1. Introduction
Staphylococci are Gram-positive, non-spore-forming bacteria with facultative anaerobic metabolism, and they are either catalase-positive or -negative, characterized by a cocci morphology that is grouped in clusters [
1,
2]. Within the genus
Staphylococcus,
S. aureus is a major etiological agent of foodborne illness due to its ability to produce heat-stable enterotoxins [
3].
S. aureus can grow across a wide temperature range, from approximately 7 °C to 48 °C (with optimal growth around 35 °C), and within a pH range from 4.2 to 9.3 (with an optimum near pH 7.0). It also exhibits high tolerance to sodium chloride, growing in concentrations up to approximately 15%. These physiological characteristics enable
S. aureus to survive in dry and osmotically stressful environments that are inhibitory to many other microorganisms [
4].
S. aureus exhibits moderate heat resistance due to its inability to produce spores (D-value of approximately 6 min at 60 °C; 1 min at 65 °C). Notably, the heat tolerance of
S. aureus can increase under certain environmental conditions, such as in the presence of elevated sodium chloride concentrations, which offer protective effects against thermal inactivation [
5]. Staphylococcal enterotoxins (SE) are tasteless, water-soluble, and highly resistant to heat (enterotoxin type A, D
121 = 28 min; enterotoxin type B, D
149 ≈ 100 min) as well as to adverse environmental conditions such as freezing. The growth of
S. aureus is typically accompanied by the production of SE, reflecting a close correlation between proliferation and toxinogenesis. The risk of acute food poisoning in healthy individuals arises when
S. aureus populations exceed 6 log colony-forming units per gram (cfu/g) in food, a threshold commonly associated with the accumulation of SE at levels of 20 ng or higher [
6,
7]. SE production has been documented predominantly under conditions where temperatures exceed 10 °C and water activity (
Aw) values are greater than 0.85, reflecting the environmental requirements for toxinogenesis [
8].
In the United States, the Food and Drug Administration (FDA) recognizes that
S. aureus does not typically grow or produce enterotoxins at
Aw values below 0.85. Thus, this threshold is widely adopted as a critical limit in food safety programs to prevent toxin formation in ready-to-eat and shelf-stable products. Similarly, SDA Ordinance No. 748 (Brazil, 2023) establishes the same
Aw threshold (≤0.85) for shelf-stable bacon. This regulatory limit is based on the growth characteristics of
S. aureus and aims to inhibit enterotoxin production, thereby ensuring the microbiological safety of the product under non-refrigerated storage conditions [
9].
Achieving low
Aw levels typically requires intensified drying processes or elevated salt concentrations, which can result in undesirable alterations to the texture of the product. Products processed under these conditions often exhibit a tougher texture, excessive saltiness, and reduced juiciness [
10]. These modifications may negatively impact consumer acceptability, particularly in markets where moist, tender, and mildly salted bacon is preferred [
11]. Although the
Aw threshold represents a critical hurdle in restricting
S. aureus growth and SE formation, it should not be regarded as the sole determinant of microbial safety in bacon. Other intrinsic and extrinsic parameters, including pH, salt concentration, storage temperature, packaging atmosphere, and the presence of competitive microbiota, also exert a significant influence on bacterial load, proliferation dynamics, and SE formation. When considered collectively, these factors operate synergistically within the framework of hurdle technology, reinforcing microbial stability and safety [
12]. Thus, a comprehensive assessment of product preservation must integrate the interaction of
Aw with additional hurdles to ensure both safety and quality. This underscores the importance of exploring complementary or alternative strategies.
This study aimed to propose a biosafe strategy to prevent the production of SE in cooked bacon under variable Aw conditions, particularly in microenvironments at the product–package interface that may favor toxinogenesis, hypothesizing that the reduction in Aw alone may not be sufficient to prevent SE formation in bacon, since SE can also be produced at the product–package interface.
2. Materials and Methods
2.1. Experimental Design
The study was carried out using three sample groups with distinct
Aw levels, designated as B1 (
Aw ≈ 0.85), B2 (
Aw ≈ 0.94), and B3 (
Aw ≈ 0.97). A defined concentration of
S. aureus (approximately 4 log cfu/g) was superficially inoculated onto the bacon samples by immersing the product for 1 min in a vessel containing a suspension of the microorganisms in sterile deionized water. The groups were subdivided into three categories:
i) blank (B1-B; B2-B; B3-B) received no additional treatment,
ii) control (B1-C; B2-C; B3-C) received 1.0% sterile distilled water, and
iii) treatment (B1-T; B2-T; B3-T) received 1.0% of the biopreservative. The packages were vacuum-sealed under controlled conditions and subsequently subjected to challenge testing in accordance with the MicroLab_ShelfLife protocol [
13]. This approach enables the simulation of realistic storage dynamics, allowing for the quantitative assessment of microbial growth potential and spoilage kinetics under defined environmental scenarios. Additionally, SE formation was assessed using the enzyme-linked immunosorbent assay (ELISA) technique.
2.2. Biopreservative Production
The biopreservative was produced at the fermentation facility of BRC Ingredientes Ltda., located in Rio Claro, São Paulo, Brazil. The fermentation was conducted using Lacticaseibacillus paracasei DTA 83. The complete genome sequence of the strain is available in the GenBank database under accession number QRBH00000000. Fermentation was conducted in a 3000 L AISI 304 stainless steel bioreactor equipped with anchor-type impellers, digital PID temperature control, pH and dissolved oxygen probes, and external cooling via a plate heat exchanger. An optimized culture medium was prepared directly in the tank using 3000 L of potable water, heat-treated at 80 °C for 5 min (1 °C/min heating rate) under 60 rpm agitation, and cooled to 36 °C. The inoculum, produced in the laboratory through a sequential 1:10 scale-up to 30 L, was cultivated at 36 °C for 18 h (log phase) and added at 1% (v/v), yielding an initial load of ≈7 log cfu/mL. Fermentation proceeded for 15 h at 36 °C with continuous inline monitoring. The final broth was heat-treated at 80 °C for 5 min and hot-filled into 20 L polypropylene containers. This heat-treatment parameter was established by the regulatory agency at the time the product was approved.
2.3. Production of Samples
Three distinct salting methods were used to produce samples of cooked bacon with various Aw. For the group with Aw ≈ 0.85 (B1), a dry curing mixture was prepared containing 20% of a commercial bacon mix composed of 52.1% sodium chloride, 34% sodium tripolyphosphate, 12% sodium erythorbate, and 1.9% sucrose (BRC Ingredientes, Rio Claro, Brazil); 3.5% curing salt (90% sodium chloride, 6% sodium nitrite, 4% sodium nitrate); 56.5% sodium chloride; and 20% sucrose. This mixture was applied to pork bellies in a 1:6 ratio, and the cuts were stored under refrigeration at 4 °C for 6 days to promote dehydration and reduce Aw. For the group with Aw ≈ 0.94 (B2), a brine was prepared consisting of 74% water and ice (1:1), 5% of the commercial bacon mix, 1% curing salt, 15% sodium chloride, and 5% sucrose. Pork bellies were immersed entirely in the brine for two days at 7 °C, followed by an additional three days of refrigerated storage to stabilize Aw. For the group with Aw > 0.97 (B3), the same brine formulation used in the group B2 was injected into the pork bellies at 20.6% of their weight (v/w), and the injected cuts were kept under refrigeration for 24 h before thermal processing.
