JMY9385, one with CR pressing extract inoculated with Yl119, and one containing 44.14 g/L SBM diluted in a 50/50 mixture of BSG and CR pressing extract, inoculated with JMY9398.
The SBM concentrate (800 g/L) was diluted 1:18 (v/v) in BSG or CR pressing extracts to obtain a final concentration of 44.14 g/L.
For all trials, precultures were incubated overnight at 28 °C and 170 rpm in a shaking incubator (New Brunswick Scientific C24, Edison, NJ, USA). After 24 h, 50 mL from each preculture was aseptically transferred into a new 500 mL baffled Erlenmeyer flask and mixed to create the co-culture. The precultures and co-cultures were then returned to the shaking incubator under the same conditions and cultured for an additional 216 h.
Every 24 h, a 1 mL sample was taken aseptically from each flask. Samples were centrifuged at 13,000 rpm for 15 min using a Micro Star 12 microcentrifuge (VWR, Rosny Sous Bois, France). The supernatants were collected and stored at −20 °C for further analysis.
2.5. Quantification of 2-PE
Filtered fermentation supernatants (0.45 μm pore size) were analyzed by high performance liquid chromatography (HPLC) (Agilent Technologies 1260 Infinity II, Santa Clara, CA, USA) equipped with a refractive index detector to quantify 2-PE. For 2-PE quantification the system was fitted with an XBridge C18 reversed phase column (250 mm length, 4.6 mm internal diameter, 5 μm film thickness; Waters, Milford, MA, USA) maintained at 35 °C. The mobile phase comprised 80% water and 20% acetonitrile and was delivered at 1.2 mL/min over a 20 min run time. Samples were diluted by a factor of 1.5 with the mobile phase, and 20 μL injections were used. Concentrations were determined from a calibration curve prepared with pure 2-PE (ACROS Organics, Thermo Scientific Chemicals, Geel, Belgium, 99% purity). For each condition all withdrawn samples were injected and 2-PE production was plotted over time.
2.6. Statistical Analysis
All fermentations were carried out in biological duplicate, and results are reported as the mean of two independent replicates ± standard deviation (SD). Statistical significance was evaluated by one-way analysis of variance (ANOVA) at the 95% confidence level using Microsoft Excel 2016.
3. Results and Discussion
Developing a culture medium composed solely of a mixture of agro-industrial by-products, without the addition of nutrients, minerals, or complex/derived ingredients, such as YE, is economically, environmentally, and technically advantageous. We hypothesized that combining two by-products could create a richer environment, where one complements the other’s nutrient, carbon, and nitrogen sources. This synergy could potentially establish an optimal medium for cultivating Y. lipolytica and enhance 2-PE production through de novo synthesis.
Since the complex components of agro-industrial by-products require specific enzymes for hydrolysis before yeast can assimilate them, two pre-cultures for each co-culture were first cultivated in shake flasks for 24 h and inoculated with the appropriate yeast strain. Specifically, JMY9398 was used for the SBM culture medium, JMY9385 for the BSG medium, and Yl119 for the CR pressing extract. These pre-cultures were incubated to allow each strain to reinitiate proliferation, activate metabolic pathways, and enter the exponential phase while simultaneously hydrolyzing the complex components of the medium, sucrose, starch, and inulin, respectively, into simpler sugars. After this pre-hydrolysis phase, different combinations of the pre-cultures were mixed to establish four distinct co-cultures.
3.1. Sugar Beet Molasses and Brewer’s Spent Grains Pressing Extract Mixed Culture Medium
The first co-culture consisted of the JMY9385 and JMY9398 strains grown in a mixed medium containing SBM and BSG (results shown in Figure 1). These two strains were selected because they overexpress AMG and SUC2 genes, respectively, which enable the hydrolysis of starch and sucrose into sugar monomers. Given that the optimal composition for the SBM medium contained 44.14 g/L SBM and 3.2 g/L YE [37], pre-cultures were prepared using 44.14 g/L SBM diluted in BSG. One pre-culture was inoculated with the JMY9385 strain for starch hydrolysis, while the other was inoculated with the JMY9398 strain for sucrose hydrolysis. The maximum 2-PE production in these individual pre-cultures was 0.8 g/L and 0.95 g/L, respectively, with no significant difference observed between them. However, combining both pre-cultures led to a 2-PE production of 1.69 g/L after 144 h, with a peak productivity of 0.0193 g2-PE/Lculture medium/h occurring after 24 h of co-culture incubation.
