Meta-Analysis of Incorporating Camelina and Its By-Products into Ruminant Diets and Their Effects on Ruminal Fermentation, Methane Emissions, Milk Yield and Composition, and Metabolic Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Risk of Bias Assesment
2.6. Statistical Analyses
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
3.4. Effects of Camelina on Ruminant Performance
3.4.1. Dry Matter Intake (DMI)
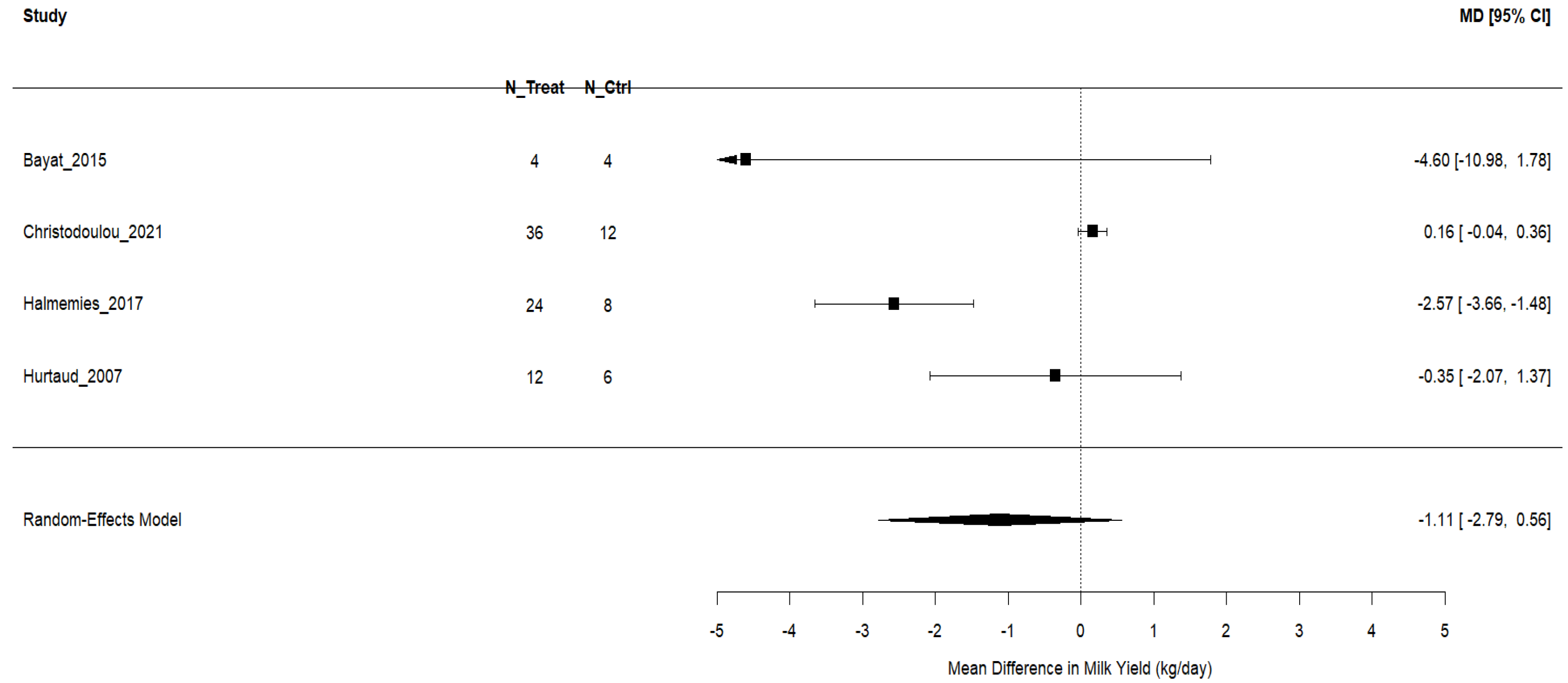
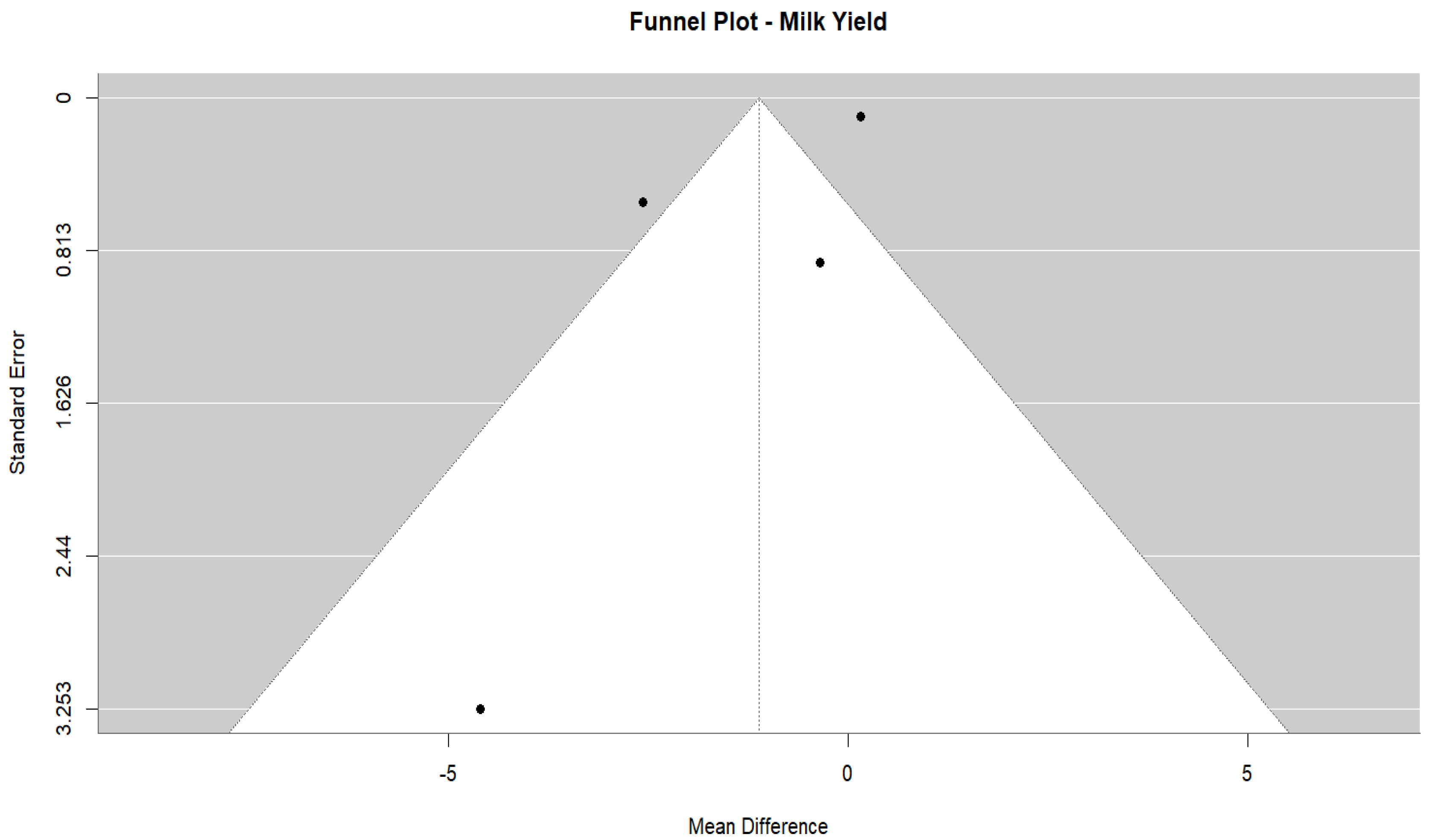
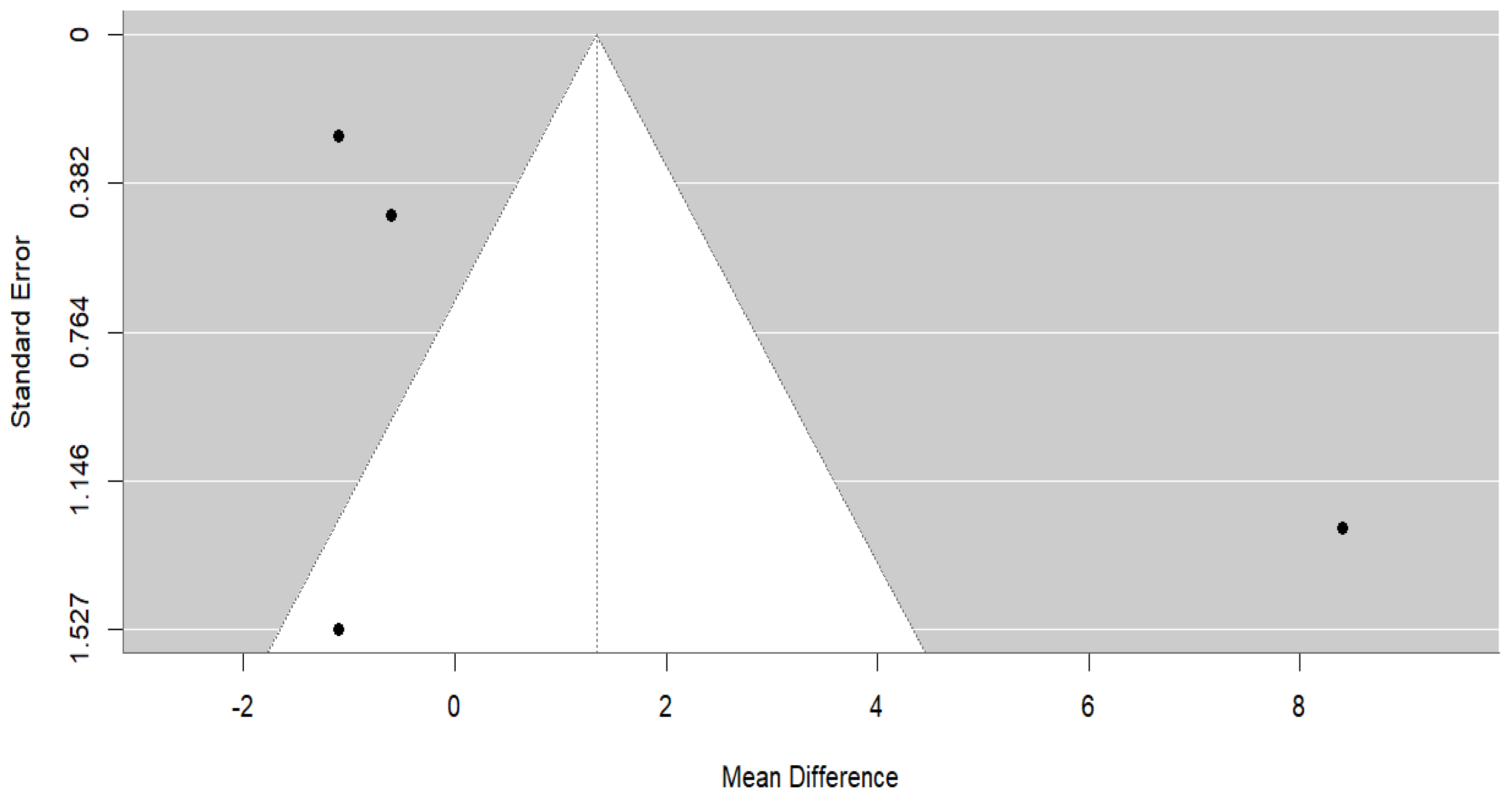
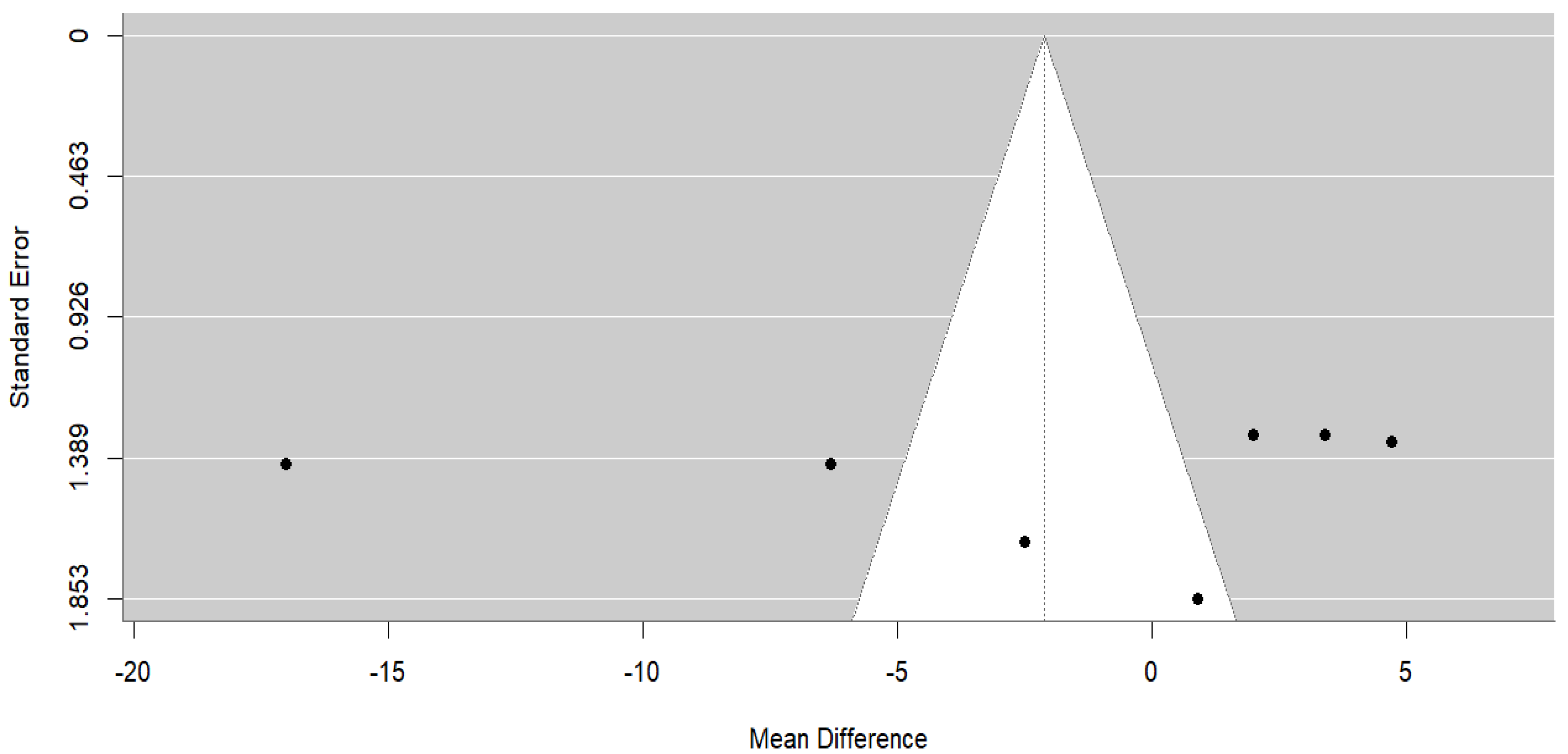
3.4.2. Milk Yield
3.5. Effects of Camelina on Milk Composition
3.5.1. Milk Protein
3.5.2. Milk Fat
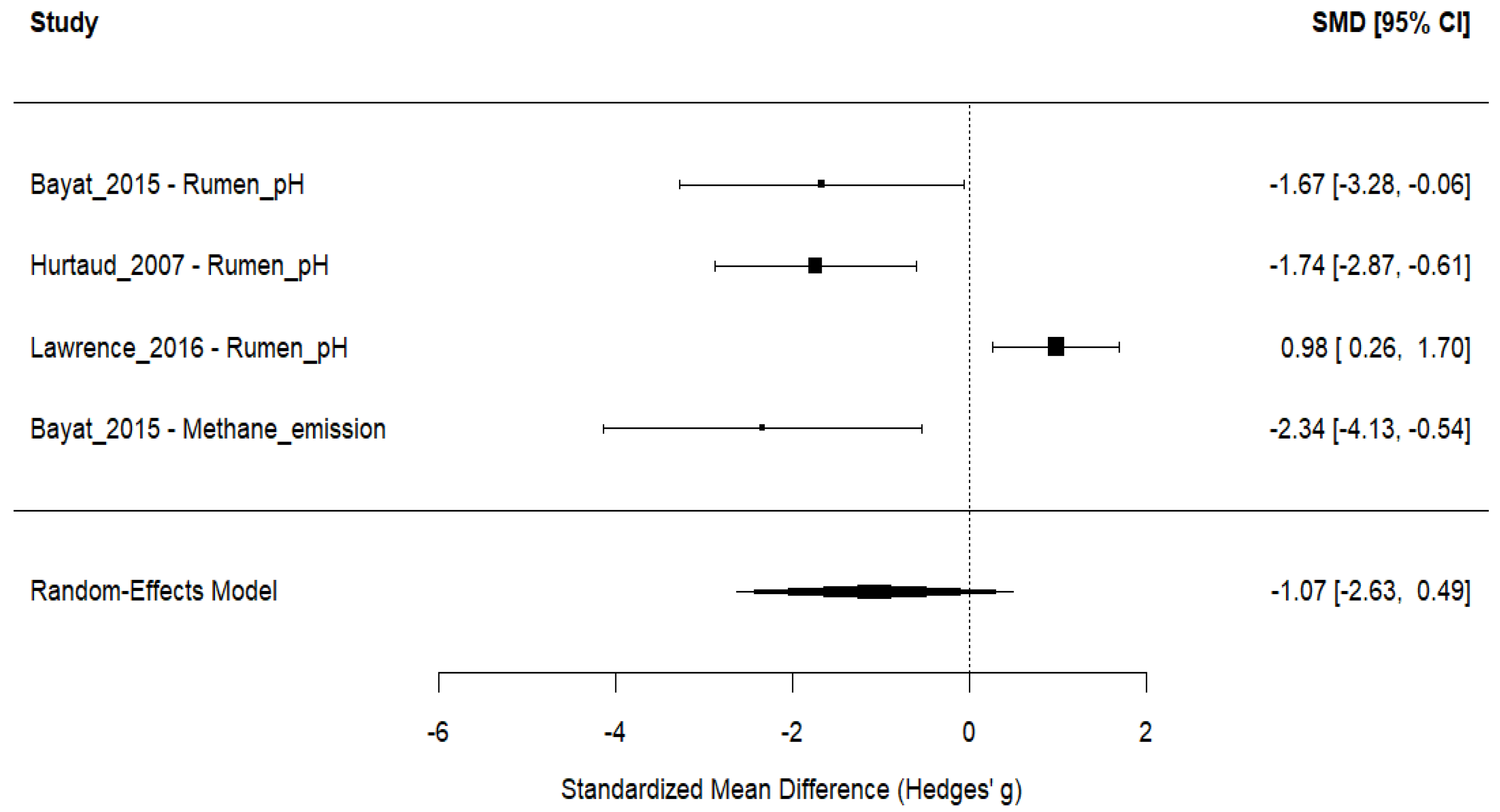
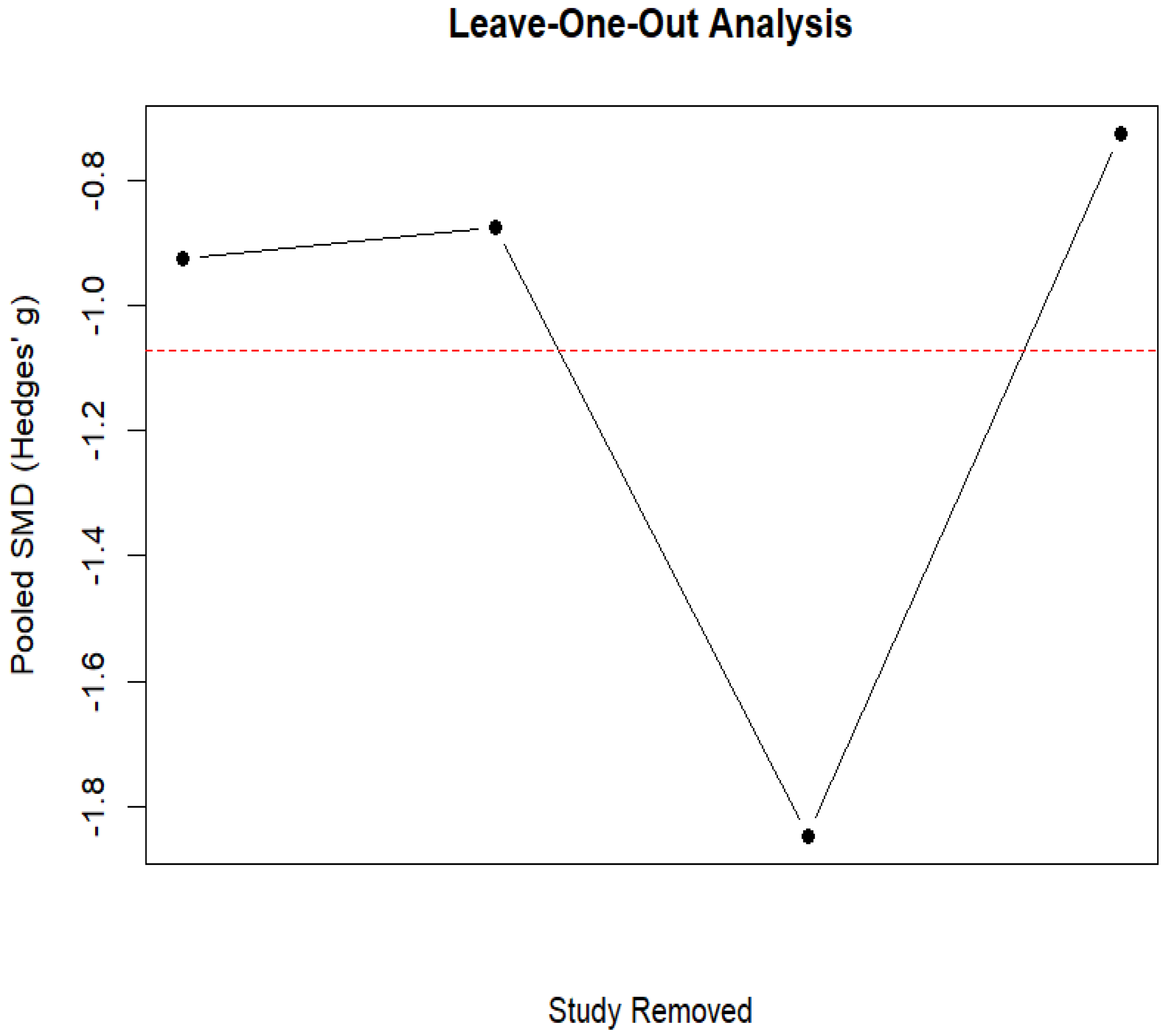
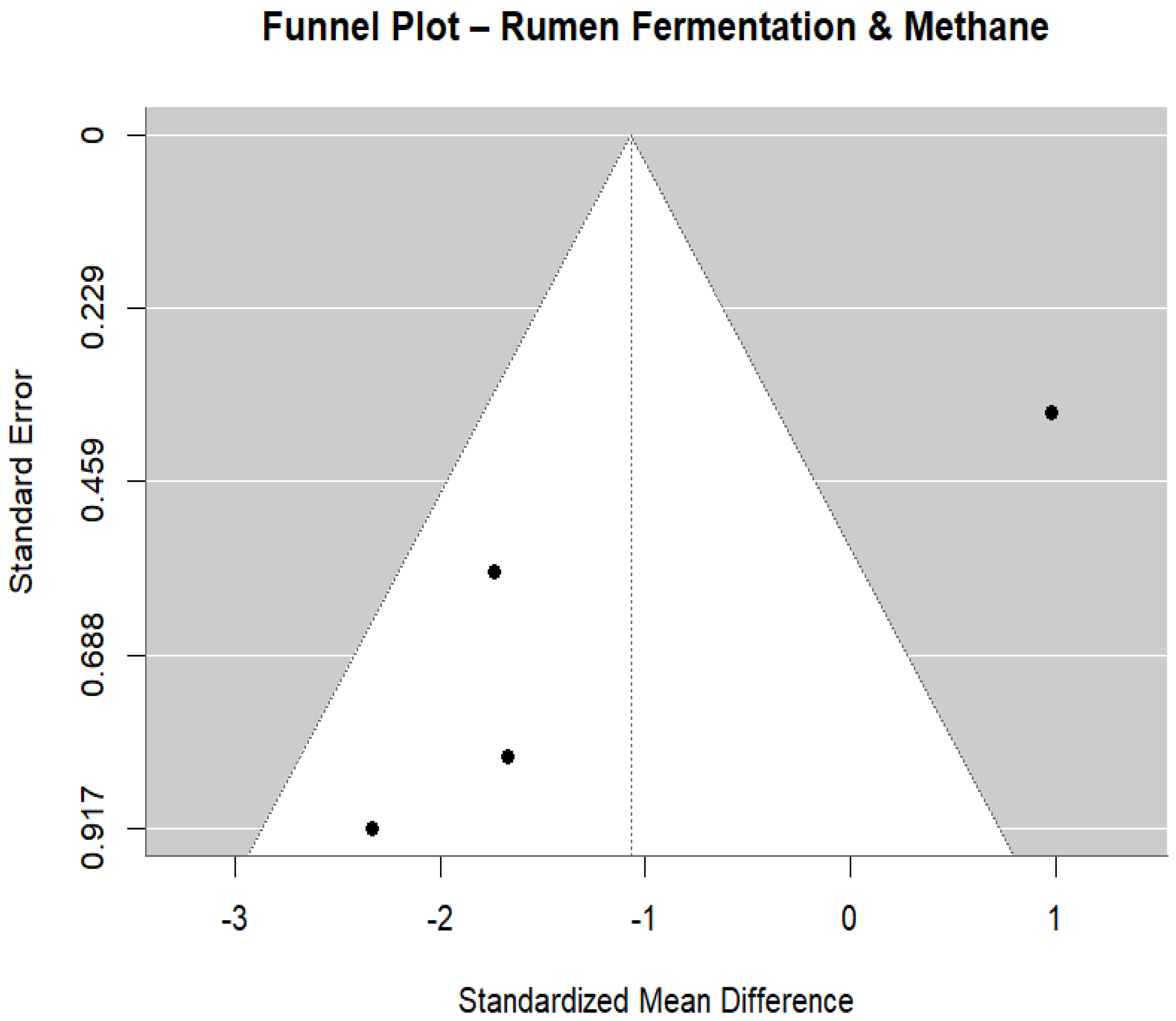
3.6. Ruminant Fermentation and Methane Emissions
3.7. Effects of Camelina on Biochemical Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalk, D.L.; Kemp, D.R.; Badgery, W.B.; Wu, J.; Zhang, Y.; Thomassin, P.J. Sustainability and future food security—A global perspective for livestock production. Land Degrad. Dev. 2019, 30, 561–573. [Google Scholar] [CrossRef]
- Vastolo, A.; Serrapica, F.; Cavallini, D.; Fusaro, I.; Atzori, A.S.; Todaro, M. Alternative and novel livestock feed: Reducing environmental impact. Front. Vet. Sci. 2024, 11, 1441905. [Google Scholar] [CrossRef]
- Rashid, M.; Archimede, H. Utilization Prospects and Limitation of Non-Conventional Feed Resources in Ruminant Ration. Ph.D. Thesis, Université des Antilles, Pointe-à-Pitre, France, 2024. [Google Scholar]
- Raza, M.A.; Din, A.M.U.; Yasin, H.S.; Gul, H.; Shah, G.A.; Zhiqi, W.; Hassan, M.J.; Bilal, M.; Gitari, H.; Iqbal, R.; et al. Maize/legume intercropping increases nutrient uptake, crop yields, land productivity, and economic profits in resource-intensive arid-irrigated areas. J. Soil Sci. Plant Nutr. 2025, 25, 8316–8332. [Google Scholar] [CrossRef]
- Iheanacho, S.; Hornburg, S.C.; Schulz, C.; Kaiser, F. Toward resilient aquaculture in Africa: Innovative and sustainable aquafeeds through alternative protein sources. Rev. Aquac. 2025, 17, e13009. [Google Scholar] [CrossRef]
- Liu, D.; Jin, J.; Qiu, X.; He, R.; Li, L.; Yang, J. Toward crop–livestock integration: A comprehensive framework for cropping system adaptation assessment to mitigate forage shortage. Environ. Dev. Sustain. 2024, in press. [Google Scholar] [CrossRef]
