Fermentation Strategies to Improve Argentinian Kefir Quality: Impact of Double Fermentation on Physicochemical, Microbial, and Functional Properties
Abstract
1. Introduction
2. Materials and Methods
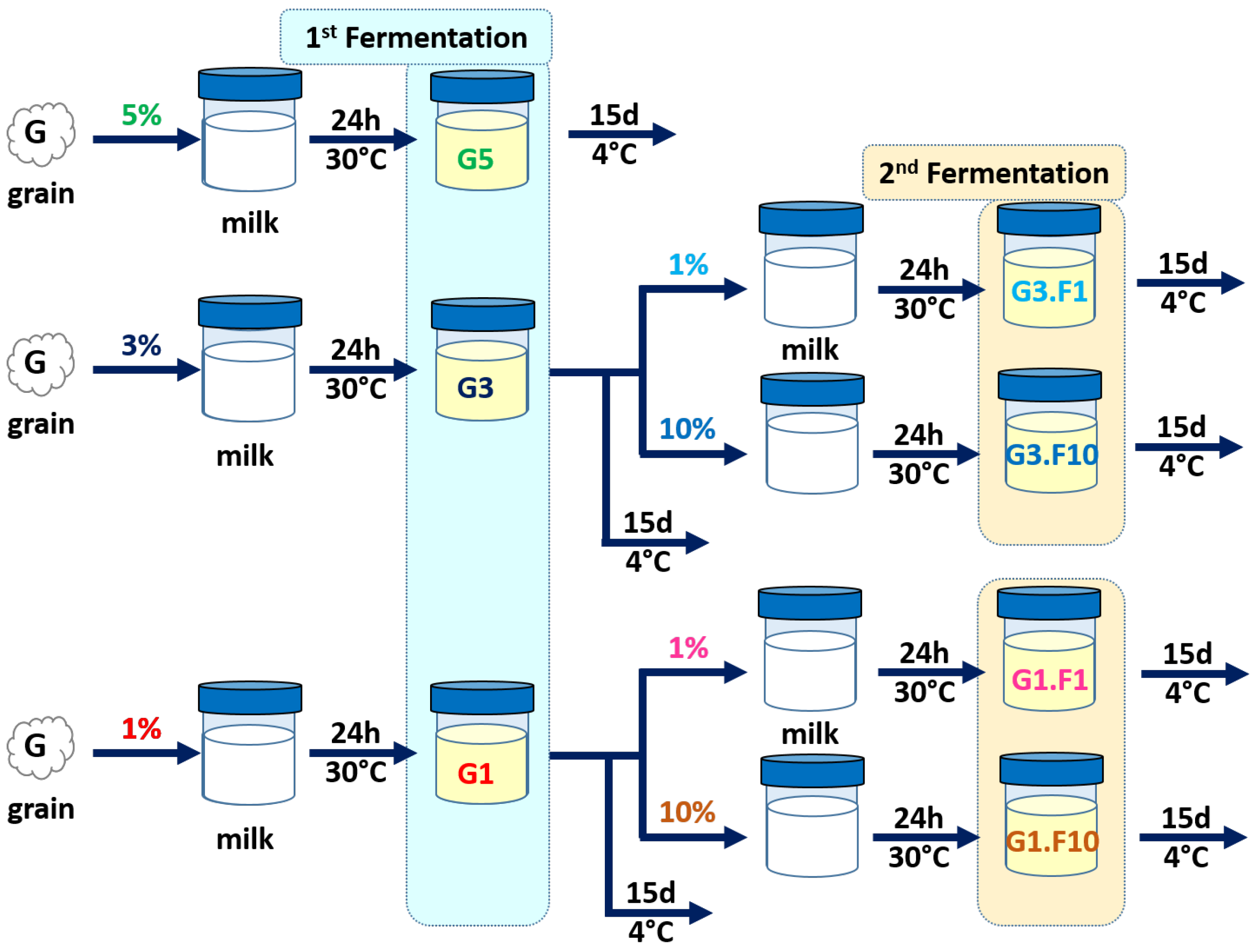
2.1. Kefir Grains and Kefir Production
2.2. Bacterial and Fungal Strains
2.3. Determination of Grain Biomass, pH, °Brix and Titratable Acidity
2.4. Determination of Protein Concentration
2.5. Determination of Sugar Concentration
2.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.7. Enumeration of Bacteria and Yeasts