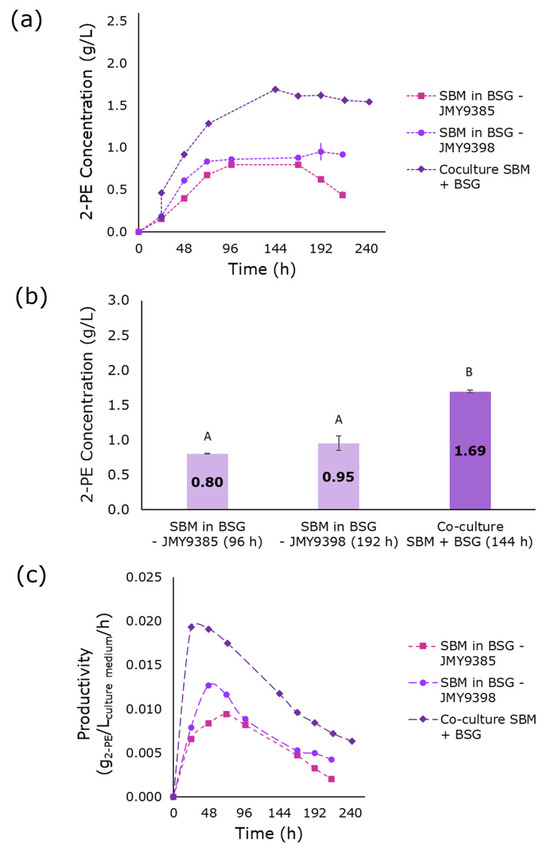
Figure 1.
2-PE production in media composed of SBM and BSG: pre-cultures and co-culture. (a) 2-PE production as a function of time. (b) Maximal production of 2-PE in the different media at various fermentation times (indicated in parentheses below each bar). (c) Volumetric productivity of 2-PE. Error bars show the standard deviation of biological replicates. Significant differences in 2-PE titers across culture media are denoted by different uppercase letters (p < 0.05). 2-PE: 2-phenylethanol; SBM: sugar beet molasses; BSG: brewer’s spent grain.
3.2. Brewer’s Spent Grains and Chicory Roots Pressing Extracts Mixed Culture Medium
The second co-culture consisted of JMY9385 and Yl119 strains grown in a mixed medium containing BSG and CR pressing extract. These two strains were selected because they overexpress AMG and INU1 genes, respectively, which enable the hydrolysis of starch and inulin into sugar monomers. As shown in Figure 2a,b the BSG pre-culture inoculated with JMY9385, expressing the AMG gene, produced a maximum of 0.72 g/L 2-PE after 168 h, with a peak productivity of 0.01 g2-PE/Lculture medium/h at 48 h (Figure 2c). Meanwhile, the CR pressing extract pre-culture resulted in a 2-PE titer of 2.32 g/L after 216 h, with a maximum productivity of 0.017 g2-PE/Lculture medium/h at 96 h. The co-culture of these two media yielded 1.71 g/L 2-PE after 145 h, with a peak productivity of 0.02 g2-PE/Lculture medium/h at 48 h. Interestingly, the 2-PE production in the co-culture was lower than that observed in the CR pressing extract pre-culture.
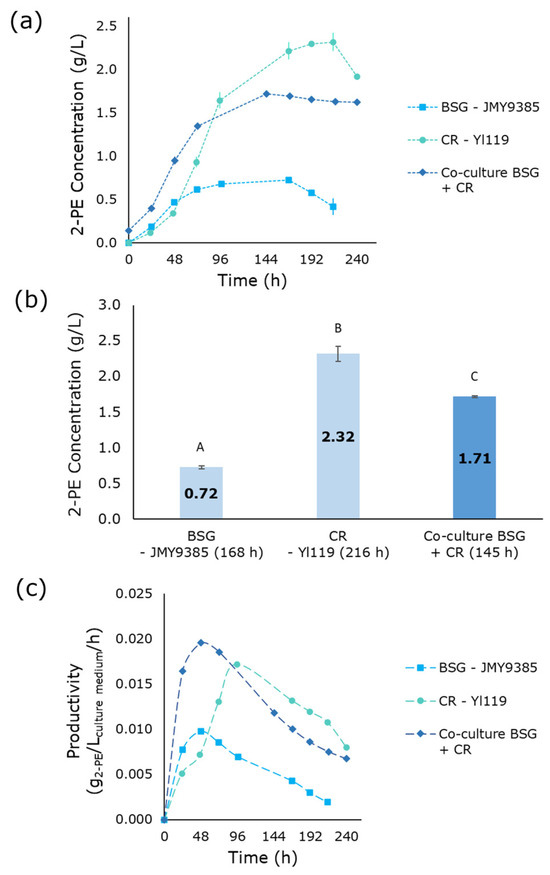
Figure 2.
2-PE production in media composed of BSG and CR: pre-cultures and co-culture. (a) 2-PE production as a function of time. (b) Maximal production of 2-PE in the different media at various fermentation times (indicated in parentheses below each bar). (c) Volumetric productivity of 2-PE. Error bars show the standard deviation of biological replicates. Significant differences in 2-PE titers across culture media are denoted by different uppercase letters (p < 0.05). 2-PE: 2-phenylethanol; BSG: brewer’s spent grain; CR: chicory roots.