- Hassan, M.U.; Vastolo, A.; Gannuscio, R.; Maniaci, G.; Mancuso, I.; Calabro, S.; Gallo, A.; Todaro, M.; Cutrignelli, M.I. Effects of feeding prickly pear by-product silage as a partial replacement of concentrate on dairy ewes: Milk characteristics, nutrient utilisation and in vitro ruminal fermentation. Anim. Feed. Sci. Technol. 2025, 324, 116330. [Google Scholar] [CrossRef]
- Malenica, D.; Kass, M.; Bhat, R. Sustainable management and valorization of agri-food industrial wastes and by-products as animal feed: For ruminants, non-ruminants and as poultry feed. Sustainability 2022, 15, 117. [Google Scholar] [CrossRef]
- Sime, A.G.; Duguma, B.S. The Role of Non-Conventional Feeds in Small Ruminant Production and Their Implication for Feed Cost Reduction in Ethiopia—A Mini Review. Vet. Med. Sci. 2025, 11, e70554. [Google Scholar] [CrossRef] [PubMed]
- Azzahra, D.; Husada, C.P.; Yanto, E. Nutritional Evaluation of Fermented Agro-Industrial Byproducts as Alternative Feed Ingredients for Sustainable Livestock Production. Feed Nutr. Res. J. 2025, 1, 1–9. [Google Scholar]
- Oancea, A.-G.; Dragomir, C.; Cismileanu, A. The effects of minor oilseeds cakes on rumen metabolism and productive performances of ruminants. Arch. Zootech. 2022, 25, 130–157. [Google Scholar] [CrossRef]
- Bilal Sadiq, M. Oilseed Meal Proteins; A Functional and Nutritional Food Ingredient. J. Culin. Sci. Technol. 2025, 23, 157–160. [Google Scholar] [CrossRef]
- Ahmed, I.; Riaz, R.; Sizmaz, Ö. Sustainable Livestock Farming with Oil Seed Crops and Their By-Products. Ank. Üniv. Vet. Fak. Derg. 2024, 71, 371–383. [Google Scholar] [CrossRef]
- Faizan, M. Enteric methane production in ruminants: Its effect on global warming and mitigation strategies—A review. Pak. J. Sci. 2024, 76, 16–38. [Google Scholar]
- Hristov, A.N.; Solomon, S. Perspective: Enteric methane mitigation and its impact on livestock hydrogen emissions. J. Dairy Sci. 2025, 108, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Palangi, V.; Lackner, M. Management of enteric methane emissions in ruminants using feed additives: A review. Animals 2022, 12, 3452. [Google Scholar] [CrossRef]