2.8. Biofilm Production of Kefir
2.9. Measurement of Antimicrobial Activity
2.10. Co-Incubation of Traditional Kefir or Double-Fermentation Kefir Products with Bacterial Pathogens
2.11. Sensory Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Acidity, pH Stability, and °Brix Dynamics in Kefir Fermentation
3.2. Microbial Composition and Fermentation Dynamics
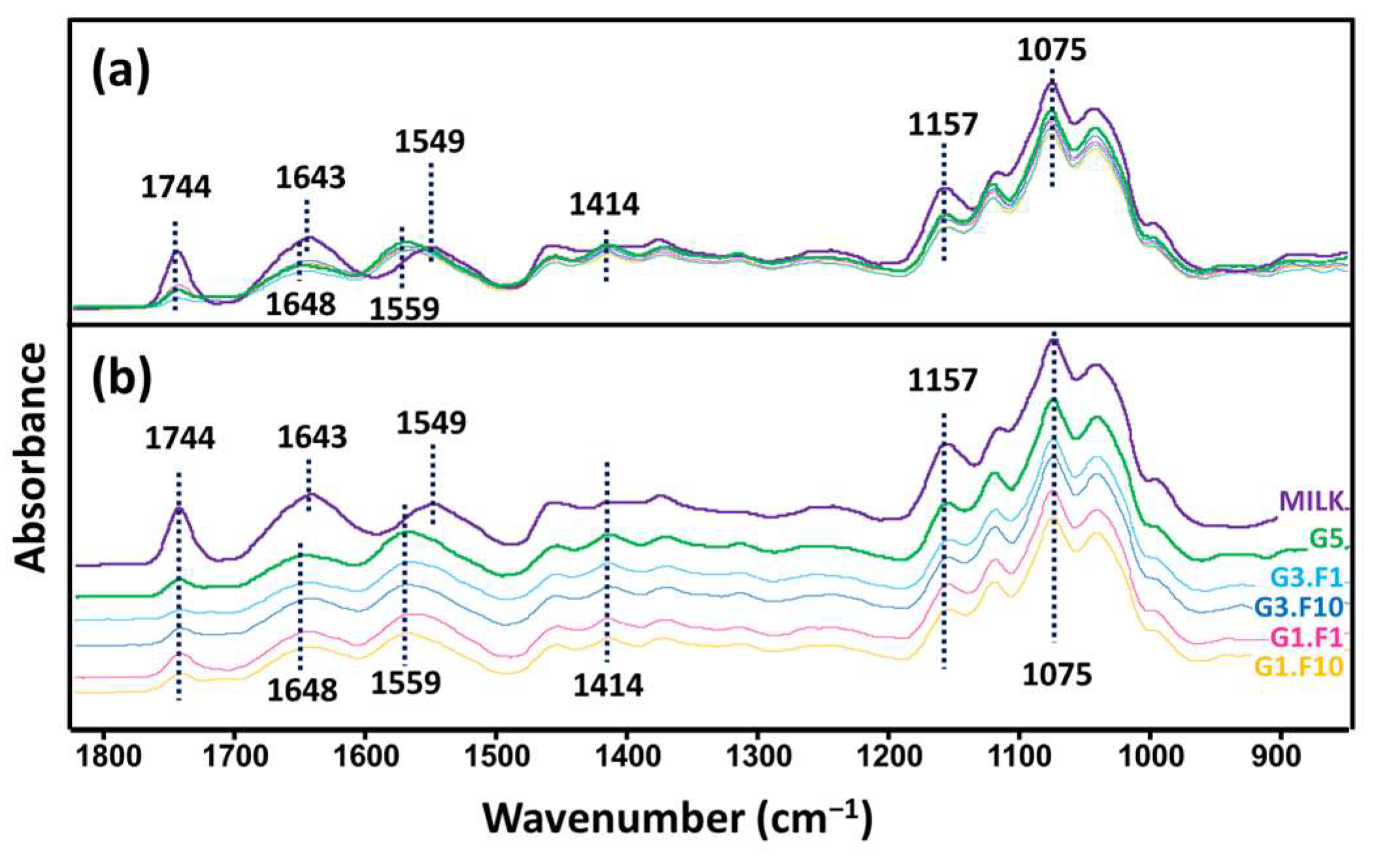
3.3. FTIR Analysis
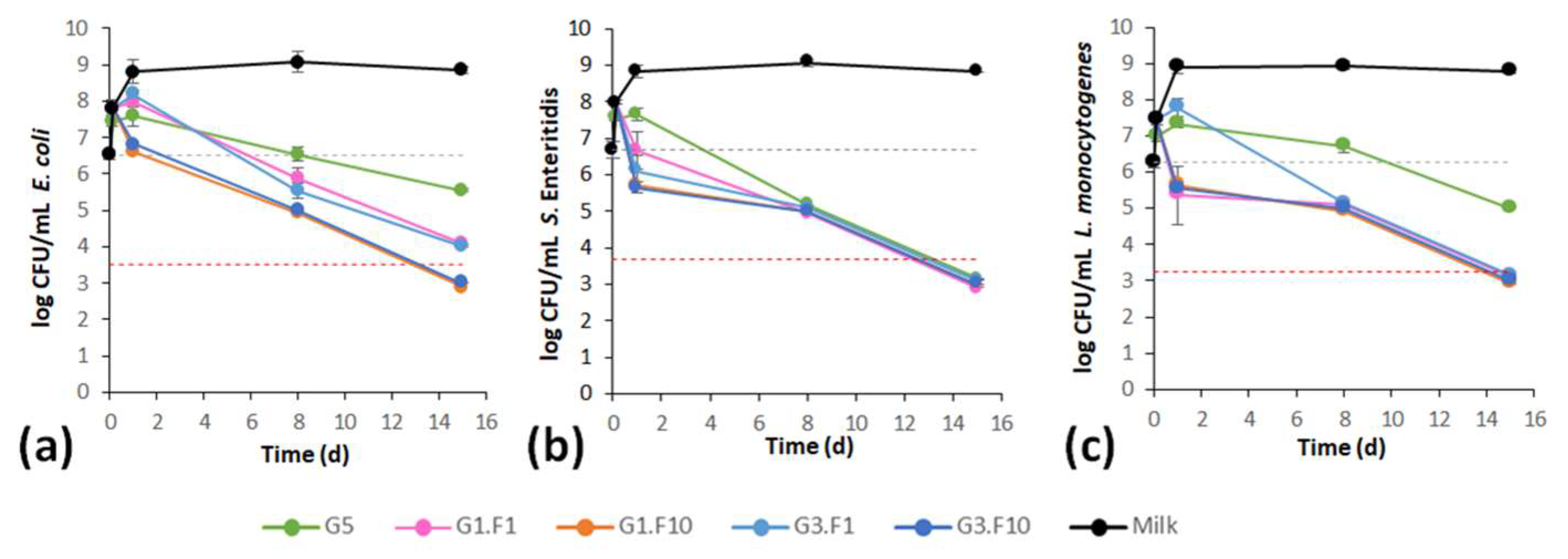
3.4. Functional Properties: Biofilm Production and Antimicrobial Activity