The presence of
S. aureus in bacon samples as well as in the biopreservative was determined according to ISO 6888-1 (2021). Briefly, 25 g of bacon or 25 mL of biopreservative were aseptically homogenized in 225 mL of sterile 0.1% peptone water using a stomacher for 60 s or by manual rotating movement, respectively. Serial decimal dilutions were prepared by transferring 1 mL into tubes containing 9 mL of the same diluent. Aliquots of 0.1 mL from appropriate dilutions were surface-spread onto Baird-Parker agar (HiMedia, Mumbai, India) plates supplemented with egg yolk tellurite emulsion, using a sterile hockey stick spreader. The plates were incubated at 37 ± 1 °C for 24–48 h, and presumptive colonies (black, shiny, convex, with a clear zone around them) were examined [
14].
S. aureus strain ATCC 25923 was obtained in lyophilized form from the American Type Culture Collection (ATCC). The complete genome sequences of the chromosome and plasmid have been deposited in DDBJ/ENA/GenBank under the accession numbers CP009361 and CP009362, respectively [
15]. Previous studies demonstrated that SE formation is strongly influenced by factors such as
Aw, pH, and temperature [
12]. Therefore, the strain represents a relevant model for assessing the risk of SE formation in bacon, particularly under post-processing scenarios favorable to
S. aureus proliferation. The culture was activated through three successive growth cycles in BHI broth (HiMedia, Mumbai, India) at 36 °C for 24 h. Cells were washed twice by centrifugation (6000×
g, 6 min), resuspended in phosphate buffer (pH 7.2), and vortexed. The suspension turbidity was adjusted with buffer to match the 0.5 McFarland standard, verified spectrophotometrically at 600 nm, corresponding to ≈1.5 × 10
8 cfu/mL.
A sterile vessel containing 5 L of deionized water was inoculated with 500 µL of the S. aureus suspension, resulting in a final concentration of approximately 4 log cfu/mL. Bacon samples, weighing approximately 100 g, were then fully immersed in this inoculated solution for 1 min to promote surface contamination. All groups were superficially contaminated with S. aureus. Following inoculation, the samples were distributed into three experimental groups based on subsequent surface treatment: blank (B1-B, B2-B, B3-B), which received no additional application; control (B1-C, B2-C, B3-C), which received 1% sterile deionized water; and treatment (B1-T, B2-T, B3-T), which received 1% of the biopreservative solution. The biopreservative or sterile deionized water was added to the package prior to inserting the bacon. The net weight of the bacon was used as the basis for calculating the volume to be added to each package.
2.4. Sample Characterization
The
Aw was measured following ISO 18787:2017 [
16]. An AquaLab Lite device (Decagon Devices, Washington, WA, USA), equipped with a dielectric humidity sensor and an infrared surface temperature sensor, was used.
Aw was determined by measuring the electrical conductivity of an electrolyte. The instrument was calibrated using standard salt solutions of potassium sulfate (K
2SO
4;
Aw = 0.973; CAS 7778-80-5) and potassium chloride (KCl;
Aw = 0.843; CAS 7447-40-7). For analysis, samples were cut into small pieces (≈0.3 cm) using a sterile blade and placed in the sample dish to ensure complete coverage of the bottom surface without voids. Stability of the device readings was verified during the analytical sequence.
Texture profile analysis (TPA) was performed in a texture analyzer (TA.XT, Stable Micro Systems, Hamilton, MA, USA) equipped with a 500 N load cell, and analysis software was used. Samples were sectioned axially into 2.0 cm
2. Six measurements were performed per sample, covering both central and peripheral areas, at ambient temperature (≈23 °C). A flat circular plate (7.5 cm diameter, P75) was used to compress the sample to 60% of its original height at a crosshead speed of 200 mm/min, in two consecutive compression cycles, following the protocol described by Desmond and Troy [
17]. The TPA parameters assessed included hardness-1 (peak force during the first compression), hardness-2 (peak force during the second compression), cohesiveness (ratio of the area under the second curve to the first, A2/A1), elasticity (height recovery between compressions), and chewiness (calculated as the product of hardness-1, elasticity, and cohesiveness) [
18]. Hardness-1 and hardness-2 were expressed in N/cm
2; elasticity in cm; and chewiness in J/cm
2.
2.5. In Vitro Efficacy of the Biopreservative
The minimal inhibitory concentration (MIC) of the biopreservative against S. aureus was determined using a modified MIC test, according to the protocol of the method Computational Microbial Density Scanning (CMDS). To assess the antimicrobial efficacy, a 10% (v/v) stock solution of the biopreservative was prepared in BHI broth (HiMedia, Mumbai, India) to ensure sufficient nutrient availability during microbial growth. The solution was sterilized by membrane filtration using a 0.22 µm pore-size filter (K18-2522PES-E, Kasvi, Curitiba, Brazil) and collected in a sterile tube. Serial two-fold dilutions were subsequently performed in the same broth.
The
S. aureus inoculum was prepared as described in
Section 2.3. Following cultivation to an estimated density of 1.5 × 10
8 cfu/mL, corresponding to the 0.5 McFarland turbidity standard, the suspension was serially diluted to reach the target concentration of approximately 6 log cfu/mL.
The antimicrobial tests were conducted in sterile 96-well microtiter plates with lids, arranged in 8 rows (A–H) and 12 columns (1–12). Columns 1 and 12 were assigned to positive and negative controls, respectively. Columns 2 through 11 were used to evaluate increasing concentrations of the biopreservative in the following sequence: 0.05%, 0.1%, 0.3%, 0.5%, 1.0%, 1.5%, 2.0%, 2.5%, 3.0%, and 3.5%. Four rows (A to D) were used to perform independent replicates of each treatment, ensuring reproducibility of the results.