- Serbester, U. The role of feed additives in enhancing ruminant performance and sustainability. In Animal Feeds and Additives; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Elwakeel, E.A.; El-Zarkouny, S.Z.; Al-Sagheer, A.A. Environmental impact of phytobiotic additives on greenhouse gas emission reduction, rumen fermentation manipulation, and performance in ruminants: An updated review. Environ. Sci. Pollut. Res. 2024, 31, 37943–37962. [Google Scholar] [CrossRef]
- de Ondarza, M.B.; de Souza, V.C.; Kebreab, E.; Tricarico, J.M. Understanding potential opportunities and risks associated with feeding supplemental rumen available fats to mitigate enteric methane emissions in lactating dairy cows. J. Dairy Sci. 2024, 107, 8072–8083. [Google Scholar] [CrossRef] [PubMed]
- Youngmark, E.C.; Kraft, J. Milk Fatty Acids as Potential Biomarkers of Enteric Methane Emissions in Dairy Cattle: A Review. Animals 2025, 15, 2212. [Google Scholar] [CrossRef] [PubMed]
- Carta, S.; Correddu, F.; Steri, R.; Zilio, D.M.; Cesarani, A.; Pulina, G.; Nudda, A. Effect of grape pomace supplementation in mid-lactation dairy ewes on production and quality of milk and methane emissions. J. Anim. Sci. 2025, 103, skaf237. [Google Scholar] [CrossRef]
- Mercês, Z.d.C.d.; Salvadori, N.M.; Evangelista, S.M.; Cochlar, T.B.; Strasburg, V.J.; da Silva, V.L.; Oliveira, V.R.d. Effectiveness, challenges, and environmental impacts of new food strategies with plant and animal protein products. Foods 2024, 13, 3217. [Google Scholar] [CrossRef]
- Ghidoli, M.; Ponzoni, E.; Araniti, F.; Miglio, D.; Pilu, R. Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges. Plants 2023, 12, 570. [Google Scholar] [CrossRef]
- Riaz, R.; Ahmed, I.; Sizmaz, O.; Ahsan, U. Use of Camelina sativa and by-products in diets for dairy cows: A Review. Animals 2022, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Waraich, E.A.; Hafeez, M.B.; Zulfiqar, U.; Ahmad, Z.; Iqbal, M.A.; Raza, A.; Slam, M.S.; Rehman, A.; Younis, U.; et al. Changing climate scenario: Perspectives of Camelina sativa as low-input biofuel and oilseed crop. In Global Agricultural Production: Resilience to Climate Change; Springer: Cham, Switzerland, 2023; pp. 197–236. [Google Scholar]
- Kidane, B.; Hunde, W.; Urugo, M.M.; Tessema, F.; Teka, T.A. Camelina Oil as Ethiopia’s Next Edible Oil: Insights into Chemical Composition and Agro-Ecological Adaptability-A Review. J. Oleo Sci. 2025, 74, 655–666. [Google Scholar] [CrossRef]
