3.1. Cell Viability with Different Processing Types
Bacterial cell viability is an important factor in establishing long term processes. The expectation for each batch cultivation is a decreasing cell viability, as the consumption of all substrates and accumulation of by-products lead to increasingly unfavourable conditions. In fed-batch processes, where substrate limitations are mitigated, cell viability also tends to decline over time due to by-product accumulation, ultimately leading to cell lysis. The aforementioned effect is particularly critical in fed-batch systems with slow-growing organisms and in batch systems with cell retention, as long-term viability directly impacts process stability. Despite the accumulation of by-products, the cells begin to lyse at a certain point. Following the completion of the lysis process, the cells were undetectable in both the biomass and viability determinations.
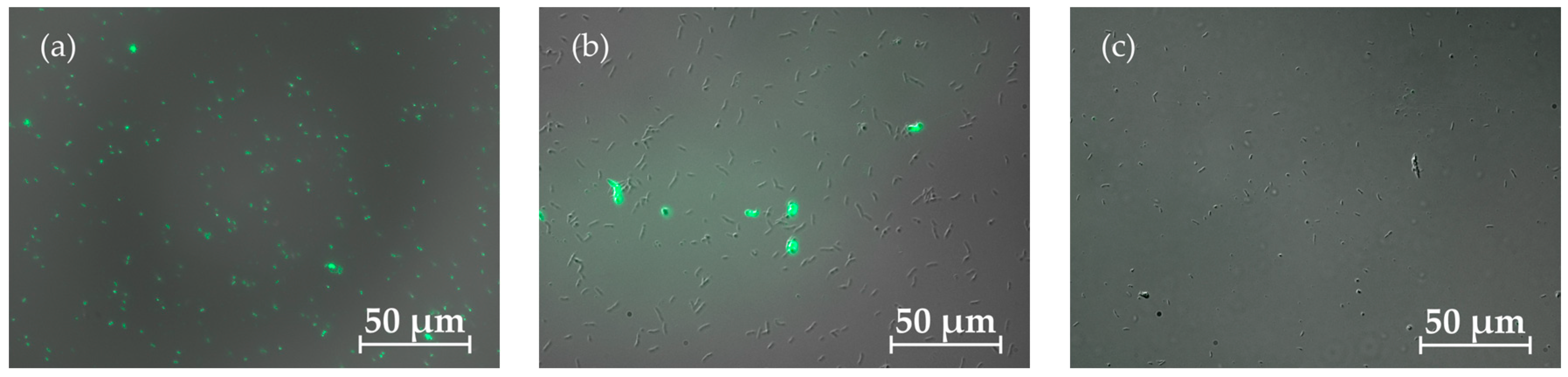
As illustrated in
Figure 2, dead (coloured) cells can be distinguished from living (uncoloured) cells. By counting the stained and unstained cells, the relative proportions of cells still alive could be determined. Samples were taken at different times for all fermentation approaches and compared with fresh dead or live controls.
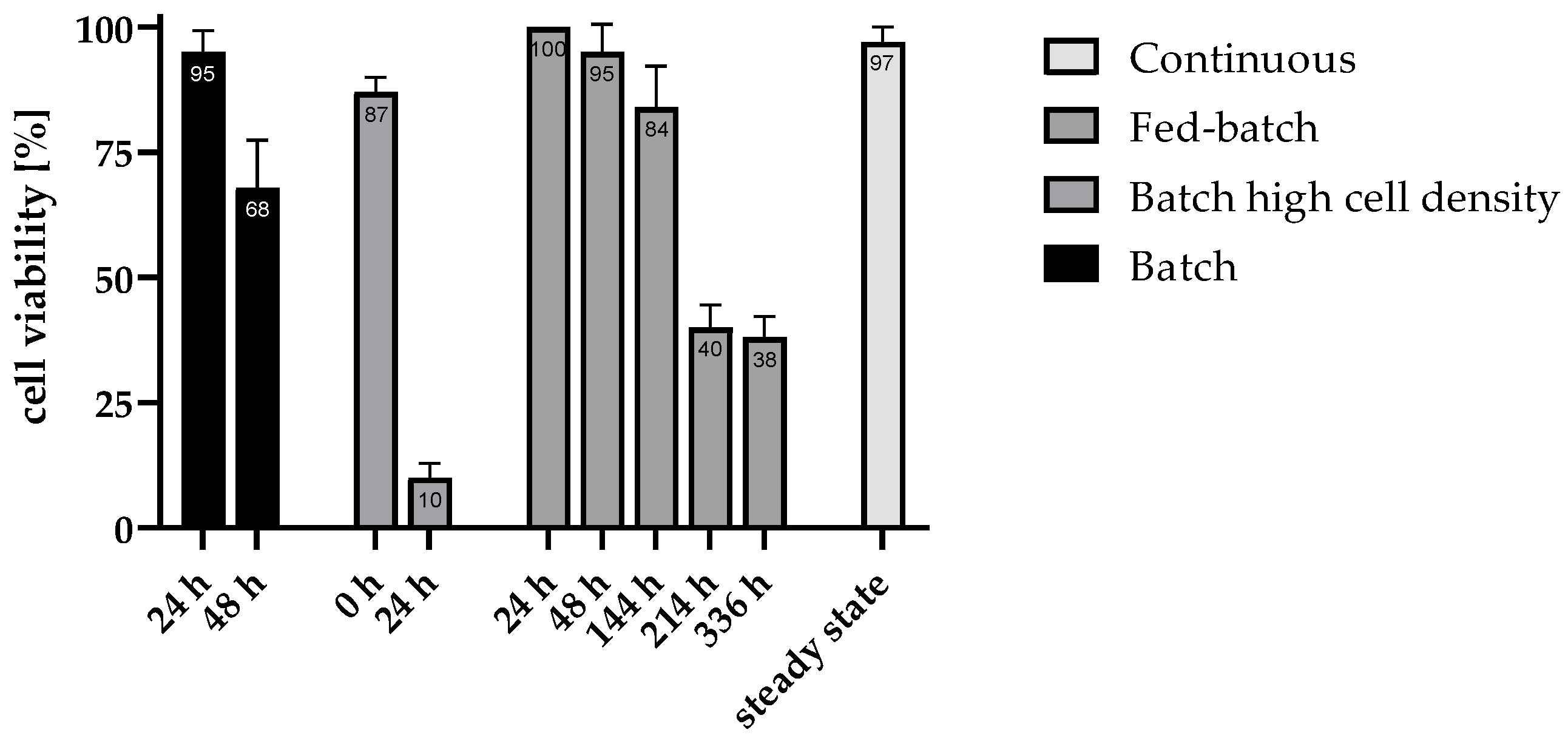
Figure 3 illustrates cell viability across different fermentation modes over time, highlighting the varying stability and resilience of each approach.