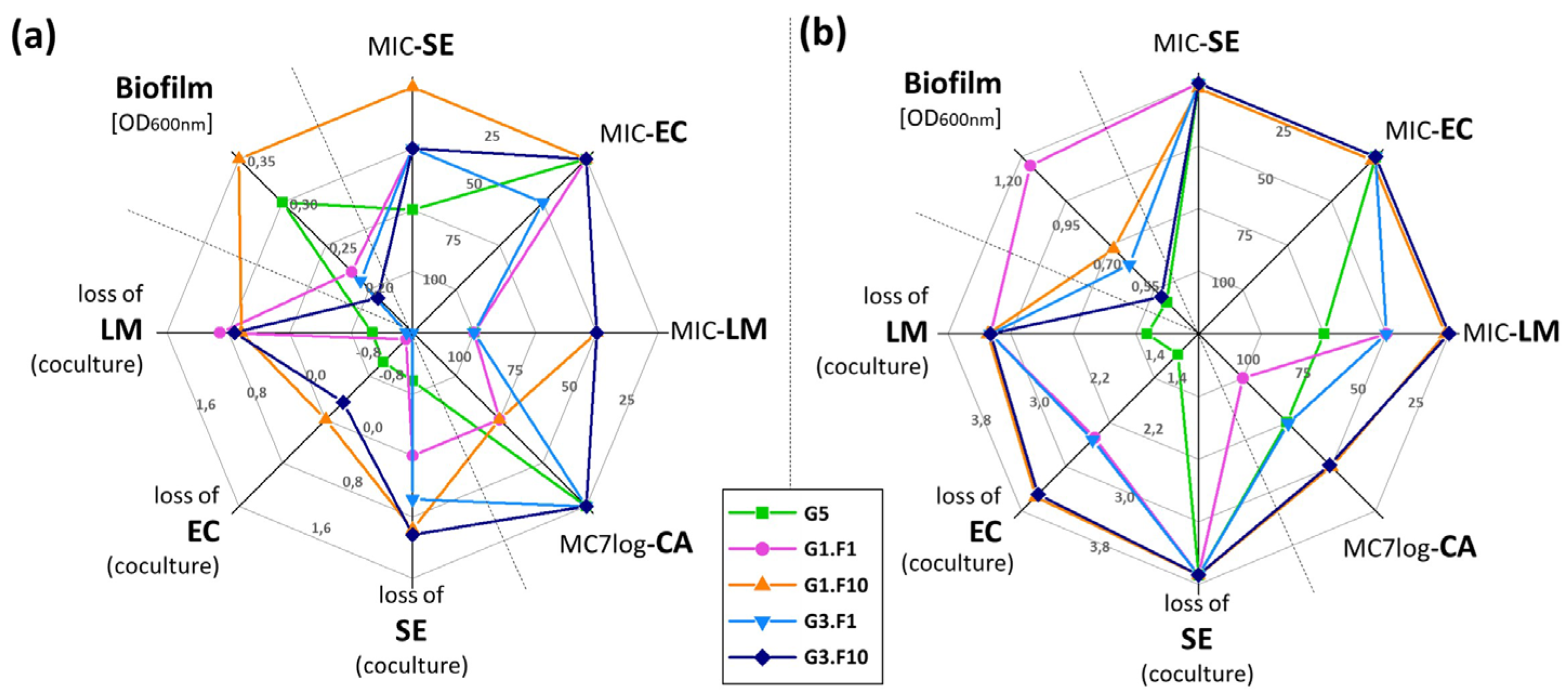
3.5. Global Analysis of Kefir Product Characteristics
3.6. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
- •
- G1: starter produced with 1% w/v kefir grains inoculated in fresh whole cow milk.
- •
- G3: starter produced with 3% w/v kefir grains inoculated in fresh whole cow milk.
- •
- G5 (traditional kefir): kefir beverage produced with 5% w/v kefir grains inoculated in fresh whole cow milk.
- •
- G3.F1: one type of double-fermentation kefir generated when starters originating from 3% w/v grain fermentations were inoculated at 1% v/v into fresh milk.
- •
- G3.F10: one type of double-fermentation kefir generated when starters originating from 3% w/v grain fermentations were inoculated at 10% v/v into fresh milk.
- •
- G1.F1: one type of double-fermentation kefir generated when starters originating from 1% w/v grain fermentations were inoculated at 1% v/v into fresh milk.
- •
- G1.F10: one type of double-fermentation kefir generated when starters originating from 1% w/v grain fermentations were inoculated at 10% v/v into fresh milk.