- Gao, M.; Irawan, A.; El-Sherbiny, M.; Szumacher-Strabel, M.; Cieślak, A.; Setiawan, M.A.; Jallal, H.; Fusaro, I.; Jayanegara, A.; Yanza, Y.R.; et al. Meta-analysis of incorporating glucosinolates into diets and their effects on ruminant performance, ruminal fermentation, methane emissions, milk composition, and metabolic biochemical attributes. Animals 2025, 15, 1480. [Google Scholar] [CrossRef]
- Guendouz, A.; Hannachi, A.; Benidir, M.; Fellahi, Z.E.A.; Frih, B. Agro-biochemical Characterisation of Camelina sativa: A review. Agric. Rev. 2022, 43, 278–287. [Google Scholar] [CrossRef]
- Delver, J.J.; Smith, Z.K. Opportunities for Camelina Meal as a Livestock Feed Ingredient. Agriculture 2024, 14, 116. [Google Scholar] [CrossRef]
- Yalçın, S.; Ramay, M.S.; Yalçınkaya, H.; Kardoğan, Ö.; Erkurt, A.; Kılınç, B.; Gebeş, E.S.; Bundur, A.; Onbaşılar, E.E.; Yalçın, S.; et al. Evaluation of high levels of solvent extracted Camelina sativa meal in diets on performance, blood indices, cecal microorganisms, and nutrient digestibility in broilers. Trop. Anim. Health Prod. 2025, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sızmaz, Ö.; Çalık, A.; Bundur, A. In vitro fermentation characteristics of camelina meal comparison with soybean meal. Livest. Stud. 2021, 61, 9–13. [Google Scholar] [CrossRef]
- Christodoulou, C.; Mavrommatis, A.; Mitsiopoulou, C.; Symeon, G.; Dotas, V.; Sotirakoglou, K.; Kotsampasi, B.; Tsiplakou, E. Assessing the optimum level of supplementation with camelina seeds in ewes’ diets to improve milk quality. Foods 2021, 10, 2076. [Google Scholar] [CrossRef]
- Tadayon, Z.; Rouzbehan, Y.; Abarghuei, M.; Rezaei, J. The effects of feeding increasing levels of camelina meal as a protein supplement on the performance of Holstein dairy cows. J. Dairy Sci. 2025, 108, 9579–9594. [Google Scholar] [CrossRef]
- Botta, C.; Tsoureki, D.; Gautam, A.; Patz, S.; Pissaridi, K.; Ferrocino, I.; Franciosa, I.; Cocolin, L.; Huson, D.H.; Rantsiou, K. The pros and cons of tracking the microbial contamination in infant food processing chain through amplicon sequencing and metagenomics. In Food System Microbiomes: Abstracts Book; 2024; pp. 1–161. Available online: https://iris.unito.it/handle/2318/1998634 (accessed on 9 October 2025).