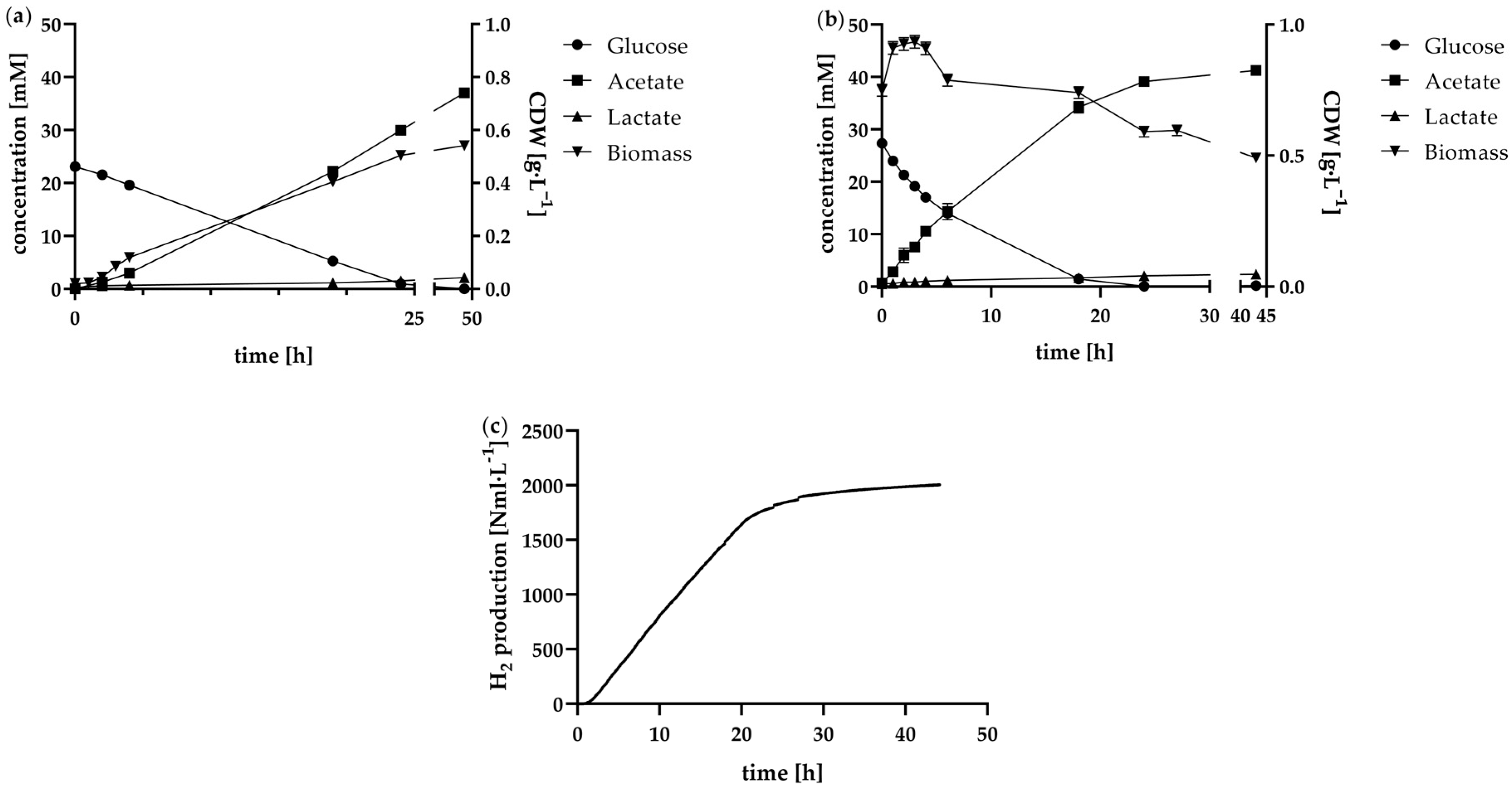
The flattening curve of biomass (
Figure 4a) combined with a high viability of 95% suggests that the batch is at the start of the stationary phase after 24 h [
33]. Subsequently, a rapid decline in cell viability was observed, with a decrease to 68% at 48 h, indicating a limited capacity to sustain cells under conditions of nutrient depletion and by-product-accumulation. This observation is supported by the available glucose, which is shown in
Figure 4a for the batch and
Figure 4b for the batch with increased inoculum density. While viability declined more rapidly in the batch with increased inoculum density compared to the batch, this was also accompanied by a more rapid depletion of nutrients. The consumption of glucose in the first hours of fermentation is correspondingly higher in the increased inoculum density batch compared to the normal batch. This initially supports a correlation with decreasing glucose concentration, viability also decreases.
In contrast, the fed-batch approach demonstrated a more gradual decline in viability, starting from 100%, slight decreasing to 95% at 48 h and further decreasing to 84% at 144 h. Nevertheless, viability dropped to 38% by the end of fed-batch fermentation, even though the glucose concentration (
Figure 5a) continued to rise after 72 h of fermentation, reaching a final concentration of 37.2 ± 0.2 mM. Thus, the glucose concentration at the end was even higher than the starting concentration of all fermentation batches. Since, in addition to glucose, the other nutrients of the modified TBGY medium were also added to the feed throughout the entire fermentation period, this suggests that the availability of nutrients alone does not guarantee high cell viability over a longer fermentation period.
