Boosting Probiotic Biomass of Lactobacillus acidophilus CCFM137 Through pH-Stat Morphological Control and Medium Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Growth Media
2.3. Species Identification Method
2.4. Growth Curve
2.5. Component Screening and Adjustment of Culture Medium
2.6. Optimization of High Cell Density Fermentation Process
2.7. Viable Cell Count Method
2.8. Cell Morphology Observation
2.9. Determination of Amino Acid Nitrogen and Reducing Sugars
2.10. Statistical Analysis
3. Results and Discussion
3.1. Species Identification
3.2. Growth in MRS Broth
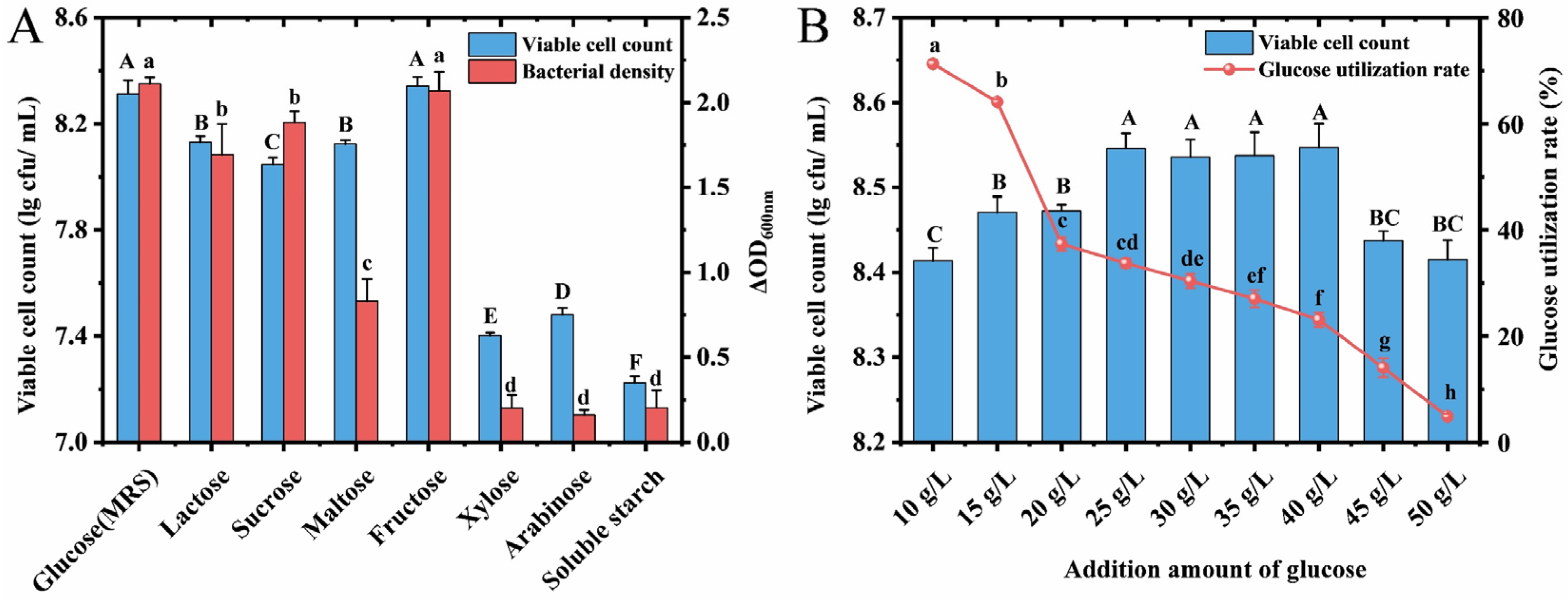
3.3. Effects of Different Carbon Sources on the Growth of L. acidophilus CCFM137
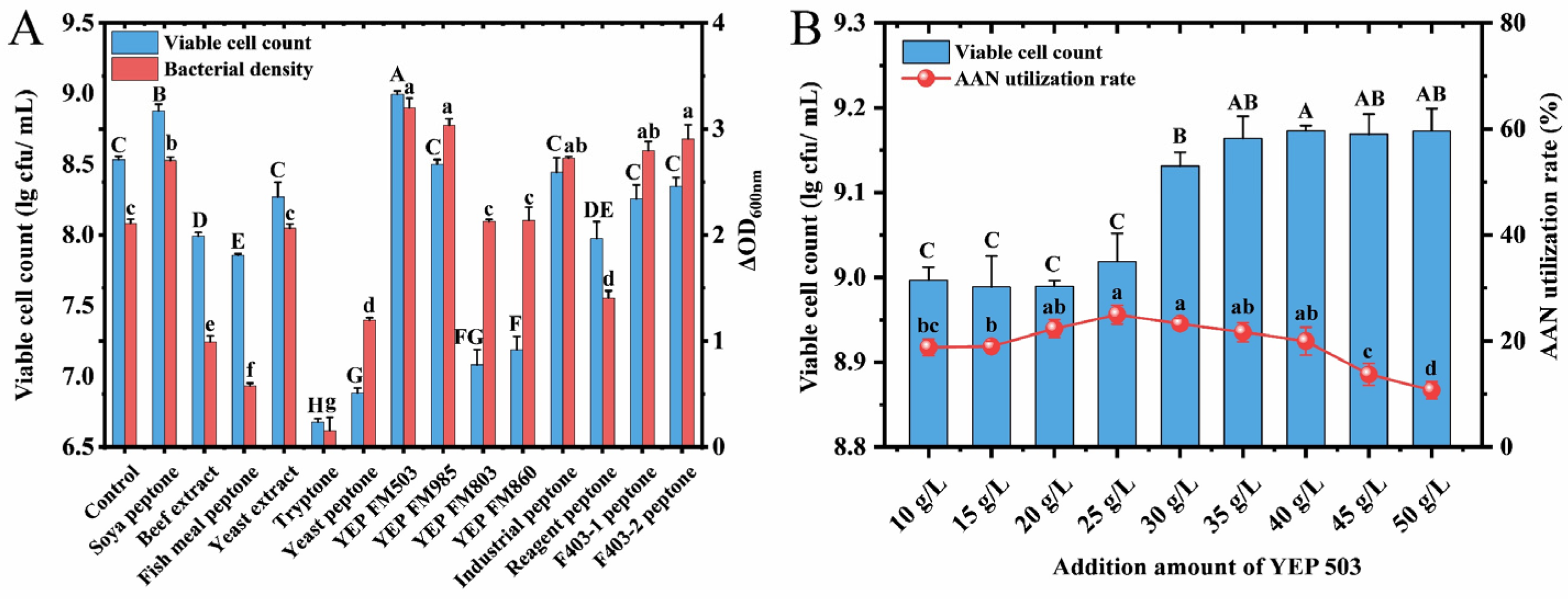
3.4. Effects of Different Nitrogen Sources on the Growth of L. acidophilus CCFM137
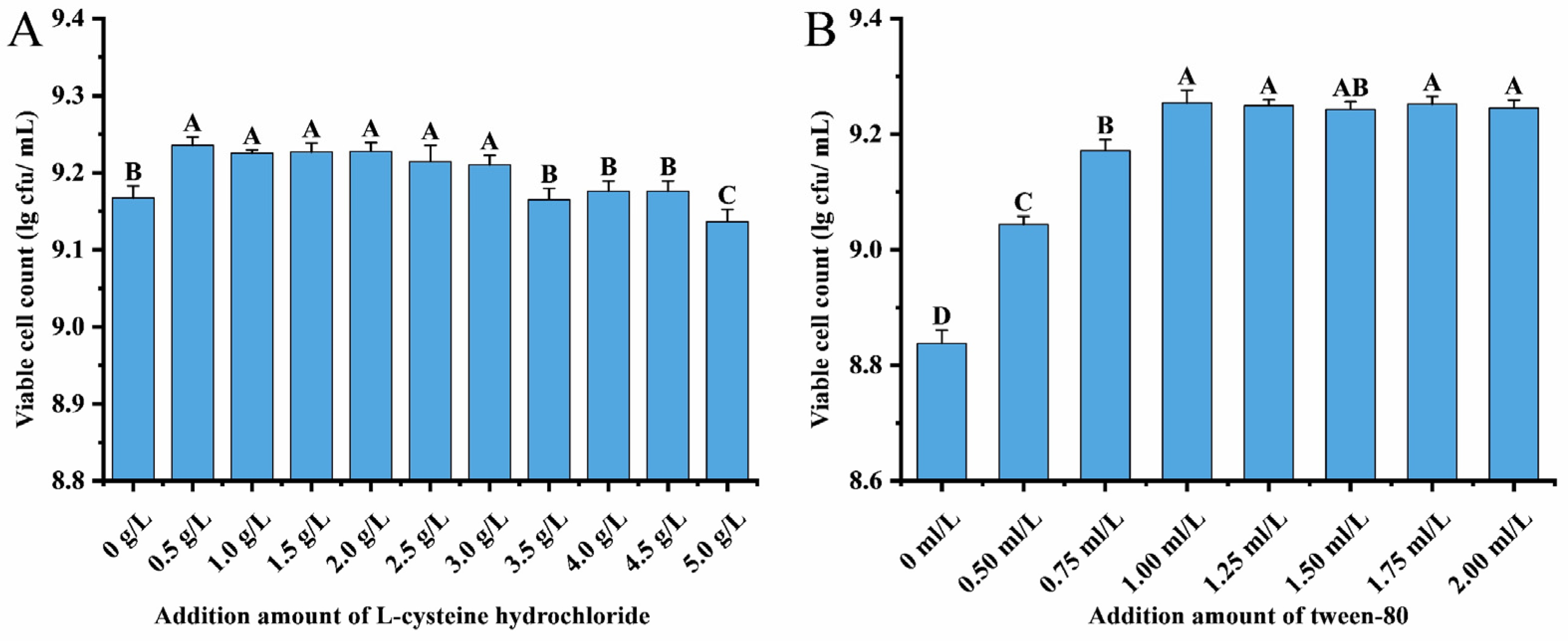
3.5. Effect of L-Cysteine Hydrochloride and Tween 80 on the Growth of L. acidophilus CCFM137
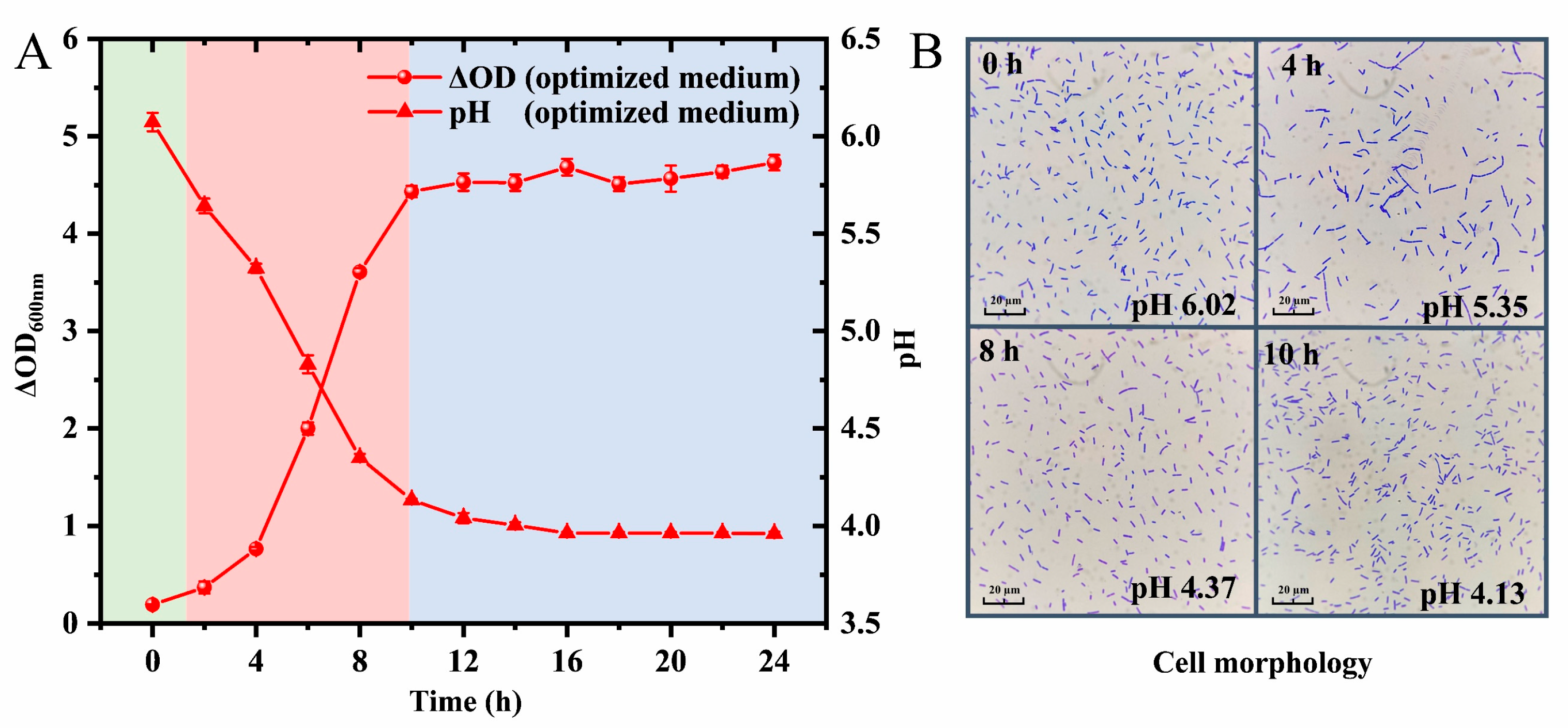
3.6. Effects of Optimized Medium on Growth Properties and Cell Morphology
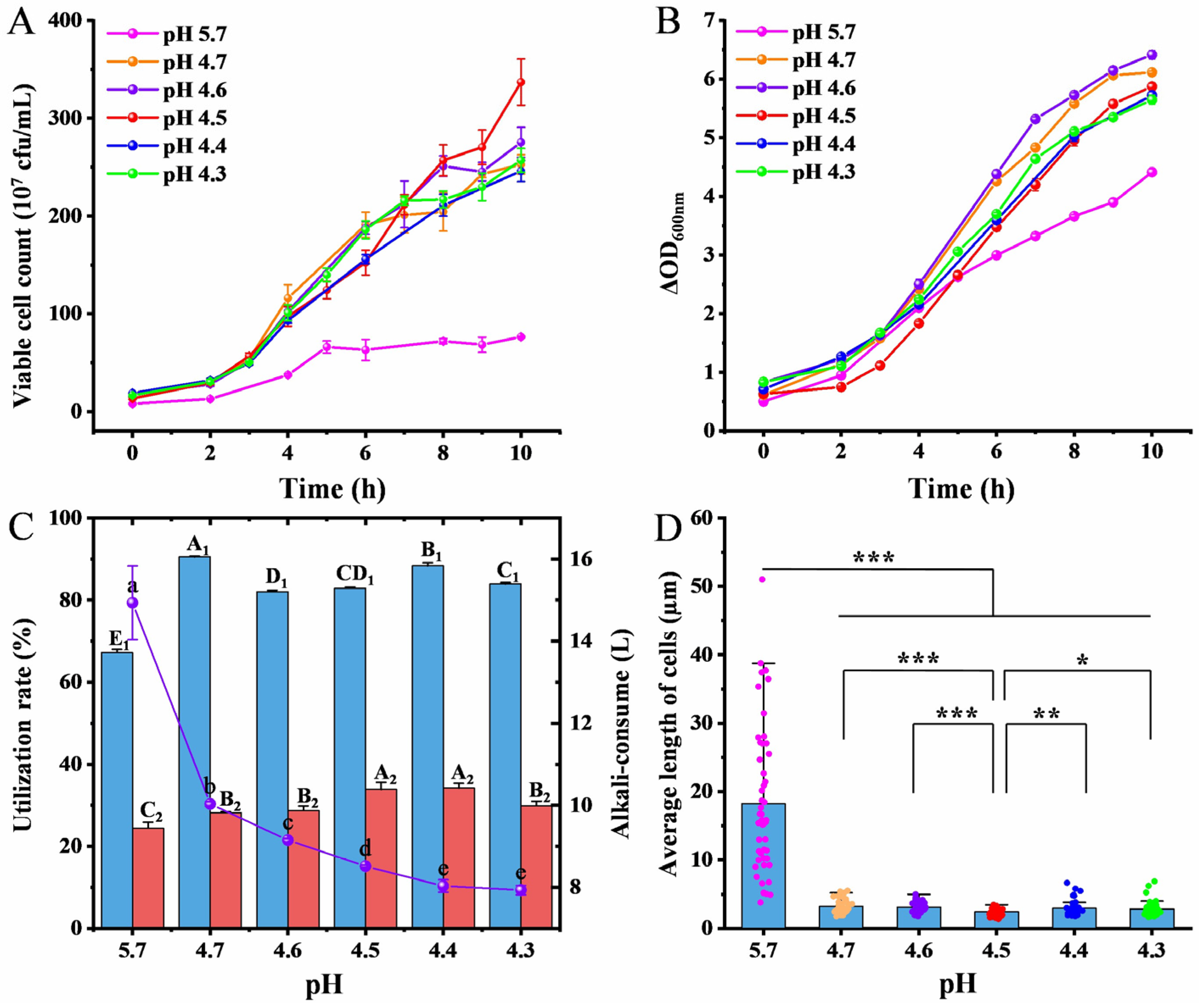
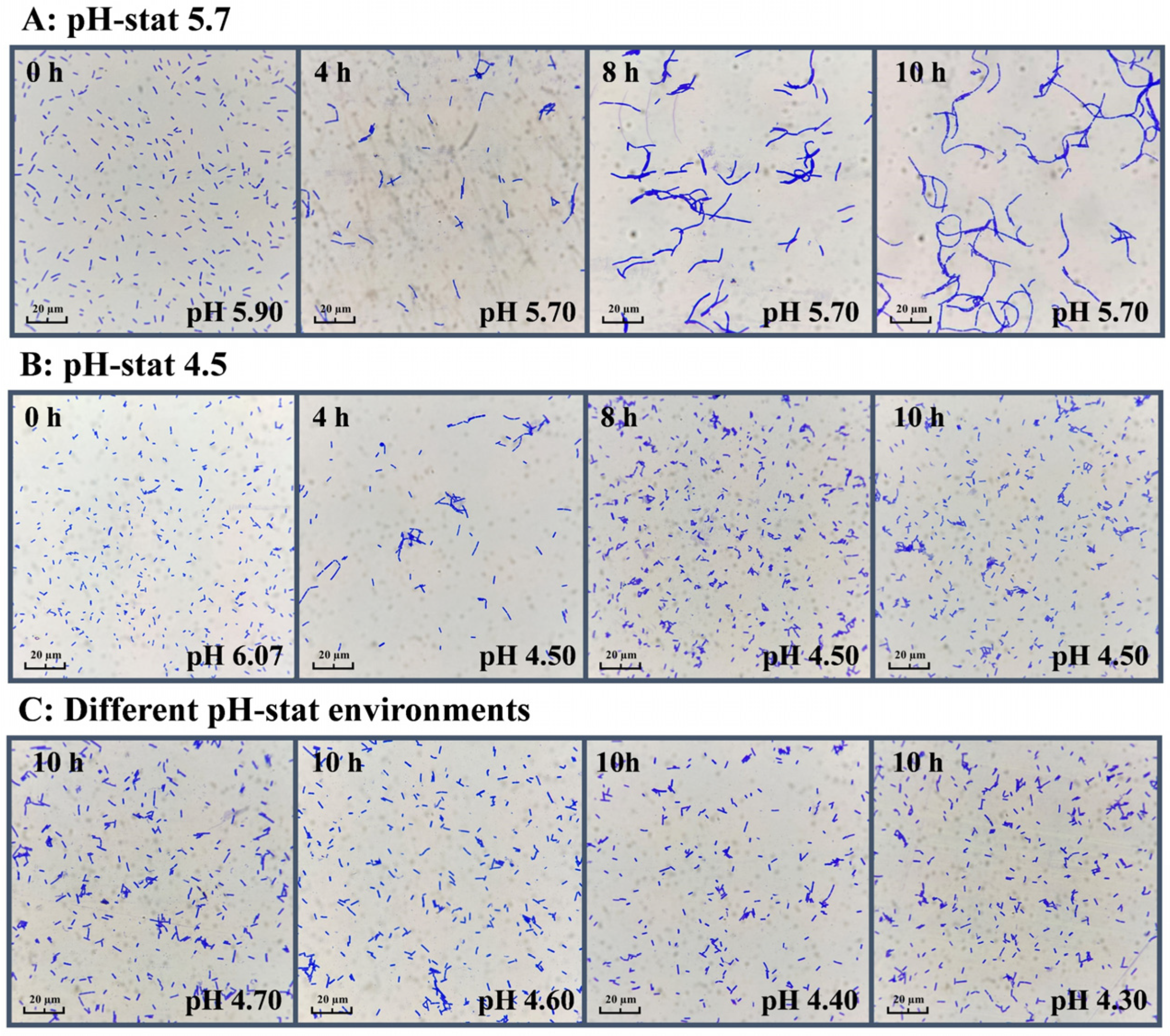
3.7. Effect of Different pH-Stat Environments on Fermentation Performance
3.8. Effect of Different pH-Stat Environments on Cell Recovery and Lyophilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Jin, X.F.; Yu, F.Q.B.; Yan, J.; Allison, M.W.; Veronika, D.; Meng, X.R.; Katherine, S.P. Culturing of A Complex Gut Microbial Community in Mucin-Hydrogel Carriers Reveals Strain- and Gene-Associated Spatial Organization. Nat. Commun. 2023, 14, 3510. [Google Scholar] [CrossRef]