- Paula, E.M.; da Silva, L.G.; Brandao, V.L.N.; Dai, X.; Faciola, A.P. Feeding canola, camelina, and carinata meals to ruminants. Animals 2019, 9, 704. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Giustini, D.; De Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
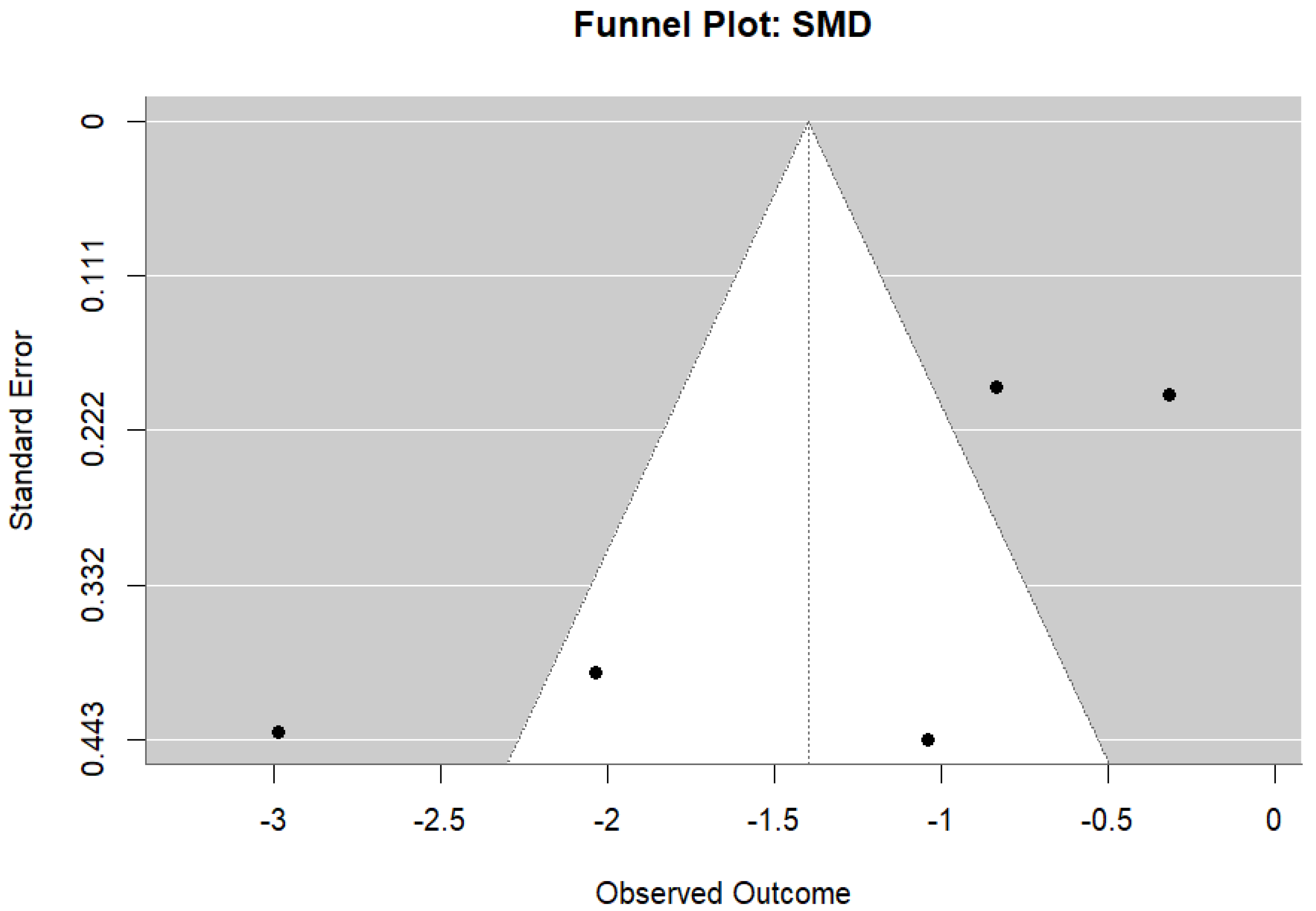
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Bayat, A.R.; Kairenius, P.; Stefanski, T.; Leskinen, H.; Comtet-Marre, S.; Forano, E.; Chaucheyras-Durand, F.; Shingfield, K.J. Effect of camelina oil or live yeasts (Saccharomyces cerevisiae) on ruminal methane production, rumen fermentation, and milk fatty acid composition in lactating cows fed grass silage diets. J. Dairy Sci. 2015, 98, 3166–3181. [Google Scholar] [CrossRef] [PubMed]
- Halmemies-Beauchet-Filleau, A.; Kokkonen, T.; Lampi, A.M.; Toivonen, V.; Shingfield, K.J.; Vanhatalo, A. Effect of plant oils and camelina expeller on milk fatty acid composition in lactating cows fed diets based on red clover silage. J. Dairy Sci. 2011, 94, 4413–4430. [Google Scholar] [CrossRef]
- Halmemies-Beauchet-Filleau, A.; Shingfield, K.J.; Simpura, I.; Kokkonen, T.; Jaakkola, S.; Toivonen, V.; Vanhatalo, A. Effect of incremental amounts of camelina oil on milk fatty acid composition in lactating cows fed diets based on a mixture of grass and red clover silage and concentrates containing camelina expeller. J. Dairy Sci. 2017, 100, 305–324. [Google Scholar] [CrossRef]
- Hurtaud, C.; Peyraud, J.L. Effects of feeding camelina (seeds or meal) on milk fatty acid composition and butter spreadability. J. Dairy Sci. 2007, 90, 5134–5145. [Google Scholar] [CrossRef]
- Lawrence, R.D.; Anderson, J.L.; Clapper, J.A. Evaluation of camelina meal as a feedstuff for growing dairy heifers. J. Dairy Sci. 2016, 99, 6215–6228. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Knight, M.I.; Moate, P.J.; Jacobs, J.L. An alternative approach for sustainable sheep meat production: Implications for food security. J. Anim. Sci. Biotechnol. 2020, 11, 83. [Google Scholar] [CrossRef]
- Salas, H.; Castillejos, L.; Faturi, C.; Ferret, A. Effects of replacing canola meal with camelina expeller on intake, total tract digestibility, and feeding behavior of beef heifers fed high-concentrate diets. Transl. Anim. Sci. 2020, 4, 922–932. [Google Scholar] [CrossRef]
- De Marzo, D.; Laudadio, V.; Khan, R.U.; Tufarelli, V.; Maiorano, G. Feeding of Camelina sativa Seeds to Light-Type Gentile di Puglia Lambs: Effect on Productive Performance and Muscle Fatty Acid Composition. Anim. Biotechnol. 2023, 34, 2360–2366. [Google Scholar] [CrossRef]
- Christodoulou, C.; Mavrommatis, A.; Loukovitis, D.; Symeon, G.; Dotas, V.; Kotsampasi, B.; Tsiplakou, E. Inclusion of Camelina sativa Seeds in Ewes’ Diet Modifies Rumen Microbiota. Animals 2023, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.X.; Weimer, P.J.; Dill-McFarland, K.A.; Brandao, V.L.N.; Suen, G.; Faciola, A.P. Camelina Seed Supplementation at Two Dietary Fat Levels Change Ruminal Bacterial Community Composition in a Dual-Flow Continuous Culture System. Front. Microbiol. 2017, 8, 2147. [Google Scholar] [CrossRef] [PubMed]