Microbial growth dynamics were monitored using a fully automated microplate spectrophotometer (Epoch2, Biotek, Agilent, Santa Clara, CA, USA) equipped with dedicated software provided by CMDS method for data acquisition and analysis. The device featured an integrated temperature control module and an orbital shaking mechanism programmed to activate prior to each reading. The incubation temperature was maintained consistently at 30 °C throughout the 24 h monitoring period. Optical density (OD) measurements were acquired at 620 nm using a fixed monochromatic filter, and readings were recorded at 30-min intervals. Prior to each measurement, the 96-well plate was automatically shaken using orbital agitation at a frequency of 282 cycles per minute (3 mm amplitude) for 5 s to ensure a uniform cell suspension and prevent sedimentation artifacts. The resulting OD values were plotted against time on a scatter graph, with time points on the x-axis and absorbance values on the y-axis, enabling real-time visualization and modeling of the microbial growth curve.
The CMDS software platform was employed to automatically calculate key microbial growth parameters, including maximum specific growth rate (Ymax.), absolute microbial growth (Xmax.), and latency duration during the adaptation (Lag) phase.
Microbial susceptibility level to the tested antimicrobial agent was interpreted based on the behavior of growth parameters in comparison to control groups: susceptible when both Xmax. and Ymax. exhibited no statistically significant difference (p > 0.95) relative to the positive control; intermediate susceptibility was assigned when either Xmax. or Ymax. showed a statistically significant reduction compared to the positive control but remained significantly higher than the negative control (p > 0.95); and resistant when both Xmax. and Ymax. showed no significant difference (p > 0.95) in comparison to the negative control. In addition to these parameters, the Lag phase was evaluated to assess the inhibitory effect of the antimicrobial on the initiation of microbial growth. The Lag parameter represents the period during which the antimicrobial agent delays the transition of the microbial population into the exponential growth phase. It was defined as the time required for the microbial biomass to reach an optical density of 0.2 absorbance units at the measurement wavelength used in the assay, indicating a delay in active proliferation due to antimicrobial exposure. The results obtained from the CMDS method were used to determine the appropriate preservative concentration to be applied in the in situ trial.
2.6. In Situ Efficacy of the Biopreservative
The challenge test to evaluate the growth of
S. aureus in bacon was conducted following the protocol established by the predictive microbiology method MicroLab_ShelfLife [
13]. Five packages of each sample group were considered to perform the method. One package from each group was analyzed immediately after production (time zero) to quantify the initial coagulase-positive staphylococci load. The microbial growth was stimulated to grow by pair incubation at low (7 °C) and high (36 °C) temperatures. Biological oxygen demand (BOD) incubators were used for precise temperature control. The doors were kept closed, except during sample withdrawals.
The method ISO 6888-1 (2021) was used for enumeration of coagulase-positive staphylococci in samples, with counts at intervals on days 4 and 8 (lower temperature) and on days 2 and 4 (upper temperature) of incubation [
14]. The colony count results for each sample group were entered into the software provided by the method to calculate microbial concentrations according to Equation (1), as well as the growth parameters
Ngrowth (Log-phase microbial growth) and
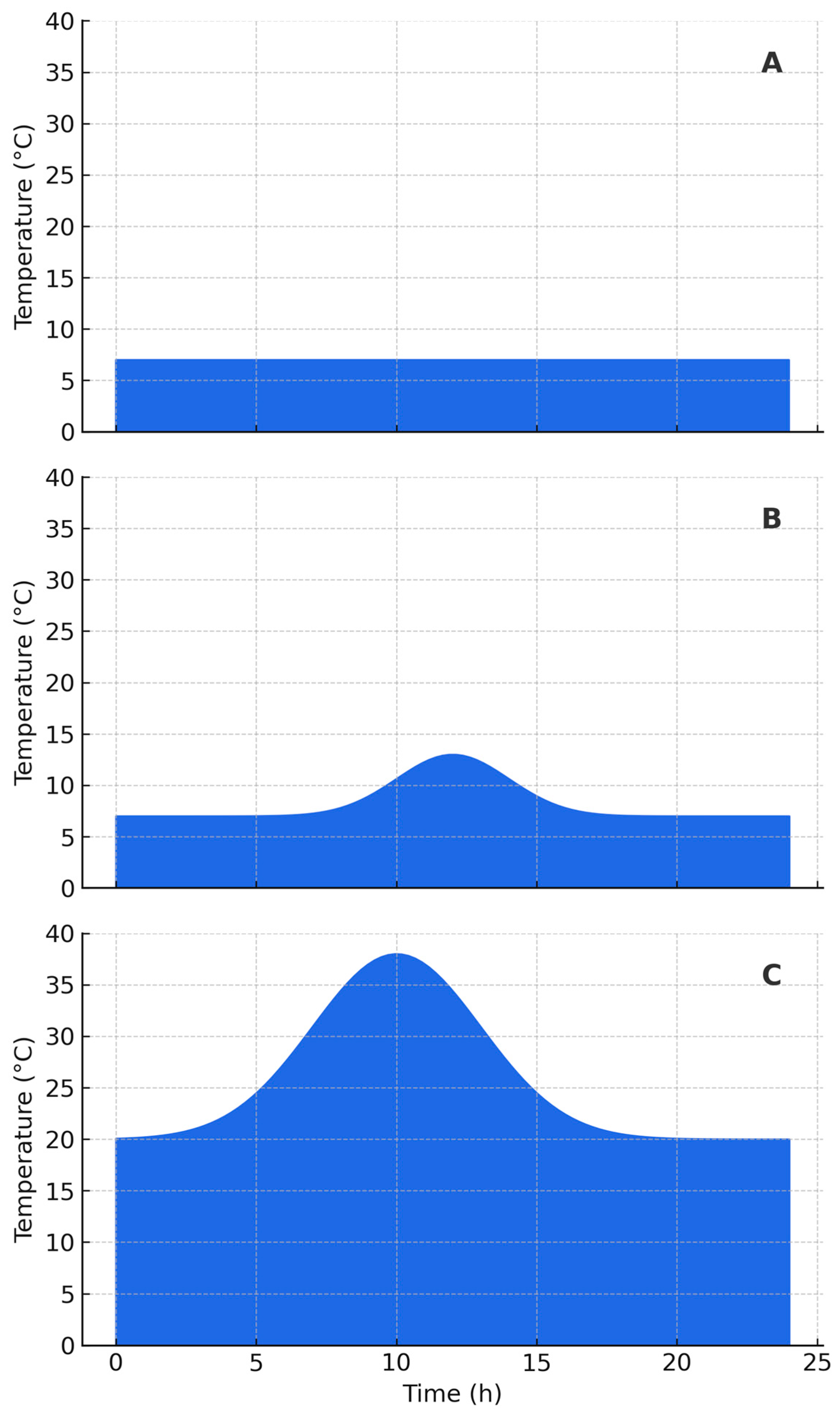
Ndeceleration (deceleration-phase microbial growth), based on Equations (2)–(6), under a dynamic daily temperature profile as illustrated in
Figure 1.
where
∑c—sum of the colonies counted on the two plates retained from two successive dilutions (at least one of which contains a minimum of 10 colonies);
V—volume of inoculum placed in each well (mL);
n1 and
n2—number of wells selected in the first dilution and number of wells selected in the second dilution, respectively; and
d–level of the first dilution retained.