Several factors could account for this outcome. First, while agro-industrial by-products provide essential nutrients, they may also contain inhibitory compounds that can negatively impact microbial growth, metabolic activity, and fermentation efficiency. Indeed, high substrate concentrations have been reported to inhibit cell growth in oleaginous yeasts such as Cryptococcus curvatus [44]. Second, the dilution of the CR pressing extract by BSG in the co-culture likely resulted in lower concentrations of readily metabolizable sugars, such as inulin and fructose, thereby reducing the productivity of the highly efficient Yl119 strain. Third, differences in substrate utilization efficiency between JMY9385 and Yl119 strains may have affected overall performance. When cultivated separately, each strain might be more efficient on its preferred substrate, whereas competition could have lowered overall efficiency in co-culture. Fourth, the initial concentrations of sugars and nitrogen sources in the fermentation medium strongly influence yeast population dynamics and metabolic performance in co-culture. Any imbalance or limitation in these nutrients may favor the dominance of one strain, leading faster resource depletion, and consequently reduced metabolite production. Moreover, during fermentation, both strains compete for essential nutrients, including nitrogen, which can alter their physiological states and shift metabolic fluxes. These interactions are often strain-dependent and can significantly affect overall 2-phenylethanol productivity [45].
Nevertheless, 2-PE productivity in the co-culture was observed to exceed that of either pre-culture alone. This suggests that the presence of multiple carbon sources and nutrients might have accelerated yeast growth and metabolism. Previous studies have reported that co-culture fermentations can lead to faster and more complete sugar depletion than monocultures, possibly due to competition between strains for substrate consumption, ultimately driving a higher overall growth rate [46].
3.3. Sugar Beet Molasses and Chicory Roots Pressing Extract Mixed Culture Medium
The third co-culture consisted of JMY9398 and Yl119 strains grown in a mixed medium containing SBM and CR pressing extract. These two strains were selected because they overexpress SUC2 and INU1 genes, respectively, which enable the hydrolysis of sucrose and inulin into sugar monomers. Fermentation in the optimized SBM medium (44.14 g/L SBM and 3.2 g/L YE) using JMY9398 resulted in 0.71 g/L 2-PE [37]. In contrast, when 44.14 g/L SBM was diluted in CR pressing extract and inoculated with JMY9398, 2.45 g/L 2-PE was produced (Figure 3a,b). The JMY9398 strain expresses the SUC2 and AMG genes, enabling the hydrolysis of sucrose and starch, respectively. However, the observed 2-PE production in this medium cannot be solely attributed to sucrose hydrolysis. It is likely that the inulin present in the CR pressing extract was also hydrolyzed and utilized by the yeast. Fructose analysis at the end of fermentation (240 h) revealed a residual fructose concentration of 229.7 ± 0.3 g/L. Previous studies have reported that S. cerevisiae invertase exhibits exo-inulinase activity, enabling the hydrolysis of inulin into fructose and glucose monomers, which can then be metabolized through glycolysis. This exo-inulinase activity varies among strains [47,48]. Overexpression of the SUC2 gene in weak inulin-fermenting strains has been shown to remarkedly increase invertase activity toward inulin, confirming that higher SUC2 expression enhances inulin degradation [49,50]. Additionally, prior fermentations in CR pressing extract yielded 2.32 g/L 2-PE after 216 h. Similarly, the SBM and CR pressing extract co-culture resulted in 2.47 g/L 2-PE production. No significant difference (p < 0.05) was observed between 2-PE production in the two pre-cultures and the co-culture. The comparable 2-PE concentrations observed across these culture media could be attributed to the compound’s toxicity to yeast cells, potentially reaching inhibitory levels. In fact, beyond a certain concentration, 2-PE starts to inhibit the growth of the producing yeast. This toxicity is species- and strain-dependent [51]. For instance, 2-PE concentrations of 2 to 3 g/L have been reported to inhibit the growth of Y. lipolytica [52]. As illustrated in Figure 3c, the co-culture exhibited the highest productivity, reaching 0.019 g2-PE/Lculture medium/h within 72 h of co-culture fermentation initiation.
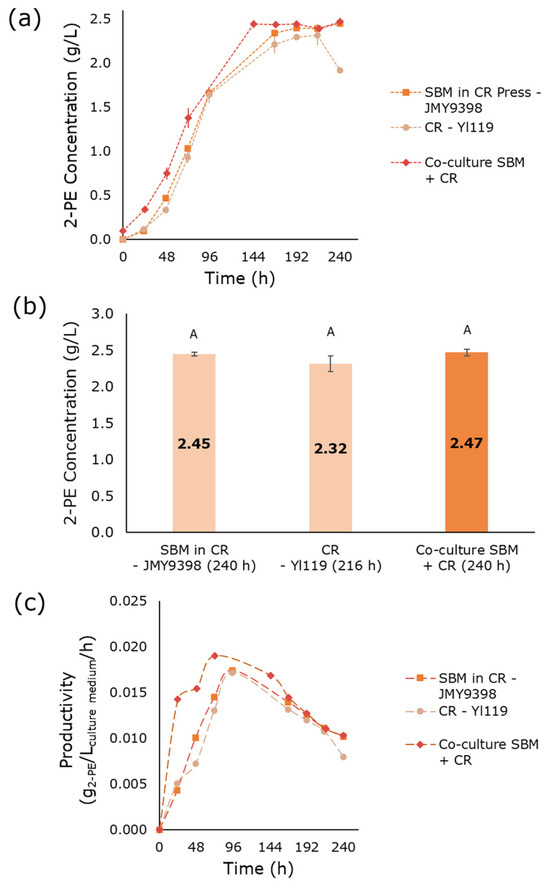
Figure 3.