High-density batch cultures exhibited high initial viability, which indicates that the process of centrifugation for preparing the fermentation approach did not damage most cells. Nonetheless the viability was reduced to 10% within the first 24 h displayed, indicates that even elevated cell concentrations cannot mitigate long-term viability loss in batch setups.
The consistently high viability of 97% observed in the continuous mode can be attributed to the absence of inhibitory by-product accumulation, as cells are continuously flushed out and replaced by freshly generated cells. This constant renewal maintains a stable and active cell population, supporting prolonged hydrogen production without the inhibitory effects seen in batch and fed-batch processes. This demonstrates that even at the lowest dilution rate tested (D = 0.03 h
−1), there was a sufficiently high nutrient supply. Since the glucose in the feed was almost completely consumed, the glucose concentration in the reactor was mostly at the lower measurement limit of the HPLC method described, which was approximately 0.5 mM. Dreschke et al. also observed that, despite very low glucose concentrations in the reactor, continuous fermentation of
T. neapolitana with active cells worked over a longer period of time [
15]. There, a constant low glucose concentration (approximately 2 mM) was also observed at a residence time of 24 h (corresponding to D = 0.0417 h
−1). The H
2 production rate and yield, which remained constant over several days, suggest that the bacteria were supplied with sufficient nutrients.
3.2. Bacterial Cell Growth
Initially, the estimation of possible dilution rates for continuous processes and feeding rate for fed batch was conducted. This was achieved by performing normal batch cultivation and batch cultivation with high cell density at the beginning. In consideration of the results obtained, the highest growth rate and substrate consumption could be calculated.
In addition to the metabolisation of glucose,
Figure 4a also provides a representation of the course of biomass formation in a normal batch mode fermentation. Based on biomass concentration measurements, the exponential growth phase between 1 h and 3 h was identified. The maximum growth rate µ
max was 0.77 ± 0.06 h
−1.
Despite this growth rate being marginally lower than the value of 0.94 h
−1 reported by Yu [
41] and Jannasch et al., who calculated a similar growth rate from a doubling time of 0.75 h (equivalent to 0.92 h
−1) [
11,
41], it is notably higher than the rate reported by Frascari et al. for suspended cells with glucose as a substrate, which was 0.024 ± 0.005 h
−1 [
42]. Differences in media composition and preculture treatments likely account for these variations, highlighting how specific cultivation conditions can highly influence the maximum growth rate.
The maximum growth rate delineates the upper limit for feasible dilution rates in continuous operation, as higher dilution rates would lead to cell washout, exceeding the rate of cellular growth [
43,
44]. However, when utilising the maximum growth rate to formulate a fed-batch or continuous process, it is imperative to acknowledge that the conditions experienced by the cells during these processes are not uniform. In the context of fed-batch fermentation, by-product accumulation like acetate is limiting the maximum growth rate. For instance, it has previously been demonstrated that the biomass growth of
T. neapolitana was reduced by 43% when 240 mM was added [
15]. Another approach to determining optimal dilution rates is to consider the maximum substrate consumption rate, since the feed medium for continuous operation was identical to that used in batch fermentation. In this study, the maximum glucose consumption rate was found to be 0.97 ± 0.10 mmol·L
−1·h
−1. It is important to note, however, that cell density at the commencement of batch fermentation at least until 4 h, is significantly lower than after 24 h of fermentation (modified
t-test according to Welch
p < 0.05). Within the first 24 h, the substrate was almost entirely depleted, similar to findings in other batch studies using a pH-controlled bioreactor with glucose as the substrate [
20,
37]. Accordingly, the biomass concentration also almost reached its maximum after 24 h at 0.50 ± 0.02 g·L
−1. It was observed that there was a paucity of metabolic activity in the following 24 h of fermentation. As demonstrated in
Figure 3, this phenomenon is also reflected in the viability of the cells during the fermentation process. While the viability just decreased to 95% in the first 24 h of fermentation, it declined to 68% in the further course. From this point on it can be concluded that at later stages of the fermentation with higher cell densities in a batch approach, optimal growth conditions were no longer present. This can also be attributed to the fact that substrate was no longer available in excess and by-products accumulated in the reactor as a result of metabolism, which can have inhibiting effects. In order to be able to simulate how high the substrate consumption rate can be with cultures of increased biomass concentration, this was also determined for the batch approach with increased cell concentration. In the high-cell-concentration batch, a glucose consumption rate of 2.58 ± 0.16 mmol·L
−1·h
−1 was determined for the period between 0 and 4 h, during which exponential growth occurred in the normal batch. This value indicates how fast the substrate consumption rate can be under optimal conditions without inhibition by by-products. The feed rate for the fed-batch and the dilution rate for the chemostat were based on these results.
In contrast to the standard batch, no exponential growth of the bacteria was observed in the high cell density batch, as illustrated in
Figure 4b. The biomass concentration exhibited an increase from 0.75 to 0.91 g·L
−1, followed by a period of stagnation and subsequent decline after 4 h. With a cell viability of 87% at the start of fermentation, the concentrated cell inoculum demonstrated high viability, indicating that the initial cell concentration was suitable for the majority of cells. This high biomass concentration, along with an increased substrate consumption rate, contributed to correspondingly elevated H
2 production rates. As illustrated in
Figure 4c, H
2 production between 1 h and 17 h exhibited a nearly linear trend. Calculated at 1 h intervals, the average H
2 production rate during this period was 89.0 ± 6.5 Nml·L
−1·h
−1. A total of 2005 ± 44 Nml·L
−1 H
2 was produced over the fermentation period of 44 h. In a 2.4 L batch fermentation, the maximum H
2 production rate reported by d’Ippolito et al. was 51 mL·L
−1·h
−1 [
20]. In a batch fermentation bioaugmented with
T. neapolitana for H
2 production by dark fermentation, the maximum H
2 production rate was 1.44 mmol·L
−1·h
−1, which is equivalent to approximately 32.3 Nml·L
−1·h
−1 [
45]. The higher production rate measured here for almost the entire period during which glucose was present in the medium indicates that the higher cell density from the outset can contribute to a consistently high hydrogen production rate. The virtual halt in further H
2 production after 17 h of fermentation is also accompanied by the depletion of glucose, as this drops from 27.4 mM to just 1.7 mM within the first 18 h, falling to 0 mM after 24 h. This highlights the connection between nutrient availability and the ability of bacteria to produce H
2 efficiently.