where
N(Tgrowth) = microbial growth rate per unit degree Celsius (log cfu/g/day/°C) in the Log-phase microbial phase (L),
N(Tdeceleration) = microbial growth rate per unit degree Celsius (log cfu/g/day/°C) in the deceleration-growth (
D) phase,
Ngrowth = daily microbial growth (log cfu/g/day) in the L phase,
Ngrowth = daily microbial growth (log cfu/g/day) in the
D phase,
α(HT) and
α(LT) = slopes at the upper temperature (
HT) and lower temperature (
LT) (°C), respectively,
n = hourly temperature ranging from 4 to 36 °C,
k = time (h). A factor named the correlation variable
FT(n) (Equation (4)) was created according to Guerra et al. [
13] based on a linear regression to mathematically model the value of
FT(n) for any mean temperature value, based on the first-degree Equation (7): y = 0.0880x + 0.8971 (7) obtained by regression. By estimating the daily population growth in the L and D phases, it is possible to predict when the population will reach 6 log cfu/g.
A higher limit of 6.0 cfu/g of S. aureus was considered to indicate the end of the durability of the challenge test.
2.7. Immunosorbent Assay for Detection of Staphylococcal Enterotoxins
The presence of SE in bacon samples was assessed using a sandwich-type enzyme-linked immunosorbent assay (ELISA), following the manufacturer’s instructions for a commercial detection kit (RIDASCREEN® SET Total, R-Biopharm AG, Darmstadt, Germany), which enables the detection of classical enterotoxins SEA, SEB, SEC, SED, and SEE. For sample preparation, 1 mL of the liquid accumulated at the bacon–package interface from packages incubated at 36 °C for 4 days was mixed with 90 mL of phosphate-buffered saline (PBS, pH 7.4) containing 0.05% Tween-20 to improve protein solubilization. The suspension was vortexed for 1 min, then gently agitated at 100 rpm for 1 h at 4 °C to extract enterotoxins. After incubation, the samples were centrifuged at 10,000× g for 10 min at 4 °C. The supernatants were filtered through a 0.22 µm sterile membrane to remove cell debris and stored at −20 °C until use.
The ELISA was conducted in 96-well microplates pre-coated with specific polyclonal or monoclonal antibodies. Standards and prepared extracts (100 µL) were pipetted into each well in duplicate and incubated at room temperature (25 °C) for 60 min to allow antigen–antibody binding. The plates were then washed five times with wash buffer to remove unbound materials. Subsequently, 100 µL of enzyme-conjugated secondary antibody solution (typically horseradish peroxidase-labeled) was added to each well and incubated for 30 min, followed by a second washing step. Then, 100 µL of the chromogenic substrate solution (tetramethylbenzidine with hydrogen peroxide) was added and incubated in the dark for 15 min to develop the color. The enzymatic reaction was stopped by the addition of 100 µL of 0.5 M sulfuric acid. Absorbance was measured at 450 nm using a microplate reader (Biotek Gen5, Agilent, Santa Clara, CA, USA) within 15 min of reaction termination. Quantification of enterotoxins was achieved by constructing a standard curve using known concentrations of SE standards provided in the kit, ranging from 0.1 to 10 ng/mL.
2.8. Statistical Treatments
All data were expressed as mean ± standard deviation (SD) of at least three independent replicates. Statistical analysis was performed using one-way analysis of variance (ANOVA) to assess significant differences among treatment groups. In cases of significant differences (p < 0.05), Tukey’s Honestly Significant Difference (HSD) test was applied for multiple pairwise comparisons among treatments. Statistical analyses were conducted using CMDS or XL-Stat software (version 2024.1, Addinsoft, Paris, France).
3. Results
Table 1 presents the physico-chemical characterization of the sample groups of Bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3).
The Aw values exhibited statistically significant differences among the sample groups (p < 0.05) and were subsequently analyzed with respect to critical threshold levels reported in the literature as permissive for SE production in bacon. Textural parameters followed a consistent pattern: hardness, cohesiveness, and chewiness were highest in B1 and progressively declined through B2 and B3. This indicates that higher Aw in B2 and B3 likely contributed to a softer and less cohesive structure. Elasticity remained relatively constant across treatments.
Table 2 summarizes the susceptibility of
S. aureus across different concentrations of the biopreservative.
The antimicrobial effect began to emerge at 0.5% biopreservative, where Ymax. was significantly reduced (p < 0.05), and Lag was notably extended, suggesting a moderate inhibitory action. From 1.0%, S. aureus displayed clear susceptibility, with substantial reductions in both Ymax. and Xmax., and dramatic increases in the Lag (up to 24 h), indicating effective suppression of bacterial proliferation. Concentrations from 1.5% to 3.5% showed similar strong inhibition, with minimal growth observed and consistently long Lag, comparable to the negative control (NC), suggesting a bacteriostatic or potentially bactericidal effect. Based on these results, a minimum concentration of 1.0% is recommended for effective control of S. aureus in bacon products.
Table 3 and
Table 4 present the experimental data from the challenge test with
S. aureus, along with the corresponding predictive safe period. These results are based on challenge tests conducted to assess the growth behavior of
S. aureus in bacon samples subjected to three different curing methods: dry salting (B1), brine immersion (B2), and injection curing (B3).
The effect of temperature on product durability is especially evident in the estimated safe period. Under refrigeration (7 °C), the safe period ranged from 10 to 22 days depending on the treatment. In contrast, at room temperature, the safe period dropped sharply to as low as 1 day in brine-cured control samples (B2-C), highlighting the inadequacy of ambient storage conditions for unpreserved products. Notably, all biopreservative-treated samples (B1-T, B2-T, B3-T) maintained exceptionally high safe periods at 7 °C (up to 745 days), even under abusive conditions (619 days), and significantly outperformed the controls under room temperature as well (up to 245 days). These findings underscore that refrigeration alone substantially reduces microbial growth and delays spoilage; however, temperature management may not be sufficient to prevent the superficial growth of S. aureus, especially on the product-package interface. Therefore, combining refrigeration with effective antimicrobial interventions is essential to ensure microbial safety, particularly in scenarios of extended distribution or intermittent temperature abuse.
The results demonstrate a clear effect of biopreservative on the inhibition of S. aureus at the product–package interface. In the control groups (C), where no biopreservative was added, there was a marked growth of S. aureus, indicating favorable conditions for pathogen development in the absence of antimicrobial intervention. In contrast, the treatment group exhibited complete inhibition of S. aureus, with no detectable growth throughout the incubation period. This pattern was consistently observed across all batches (B1-T, B2-T, B3-T), suggesting that the antimicrobial effect was robust and reproducible, regardless of potential variations in initial microbiota or processing differences. These findings highlight the importance of incorporating the biopreservative that creates an inhospitable environment for S. aureus at the product-package interface, through the combined action of lactic acid and bacteriocins, to prevent SE production. This mechanism is particularly relevant in products at risk of S. aureus contamination during post-cooking handling, when thermal barriers are no longer effective.