- Brandao, V.L.N.; Silva, L.G.; Paula, E.M.; Monteiro, H.F.; Dai, X.; Lelis, A.L.J.; Faccenda, A.; Poulson, S.R.; Faciola, A.P. Effects of replacing canola meal with solvent-extracted camelina meal on microbial fermentation in a dual-flow continuous culture system. J. Dairy Sci. 2018, 101, 9028–9040. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.H.; Khan, N.A.; Wang, Z.S.; Yu, P.Q. Moist and dry heating-induced changes in protein molecular structure, protein subfractions, and nutrient profiles in camelina seeds. J. Dairy Sci. 2014, 97, 446–457. [Google Scholar] [CrossRef] [PubMed]
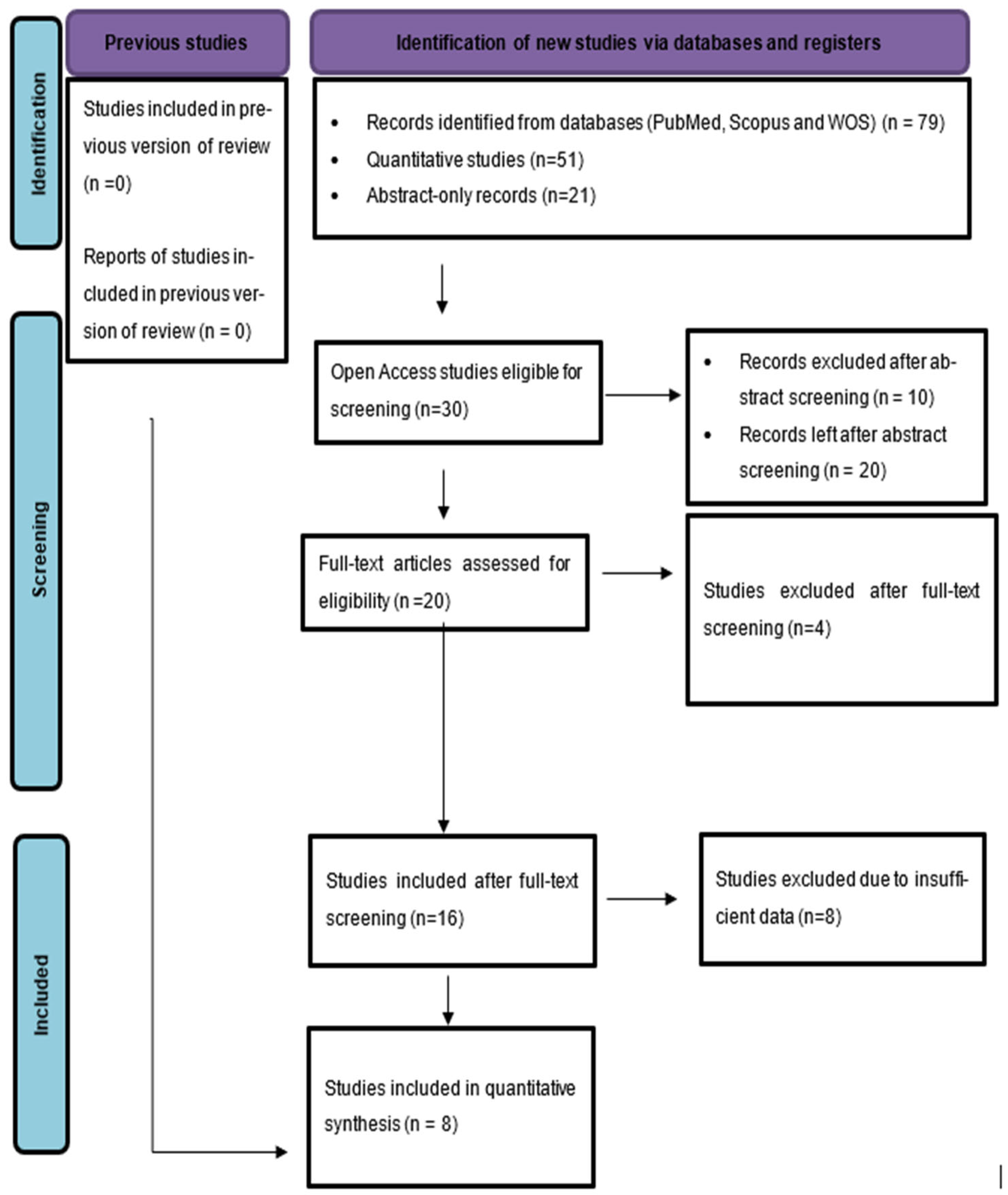
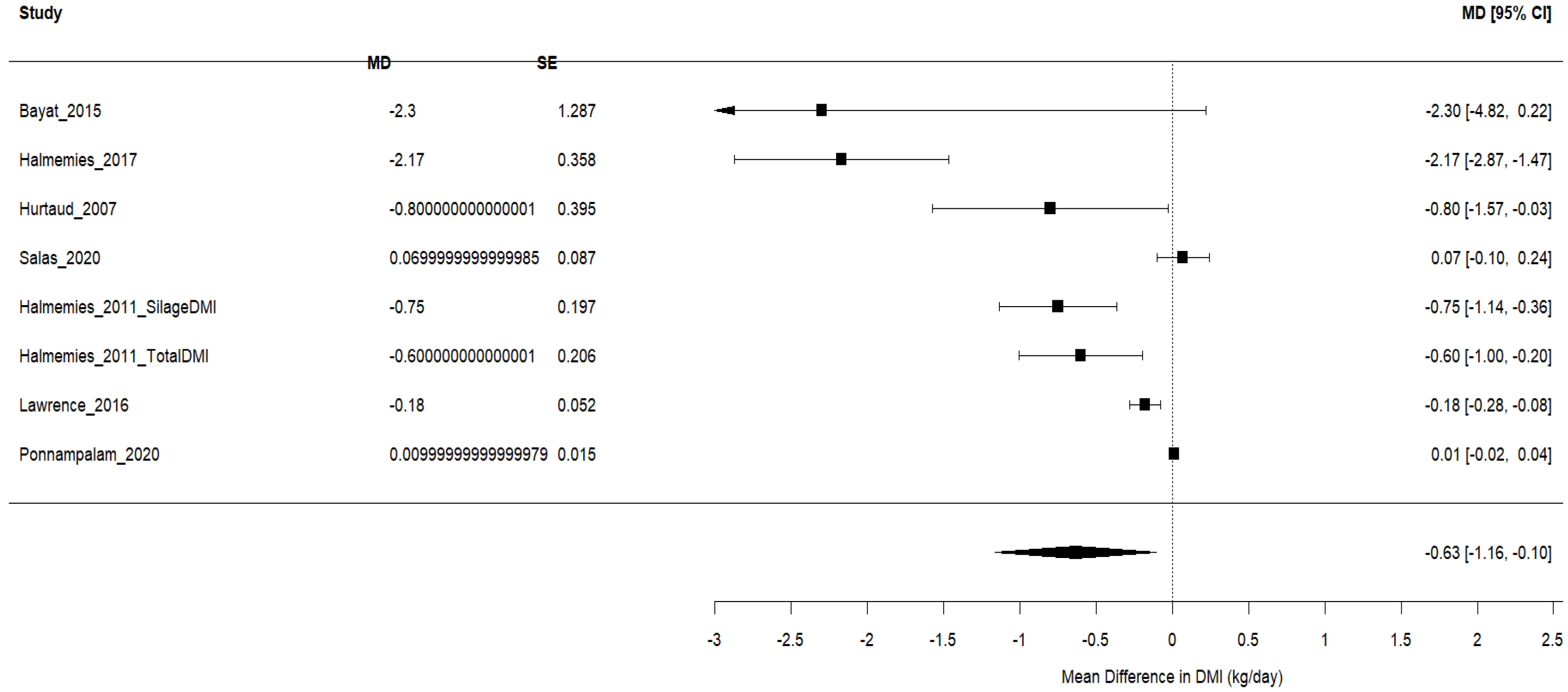
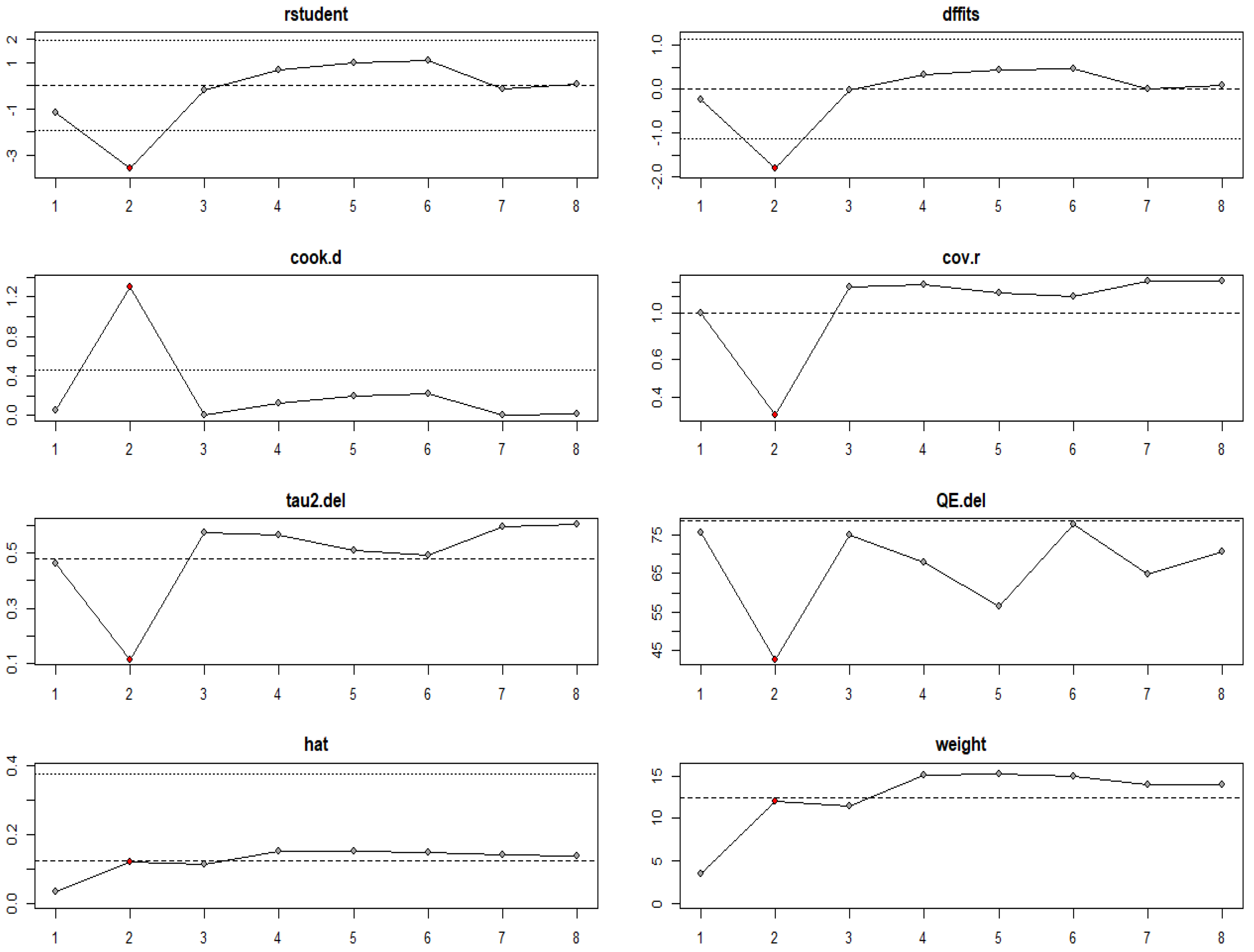
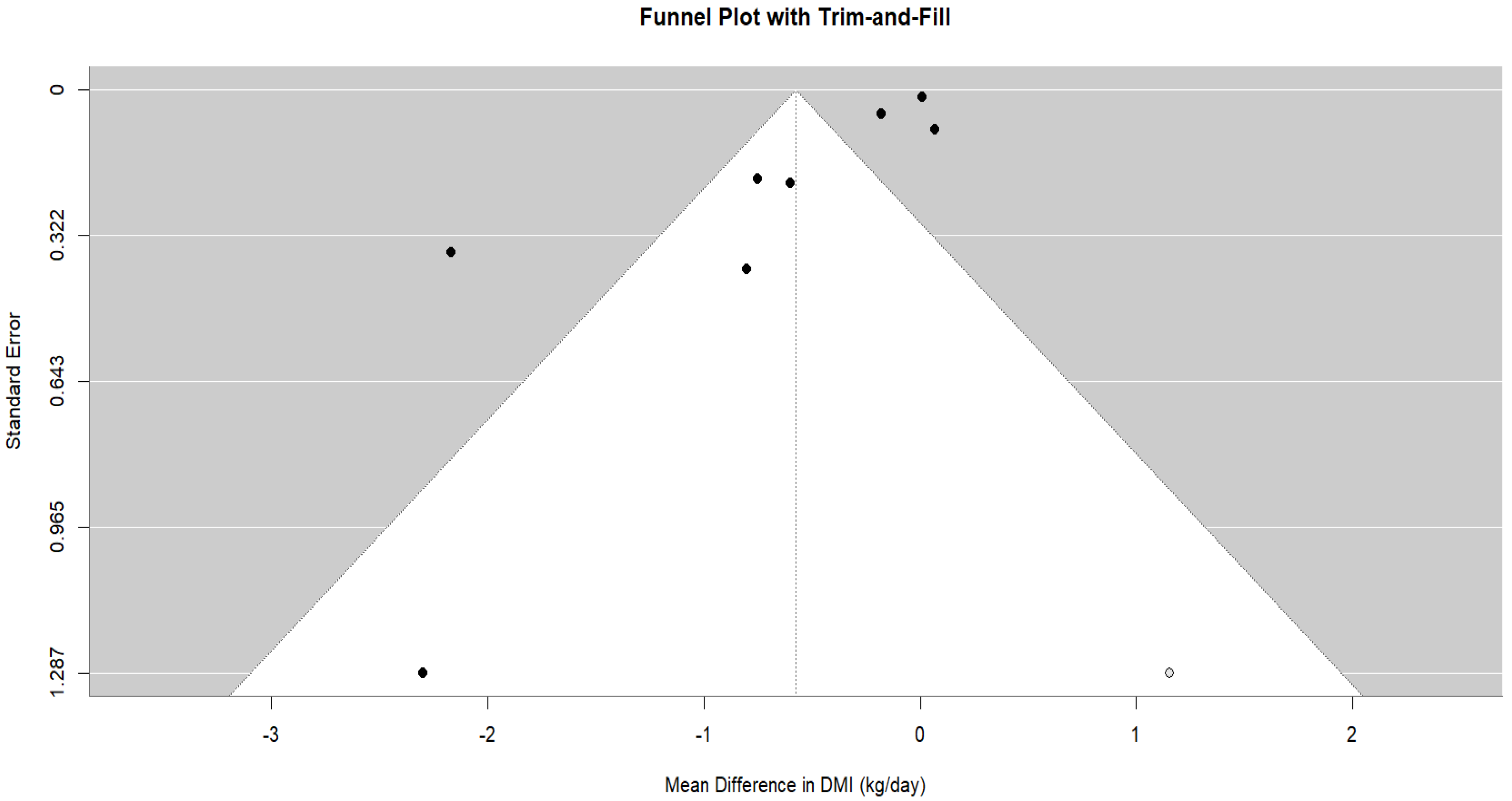




| Study ID | Animal and Experimental Design | Intervention Details (Including Dosage) | Comparator (Control) | Outcomes |
|---|---|---|---|---|
| Bayat et al. [44] | 4 Finnish Ayrshire dairy cows; multiparous; fitted with rumen cannulas; 53 ± 7 DIM; ~711 kg BW; housed in individual tie stalls; milked twice daily; allocated in a 4 × 4 Latin square design; four 42-day periods (23 d adaptation, 5 d sampling, 14 d washout); diet fed as TMR. | Camelina oil (60 g/kg DM, 6% of DM), replacing concentrate; provided 4× daily. Compared with two live yeast strains (0.5 g/d, 1010 cfu/d) directly into the rumen. | Basal TMR (50:50 forage:concentrate, grass silage-based); no added Camelina oil or yeast; nutrient and energy requirements met. | Intake (DM, OM, CP, fiber, starch, FAs); digestibility (OM, CP, NDF, ADF, starch); fermentation (pH, ammonia-N, VFA); gas emissions (methane, CO2); N balance; microbial populations (qPCR); milk production and composition (yield, ECM, fat, protein, lactose); milk FAs (SFA, MUFA, PUFA, CLA); efficiency metrics (milk N/N intake, CH4 intensity, energy use). |
| Christodoulou et al. [32] | 48 Chios ewes, 2–4 yrs, 55 ± 6.5 kg, 67 ± 8 DIM; 4 groups (n = 12); 60-day RCT; groups housed, individually fed. | Camelina seed in concentrate at 6%, 11%, and 16% of DM; partially replaced soybean meal and maize; offered 2× daily after milking; concentrate intake 1.5 kg/ewe/day. | Standard concentrate without Camelina; all diets isonitrogenous and comparable in energy. | Milk: ↓ fat (decrease) (CSS16), ↑ increase in total solids (CSS11), no effect on yield, protein, lactose, SNF, SCC, FCM, ECM. FA: ↓ SFA (C14:0, C16:0), ↑ PUFA (ALA, CLA), ↑ MUFA, ↓ ω6/ω3, ↓ atherogenic and thrombogenic indices, ↑ health index. Blood FA: ↓ C14:0, C15:0, C16:0, ↑ C18:2 n-6, ↑ ALA. Plasma: ↑ SOD (CSS16), ↑ CAT (CSS11, CSS16), ↑ MDA (CSS11), ↑ PC (CSS16), ↑ FRAP (CSS16), ↑ ABTS (CSS6, CSS11). Milk oxidative: ↑ SOD, CAT, GSH-Px, ABTS, FRAP (CSS16), ↓ MDA, PC (CSS16). |