In order to determine what was produced in the process of fermentation in addition to the products already mentioned, further by-product formation analysis was conducted. As already known from
T. neapolitana, alanine was detected. However, another amino acid, namely glutamate, which has not been described as a by-product previously, was also detected. An increase in alanine (
Figure S1) and glutamate (
Figure S2) concentration could be observed. Both batch approaches ended up with comparable amino acid concentrations. The concentration of alanine attained its maximum at 0.84 ± 0.01 mM, while highest recorded glutamate concentration was 0.67 ± 0.08 mM. Alanine as by-product is known from
T. neapolitana in low amounts. However, little is known so far about glutamate production. Nonetheless, a glutamate dehydrogenase has been described previously for a closely related species,
T. maritima [
46]. The concentrations measured here are comparatively low compared to the main by-products produced. Others with considerably higher production rates, such as
Corynebacterium glutamicum, have already been established for the industrial production of amino acids [
47]. Here, the analysis of the amino acids produced is primarily used to determine the products into which the glucose is metabolised, given that all by-products can affect the possible H
2 yield.
3.3. Fed-Batch
The fed-batch was initially permitted to run for 26 h in batch mode. Subsequently, he feed was operated at a rate of 0.7 mL·h
−1. At 20-fold glucose feed concentration, this corresponded to a feed of 0.56 mmol
glucose·L
−1·h
−1. The maximum H
2 production rate during fed-batch was 49.2 ± 1.4 Nml·L
−1·h
−1, which was maintained for 4 h during this first feed phase. Following a 35 h of feed supply, the feed was halted in order to ascertain that
T. neapolitana was able to completely metabolise the glucose present (
Figure 5a). As the glucose in the reactor was completely consumed, H
2 production dropped rapidly, as evident from the decline in the H
2 production curve (
Figure 5b). At 73 h, following the complete depletion of glucose in the reactor, the feed rate was increased to 1 mL·h
−1, corresponding to 0.793 mmol·L
−1·h
−1 of glucose. This adjustment initially led to an increase in hydrogen production, indicating that substrate availability directly influences H
2 output. However, despite the increased glucose feed rate, there was no further increase in biomass concentration. Instead, it slightly decreased and subsequently turned stationary. This suggests that a threshold in cell growth was reached, possibly due to limitations from by-product accumulation (e.g., acetate and lactate) or other inhibitory effects specific to the metabolic environment.
One potential explanation for this phenomenon for this is the accumulation of by-products such as acetate and lactate. The concentrations of these two components were similar to the batch approach in the first 48 h but then increased accordingly (
Figure 5a). The inhibitory effect of acetate had already been demonstrated by Dreschke et al. [
15]. In addition to the aforementioned reduction in maximum growth rate due to increased acetate concentrations, this study also showed that H
2 production decreases under these conditions. By increasing the acetate concentration to up to 240 mM at the start of fermentation, H
2 production gradually decreased to a maximum reduction of 45% compared to a batch without added acetate. Another recent study focussed on the inhibition of acetate on hydrogen production by dark fermentation in a bacterial consortium. Here, increased acetate concentrations led to the lactate metabolic pathway being favoured and the composition of the dominant bacteria changed considerably [
34]. After 144 h, the concentration of acetate already exceeded with 84.9 ± 0.5 mM more than twice that of the batch approach at the end of fermentation (37.0 ± 0.2 mM). This phenomenon was reflected in the productivity of the cells. As demonstrated in
Figure 5a, there was an increase in glucose concentration, which was accompanied by a decrease in glucose consumption rate. Conversely, H
2 production exhibited a marked decline, accompanied by a modest decrease in biomass within the reactor. As the cells were no longer able to consume all the glucose supplied from the feed, the feed rate was consequently reduced from 1 mL·h
−1 to 0.7 mL·h
−1 after 146 h of fermentation time, corresponding to a glucose supply of 0.556 mmol
glucose·L
−1·h
−1. A similar trend emerged as the fermentation time progressed. The glucose concentration in the reactor continued to increase, indicating that the glucose consumption rate was still below the feed rate. The by-products acetate and lactate continued to increase, possibly leading to increasing inhibition effects. The H
2 formation rate decreased to an average of 10.0 ± 3.0 Nml·L
−1·h
−1 between 146 h and 217 h. Given that the pH value of the reactor was consistently maintained at 7.35, the inhibitory effect due to the accumulation of the acids produced cannot be attributed to pH reduction. In this case, the increasing ionic strength in the reactor may potentially exert deleterious effects on the bacteria. During this period, a slight decrease in biomass was observed, and viability fell from around 84% to 40%. As this occurred despite continuous feeding and the associated nutrient supply, it could also be explained by the aforementioned effect of individual cells losing their ability to proliferate after a certain fermentation time, regardless of the nutrient supply available [
33]. Due to the continuing increase in glucose concentration, the feed rate was further adjusted downwards from time 217 h, being changed to 0.5 mL·h
−1, corresponding to 0.396 mmol
glucose·L
−1·h
−1. During this period of fermentation, H
2 was still produced, but at a lower production rate of 5.4 ± 3.1 Nml·L
−1·h
−1. Even at the lower feed rate, the rate of glucose metabolism was found to be less than the rate of glucose supply.