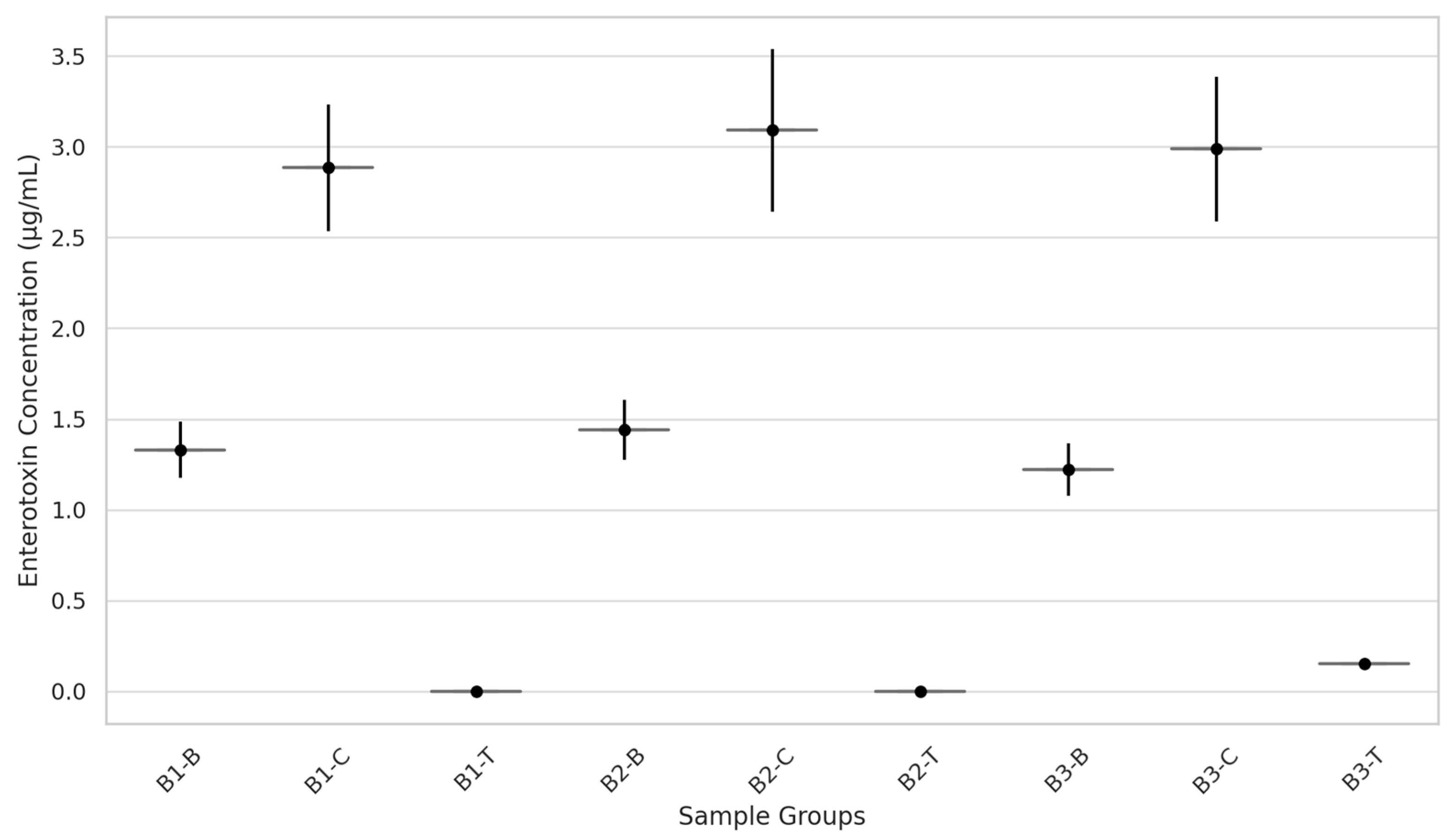
Figure 2 presents SE production in bacon incubated at 36 °C for 4 days.
The results indicate a clear association between S. aureus growth and SE production, with the highest SE levels observed in control groups (B1-C, B2-C, and B3-C), which also presented the highest bacterial counts. Intermediate SE concentrations were found in the baseline groups (B1-B, B2-B, B3-B), suggesting partial growth control but insufficient suppression to prevent toxin formation, indicating that Aw alone was not sufficient to inhibit toxinogenesis. Notably, no detectable SE production occurred in the biopreservative-treated groups (B1-T, B2-T, B3-T), regardless of the Aw of the samples.
4. Discussion
During the thermal processing of bacon, internal product temperatures commonly exceed 70 °C, a threshold sufficient to eliminate the vegetative forms of
S. aureus [
19]. Therefore, the presence of this microorganism in thermally processed products such as cooked bacon is not attributed to survival through heat treatment, but rather to post-processing contamination. This may occur through direct contact with contaminated surfaces, utensils, or handlers, as well as through exposure to aerosols or packaging materials in inadequately sanitized environments. The detection of
S. aureus in the final product is thus a strong indicator of failures in hygiene or cross-contamination events after cooking. The main sources of
S. aureus in bacon are primarily associated with human handling and environmental contamination during the post-cooking stages of processing. Airborne particles and condensation droplets in poorly ventilated or humid processing rooms may also contribute to indirect contamination, particularly when the product surface remains moist, as often observed at the product–package interface [
20]. Moreover, the accumulation of residual moisture in vacuum or modified atmosphere packaging can create favorable microenvironments for the reactivation and growth of
S. aureus, if the necessary inhibitory barriers are not maintained [
13].
In this study, two critical factors were identified as determinants for the growth of S. aureus and the subsequent SE production: temperature abuse during storage and high humidity conditions within the packaging environment. Temperature deviations, particularly when the product remains within the mesophilic growth range of S. aureus (20–40 °C) for an extended period, can allow for rapid bacterial multiplication. Concurrently, elevated relative humidity and moisture accumulation inside the package create a microenvironment conducive to bacterial survival and metabolic activity, especially at the product–package interface. These combined factors establish favorable conditions not only for S. aureus proliferation but also for the expression of SE genes, potentially leading to toxin accumulation even before the product reaches the consumer. These findings underscore the need for the incorporation of targeted antimicrobial barriers, particularly in high-risk zones within the packaging system.