2-PE production in media composed of SBM and CR: pre-cultures and co-culture. (a) 2-PE production as a function of time. (b) Maximal production of 2-PE in the different media at various fermentation times (indicated in parentheses below each bar). (c) Volumetric productivity of 2-PE. Error bars show the standard deviation of biological replicates. Significant differences in 2-PE titers across culture media are denoted by different uppercase letters (p < 0.05). 2-PE: 2-phenylethanol; SBM: sugar beet molasses; CR: chicory roots.
3.4. Mixed Culture Medium of Sugar Beet Molasses, Chicory Roots, and Brewer’s Spent Grains Pressing Extracts
The final co-culture consisted of JMY9398, Yl119, and JMY9385 strains grown in a mixed medium containing SBM, CR pressing extract, and BSG, yielding 2.29 g/L 2-PE after 240 h of starting the co-culture fermentation (Figure 4a,b). The pre-culture of SBM diluted 1:1 with CR pressing extract and BSG resulted in 2.38 g/L 2-PE after 240 h. At the end of fermentation, 106.5 ± 2.2 g/L of fructose remained in the medium. No significant difference (p < 0.05) was observed in 2-PE production between the SBM pre-culture, the CR pressing extract pre-culture, and the co-culture. However, 2-PE production was significantly lower in the BSG pre-culture, yielding 0.72 g/L. Among all pre-cultures and co-cultures, this co-culture’s highest productivity was observed, reaching 0.021 g2-PE/Lculture medium/h after 72 h (Figure 4c).
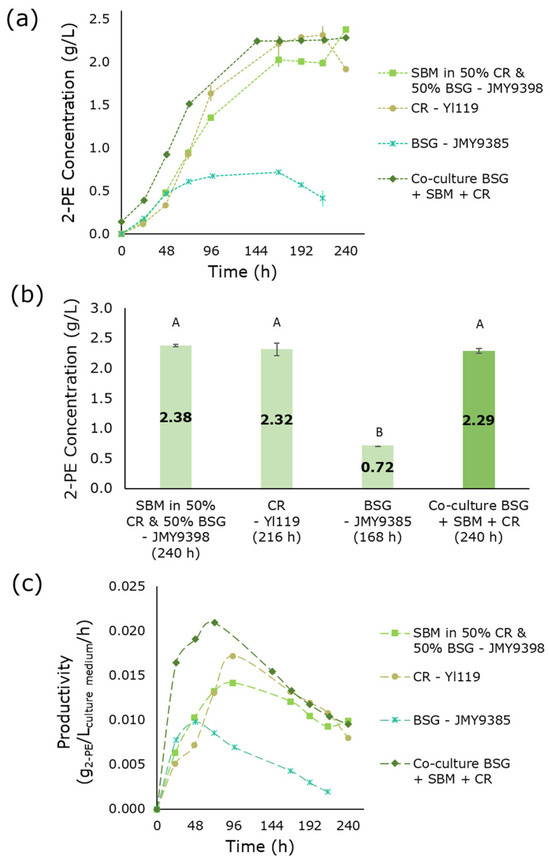
Figure 4.
2-PE production in media composed of SBM, CR, and BSG: pre-cultures and co-culture. (a) 2-PE production as a function of time. (b) Maximal production of 2-PE in the different media at various fermentation times (indicated in parentheses below each bar). (c) Volumetric productivity of 2-PE. Error bars show the standard deviation of biological replicates. Significant differences in 2-PE titers across culture media are denoted by different uppercase letters (p < 0.05). 2-PE: 2-phenylethanol; SBM: sugar beet molasses; CR: chicory roots; BSG: brewer’s spent grain.
A study by Castillo et al. (2023) investigated 2-PE production via co-culture fermentation of K. marxianus and Debaryomyces hansenii in a medium composed of cheese whey supplemented with nitrogen-rich agri-food residues, such as crab head shells, residual soy cake, and brewer’s spent yeast [53]. In flask fermentations, the highest 2-PE concentration (0.67 g/L after 48 h fermentation) was achieved in whey medium supplemented with 3 g/L L-Phe (WM), with a productivity of 17 mg2-PE/Lculture medium/h. At the bioreactor scale (2 L), the highest 2-PE production was observed in a whey medium enriched with a brewer’s spent yeast (WBSY) medium, reaching 1.8 g/L after 48 h with a productivity of 0.04 g2-PE/L/h, compared to a 2-PE production of 0.69 g/L after 31 h in WM. Notably, WBSY fermentation in the bioreactor exhibited 2.35-fold higher productivity than WM bioreactor fermentation and 12.12-fold higher productivity than WBSY flask fermentation, highlighting the critical role of aeration [53]. This finding is particularly relevant to fermentations involving the strictly aerobic yeast Y. lipolytica. Scaling up to a bioreactor with controlled aeration could enhance yeast activity, potentially leading to higher 2-PE production.
A comparison of studies on 2-PE production using Y. lipolytica was previously conducted by Mitri et al. (2024) [37]. Their analysis showed that most studies to date using this yeast have employed either synthetic media or media supplemented with the precursor [37].