Interestingly, the concentrations of the by-products alanine and glutamate, which are depicted in
Figure 5c, show different courses. The concentration of the analysed amino acids was found to be above 0 mM at the commencement of fermentation, mainly due to the addition of yeast extract to the media. In this instance, an increase in concentration was observed from the start of fermentation until after 144 h fermentation time. Alanine increased from 0.18 ± 0.03 mM to 1.99 ± 0.04 mM and glutamate from 0.13 ± 0.03 mM to 1.05 ± 0.07 mM. Unlike the continuous accumulation observed for acetate and lactate, glutamate concentration stabilised, remaining relatively constant until the end of the fermentation process. Conversely, alanine levels exhibited a decline, indicating a transition from synthesis to utilisation. Considering the initial amino acid concentrations (e.g., 0.18 mM alanine from yeast extract at 0 h) and the low amino acid input from the feed, which primarily contained glucose in concentrated form, it is likely that the ongoing addition of amino acids was minimal. Accordingly, it is conceivable that
T. neapolitana consumed rather than produced amino acids in the later course of the fermentation, possibly as an adaptive response to prolonged fermentation conditions or limited nutrient availability.
An additional indication that the cells may have experienced inhibition in their metabolic pathway toward acetate and H
2 is the accumulation of another by-product ethanol. While ethanol could not be quantified in all batch or continuous approaches (c < 0.1 g·L
−1), the fed-batch showed an increasing ethanol concentration in the reactor over the course of 72 h fermentation time. The concentration profile is displayed in
Table 2 and reached its maximum of 0.61 ± 0.04 g·L
−1 at the end of fermentation. Although
T. neapolitana possesses the necessary enzymatic system required for ethanol production, previous studies indicate that the activity of these enzymes is relatively low [
21]. This finding indicates that ethanol accumulation may occur under specific conditions, such as those present in the fed-batch process, where inhibition of primary pathways (e.g., acetate and hydrogen production) prompts the cells to redirect metabolism toward ethanol as an alternative by-product. H
2 and CO
2 are unlikely to cause product inhibition, as they readily escape as gases and are continuously expelled through N
2 sparging. However, dissolved by-products that accumulate in the liquid phase can reach inhibitory concentrations, potentially leading to a metabolic shift towards ethanol production. This accumulation of liquid-phase inhibitors may alter the metabolic balance, favouring ethanol as an alternative by-product under higher concentration conditions. As H
2 is produced in the same metabolic pathway as acetate, inhibition of acetate production also results in lower H
2 production. It would therefore be advisable to plan for in situ product removal of acetate if longer lasting H
2 production in the fed-batch is intended.
In addition to the expected results that in the course of a batch process, the viability decreased with time and the running out of the substrate, the same could also be determined for the fed-batch. In this instance, the consumption of the substrate was not the limiting factor that caused the viability to decrease over time (
Figure 3). The inhibition caused by the accumulation of by-products inhibited the cells in their metabolism and growth. In this context, it is plausible to hypothesise that the ionic strength of the medium, rather than merely its by-products, was the pivotal factor. This naturally also increased due to the accumulation of by-products. Various experiments with different buffer concentrations have already shown that increased buffer concentrations may exert inhibitory effects on
T. neapolitana [
48]. Thus, by-product enrichment and the associated increase in ionic strength could also have a negative effect on
T. neapolitana. In order to better define the influence of ionic strength, the osmolarity of the batch fermentation preparations would have to be compared with that of the fed-batch. Since it is not possible to analyse the extent to which the individual media components are completely or only partially bound in the production of biomass and metabolites, the osmolarity at the start of fermentation can be compared with the potential change due to glucose metabolism and NaOH addition. Neglecting yeast extract, which is not clearly defined, the osmolarity in the modified TBGY medium used here is 804 mOsmol·L
−1. The metabolism of glucose to the dissolved products and the addition of NaOH resulted in a theoretical increase in osmolarity of 142 mOsmol·L
−1 in the batch. The calculation took into account that the acids produced and the NaOH added dissociate in aqueous solutions and form the corresponding salts under fermentation conditions. This avoided double counting of the dissociating substances. In the fed-batch, the increase in ionic strength is greater. This phenomenon can be attributed, firstly, to the elevated glucose concentration in the feed, which is 20 times higher than in the initial medium. Secondly, it is due to the presence of higher concentrations of metabolic products in general, as well as a greater utilisation of NaOH for pH regulation. This resulted in a theoretical increase in osmolarity of 342 mOsmol·L
−1 over the entire fermentation period in the fed-batch process. In any case, it can be deduced from the viability profile that a fed-batch can no longer maintain high viability rates over several weeks under the tested conditions. Whilst the viability remained relatively high at 84% after 144 h fermentation time, it decreased to only 40% after 214 h. After 336 h, viability was at its lowest at 38%. It is important to note that the staining method was only able to differentiate between living and dead cells. Accordingly, cells that died and were completely lysed during fermentation could not be detected. As the fermentation lasted a total of two weeks and low cell viabilities were present at later times, it can be assumed that several cells were already lysed during fermentation.