Inadequately refrigerated, undercooked, or incompletely reheated foods are the most associated with
S. aureus food poisoning [
21]. For shelf-stable bacon, strict management of
Aw is mandatory to ensure a safe product and to comply with regulatory limits that aim to inhibit
S. aureus growth and SE production [
22].
These results demonstrate that, even when bacon exhibits an Aw around 0.85, a value traditionally considered below the threshold for active growth of S. aureus, the production of SE may still occur, particularly in scenarios involving recontamination after cooking. This finding highlights the fact that SE synthesis can take place under marginal Aw conditions, especially when accompanied by other permissive factors. The risk becomes even more pronounced in the presence of visible water accumulation at the product–package interface. These observations underscore the complexity of microbial risk in vacuum-packaged bacon.
The indirect application of 1.0% biopreservative at the product–packaging interface proved highly effective in preventing both the growth of
S. aureus and the subsequent production of SE. Acidification and the action of thermostable bacteriocins, as paracaseicin A and B, caseicin A and B, and lactocin 705 at the product–package interface, constitute the primary hurdle responsible for the antimicrobial efficacy of the biopreservative, creating an unfavorable microenvironment for the growth of
S. aureus and consequently inhibiting SE formation [
23]. This targeted intervention at the interface region, recognized as a critical point of vulnerability due to localized humidity and potential for recontamination, successfully established a protective antimicrobial barrier, even under conditions favorable to toxinogenesis. Guerra (2024) reported that the addition of the biopreservative at the product–package interface in vacuum-packaged meat products lowered the pH to a level that inhibited spoilage microbial growth, thereby extending shelf life [
24]. By acting precisely where moisture tends to accumulate, the biopreservative inhibited the metabolic activity of
S. aureus, thereby interrupting the SE synthesis pathway, which is typically upregulated under stress-related sublethal conditions. The results suggest that such localized biopreservative strategies can be highly advantageous in cooked meat products, where post-lethality exposure to contamination cannot always be fully eliminated. This approach reinforces the concept of hurdle technology, where physical, chemical, and biological interventions are strategically combined to suppress microbial hazards. Moreover, it demonstrates that the interface may serve as a critical control point, complementing thermal processing and improving food safety without altering product quality or requiring systemic preservative dispersion. However, additional hurdle strategies should be applied in conjunction with the biopreservative, as part of the preventive measures defined within food safety management systems. In this context, the implementation of Hazard Analysis and Critical Control Points (HACCP), Good Manufacturing Practices (GMP), and Good Hygiene Practices (GHP) is fundamental.
Although the results of this study demonstrated the potential of the biopreservative to inhibit the production of SE in bacon, the findings were limited to the sample group produced specifically for this experiment. Therefore, it is strongly recommended that the study be repeated on a case-by-case basis to validate the safety of bacon produced at an industrial scale. Nevertheless, the time required to obtain results and the costs associated with the analyses may represent barriers to the implementation of this protocol in the industry.
5. Conclusions
This study highlights the complexity of S. aureus control in shelf-stable bacon, where contamination risks persist even after effective thermal inactivation. While vegetative cells of S. aureus are eliminated during cooking, recontamination during post-lethality handling remains a significant hazard, particularly in environments where moisture accumulates. Crucially, the findings demonstrate that SE production is not solely dependent on the global Aw of the product. Even when Aw values are near 0.85—traditionally considered below the threshold for S. aureus activity—toxin synthesis can still occur at the product–package interface. This microenvironment, often characterized by limited oxygen diffusion and water accumulation from condensation or residual vapors, provides favorable conditions for bacterial metabolism and the expression of SE. Therefore, Aw values may not adequately reflect microbial risks in packaged meat products, emphasizing the need for localized control strategies.
The indirect application of 1.0% biopreservative at the interface was shown to be highly effective in preventing both S. aureus growth and SE production. This targeted antimicrobial approach, focused on a known zone of vulnerability, supports the principles of hurdle technology and offers a practical, non-intrusive method for enhancing microbial safety without altering product quality.
6. Patents
A patent application has been filed for the technology described herein, BR 10 2024 022575 9.
Author Contributions
Conceptualization, C.A.G. and A.F.G.; methodology, A.F.G.; validation, A.F.G.; formal analysis, A.F.G.; investigation, C.A.G., E.P.B.J., and L.G.d.O.A.; writing—original draft preparation, A.F.G.; writing—review and editing, A.F.G.; supervision, A.F.G.; project administration, A.F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the findings of this study are contained within the article.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Council for Scientific and Technological Development (CNPq, Brazil) and from BRC Ingredients Ltda (under the leadership of Lucas Marques Costa, CEO). During the preparation of this manuscript, the authors used ChatGPT (OpenAI, GPT-4, July 2025 version) to assist in the writing and editing of scientific text, including language refinement, sentence structure, and formatting suggestions. The authors have thoroughly reviewed, verified, and edited all AI-generated content and take full responsibility for the accuracy and integrity of the final version of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SE | Staphylococcal enterotoxins |
| Aw | Water activity |
| pH | Potential of hydrogen |
| OD | Optical density |
| Ymax. | Maximum specific growth rate |
| Xmax. | Absolute microbial growth |
| Lag | Latency duration during the adaptation |
References
- Todd, E.C.D. Bacteria: Staphylococcus aureus. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 530–534. ISBN 978-0-12-378613-5. [Google Scholar]
- Touaitia, R.; Mairi, A.; Ibrahim, N.A.; Basher, N.S.; Idres, T.; Touati, A. Staphylococcus aureus: A Review of the Pathogenesis and Virulence Mechanisms. Antibiotics 2025, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Cieza, M.Y.; Bonsaglia, E.C.; Rall, V.L.; Santos, M.V.; Silva, N.C. Staphylococcal Enterotoxins: Description and Importance in Food. Pathogens 2024, 13, 676. [Google Scholar] [CrossRef] [PubMed]