In contrast, comparing the 2-PE yields obtained in this study with those reported in the literature under similar conditions, such as flask-scale or fed-batch fermentations using yeasts and agro-industrial by-products as substrates, is challenging. Most reported studies, whether using Y. lipolytica or other yeast and fungi species, typically rely on synthetic media or supplement agro-industrial by-product-based media with L-Phe, often under batch or fed-batch fermentation conditions. Table 3 summarizes several studies that produced 2-PE using agro-industrial by-products. This comparison highlights the novelty of the present work, which demonstrates 2-PE production by Y. lipolytica strains in mixed-culture media, primarily through de novo synthesis and without supplementing the medium with the precursor L-Phe. Such an approach is rare among published studies.

Table 3.
Summary of 2-PE production through fungal fermentation using agro-industrial by-product substrates.
Figure 5 summarizes the maximum productivities achieved across six pre-cultures and the four co-cultures. The highest productivities were observed in the co-cultures, reaching approximately 0.02 g2-PE/Lculture medium/h. This was followed by the SBM diluted in CR pressing extract and the CR pressing extract medium, both exceeding 0.017 g2-PE/Lculture medium/h.
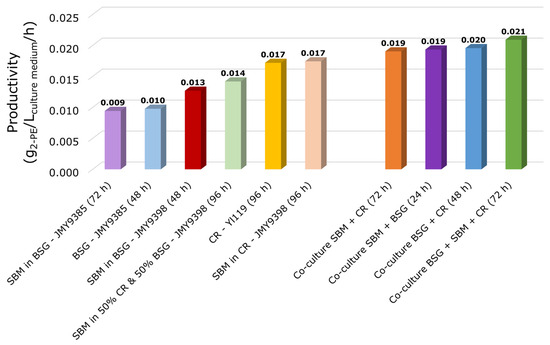
Figure 5.
Maximum 2-PE productivity in different pre-cultures and co-culture. Peak times are indicated in parentheses below each bar. 2-PE: 2-phenylethanol; SBM: sugar beet molasses; CR: chicory roots; CR: chicory roots pressing extract; BSG: brewer’s spent grain; CG: crude glycerol; YE: yeast extract.
A previous study demonstrated that 2-PE production is enhanced when brewer’s spent yeast is used as a renewable nitrogen source for media supplementation rather than YE or peptone [53], reinforcing the potential of sustainable microbial fermentation for value-added compound production.
Co-culture fermentation using Y. lipolytica and agro-industrial by-products presents a promising approach for the sustainable bioproduction of 2-PE. However, the current co-culture experiments represent only an initial step in exploring this strategy. This study did not include detailed monitoring of yeast growth dynamics, elemental characterization of the agro-industrial by-products, assessment of potential substrate inhibition, or an evaluation of substrate utilization over the course of fermentation, which limits the understanding of microbial interactions and fermentation efficiency. Future research should aim to optimize fermentation conditions and investigate different combinations of agro-industrial by-products to further enhance 2-PE production. Conducting additional control experiments and systematically monitoring yeast growth and sugar consumption will provide deeper insights into the underlying biological processes. Moreover, scaling up the co-culture system to a bioreactor with controlled parameters, particularly oxygenation, could substantially improve 2-PE synthesis. Refining these aspects will contribute to the development of a cost-effective, scalable, and sustainable bioprocess for industrial 2-PE production.
4. Conclusions
This paper demonstrated the potential of agro-industrial by-products as substrates for cost-effective 2-PE production, addressing the need for a sustainable, economical, and environmentally friendly bioprocess for the de novo synthesis of 2-PE in Y. lipolytica. Co-substrate fermentations were carried out using mixed agro-industrial by-product media and a co-culture of Y. lipolytica strains, resulting in the production of 2.5 g/L of 2-PE in a medium composed of a mixture of sugar beet molasses and chicory root pressing extract. This represents one of the highest values reported in the literature for de novo 2-PE synthesis in Y. lipolytica, achieved without supplementation of either the precursor or synthetic/complex ingredients.