- Wang, M.X.; Chen, Y.X.; Wang, Y.Y.; Li, Y.; Zhang, X.J.; Zheng, H.; Ma, F.L.; Ma, C.H.; Lu, B.Y.; Xie, Z.H.; et al. Beneficial changes of gut microbiota and metabolism in weaned rats with Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 supplementation. J. Funct. Foods 2018, 48, 252–265. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yu, B.H.; Zhou, J.; Zhang, J.Y.; Zhang, R.L.; Xie, J.; Wang, Q.L.; Zhao, S.L. Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci. Rep. 2019, 9, 20128. [Google Scholar] [CrossRef]
- Lau, H.C.-H.; Zhang, X.; Ji, F.F.; Lin, Y.F.; Liang, W.; Li, Q.; Chen, D.Y.; Fong, W.; Kang, X.; Liu, W.X.; et al. Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid. EBioMedicine 2024, 100, 104952. [Google Scholar] [CrossRef]
- Masoumi, S.J.; Mehrabani, D.; Saberifiroozi, M.; Fattahi, M.R.; Moradi, F.; Najafi, M. The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food Sci. Nutr. 2021, 9, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.; Liao, Y.R.; Zhu, J.R.; Zhang, J.; Wu, Z.H.; Li, X.; Tong, P.; Chen, H.; Wang, S.; Liu, Z. Screening of anti-allergy Lactobacillus and its effect on allergic reactions in BALB/c mice sensitized by soybean protein. J. Funct. Foods 2021, 87, 104858. [Google Scholar]
- Kim, H.R.; Seo, E.S.; Oh, S.Y.; Seo, M.Y.; Byun, K.H.; Kim, B.-Y. Anti-obesity effects of multi-strain probiotics in mice with high-carbohydrate diet-induced obesity and the underlying molecular mechanisms. Nutrients 2022, 14, 5173. [Google Scholar] [CrossRef]
- Schmitt, J.D.; De Faria, L.O.; Simes, M.; Kottwitz, L.B.M. Evaluation of the Probiotic Profile of the Lactobacillus acidophilus Used in Pharmaceutical and Food Applications. Acta Sci. Health Sci. 2018, 40, e36664. [Google Scholar]
- Huang, Z.; Zhou, X.Y.; Stanton, C.; Ross, R.P.; Zhao, J.X.; Zhang, H.; Yang, B. Comparative genomics and specific functional characteristics analysis of Lactobacillus acidophilus. Microorganisms 2021, 9, 1992. [Google Scholar] [CrossRef]