- Notermans, S.; Heuvelman, C.J. Combined Effect of Water Activity, pH and Sub-Optimal Temperature on Growth and Enterotoxin Production of Staphylococcus aureus. J. Food Sci. 1983, 48, 1832–1835. [Google Scholar] [CrossRef]
- Nema, V.; Agrawal, R.; Kamboj, D.V.; Goel, A.K.; Singh, L. Isolation and Characterization of Heat Resistant Enterotoxigenic Staphylococcus aureus from a Food Poisoning Outbreak in Indian Subcontinent. Int. J. Food Microbiol. 2007, 117, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Schelin, J.; Wallin-Carlquist, N.; Thorup Cohn, M.; Lindqvist, R.; Barker, G.C. The Formation of Staphylococcus aureus Enterotoxin in Food Environments and Advances in Risk Assessment. Virulence 2011, 2, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and Food Poisoning. Genet. Mol. Res. 2003, 2, 63–76. [Google Scholar] [PubMed]
- Cheng, C.; Liu, B.; Tian, M.; Fang, T.; Li, C. Application of Interaction Models in Predicting the Simultaneous Growth of Staphylococcus aureus and Different Concentrations of Background Microbiota in Chinese-Style Braised Beef. Meat Sci. 2023, 200, 109162. [Google Scholar] [CrossRef] [PubMed]
- Brazil. Portaria SDA nº 748, de 8 de Fevereiro de 2023; Aprova o Regulamento Técnico de Identidade e Qualidade do Bacon; Diário Oficial da União: Brasília, DF, Brazil, 2023. [Google Scholar]
- Delgado-Pando, G.; Fischer, E.; Allen, P.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Salt Content and Minimum Acceptable Levels in Whole-Muscle Cured Meat Products. Meat Sci. 2018, 139, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, E.; Soletti, I.; Martins, M.M.; Menegali, B.S.; Merlo, T.C.; Selani, M.M.; Teixeira, A.C.B.; Silva, F.G.; Contreras-Castillo, C.J. Understanding Consumers’ Dynamic Sensory Perception for Bacon Smoked with Different Brazilian Woods. Meat Sci. 2019, 154, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Donati, E.; Ramundi, V.; Nicoletti, I.; Righetti, L.; Cimini, S.; De Gara, L.; Mariani, F. Bioprospecting of Six Polyphenol-Rich Mediterranean Wild Edible Plants Reveals Antioxidant, Antibiofilm and Bactericidal Properties against Methicillin Resistant Staphylococcus aureus. Sci. Rep. 2025, 15, 28765. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.A.; Costa, L.M.; de Oliveira, V.S.; de Paula, B.P.; Junior, W.J.F.L.; Luchese, R.H.; Corich, V.; Giacomini, A.; Guerra, A.F. Correlation Between Natural Microbial Load and Formation of Ropy Slime Affecting the Superficial Color of Vacuum-Packaged Cooked Sausage. Meat Sci. 2023, 201, 109197. [Google Scholar] [CrossRef] [PubMed]
- ISO 6888-1:2021; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Technique Using Baird-Parker Agar Medium. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- Treangen, T.J.; Maybank, R.A.; Enke, S.; Friss, M.B.; Diviak, L.F.; David, D.K.; Koren, S.; Ondov, B.; Phillippy, A.M.; Bergman, N.H.; et al. Complete Genome Sequence of the Quality Control Strain Staphylococcus aureus subsp. aureus ATCC 25923. Genome Announc. 2014, 2, e01110–e01114. [Google Scholar] [CrossRef] [PubMed]
- ISO 18787:2017; Foodstuffs—Determination of Water Activity. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Desmond, E.M.; Troy, D.J. Effect of Lactic and Citric Acid on Low-Value Beef Used for Emulsion-Type Meat Products. LWT-Food Sci. Technol. 2001, 34, 374–379. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture Profile of Ripening Pears. J. Food Sci. 1968, 33, 223–226. [Google Scholar] [CrossRef]
- Campbell, J.A.; Dickson, J.S.; Cordray, J.C.; Olson, D.G.; Mendonca, A.F.; Prusa, K.J. Survival of Methicillin-Resistant Staphylococcus aureus during Thermal Processing of Frankfurters, Summer Sausage, and Ham. Foodborne Pathog. Dis. 2013, 11, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Boto, D.; D’Arrigo, M.; García-Lafuente, A.; Bravo, D.; Pérez-Baltar, A.; Gaya, P.; Medina, M.; Arqués, J.L. Staphylococcus aureus in the Processing Environment of Cured Meat Products. Foods 2023, 12, 2161. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. Biomed. Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Pérez-Rodríguez, F.; Carrasco, E.; Fuentes-Alventosa, J.M.; García-Gimeno, R.M.; Zurera, G. Modelling the Growth Boundaries of Staphylococcus aureus: Effect of Temperature, pH and Water Activity. Int. J. Food Microbiol. 2009, 133, 186–194. [Google Scholar] [CrossRef]
- Huang, T.; Li, Z.; Qu, X.; Yao, G.; Kwok, L.-Y.; He, Q.; Zhang, H. Preliminary Purification and Partial Characterization of a Functional Bacteriocin of Lacticaseibacillus paracasei Zhang and Mining for Its Gene Cluster. Probiotics Antimicrob. Proteins 2025, 17, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.F. Update on the Use and Abuse of Preservatives in Meat Products. Food Control 2024, 166, 110775. [Google Scholar] [CrossRef]
Figure 1.
Dynamic temperature profiles based on hourly variation over a period of one day to simulate realistic temperature conditions during logistics for distribution and display for sale. (A)—constant refrigeration at 7 °C; (B)—refrigeration with excessive temperature; (C)—room temperature.
Figure 1.
Dynamic temperature profiles based on hourly variation over a period of one day to simulate realistic temperature conditions during logistics for distribution and display for sale. (A)—constant refrigeration at 7 °C; (B)—refrigeration with excessive temperature; (C)—room temperature.
Figure 2.
Production of staphylococcal enterotoxins (SE) in bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3), subdivided in blank group (B1-B, B2-B, B3-B), which received no additional application, control group (B1-C, B2-C, B3-C), which received 1.0% sterile deionized water, and treatment group (B1-T, B2-T, B3-T), which received 1.0% of the biopreservative solution.
Figure 2.
Production of staphylococcal enterotoxins (SE) in bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3), subdivided in blank group (B1-B, B2-B, B3-B), which received no additional application, control group (B1-C, B2-C, B3-C), which received 1.0% sterile deionized water, and treatment group (B1-T, B2-T, B3-T), which received 1.0% of the biopreservative solution.
Table 1.
Characterization of the bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3) (mean ± standard deviation).
Table 1.
Characterization of the bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3) (mean ± standard deviation).
| Parameters | B1 | B2 | B3 |
|---|
| Aw | 0.86 ± 0.012 c | 0.94 ± 0.006 b | 0.97 ± 0.003 a |
| TPA | Hardness-1 (N/cm2) | 22.512 ± 1.123 a | 18.354 ± 0.927 b | 14.163 ± 0.794 c |
| Hardness-2 (N/cm2) | 20.854 ± 1.048 a | 14.974 ± 0.946 b | 13.587 ± 0.684 b |
| Cohesiveness | 0.526 ± 0.03 a | 0.483 ± 0.024 b | 0.434 ± 0.025 b |
| Elasticity (cm) | 1.455 ± 0.074 | 1.521 ± 0.086 | 1.647 ± 0.087 |
| Chewiness (J/cm2) | 17.229 ± 1.104 a | 13.483 ± 1.599 b | 10.123 ± 0.904 b |
Table 2.
Microbial susceptibility (mean ± standard deviation) level to the tested antimicrobial agent based on the behavior of growth, based on the maximum specific growth rate (Ymax.), absolute microbial growth (Xmax.), and latency duration during the adaptation (Lag) phase.