Author Contributions
Conceptualization, M.K. and N.L.; methodology, S.M. and T.R.; validation, N.L. and M.K.; formal analysis, N.L., M.K., T.R. and S.M.; investigation, S.M., N.L., T.R. and M.K.; resources, M.K.; data curation, N.L.; writing—original draft preparation, S.M.; writing—review and editing, N.L., M.K., T.R., R.G.M. and S.M.; visualization, S.M., N.L. and M.K.; supervision, M.K., R.G.M., T.R. and N.L.; project administration, M.K. and N.L.; funding acquisition, M.K., R.G.M., N.L. and T.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education, Research and Innovation (France), by The Research Council of the Saint Joseph University of Beirut, FS-165 (Lebanon), and by Ecole Supérieure de Chimie Organique et Minérale (Compiègne, France).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2-PE | 2-Phenylethanol |
| SBM | Sugar beet molasses |
| BSG | Brewer’s spent grains |
| CR | Chicory roots |
| CG | Crude glycerol |
| YE | Yeast extract |
| HPLC | High performance liquid chromatography |
| AAA | Aromatic amino acid |
| AMG | Amyloglucosidase |
| 4HPPD | 4-Hydroxyphenylpyruvate dioxygenase |
| LB | Luria Bertani |
| YPD | Yeast Peptone Dextrose |
| YNB | Yeast Nitrogen Base |
| L-Phe | L-Phenylalanine |
References
- Karataş, E. Overview of Yarrowia lipolytica: History, Taxonomy, Characteristics, and Reproduction. In Yarrowia lipolytica Yeast: From Metabolic Engineering to Biotechnological Applications; Koubaa, M., Mitri, S., Louka, N., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 1–32. [Google Scholar]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A Beneficious Yeast in Biotechnology as a Rare Opportunistic Fungal Pathogen: A Minireview. World J. Microbiol. Biotechnol. 2019, 35, 10. [Google Scholar] [CrossRef]
- López-Trujillo, J.; Mellado-Bosque, M.; Ascacio-Valdés, J.A.; Prado-Barragán, L.A.; Hernández-Herrera, J.A.; Aguilera-Carbó, A.F. Temperature and PH Optimization for Protease Production Fermented by Yarrowia lipolytica from Agro-Industrial Waste. Fermentation 2023, 9, 819. [Google Scholar] [CrossRef]
- Park, Y.K.; Ledesma-Amaro, R. What Makes Yarrowia lipolytica Well Suited for Industry? Trends Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Li, Z.; Wang, X.; Gao, R.; Zhou, X.; Cheng, S.; Men, Y.; Zheng, L. Approaches to Improve the Lipid Synthesis of Oleaginous Yeast Yarrowia lipolytica: A Review. Renew. Sustain. Energy Rev. 2021, 149, 111386. [Google Scholar] [CrossRef]
- Vandermies, M.; Fickers, P. Bioreactor-Scale Strategies for the Production of Recombinant Protein in the Yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef]
- Carsanba, E.; Agirman, B.; Papanikolaou, S.; Fickers, P.; Erten, H. Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation. Fermentation 2023, 9, 687. [Google Scholar] [CrossRef]
- Deken, R.H. The Crabtree Effect: A Regulatory System in Yeast. J. Gen. Microbiol. 1966, 44, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Mesquita, D.P.; Cordeiro, A.; Belo, I.; Ferreira, E.C.; Amaral, A.L. Monitoring Biotechnological Processes through Quantitative Image Analysis: Application to 2-Phenylethanol Production by Yarrowia lipolytica. Process Biochem. 2023, 129, 221–229. [Google Scholar] [CrossRef]
- Dias, B.; Fernandes, H.; Belo, I.; Lopes, M. Exploring Lipids and Citric Acid Production by Yarrowia lipolytica in Lignocellulosic Biomass Hydrolysate-Mimicking Media: Effect of Cultivation Operation Modes. Process Biochem. 2025, 159, 25–32. [Google Scholar] [CrossRef]
- Naveira-Pazos, C.; Veiga, M.C.; Kennes, C. Synthetic Media, Agro-Industrial by-Products, and Culture Conditions Used in Biotechnological Applications of Yarrowia lipolytica. In Yarrowia lipolytica Yeast: From Metabolic Engineering to Biotechnological Applications; Koubaa, M., Mitri, S., Louka, N., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 165–210. [Google Scholar]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Theodosiou, E. Engineering Strategies for Efficient Bioconversion of Glycerol to Value-Added Products by Yarrowia lipolytica. Catalysts 2023, 13, 657. [Google Scholar] [CrossRef]
- Darvishi, F.; Ariana, M.; Marella, E.R.; Borodina, I. Advances in Synthetic Biology of Oleaginous Yeast Yarrowia lipolytica for Producing Non-Native Chemicals. Appl. Microbiol. Biotechnol. 2018, 102, 5925–5938. [Google Scholar] [CrossRef]
- de Lima, L.A.; Ventorim, R.Z.; Bianchini, I.A.; de Queiroz, M.V.; Fietto, L.G.; da Silveira, W.B. Obtainment, Selection and Characterization of a Mutant Strain of Kluyveromyces marxianus That Displays Improved Production of 2-Phenylethanol and Enhanced DAHP Synthase Activity. J. Appl. Microbiol. 2021, 130, 878–890. [Google Scholar] [CrossRef]