- Su, X.; Menghe, B.L.G.; Zhang, H.P.; Liu, W.J. In Vitro Evaluation of Intestinal Transport and High-Density Fermentation of Lactobacillus acidophilus. Metabolites 2023, 13, 1077. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, X.; Jin, H.; Man, C.; Jiang, Y. The effect of optimized carbon source on the synthesis and composition of exopolysaccharides produced by Lactobacillus paracasei. J. Dairy Sci. 2021, 104, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Goderska, K.; Nowak, J.; Czarnecki, Z. Comparison of growth of Lactobacillus acidophilus and Bifidobacterium bifidum species in media supplemented with selected saccharides including prebiotics. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–20. [Google Scholar]
- Zang, J.; Wang, T.; Piotr, D.; Zhao, H.; Zhang, B. Increasing lactose concentration is a strategy to improve the proliferation of Lactobacillus helveticus in milk. Food Sci. Nutr. 2021, 9, 1050–1060. [Google Scholar] [CrossRef]
- Karim, G.; Darhabi, H.K.; Habibollah, M. The effect of fructose, prolin, initial doses and different temperatures on the growth and metabolism of Lactobacillus acidophilus La5. J. Anim. Vet. Adv. 2009, 8, 2432–2437. [Google Scholar]
- Tatsuo, M.; Yoshiaki, D.; Motoyuki, Y.; Takeshi, S.; Takashi, Y. Multiple nutritional requirements of lactobacilli: Genetic lesions affecting amino acid biosynthetic pathways. J. Bacteriol. 1981, 148, 64–71. [Google Scholar] [CrossRef]
- Qiao, Y.L.; Yin, B.S.; Zhou, W.; Wang, M.-J.; Chang, Z.; Zhou, J.P.; Yue, M.X.; Chen, J.X.; Liu, F.; Feng, Z. Strategies for enhancing acid resistance of Lactobacillus acidophilus through analyzing nutrient consumption patterns. J. Sci. Food Agric. 2024, 104, 5982–5990. [Google Scholar] [CrossRef]