- •
- CFU: colony forming unit
- •
- CFS: cell free supernatants
- •
- FTIR: Fourier Transform Infrared spectroscopy
References
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Stroher, J.A.; Oliveira, W.d.C.; de Freitas, A.S.; Salazar, M.M.; da Silva, L.d.F.F.; Bresciani, L.; Flôres, S.H.; Malheiros, P.d.S. A Global Review of Geographical Diversity of Kefir Microbiome. Fermentation 2025, 11, 150. [Google Scholar] [CrossRef]
- Gamba, R.R.; Caro, C.A.; Martínez, O.L.; Moretti, A.F.; Giannuzzi, L.; De Antoni, G.L.; Peláez, A.L. Antifungal effect of kefir fermented milk and shelf-life improvement of corn arepas. Int. J. Food Microbiol. 2016, 235, 85–92. [Google Scholar] [CrossRef]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašić, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F.; et al. Study of kefir drinks produced by backslopping method using kefir grains from Bosnia and Herzegovina: Microbial dynamics and volatilome profile. Food Res. Int. 2020, 137, 109369. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeong, D.; Song, K.Y.; Seo, K.H. Comparison of traditional and backslopping methods for kefir fermentation based on physicochemical and microbiological characteristics. LWT 2018, 97, 503–507. [Google Scholar] [CrossRef]
- Korsak, N.; Taminiau, B.; Leclercq, M.; Nezer, C.; Crevecoeur, S.; Ferauche, C.; Detry, E.; Delcenserie, V.; Daube, G. Short communication: Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 2015, 98, 3684–3689. [Google Scholar] [CrossRef]
- Elgarhy, M.R.; Omar, M.M.; Abou Ayana, I.A.A.; Khalifa, S.A. Kefir Production From Cow’s And Buffalo’s Milk Under Egyptian Conditions. Zagazig J. Agric. Res. 2018, 45, 227–238. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kim, H.; Seo, K.H. Modern perspectives on the health benefits of kefir in next-generation sequencing era: Improvement of the host gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 1782–1793. [Google Scholar] [CrossRef]
- Solanki, K.K.; Ghosh, B.C.; Kumawat, A. Shelf-life study of fortified sweetened milk kefir during storage. Pharma Innov. J. 2023, 12, 2514–2521. [Google Scholar]
- Özcan, T.; Sahin, S.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L. Assessment of antioxidant capacity by method comparison and amino acid characterisation in buffalo milk kefir. Int. J. Dairy Technol. 2019, 72, 65–73. [Google Scholar] [CrossRef]
- González-Orozco, B.D.; Kosmerl, E.; Jiménez-Flores, R.; Alvarez, V.B. Enhanced probiotic potential of Lactobacillus kefiranofaciens OSU-BDGOA1 through co-culture with Kluyveromyces marxianus bdgo-ym6. Front. Microbiol. 2023, 14, 1236634. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef]
- Savastano, M.L.; Pati, S.; Bevilacqua, A.; Corbo, M.R.; Rizzuti, A.; Pischetsrieder, M.; Losito, I. Influence of the production technology on kefir characteristics: Evaluation of microbiological aspects and profiling of phosphopeptides by LC-ESIQTOF-MS/MS. Food Res. Int. 2020, 129, 108853. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Lama, S. Probiotic properties of yeasts in traditional fermented foods and beverages. J. Appl. Microbiol. 2022, 132, 3533–3542. [Google Scholar] [CrossRef]
- Laureys, D.; Leroy, F.; Vandamme, P.; De Vuyst, L. Backslopping Time, Rinsing of the Grains During Backslopping, and Incubation Temperature Influence the Water Kefir Fermentation Process. Front. Microbiol. 2022, 13, 871550. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wang, Y.; Tang, N.; Xiao, L.; Tian, J.; Rui, X.; Li, W. Yeast cell wall polysaccharides in Tibetan kefir grains are key substances promoting the formation of bacterial biofilm. Carbohydr. Polym. 2023, 300, 120247. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001, 68, 639–652. [Google Scholar] [CrossRef]
- Gamba, R.R.; Yamamoto, S.; Abdel-Hamid, M.; Sasaki, T.; Michihata, T.; Koyanagi, T.; Enomoto, T. Chemical, Microbiological, and Functional Characterization of Kefir Produced from Cow’s Milk and Soy Milk. Int. J. Microbiol. 2020, 2020, 7019286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Y.; Ma, Y.; Feng, Z.-M.; Yu, F.-J.; Liu, C.-Y.; Yi, Y.-S. Bicinchoninic Acid Method for Determination of Protein Content in Milk. Food Sci. 2010, 31, 151–154. [Google Scholar] [CrossRef]
- Başkan, K.S.; Tütem, E.; Akyüz, E.; Özen, S.; Apak, R. Spectrophotometric total reducing sugars assay based on cupric reduction. Talanta 2016, 147, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, K.T.; Pereira, M.G.V.; Dias, D.R.; Schwan, R.F. Microbial communities and chemical changes during fermentation of sugary Brazilian kefir. World J. Microbiol. Biotechnol. 2010, 26, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Senda, S.; Nomura, N.; Tokuda, H.; Uchiyama, H. Biofilm formation by lactic acid bacteria and resistance to environmental stress. J. Biosci. Bioeng. 2008, 106, 381–386. [Google Scholar] [CrossRef]
- Vázquez, M.B.; Curia, A.; Hough, G. Sensory descriptive analysis, sensory acceptability and expectation studies on biscuits with reduced added salt and increased fiber. J. Sens. Stud. 2009, 24, 498–511. [Google Scholar] [CrossRef]
- ISO Standard No.6658; Sensory Analysis—Methodology—General Guidance. International Organization for Standardization:: Geneva, Switzerland, 2017.