Table 2.
Microbial susceptibility (mean ± standard deviation) level to the tested antimicrobial agent based on the behavior of growth, based on the maximum specific growth rate (Ymax.), absolute microbial growth (Xmax.), and latency duration during the adaptation (Lag) phase.
| Treatment | Ymax. | Xmax. | Lag | Conclusion |
|---|
| PC * | 0.118 ± 0.020 a | 1.334 ± 0.066 a | 2.3 ± 0.0 | Resistant |
| 0.05% | 0.124 ± 0.003 a | 1.338 ± 0.025 a | 2.0 ± 0.0 | Resistant |
| 0.1% | 0.127 ± 0.013 a | 1.345 ± 0.009 a | 2.3 ± 0.4 | Resistant |
| 0.3% | 0.124 ± 0.003 a | 1.380 ± 0.003 a | 2.5 ± 0.0 | Resistant |
| 0.5% | 0.089 ± 0.001 b | 1.230 ± 0.049 a | 3.8 ± 0.4 | Intermediate |
| 1.0% | 0.051 ± 0.004 b | 0.701 ± 0.810 b | 19.3 ± 6.7 | Susceptible |
| 1.5% | 0.041 ± 0.002 b | 0.053 ± 0.074 b | 24.0 ± 0.0 | Susceptible |
| 2.0% | | 0.105 ± 0.001 b | 24.0 ± 0.0 | Susceptible |
| 2.5% | | 0.104 ± 0.004 b | 24.0 ± 0.0 | Susceptible |
| 3.0% | | 0.104 ± 0.001 b | 24.0 ± 0.0 | Susceptible |
| 3.5% | | 0.074 ± 0.104 b | 24.0 ± 0.0 | Susceptible |
| NC ** | | 0.071 ± 0.94 b | 24.0 ± 0.0 | Susceptible |
Table 3.
Experimental data of the challenge test for Staphylococcus aureus (ATCC 25923) growth in bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3), subdivided in blank group (B1-B, B2-B, B3-B), which received no superficial additional application at the product-package interface, control group (B1-C, B2-C, B3-C), which received 1.0% sterile deionized water at the product-package interface, and treatment group (B1-T, B2-T, B3-T), which received 1.0% of the biopreservative solution at the product-package interface.
Table 3.
Experimental data of the challenge test for Staphylococcus aureus (ATCC 25923) growth in bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3), subdivided in blank group (B1-B, B2-B, B3-B), which received no superficial additional application at the product-package interface, control group (B1-C, B2-C, B3-C), which received 1.0% sterile deionized water at the product-package interface, and treatment group (B1-T, B2-T, B3-T), which received 1.0% of the biopreservative solution at the product-package interface.
| | Treatments |
|---|
| | Samples
Incubation | Dry Salting
(Aw * ≈ 0.85) | Brine Immersion
(Aw * ≈ 0.94) | Injection Curing
(Aw * ≈ 0.97) |
|---|
| | °C | Days | B1-B | B1-C | B1-T | B2-B | B2-C | B2-T | B3-B | B3-C | B3-T |
|---|
Colony counting
(Log cfu/g) | | 0 | 3.71 | 3.88 | 3.79 | 3.66 | 3.76 | 3.81 | 3.66 | 3.76 | 3.81 |
| 7 | 4 | 3.89 | 3.99 | 3.80 | 3.95 | 4.01 | 3.81 | 3.95 | 4.01 | 3.80 |
| 8 | 4.37 | 4.45 | 3.82 | 4.42 | 4.14 | 3.81 | 4.42 | 4.14 | 3.79 |
| 36 | 4 | 4.70 | 4.80 | 3.80 | 4.59 | 4.66 | 3.80 | 4.59 | 4.66 | 3.82 |
| 8 | 6.49 | 6.61 | 3.81 | 6.42 | 5.12 | 3.80 | 6.42 | 5.12 | 3.85 |
Table 4.
Predictive safe period obtained on the basis of experimental data for Staphylococcus aureus (ATCC 25923) growth in bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3), subdivided in blank group (B1-B, B2-B, B3-B), which received no superficial additional application at the product-package interface, control group (B1-C, B2-C, B3-C), which received 1.0% sterile deionized water at the product-package interface, and treatment group (B1-T, B2-T, B3-T), which received 1.0% of the biopreservative solution at the product-package interface.
Table 4.
Predictive safe period obtained on the basis of experimental data for Staphylococcus aureus (ATCC 25923) growth in bacon produced by dry salting (B1), brine immersion (B2), and injection curing (B3), subdivided in blank group (B1-B, B2-B, B3-B), which received no superficial additional application at the product-package interface, control group (B1-C, B2-C, B3-C), which received 1.0% sterile deionized water at the product-package interface, and treatment group (B1-T, B2-T, B3-T), which received 1.0% of the biopreservative solution at the product-package interface.
| | | Treatments |
|---|
| | Temperature
Profile | Dry Salting
(Aw **** ≈ 0.85) | Brine Immersion
(Aw **** ≈ 0.94) | Injection Curing
(Aw **** ≈ 0.97) |
|---|
| Parameters | B1-B | B1-C | B1-T | B2-B | B2-C | B2-T | B3-B | B3-C | B3-T |
|---|
Ngrowth
(Log cfu/g/day) * | 7 °C | 0.2982 | 0.2771 | 0.0000 | 0.3064 | 0.2078 | 0.0000 | 0.4839 | 0.8433 | 0.0001 |
| 7 °C (abusive) | 0.3429 | 0.3214 | 0.0000 | 0.3473 | 0.2360 | 0.0000 | 0.5839 | 0.9493 | 0.0002 |
| Room | 0.4800 | 0.4572 | 0.0000 | 0.4724 | 0.3224 | 0.0000 | 0.6574 | 1.4323 | 0.0000 |
Ndeceleration
(Log cfu/g/day) ** | 7 °C | 0.0983 | 0.0888 | 0.0000 | 0.1057 | 0.0712 | 0.0000 | 0.2833 | 0.4432 | 0.0000 |
| 7 °C (abusive) | 0.1375 | 0.1310 | 0.0000 | 0.1354 | 0.0924 | 0.0000 | 0.2847 | 0.5943 | 0.0000 |
| Room | 0.1223 | 0.1146 | 0.0000 | 0.1239 | 0.842 | 0.0000 | 0.2483 | 0.6432 | 0.0000 |
Safe period ***
(days) *** | 7 °C | 21 | 12 | n.d | 22 | 11 | n.d | 20 | 10 | 745 |
| 7 °C (abusive) | 18 | 10 | n.d | 18 | 10 | n.d | 17 | 10 | 619 |
| Room | 15 | 7 | n.d | 1 | 7 | n.d | 14 | 7 | 245 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).