- Senz, M.; Lengerich, B.V.; Bader, J.; Ståhl, U. Control of Cell Morphology of Probiotic Lactobacillus acidophilus for Enhanced Cell Stability During Industrial Processing. Int. J. Food Microbiol. 2015, 192, 34–42. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Yin, B.S.; Liu, F.; Zhou, W.; Wang, M.-J.; Chang, Z.; Zhou, J.P.; Yue, M.X.; Chen, J.X.; Feng, Z. Effect of The Initial ph of The Culture Medium on The Nutrient Consumption Pattern of Bifidobacterium animalis subsp. lactis Bb12 and The Improvement of Acid Resistance By Purine and Pyrimidine Compounds. J. Appl. Microbiol. 2024, 135, lxae02. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Liew, S.L. Growth Medium Optimization for Biomass Production of a Probiotic Bacterium, Lactobacillus rhamnosus ATCC 7469. J. Food Biochem. 2012, 37, 536–543. [Google Scholar] [CrossRef]
- Cui, S.M.; Zhao, J.X.; Liu, X.M.; Chen, Y.Q.; Zhang, H.; Chen, W. Maximum-Biomass Prediction of Homofermentative Lactobacillus. J. Biosci. Bioeng. 2016, 122, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.K.; Pack, M.Y. Effect of Environmental ph on Chain Length of Lactobacillus bulgaricus. J. Bacteriol. 1980, 144, 865–868. [Google Scholar] [CrossRef] [PubMed]
- De, M.J.C.; Rogosa, M.; Elisabeth, S.M. A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Mao, B.Y.; Li, D.Y.; Zhao, J.X.; Liu, X.M.; Gu, Z.B.; Chen, Y.Q.; Zhang, H.; Chen, W. In Vitro Fermentation of Lactulose by Human Gut Bacteria. J. Agric. Food Chem. 2014, 62, 10970–10977. [Google Scholar] [CrossRef]
- Fan, X.; Shi, Y.Y.; Li, R.; Yang, R.; Yang, X.; Feng, H.; Zhang, H.; Chen, W. Preliminary Study on The Effect of Pre-Freezing Methods on Lyophilization Quality and Storage Stability of Probiotics. Dry. Technol. 2024, 42, 1480–1492. [Google Scholar] [CrossRef]