- ISO Standard No. 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO Standard No. 22935-2:2009; Milk and Milk Products—Sensory Analysis. ISO: Geneva, Switzerland, 2009.
- Moretti, A.F.; Gamba, R.R.; Costa, M.; De Antoni, G.; Peláez, Á.L. Protective Effect of Lyophilization on Fermentative, Microbiological and Sensory Properties of Kefir. Int. J. Biochem. Pharmacol. 2019, 1, 5–11. [Google Scholar] [CrossRef]
- Hough, G. Course: Sensory Food Assessment Workshop; CIDCA, Centro de Investigación en Criotecnología de Alimentos, Food Sensory Evaluation Department, Instituto Superior Experimental de Tecnología Alimentaria (ISETA): Nueve de Julio, Argentina, 2006; p. 108. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Codex Standard for Fermented Milk, 2nd ed.; FAO: Rome, Italy, 2003; Volume 243. [Google Scholar]
- Código Alimentario Argentino (CAA). Capítulo VIII: Leche, Productos Lácteos y Derivados Grasos, Artículo 553 (Límites de Acidez). Ministerio de Agricultura, Ganadería y Pesca de la Nación, 2023. Available online: https://alimentosargentinos.magyp.gob.ar/contenido/marco/CAA/Capitulo_08.htm (accessed on 1 August 2025).
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef]
- Grønnevik, H.; Falstad, M.; Narvhus, J.A. Microbiological and chemical properties of Norwegian kefir during storage. Int. Dairy J. 2011, 21, 601–606. [Google Scholar] [CrossRef]
- Alves, E.; Ntungwe, E.N.; Gregório, J.; Rodrigues, L.M.; Pereira-Leite, C.; Caleja, C.; Pereira, E.; Barros, L.; Aguilar-Vilas, M.V.; Rosado, C.; et al. Characterization of Kefir Produced in Household Conditions: Physicochemical and Nutritional Profile, and Storage Stability. Foods 2021, 10, 1057. [Google Scholar] [CrossRef]
- Nicolaou, N.; Xu, Y.; Goodacre, R. Fourier transform infrared spectroscopy and multivariate analysis for the detection and quantification of different milk species. J. Dairy Sci. 2010, 93, 5651–5660. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P. Moreno, F.J., Sanz, L.M., Eds.; Biosynthesis and bioactivity of exopolysaccharides produced by probiotic bacteria. In Food Oligosaccharides; Wiley: Hoboken, NJ, USA, 2014; pp. 118–133. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Tian, Z.; He, C.; Yang, Y.; Yang, Z. Characterization of Biofilm Formation by Lactobacillus plantarum WCFS1 and Its Influence on the Growth of Escherichia coli and Salmonella Typhimurium. LWT Food Sci. Technol. 2021, 136, 110331. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Jing, X.; Yu, P.; Zhang, Y.; Yi, H.; Zhang, L. Enhancement of Biofilm Formation and Antioxidant Activity of Lactobacillus plantarum Strains by Co-Culture with Yeast. Food Res. Int. 2018, 102, 559–564. [Google Scholar] [CrossRef]