| Halmemies-Beauchet-Filleau et al. [45] | 5 Finnish Ayrshire cows; tie stall; 5 × 5 Latin square, 21-d periods; 115 ± 5 DIM; avg. milk yield 33.5 kg/d; red clover silage-based; 12 kg/d concentrate + ad lib silage. | Camelina oil (2.9%) or expeller (20% concentrate, 350 g lipid/d); diets isonitrogenous/isoenergetic. | Control with no added lipid; also included rapeseed/sunflower oil; all diets isonitrogenous. | Milk yield/composition unaffected; FA profile: ↓ SFA (12:0, 14:0, 16:0), ↑ MUFA, PUFA, CLA; ↑ trans-11 18:1, cis-9, trans-11 CLA with CE vs. CO; intake, digestibility, plasma mostly unaffected; lower milk 18:3n-3 in CE than CO; CE ↑ trans FA intermediates (incomplete biohydrogenation). |
| Halmemies-Beauchet-Filleau et al. [46] | 8 Finnish Ayrshire cows, multiparous, mid-lactation (91 ± 16.5 DIM); individual tie stalls; 4 × 4 Latin square; 21-d periods; 4 rumen-cannulated. | Camelina oil 0, 2, 4, 6% of concentrate DM in expeller-cereal base; 12 kg/d concentrate; ad libitum grass/red clover silage (1:1 DM). | Basal diet without oil; all diets isoenergetic and contained Camelina expeller. | Milk yield, ECM, fat/protein/lactose yield; rumen pH, ammonia-N, VFA, protozoa; digestibility (DM, OM, NDF, fat, N); plasma NEFA, glucose, insulin, BHB, acetic acid; sensory score (1–5); detailed milk FA profile (CLA, trans FA, PUFA, SFA); FA secretion in milk. |
| Hurtaud and Peyraud [47] | 6 Holstein cows; double 3 × 3 Latin square; lactating; individually housed; 3 × 4-week periods; 12 experimental units. | Camelina seed (630 g/d) or meal (2 kg/d); both ~240 g PUFA/day; corn silage-based diet. | Corn silage + high-energy concentrate + soybean meal; isoenergetic/isonitrogenous. | Milk: fat %, protein %, lactose %, FCM, CLA, FA profile (trans-10 C18:1, cis-9, trans-11 CLA). Rumen: pH, acetate, propionate, butyrate, C2:C3. Plasma: glucose, NEFA, glycerol, urea, α-amino N. Butter: churning, hardness, spreadability. DMI, energy/protein balance, short-/medium-chain FA. |
| Lawrence et al. [48] | 42 heifers (33 Holstein, 9 Brown Swiss), ~145 d old, ~172 kg; 3 groups (13, 13, 12); pens (6/pen, straw bedding); 12-wk feeding after 2-wk adaptation; randomized block. | 10% Camelina meal (cold-pressed); 60% grass hay + 40% concentrate; isonitrogenous, limit-fed 2.65% BW. | 10% DDGS and 10% linseed meal; all diets isonitrogenous, energy matched. | DMI, ADG, gain:feed, body weights/length, BCS, frame, rumen (pH, NH3-N, VFA), blood (glucose, cholesterol, triglycerides, PUN), hormones (IGF-1, insulin, T3, T4), total-tract digestibility (DM, OM, CP, NDF, ADF). |
| Ponnampalam et al. [49] | 160 sheep (80 Composite, 80 Merino); Composite (~4 mo), Merino (~15 mo); 20 pens (10/group); 8–10 wks; 2 × 3 factorial. | Camelina hay (CAMH), Camelina meal (CAMM) pellets; full pelleted diets, ad libitum; 2-wk adaptation; ≥150 g/d LWG. | Control pellet (CONT) with conventional summer feeds; similar ME (10–11 MJ/kg DM), CP (14–15%); isocaloric/isonitrogenous. | Final LWG: Composites: 17.6–20.3 kg, Merinos: 10.7–12.9 kg; Dressing %: Composites (CAMH: 48.1%), Merinos (CAMM: 45.8%); hot carcass weight: CAMH/CAMM ~5% ↑ vs. CONT in Composites; carcass fat (GR): unaffected; methane (g CH4/kg DMI): lower CAMH/CAMM; profit/lamb higher with CAMH/CAMM. |