- Rahayu, H.M.; Setiadi, A.E. Isolation and Characterization of Indigenous Lactic Acid Bacteria from Pakatikng Rape, Dayak’s Traditional Fermented Food. J. Penelit. Pendidik. IPA (JPPIPA) 2023, 9, 920–925. [Google Scholar] [CrossRef]
- Chen, S.W.; Niu, H.Y.; Wu, Y.F.; Sun, J.L.; Han, X.; Zhang, L.W. Influence of Lactic Acid on Cell Cycle Progressions in Lactobacillus bulgaricus During Batch Culture. Appl. Biochem. Biotechnol. 2020, 193, 912–927. [Google Scholar] [CrossRef]
- Schaan, K.; Hughes, P. A comparison of free amino nitrogen and yeast-assimilable nitrogen measurement methods for use in alcoholic fermentation of whey. J. Dairy Sci. 2024, 107, 6592–6601. [Google Scholar] [CrossRef]
- Zohri, A.A.; Abdelazim, M.; Ibrahim, S. 2-Aminoethanaminium 2-(ethoxycarbonyl)-4,6-dinitrophenolate as a greener route in reducing sugar quantification. MethodsX 2018, 5, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, I.; Kitahara, K. Effect of several vitamins on the cell division and the growth of Lactdbacillus delbrueckii. J. Vitaminol. 1962, 8, 115–120. [Google Scholar] [CrossRef]
- Jeener, H.; Jeener, R. Cytological study of Thermobacterium acidophilus R26 cultured in absence of deoxyribonucleosides or uracil. Exp. Cell Res. 1952, 3, 675–680. [Google Scholar] [CrossRef]
- Mary, B. The Classification of Lactobacilli by means of Physiological Tests. J. Gen. Microbiol. 1953, 9, 234–248. [Google Scholar] [CrossRef][Green Version]
- Shalini, S.; Mital, B.K.; Shweta, G. Effect of casitone and fructose on the growth of Lactobacillus acidophilus and its survival during storage. Int. J. Food Microbiol. 1994, 21, 271–276. [Google Scholar] [CrossRef]