3.4. Continuous Fermentation
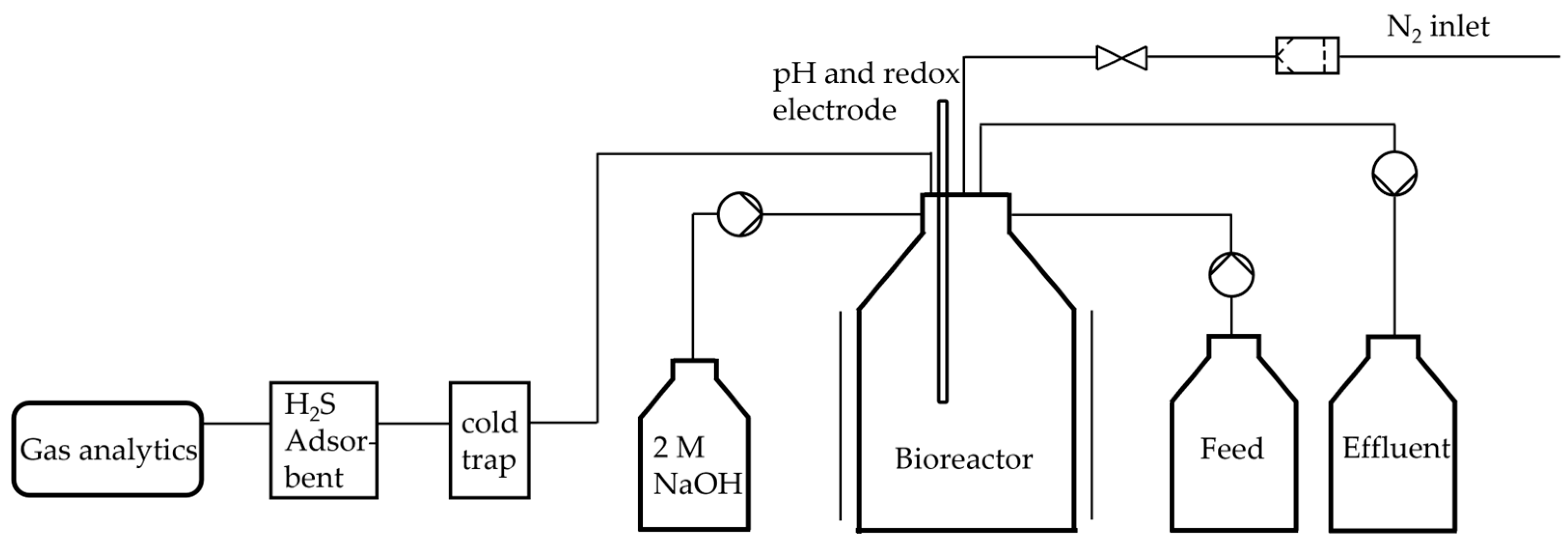
Due to the limitations observed in the fed-batch system, particularly with regard to extended fermentation times, a continuous bioreactor was implemented following the setup outlined in
Figure 1. The continuous supply of fresh medium and removal of used medium ensured that no by-products could accumulate in the medium in higher concentrations. The dilution rates of 0.03 h
−1, 0.05 h
−1, 0.07 h
−1 and 0.1 h
−1 that were examined in this study were derived from the maximum observed glucose consumption rates of the batch runs. Since the feed had the same composition as the TBGY media described, the glucose feed rates were accordingly 0.833 mmol
glucose·L
−1·h
−1, 1.388 mmol
glucose·L
−1·h
−1, 1.943 mmol
glucose·L
−1·h
−1 and 2.775 mmol
glucose·L
−1·h
−1. The glucose concentration in the reactor increased from 0.61 ± 0.28 mM to 5.99 ± 0.17 mM at a dilution rate of 0.1 h
−1. This indicates that the critical dilution rate was already exceeded. Accordingly, a steady state could not be achieved. Steady state was observed for all other tested dilution rates. The steady state was characterised by the fact that the glucose concentration in the reactor remained constantly low and fluctuated by less than 0.5 mM. Consequently, it can be deduced that the substrate provided was almost fully utilised. The highest glucose consumption rate in chemostat mode was 2.19 ± 0.05 mmol
glucose·L
−1·h
−1. This observation is consistent with the results of the maximum glucose consumption rate, which could be determined in batch fermentations. Here, a glucose consumption rate of 2.58 ± 0.16 mmol
glucose·L
−1·h
−1 could only be determined for the batch with an increased starting cell concentration under optimum conditions. This is marginally lower than the glucose supply rate at the 0.1 h
−1 dilution rate in continuous operation. Theoretically, based on the maximum growth rate, which was µ
max = 0.77 ± 0.06 h
−1 during the batch fermentation in exponential growth phase, even considerably higher dilution rates would be conceivable. Several factors likely contribute to the observed discrepancy between the maximum growth rate and the achievable dilution rate in continuous operation. One primary factor is the significantly higher cell density in the steady state of the continuous reactor through all dilution rates compared to the exponential growth phase in batch mode (modified
t-test according to Welch
p < 0.05). The biomass grew between 0.023 ± 0.009 g·L
−1 and 0.085 ± 0.011 g·L
−1 during the exponential growth phase. The continuous reactor with a dilution rate of 0.03 h
−1 had an average biomass concentration of 0.550 ± 0.065 g·L
−1 during the steady state. The elevated biomass concentration could therefore prevent the cells from continuing to grow at the maximum possible growth rate, as the availability of nutrients might be limited, or the cell density itself inhibits further growth. This also indicates that, as already mentioned, the maximum growth rate can vary greatly under different conditions and is not consistent over extended periods. Accordingly, the specific growth rate can be lower for a longer period of time. With an experimentally determined maximum dilution rate D = 0.07 h
−1, this is noticeably higher than the prognosed optimal dilution rate of D = 0.041 h
−1 predicted by Frascari et al. for dissolved cells, which is based on substrate to product and biomass conversion rate and yield [
42]. The results are displayed in
Figure 6a–d.