- Piermaria, J.A.; Pinotti, A.; García, M.A.; Abraham, A.G. Films Based on Kefiran, an Exopolysaccharide Obtained from Kefir Grain: Development and Characterization. Food Hydrocoll. 2009, 23, 684–690. [Google Scholar] [CrossRef]
- Barukčić, I.; Gracin, L.; Režek Jambrak, A.; Božanić, R. Comparison of chemical, rheological and sensory properties of kefir produced by kefir grains and commercial kefir starter. Mljekarstvo 2017, 67, 169–176. [Google Scholar] [CrossRef]

| Kefir Products | Time (Days) | pH | °Brix | TTA (%) 1 | Sugar Remnants (%) |
|---|---|---|---|---|---|
| G5 | 0 | 6.76 ± 0.06 **** | 12.2 ± 0.1 *** | 0.15 ± 0.01 ***** 2 | 100.0 ± 1.6 ** |
| 1 | 4.43 ± 0.03 *** | 6.2 ± 0.1 * | 0.87 ± 0.06 * | 55.7 ± 1.4 * | |
| 8 | 4.31 ± 0.01 ** | 6.2 ± 0.1 * | 0.95 ± 0.00 ** | 53.3 ± 25.8 * | |
| 15 | 4.32 ± 0.01 ** | 6.3 ± 0.1 * | 0.96 ± 0.01 ** | 54.5 ± 10.7 * | |
| G3.F1 | 0 | 6.74 ± 0.04 **** | 12.1 ± 0.2 **** | 0.15 ± 0.01 ***** 2 | 100.0 ± 1.6 ** |
| 1 | 4.50 ± 0.04 *** | 6.4 ± 0.1 * | 0.83 ± 0.05 * | 59.7 ± 1.3 * | |
| 8 | 4.30 ± 0.02 ** | 6.9 ± 0.1 ** | 0.94 ± 0.01 ** | 63.2 ± 6.9 * | |
| 15 | 4.27 ± 0.01 ** | 7.0 ± 0.1 ** | 0.93 ± 0.02 ** | 70.3 ± 7.1 * | |
| G3.F10 | 0 | 6.75 ± 0.06 **** | 12.2 ± 0.1 **** | 0.15 ± 0.01 ***** 2 | 100.0 ± 1.6 ** |
| 1 | 4.19 ± 0.04 ** | 6.7 ± 0.1 ** | 0.87 ± 0.02 * | 52.0 ± 23.3 * | |
| 8 | 3.90 ± 0.03 * | 6.6 ± 0.1 ** | 1.02 ± 0.00 ** | 51.8 ± 14.0 * | |
| 15 | 3.88 ± 0.03 * | 6.8 ± 0.1 ** | 1.09 ± 0.01 *** | 52.8 ± 12.4 * | |
| G1.F1 | 0 | 6.74 ± 0.05 **** | 12.2 ± 0.1 **** | 0.15 ± 0.01 ***** 2 | 100.0 ± 1.6 ** |
| 1 | 4.43 ± 0.01 *** | 6.6 ± 0.1 ** | 0.85 ± 0.05 * | 60.5 ±1 3.0 * | |
| 8 | 4.26 ± 0.03 ** | 6.9 ± 0.1 ** | 0.91 ± 0.01 ** | 60.6 ± 6.4 * | |
| 15 | 4.21 ± 0.01 ** | 7.1 ± 0.1 ** | 0.95 ± 0.00 ** | 58.5 ± 6.0 * | |
| G1.F10 | 0 | 6.76 ± 0.06 **** | 12.2 ± 0.2 **** | 0.15 ± 0.01 ***** 2 | 100.0 ± 1.6 ** |
| 1 | 4.12 ± 0.03 ** | 6.6 ± 0.1 ** | 1.00 ± 0.05 ** | 55.0 ± 14.5 * | |
| 8 | 3.94 ± 0.03 * | 6.6 ± 0.2 ** | 1.09 ± 0.01 *** | 56.5 ± 21.2 * | |
| 15 | 3.87 ± 0.04 * | 6.8 ± 0.2 ** | 1.21 ± 0.01 **** | 65.9 ± 10.5 * |
| Kefir Products | Time (Days) | LAB 1 | Yeasts 2 | AAB |
|---|---|---|---|---|
| G5 | 1 | 8.21 ± 0.10 * | 7.16 ± 0.02 *** | 7.44 ± 0.06 * |
| 8 | 8.11 ± 0.01 * | 5.98 ± 0.10 ** | 7.44 ± 0.30 * | |