- Yang, S.; Hou, M.; Tan, W.; Chen, Y.; Li, H.; Song, J.; Wang, X.; Ren, J.; Gao, Z. Lactic acid bacteria sequential fermentation improves viable counts and quality of fermented apple juice via generating two logarithmic phases. Food Chem. 2025, 464, 141635. [Google Scholar] [CrossRef]
- Chen, H.; Niu, J.; Qin, T.; Ma, Q.; Wang, L.; Shu, G. Optimization of the medium for Lactobacillus acidophilus by Plackett-Burman and steepest ascent experiment. Acta Sci. Pol. Technol. Aliment. 2015, 14, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Roslinda, B.A.M.; Atim, A.; Jikan, S.S.; Mohd Fuzi, S.F.Z. Effects of Different Carbon Sources for High Level Lactic Acid Production by Lactobacillus casei. Appl. Mech. Mater. 2015, 695, 220–223. [Google Scholar] [CrossRef]
- Sionek, B.; Szydowska, A.; Trzskowska, M.; Kooyn-Krajewska, D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Bircher, L.; Sourabié, A.M.; Paurevic, M.; Hochuli, J.; Geirnaert, A.; Navas, C.; Drogue, B.; Lacroix, C. Faecalibacterium duncaniae A2-165 growth is strongly promoted by yeast extract and vitamin B5 in cGMP medium. Microb. Biotechnol. 2024, 17, e14374. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Li, H.; Zhang, C.; Zhang, J.; Uddin, J.; Liu, X. Effect of soybean oligopeptide on the growth and metabolism of Lactobacillus acidophilus JCM 1132. RSC Adv. 2020, 10, 16737–16748. [Google Scholar] [CrossRef]
- Rosario, I.S.; María Teresa, J.-M.; Emma, M.L.; Enrique, P.; Aurelio, L.M. Growth and viability of Lactobacillus acidophilus NRRL B-4495, Lactobacillus casei NRRL B-1922 and Lactobacillus plantarum NRRL B-4496 in milk supplemented with cysteine, ascorbic acid and tocopherols. Int. Dairy J. 2019, 97, 15–24. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; Chen, J.; Zhang, Z.; Hu, W. Enhancement effect of l-cysteine on lactic acid fermentation production. Biotechnol. J. 2023, 18, e2300110. [Google Scholar] [CrossRef]