| Salas et al. [50] | 24 Simmental beef heifers (initial BW ≈ 295 kg), pens (3/pen), 4 × 4 replicated Latin square; 28-d periods × 4 (112 d); competitive feeding (12.5 m2 pens). | Camelina expeller (CE) replacing canola meal (CM) at 0%, 3%, 6%, and 9% of diet DM; 90:10 concentrate:straw TMR; isoenergetic (2.8 Mcal ME/kg DM), isonitrogenous (13% CP); CE: 0%, 2.7%, 5.4%, 8.1% (concentrate). | Diet with 15.8% canola meal, 0% CE; isoenergetic/isonitrogenous. | DMI, OM, CP, NDF intake: no significant effect; digestibility: DM, OM, CP, NDF unaffected; feeding behavior: ↑ intake of long particles (p = 0.015), ↓ sorting in 6CE/9CE; chewing activity: no sig. differences; ruminating/chewing ↑ numerically. |
| Study ID | RoB Tool Used | Overall Risk of Bias |
|---|---|---|
| Bayat et al. [44] | SYRCLE’s RoB Tool | Moderate |
| Christodoulou et al. [32] | SYRCLE’s RoB Tool | Low |
| Halmemies-Beauchet-Filleau, Kokkonen [45] | SYRCLE’s RoB Tool | Moderate |
| Halmemies-Beauchet-Filleau et al. [46] | SYRCLE’s RoB Tool | Moderate |
| Hurtaud and Peyraud [47] | SYRCLE’s RoB Tool | Moderate |
| Lawrence et al. [48] | SYRCLE’s RoB Tool | Moderate |
| Ponnampalam et al. [49] | SYRCLE’s RoB Tool | Moderate |
| Salas et al. [50] | SYRCLE’s RoB Tool | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, R.; Waqas, M.; Ahmed, I.; Nouman, H.M.; Imtiaz, B.; Ul Hassan, M.; Todaro, M.; Gannuscio, R.; Tahir, M.N.; Sizmaz, O. Meta-Analysis of Incorporating Camelina and Its By-Products into Ruminant Diets and Their Effects on Ruminal Fermentation, Methane Emissions, Milk Yield and Composition, and Metabolic Profile. Fermentation 2025, 11, 593. https://doi.org/10.3390/fermentation11100593
Riaz R, Waqas M, Ahmed I, Nouman HM, Imtiaz B, Ul Hassan M, Todaro M, Gannuscio R, Tahir MN, Sizmaz O. Meta-Analysis of Incorporating Camelina and Its By-Products into Ruminant Diets and Their Effects on Ruminal Fermentation, Methane Emissions, Milk Yield and Composition, and Metabolic Profile. Fermentation. 2025; 11(10):593. https://doi.org/10.3390/fermentation11100593
Chicago/Turabian StyleRiaz, Roshan, Muhammad Waqas, Ibrar Ahmed, Hafiz Muhammad Nouman, Beenish Imtiaz, Mahmood Ul Hassan, Massimo Todaro, Riccardo Gannuscio, Muhammad Naeem Tahir, and Ozge Sizmaz. 2025. "Meta-Analysis of Incorporating Camelina and Its By-Products into Ruminant Diets and Their Effects on Ruminal Fermentation, Methane Emissions, Milk Yield and Composition, and Metabolic Profile" Fermentation 11, no. 10: 593. https://doi.org/10.3390/fermentation11100593
APA StyleRiaz, R., Waqas, M., Ahmed, I., Nouman, H. M., Imtiaz, B., Ul Hassan, M., Todaro, M., Gannuscio, R., Tahir, M. N., & Sizmaz, O. (2025). Meta-Analysis of Incorporating Camelina and Its By-Products into Ruminant Diets and Their Effects on Ruminal Fermentation, Methane Emissions, Milk Yield and Composition, and Metabolic Profile. Fermentation, 11(10), 593. https://doi.org/10.3390/fermentation11100593