| 15 | 8.10 ± 0.10 * | 5.48 ± 0.00 * | 7.48 ± 0.00 * | |
| G3.F1 | 1 | 9.04 ± 0.14 ** | 5.86 ± 0.15 ** | 7.76 ± 0.03 * |
| 8 | 9.15 ± 0.17 ** | 5.54 ± 0.18 ** | 7.63 ± 0.04 * | |
| 15 | 8.60 ± 0.16 * | 4.95 ± 0.00 * | 8.35 ± 0.38 ** | |
| G3.F10 | 1 | 9.31 ± 0.06 *** | 5.70 ± 0.19 ** | 7.24 ± 0.09 * |
| 8 | 9.11 ± 0.19 ** | 5.94 ± 0.05 ** | 7.35 ± 0.21 * | |
| 15 | 8.24 ± 0.55 * | 5.70 ± 0.06 ** | 7.60 ± 0.24 * | |
| G1.F1 | 1 | 9.04 ± 0.08 ** | 5.18 ± 0.49 * | 7.74 ± 0.06 * |
| 8 | 9.10 ± 0.02 ** | 5.35 ± 0.07 * | 7.65 ± 0.07 * | |
| 15 | 8.40 ± 0.12 * | 4.92 ± 0.09 * | 7.60 ± 0.00 * | |
| G1.F10 | 1 | 9.15 ± 0.05 ** | 5.94 ± 0.09 ** | 7.40 ± 0.26 * |
| 8 | 9.01 ± 0.08 ** | 5.65 ± 0.07 ** | 7.57 ± 0.11 * | |
| 15 | 8.80 ± 0.23 ** | 5.24 ± 0.09 * | 7.10 ± 0.12 * |
| Kefir Products | Time (Days) | A600nm |
|---|---|---|
| G5 | 1 | 0.30 ± 0.12 * |
| 8 | 0.44 ± 0.11 * | |
| 15 | 0.48 ± 0.06 * | |
| G3.F1 | 1 | 0.21 ± 0.11 * |
| 8 | 0.50 ± 0.18 * | |
| 15 | 0.69 ± 0.15 ** | |
| G3.F10 | 1 | 0.19 ± 0.10 * |
| 8 | 0.36 ± 0.18 * | |
| 15 | 0.51 ± 0.17 * | |
| G1.F1 | 1 | 0.22 ± 0.10 * |
| 8 | 0.72 ± 0.32 ** | |
| 15 | 1.25 ± 0.20 *** | |
| G1.F10 | 1 | 0.35 ± 0.15 * |
| 8 | 0.47 ± 0.15 * | |
| 15 | 0.78 ± 0.14 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamba, R.R.; Ibáñez, A.; Sampaolesi, S.; Mobili, P.; Golowczyc, M.A. Fermentation Strategies to Improve Argentinian Kefir Quality: Impact of Double Fermentation on Physicochemical, Microbial, and Functional Properties. Fermentation 2025, 11, 584. https://doi.org/10.3390/fermentation11100584
Gamba RR, Ibáñez A, Sampaolesi S, Mobili P, Golowczyc MA. Fermentation Strategies to Improve Argentinian Kefir Quality: Impact of Double Fermentation on Physicochemical, Microbial, and Functional Properties. Fermentation. 2025; 11(10):584. https://doi.org/10.3390/fermentation11100584
Chicago/Turabian StyleGamba, Raúl Ricardo, Andrea Ibáñez, Sofía Sampaolesi, Pablo Mobili, and Marina Alejandra Golowczyc. 2025. "Fermentation Strategies to Improve Argentinian Kefir Quality: Impact of Double Fermentation on Physicochemical, Microbial, and Functional Properties" Fermentation 11, no. 10: 584. https://doi.org/10.3390/fermentation11100584
APA StyleGamba, R. R., Ibáñez, A., Sampaolesi, S., Mobili, P., & Golowczyc, M. A. (2025). Fermentation Strategies to Improve Argentinian Kefir Quality: Impact of Double Fermentation on Physicochemical, Microbial, and Functional Properties. Fermentation, 11(10), 584. https://doi.org/10.3390/fermentation11100584