- Dave, R.I.; Shah, N.P. Ingredient supplementation effects on viability of probiotic bacteria in yogurt. J. Dairy Sci. 1998, 81, 2804–2816. [Google Scholar] [CrossRef]
- Partanen, L.; Marttinen, N.; Alatossava, T. Fats and fatty acids as growth factors for Lactobacillus delbrueckii. Syst. Appl. Microbiol. 2001, 24, 500–506. [Google Scholar] [CrossRef]
- Elvina, P.; Chaitali, D.; Trung Kien, T.; Oliver, A.H.J.; Bee, K.M. Morphological and ultrastructural changes in Lactobacillus plantarum B21 as an indicator of nutrient stress. LWT 2018, 92, 556–563. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, X.; Yang, D.; Si, T.; Pan, S.; Yang, F. Yield improvement of exopolysaccharides by screening of the Lactobacillus acidophilus ATCC and optimization of the fermentation and extraction conditions. EXCLI J. 2016, 15, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Matthew, B.; Sue, P.; Julian, R.M.; Eshwar, M. The Life History of Lactobacillus acidophilusas A Probiotic: A Tale of Revisionary Taxonomy, Misidentification and Commercial Success. FEMS Microbiol. Lett. 2013, 349, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.; Saima, Y.; Ikram ul, H. Production of Probiotic Mozzarella Cheese by Incorporating Locally Isolated Lactobacillus acidophilus. Ann. Microbiol. 2020, 70, 240–251. [Google Scholar] [CrossRef]
- Rao, N.S.; Lundberg, L.; Palmkron, S.B.; Håkansson, S.; Bergenståhl, B.; Carlquist, M. Flow Cytometric Analysis Reveals Culture Condition Dependent Variations in Phenotypic Heterogeneity of Limosilactobacillus reuteri. Sci. Rep. 2021, 11, 23567. [Google Scholar] [CrossRef]