- Yan, W.; Gao, H.; Jiang, W.; Jiang, Y.; Lin, C.S.K.; Zhang, W.; Xin, F.; Jiang, M. The De Novo Synthesis of 2-Phenylethanol from Glucose by the Synthetic Microbial Consortium Composed of Engineered Escherichia coli and Meyerozyma guilliermondii. ACS Synth. Biol. 2022, 11, 4018–4030. [Google Scholar] [CrossRef]
- Braga, A.; Freitas, B.; Cordeiro, A.; Belo, I. Valorization of Crude Glycerol as Carbon Source for the Bioconversion of L-Phenylamine to 2-Phenylethanol by Yarrowia Species. J. Chem. Technol. Biotechnol. 2021, 96, 2940–2949. [Google Scholar] [CrossRef]
- Kong, S.; Pan, H.; Liu, X.; Li, X.; Guo, D. De Novo Biosynthesis of 2-Phenylethanol in Engineered Pichia pastoris. Enzyme Microb. Technol. 2020, 133, 109459. [Google Scholar] [CrossRef]
- Souza, M.A.d.; Vilas-Boas, I.T.; Leite-da-Silva, J.M.; Abrahão, P.d.N.; Teixeira-Costa, B.E.; Veiga-Junior, V.F. Polysaccharides in Agro-Industrial Biomass Residues. Polysaccharides 2022, 3, 95–120. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Ramos, C.L.; Kuddus, M.; Rodriguez-Couto, S.; Srivastava, N.; Ramteke, P.W.; Mishra, P.K.; Molina, G. Enzymatic Potential for the Valorization of Agro-Industrial by-Products. Biotechnol. Lett. 2020, 42, 1799–1827. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From Waste to Sustainable Industry: How Can Agro-Industrial Wastes Help in the Development of New Products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Makambai kyzy, A.; Mazhitova, A. Biotechnological Valorization of Sugar Beet Wastes into Value-Added Products. MANAS J. Eng. 2023, 11, 136–144. [Google Scholar] [CrossRef]
- Schmid, M.T.; Song, H.; Raschbauer, M.; Emerstorfer, F.; Omann, M.; Stelzer, F.; Neureiter, M. Utilization of Desugarized Sugar Beet Molasses for the Production of Poly(3-Hydroxybutyrate) by Halophilic Bacillus megaterium Uyuni S29. Process Biochem. 2019, 86, 9–15. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial Production of Value-Added Bioproducts and Enzymes from Molasses, a by-Product of Sugar Industry. Food Chem. 2021, 346, 128860. [Google Scholar] [CrossRef]
- El Kantar, S.; Koubaa, M. Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 284. [Google Scholar] [CrossRef]
- Oliver, G.; Colicchio, T. The Oxford Companion to Beer; The Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of Brewers’ Spent Grain for the Production of Lipids by Oleaginous Yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Brewing Process Development by Integration of Edible Filamentous Fungi to Upgrade the Quality of Brewer’s Spent Grain (BSG). BioResources 2021, 16, 1686. [Google Scholar] [CrossRef]
- Tsouko, E.; Pilafidis, S.; Dimopoulou, M.; Kourmentza, K.; Sarris, D. Bioconversion of Underutilized Brewing By-Products into Bacterial Cellulose by a Newly Isolated Komagataeibacter rhaeticus Strain: A Preliminary Evaluation of the Bioprocess Environmental Impact. Bioresour. Technol. 2023, 387, 129667. [Google Scholar] [CrossRef] [PubMed]
- Pabbathi, N.P.P.; Velidandi, A.; Pogula, S.; Gandam, P.K.; Baadhe, R.R.; Sharma, M.; Sirohi, R.; Thakur, V.K.; Gupta, V.K. Brewer’s Spent Grains-Based Biorefineries: A Critical Review. Fuel 2022, 317, 123435. [Google Scholar] [CrossRef]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a Food Ingredient—Nutritional Composition, Bioactivity, Safety, and Health Claims: A Review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- Massoud, M.; Amin, W.; Elgindy, A.A. Chemical and Technological Studies on Chicory (Cichorium intybus L.) and Its Applications in Some Functional Food. J. Adv. Agric. Res. Fac. Ag. Saba Basha 2009, 14, 735–755. [Google Scholar]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 1, 7343928. [Google Scholar] [CrossRef]
- Zhu, Z.; Bals, O.; Grimi, N.; Vorobiev, E. Pilot Scale Inulin Extraction from Chicory Roots Assisted by Pulsed Electric Fields. Int. J. Food Sci. Technol. 2012, 47, 1361–1368. [Google Scholar] [CrossRef]
- Grigs, O.; Didrihsone, E.; Bolmanis, E. Investigation of a Broad-Bean Based Low-Cost Medium Formulation for Bacillus subtilis MSCL 897 Spore Production. Fermentation 2023, 9, 390. [Google Scholar] [CrossRef]
- Mitri, S.; Louka, N.; Rossignol, T.; Maroun, R.G.; Koubaa, M. Bioproduction of 2-Phenylethanol by Yarrowia lipolytica on Sugar Beet Molasses as a Low-Cost Substrate. Fermentation 2024, 10, 290. [Google Scholar] [CrossRef]
- Mitri, S.; Louka, N.; Rossignol, T.; Maroun, R.G.; Koubaa, M. Brewer’s Spent Grain and Crude Glycerol: Sustainable Substrates for 2-Phenylethanol Production by Yarrowia lipolytica. Futur. Foods 2025, 11, 100569. [Google Scholar] [CrossRef]