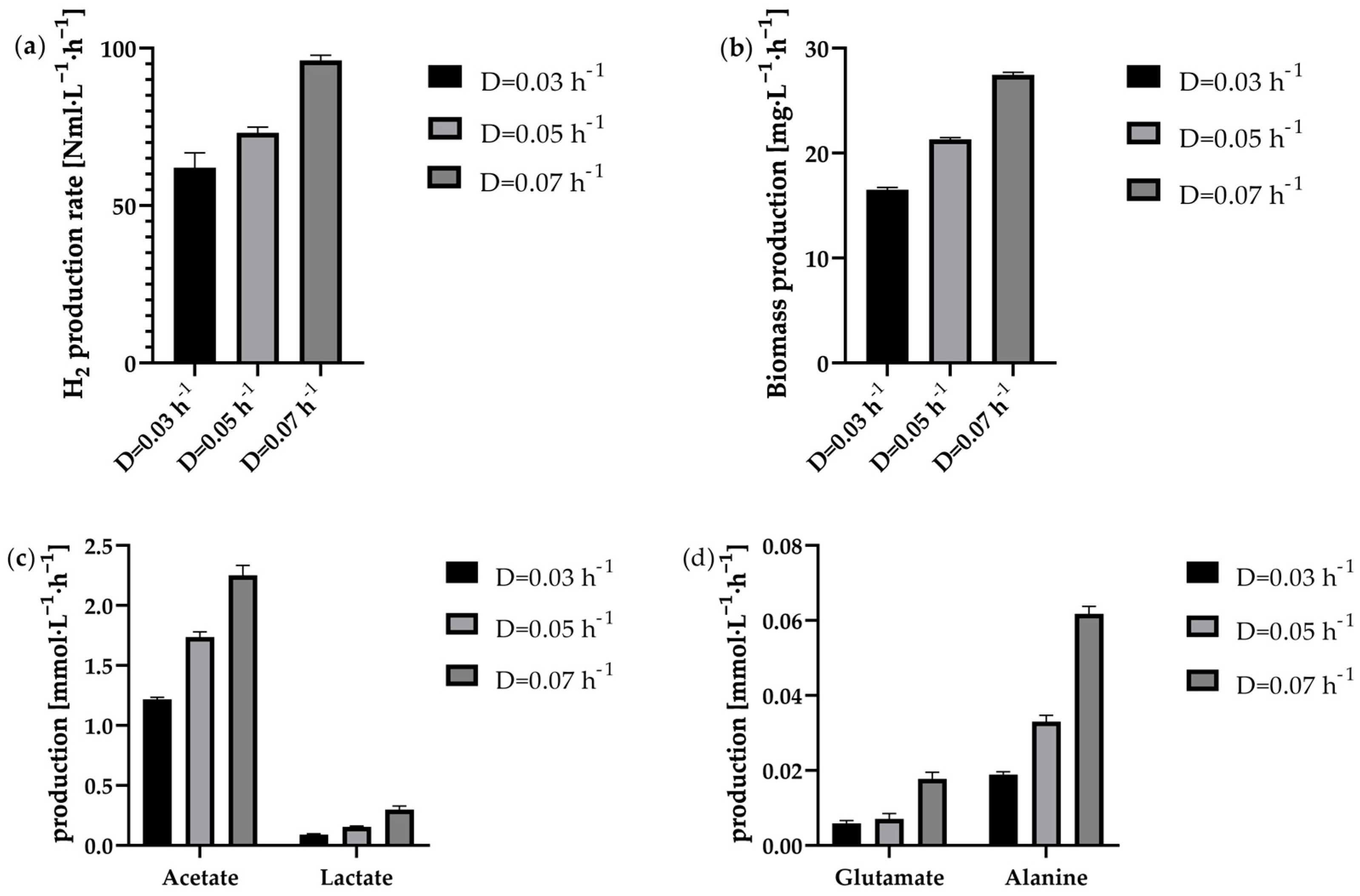
The H2 production rate was found to be consistent overall, indicating a stable steady state. Concurrently, the biomass concentration remained constant. So was the viability of the cells in continuous operation very high at steady state for all dilution rates tested and averaged 97 ± 3%. Thus, in contrast to the batch and fed-batch approaches, there was no recognisable downward trend over the entire steady state period. This indicates that suitable fermentation conditions were generally available for the cells at all different dilution rates. But as the cells are also permanently rinsed out during the continuous process, the cells are only in the bioreactor for a certain time depending on the respective dilution rate. In this case, the residence time was 33.3 h (D = 0.03 h−1), 20.0 h (D = 0.05 h−1) or 14.3 h (D = 0.07 h−1). For periods of time in this range, a comparably high viability was also observed at the start of fermentation in the batch or fed-batch. Nonetheless, it can be hypothesised that, during the fermentation process, a proportion of cells underwent lysis. However, upon their demise, they became undetectable.
Figure 6a–d illustrate that both biomass and metabolic production rates increased with increasing dilution rates, suggesting that the cells can utilise the higher nutrient supply. This is also reflected by the fact that the supplied glucose was almost entirely consumed in all cases. The increasing production rates of alanine and glutamate also suggest that nitrogen availability could increase with higher dilution rates, since amino acid production requires energy-rich carbohydrates, such as glucose, as well as usable nitrogen sources. In this case, alanine is synthesised from the glycolysis end product pyruvate via transamination [
20]. Another indication is that the sustained high viability of the cells, which remained at a constant level close to 100% compared to batch and fed-batch approaches, resulted from a higher nutrient supply. As depicted in
Figure 6a, the H
2 production rate increased with increasing dilution rate. While at D = 0.03 h
−1 it was 62.1 ± 4.7 Nml·L
−1·h
−1, at D = 0.07 h
−1 it was even 96.1 ± 1.7 Nml·L
−1·h
−1 at steady state. An almost comparable increase in biomass production was also observed at higher dilution rates (
Figure 6b). Reasons for this phenomenon pertains to the enhanced availability of glucose at elevated dilution rates. For the by-products analysed here, a trend of increasing production rate with increasing dilution rate could also be observed. However, the ratio of acetate to lactate production shifted slightly in favour of lactate as the dilution rate increased. While at the lowest dilution rate D = 0.03 h
−1 the ratio was 13.4 acetate: 1 lactate, at D = 0.05 h
−1 this ratio fell to 11.1 acetate: 1 lactate. At D = 0.07 h
−1, the ratio was even decreased to 7.5 acetate: 1 lactate. While all of the dilution rates tested in a steady-state condition displayed high levels of viability, a consistent H
2 production rate and nearly complete glucose consumption, a transition in the acetate-to-lactate ratio was observed as the dilution rate increased. In order to achieve a more comprehensive understanding of the underlying causes of this phenomenon, future research initiatives should incorporate metabolic flux analyses as a crucial component within their experimental designs. It is imperative to minimise the lactate production rate, given the competition between the metabolic pathways to lactate and H
2 [
14]. However, it has been demonstrated that the lactate production does not necessarily have to reduce the H
2 yield, as
T. neapolitana, or the strain
T. neapolitana cf capnolactica (DSM33003) used in some cases, appears to be able to form lactate from acetate and CO
2 without negatively affecting H
2 production [
16,
49]. Activating this metabolic pathway could potentially be used to achieve higher H
2 production rates. Dipasquale et al. report that gassing the reactor with CO
2 instead of N
2 increased the maximum H
2 production rate from 34.6 ± 4.7 mL·L
−1·h
−1 to 67.0 ± 15.6 mL·L
−1·h
−1 [
16]. At the same time, glucose was also consumed more quickly during this period. Higher glucose consumption rates would suggest that higher dilution rates can also be set in the continuous process. However, it must be noted that gassing with CO
2 leads to an increased NaOH supply due to the drop in pH value. In fed-batch processes, this could lead to decreasing performance over a longer period of time, as the ionic strength increases even more with time. In continuous processes, on, however, the effect is expected to be less noticeable, as the medium is continuously renewed. However, the shift in the ratio of acetate to lactate produced towards lactate also suggests that the nutrient supply is noticeably higher at higher dilution rates. The breakdown of pyruvate to acetate enables the generation of an additional ATP molecule via acetate kinase [
13,
50]. However, if pyruvate is now reduced to lactate, no energy is generated in the form of ATP. Nevertheless, NAD
+ can be regenerated in this way, which is otherwise provided by hydrogen synthesis. Since the yield of hydrogen remained very similar at all dilution rates, the increased ratio of lactate to acetate could indicate that the cells prioritise the regeneration of NAD
+ at higher nutrient supply levels due to a possible excess of NADH. However, further experiments on metabolic analysis would be necessary to validate this hypothesis.