- Weart, R.B.; Lee, A.; Chien, A.-C.; Haeusser, D.P.; Hill, N.S.; Levin, P.A. A Metabolic Sensor Governing Cell Size in Bacteria. Cell 2007, 130, 335–347. [Google Scholar] [CrossRef]
- Silva, J.; Carvalho, A.S.; Ferreira, R.; Vitorino, R.; Amado, F.; Domingues, P.; Teixeira, P.; Gibbs, P. Effect of The pH of Growth on The Survival of Lactobacillus delbrueckii subsp. bulgaricus to Stress Conditions During Spray-Drying. J. Appl. Microbiol. 2005, 98, 775–782. [Google Scholar] [CrossRef]
- Cui, S.M.; Pan, Z.F.; Wu, S.; Mao, B.Y.; Tang, X.; Zhang, Q.X.; Zhang, H.; Zhao, J.X. Improvement of The Lyophilization Survival Rate of Lactobacillus casei Via Regulation of its Surface Substances. Foods 2022, 11, 3468. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.-Q.; Shi, Y.-Y.; Yang, R.; Li, R.; Hang, F.; Zhang, H. Boosting Probiotic Biomass of Lactobacillus acidophilus CCFM137 Through pH-Stat Morphological Control and Medium Optimization. Fermentation 2025, 11, 564. https://doi.org/10.3390/fermentation11100564
Yan S-Q, Shi Y-Y, Yang R, Li R, Hang F, Zhang H. Boosting Probiotic Biomass of Lactobacillus acidophilus CCFM137 Through pH-Stat Morphological Control and Medium Optimization. Fermentation. 2025; 11(10):564. https://doi.org/10.3390/fermentation11100564
Chicago/Turabian StyleYan, Shao-Quan, Yang-Yang Shi, Rui Yang, Rui Li, Feng Hang, and Hao Zhang. 2025. "Boosting Probiotic Biomass of Lactobacillus acidophilus CCFM137 Through pH-Stat Morphological Control and Medium Optimization" Fermentation 11, no. 10: 564. https://doi.org/10.3390/fermentation11100564
APA StyleYan, S.-Q., Shi, Y.-Y., Yang, R., Li, R., Hang, F., & Zhang, H. (2025). Boosting Probiotic Biomass of Lactobacillus acidophilus CCFM137 Through pH-Stat Morphological Control and Medium Optimization. Fermentation, 11(10), 564. https://doi.org/10.3390/fermentation11100564