- Larroude, M.; Nicaud, J.M.; Rossignol, T. Yarrowia lipolytica Chassis Strains Engineered to Produce Aromatic Amino Acids via the Shikimate Pathway. Microb. Biotechnol. 2021, 14, 2420–2434. [Google Scholar] [CrossRef] [PubMed]
- Larroude, M.; Onésime, D.; Rué, O.; Nicaud, J.M.; Rossignol, T. A Yarrowia lipolytica Strain Engineered for Pyomelanin Production. Microorganisms 2021, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Hapeta, P.; Rakicka, M.; Dulermo, R.; Gamboa-Meléndez, H.; Cruz-Le Coq, A.M.; Nicaud, J.M.; Lazar, Z. Transforming Sugars into Fat—Lipid Biosynthesis Using Different Sugars in Yarrowia lipolytica. Yeast 2017, 34, 293–304. [Google Scholar] [CrossRef]
- Larroude, M.; Park, Y.K.; Soudier, P.; Kubiak, M.; Nicaud, J.M.; Rossignol, T. A Modular Golden Gate Toolkit for Yarrowia lipolytica Synthetic Biology. Microb. Biotechnol. 2019, 12, 1249–1259. [Google Scholar] [CrossRef]
- Park, Y.K.; Sellés Vidal, L.; Bell, D.; Zabret, J.; Soldat, M.; Kavšček, M.; Ledesma-Amaro, R. Efficient Synthesis of Limonene Production in Yarrowia lipolytica by Combinatorial Engineering Strategies. Biotechnol. Biofuels Bioprod. 2024, 17, 94. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting Crude Glycerol Derived from Yellow Grease to Lipids through Yeast Fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Bordet, F.; Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Yeast-Yeast Interactions: Mechanisms, Methodologies and Impact on Composition. Microorganisms 2020, 8, 600. [Google Scholar] [CrossRef]
- Valdez Castillo, M.; Tahmasbi, H.; Pachapur, V.L.; Brar, S.K.; Vuckovic, D.; Sitnikov, D.; Arriaga, S.; Blais, J.F.; Avalos Ramirez, A. Production of Aroma and Flavor-Rich Fusel Alcohols by Cheese Whey Fermentation Using the Kluyveromyces marxianus and Debaryomyces hansenii Yeasts in Monoculture and Co-Culture Modes. J. Chem. Technol. Biotechnol. 2021, 96, 2354–2367. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Z.C.; Wang, X.; Li, L.L.; Yang, L.; Tang, W.Z.; Yu, Z.M.; Li, X. Invertase Suc2-Mediated Inulin Catabolism Is Regulated at the Transcript Level in Saccharomyces cerevisiae. Microb. Cell Fact. 2015, 14, 59. [Google Scholar] [CrossRef]
- Wang, S.A.; Li, F.L. Invertase SUC2 Is the Key Hydrolase for Inulin Degradation in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2013, 79, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, W.J.; Liu, N.N.; Tan, M.J.; Liu, G.L.; Chi, Z.M. Role of SUC2 Gene and Invertase of Saccharomyces Sp. W0 in Inulin Hydrolysis. J. Mol. Catal. B Enzym. 2015, 111, 71–78. [Google Scholar] [CrossRef]
- Naumov, G.I.; Naumova, E.S. Invertase Overproduction May Provide for Inulin Fermentation by Selection Strains of Saccharomyces cerevisiae. Microbiology 2015, 84, 130–134. [Google Scholar] [CrossRef]
- Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.M.; Louka, N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods 2022, 11, 109. [Google Scholar] [CrossRef]
- Braga, A.; Oliveira, A.; Freitas, B.; Nagy, E.; Nguyen, D.Q.; Belo, I. Yarrowia lipolytica as a Potential Producer of 2-Phenylethanol from L-Phenylalanine Biotransformation. In Proceedings of the 13th International Chemical and Biological Engineering Conference—CHEMPOR, Aveiro, Portugal, 2–4 October 2018; Volume 2018, pp. 41–42. [Google Scholar]
- Valdez Castillo, M.; Brar, S.K.; Arriaga, S.; Blais, J.F.; Heitz, M.; Avalos Ramirez, A. Co-Fermentation of Agri-Food Residues Using a Co-Culture of Yeasts as a New Bioprocess to Produce 2-Phenylethanol. Molecules 2023, 28, 5536. [Google Scholar] [CrossRef]
- Alonso-Vargas, M.; Téllez-Jurado, A.; Gómez-Aldapa, C.A.; Ramírez-Vargas, M.D.R.; Conde-Báez, L.; Castro-Rosas, J.; Cadena-Ramírez, A. Optimization of 2-Phenylethanol Production from Sweet Whey Fermentation Using Kluyveromyces marxianus. Fermentation 2022, 8, 39. [Google Scholar] [CrossRef]
- Drężek, K.; Kozłowska, J.; Detman, A.; Mierzejewska, J. Development of a Continuous System for 2-Phenylethanol Bioproduction by Yeast on Whey Permeate-Based Medium. Molecules 2021, 26, 7388. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewska, J.; Dąbkowska, K.; Chreptowicz, K.; Sokołowska, A. Hydrolyzed Corn Stover as a Promising Feedstock for 2-Phenylethanol Production by Nonconventional Yeast. J. Chem. Technol. Biotechnol. 2019, 94, 777–784. [Google Scholar] [CrossRef]
- Tong, Q.; Yang, L.; Zhang, J.; Zhang, Y.; Jiang, Y.; Liu, X.; Deng, Y. Comprehensive Investigations of 2-Phenylethanol Production by the Filamentous Fungus Annulohypoxylon stygium. Appl. Microbiol. Biotechnol. 2024, 108, 374. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).