An examination of the yields for all stable dilution rates showed hardly any differences in terms of product yields. As illustrated in
Figure 7a, the H
2 yield at steady state for the dilution rates tested was between 2.7 ± 0.1 mol
H2·mol
glucose−1 (D = 0.05 h
−1) and 3.0 ± 0.1 mol
H2·mol
glucose−1 (D = 0.07 h
−1). Furthermore, the yields demonstrated stability during the steady-state period, indicating consistent physiological responses. The yields for acetate and lactate also showed hardly any differences. Only the yields of the produced amino acids alanine and glutamate showed a slightly increased yield at the dilution rate of D = 0.07 h
−1 (
Figure 7b). The calculated yield of 0.044 ± 0.002 mol
alanine·mol
glucose−1 is comparable to the previously reported alanine yield [
51]. However, among other things, production of alanine ensured the H
2 yield was below the theoretical maximum. It has previously been demonstrated that both pyruvate and reduction equivalents are necessary for the production of alanine [
16,
20,
52]. As a consequence, these components are not available to the maximum possible extent for H
2 production. Conversely, it has been demonstrated that alanine, amongst other amino acids, can function as a nutrient source [
53], a finding that is consistent with the results obtained from the fed batch process previously outlined. Accordingly, the use of pyruvate and reduction equivalents for alanine production may be counteracted by specifically adding small amounts of alanine to the fermentation.
One potential approach to enhance the system would be to implement a membrane that facilitates the retention of cells within the bioreactor. It has already been shown that an increase in the biomass concentration can result in accelerated glucose metabolism [
51]. Higher biomass concentration could also overcome possible inhibition because of higher glucose concentration in the feed. As shown by Dreschke et al. increased glucose concentration from 11.1 to 41.6 mM in the feed, reduced noticeably the hydrogen yield [
15]. An enhanced glucose metabolism would confer numerous benefits, especially with regard to scale-up. In this way, higher throughputs could also be achieved with lower volumes, thereby rendering the entire process more cost-effective.
Another option for optimising the continuous system to enable an increase in the dilution rate would be to immobilise the cells in the reactor. Successful approaches for this have already been demonstrated [
38,
42]. This would serve to prevent a washout, even at higher dilution rates. However, it would have to be ensured that the substrate is as favourably accessible as possible for the cells and is metabolised expeditiously.
In addition to the individual production rates in a continuous process, the carbon balance is an important aspect to consider. This can provide information on whether all the main products produced have already been identified and quantified. Furthermore, an overview of the proportions of the products produced can provide information on whether contamination or a shift in metabolism has occurred over time.
The carbon balance, which is illustrates in
Figure 8, refers to the substrate consumption and the resulting products in continuous operation at steady state. Empirical analysis of
T. maritima revealed a carbon content of 45.9% of the total cell dry weight [
54]. In this study, a C content of 38.7 ± 0.2% was determined experimentally for
T. neapolitana. For this purpose, the dried cell biomass from the fermenter was analysed as part of a C-H-N analysis using a Vario EL cube elemental analyser. It is initially noticeable that the overall balance of 102.8% ± 7.4% exceeds the theoretical maximum. Compared to the carbon balance of Munro et al. [
18] the value is quite similar, though. This could be explained by the assumption that the small amounts of yeast extract present in the media were also used to synthesise biomass. As the composition of yeast extract is not well defined, it is difficult to estimate the extent to which carbon from this source has been utilised. Nonetheless, given that the modified TBGY medium contains 0.5 g·L
−1 of yeast extract and 5 g·L
−1 of glucose, a maximum deviation of up to 10% cannot be ruled out in theory. However, the balance suggests that all carbon-containing products that are formed in noticeable quantities have been found and quantified here. In conclusion, the analysis revealed that acetate constituted the predominant carbon fraction, accounting for 54.0% of the total carbon content. This was followed by CO
2 (27.3%) and biomass (11.1%). As acetate is produced in the same metabolic pathway as H
2, a correspondingly high acetate production is also to be expected with a high H
2 production [
52,
55]. Given that the carbon utilised at the inception of the process could be recovered in the form of various products, potential further utilisation options are quite calculable. For instance, the biomass can be used as a favourable substrate for further fermentation processes. The utilisation of CO
2 gas in this manner paves the way for the subsequent production of methanol from CO
2 and H
2. Methanol has been considered a viable solution for the purpose of both capturing CO
2 and enabling the H
2 to be stored and transported more easily [
56].