The Effects of Substrates and Sonication Methods on the Antioxidant Activity of Kefir Postbiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture Preparation
2.2. Ultrasonic Treatments
2.3. Postbiotics Obtention
2.4. Hydrogen Potential Determination
2.5. Antioxidant Activity (AA)
2.6. Total Water-Soluble Protein Content
2.7. Proteolysis Assessment
2.8. Cell Membrane Permeability
2.9. Statistical Analysis
3. Results
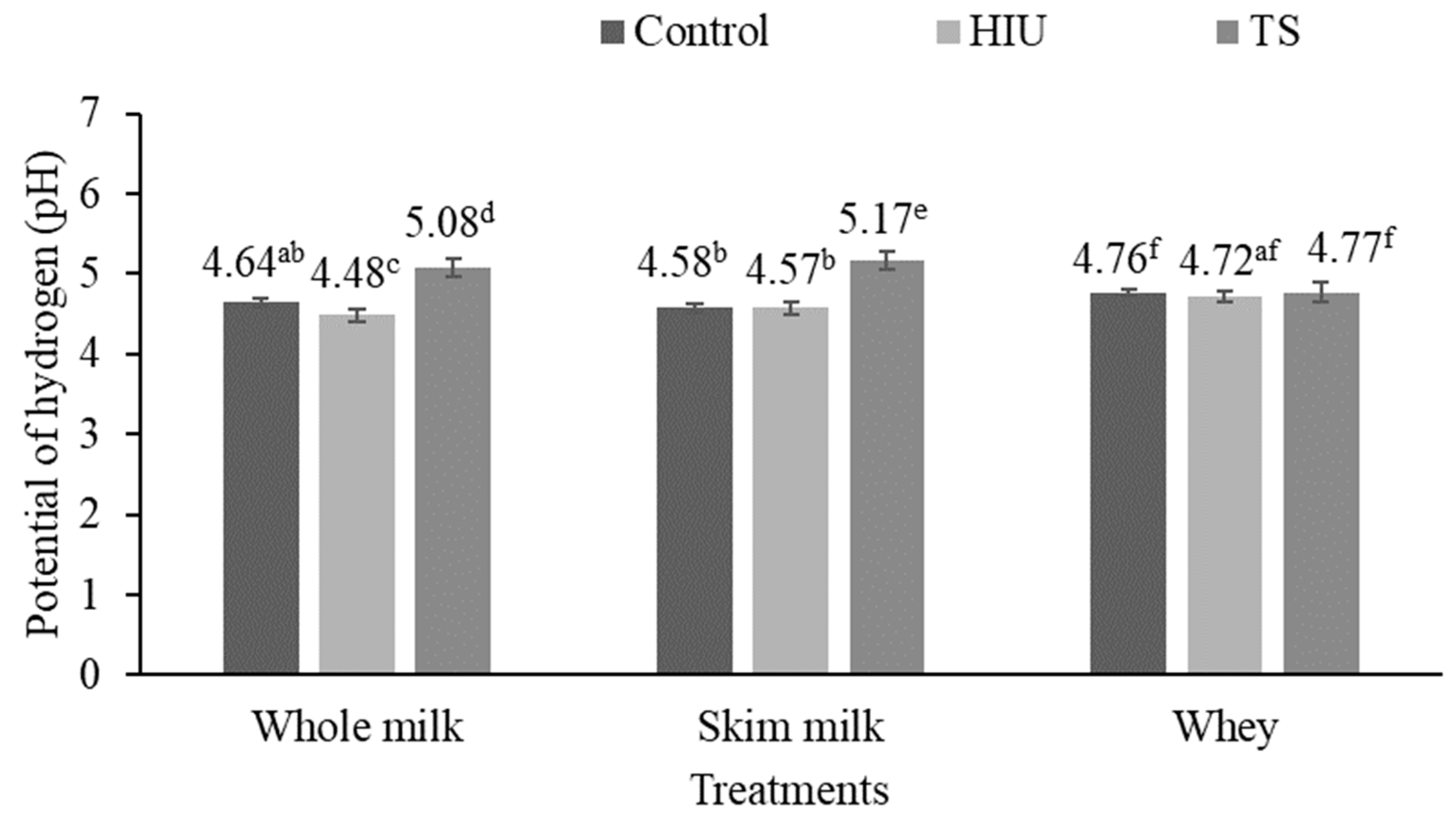
3.1. Potential of Hydrogen (pH)
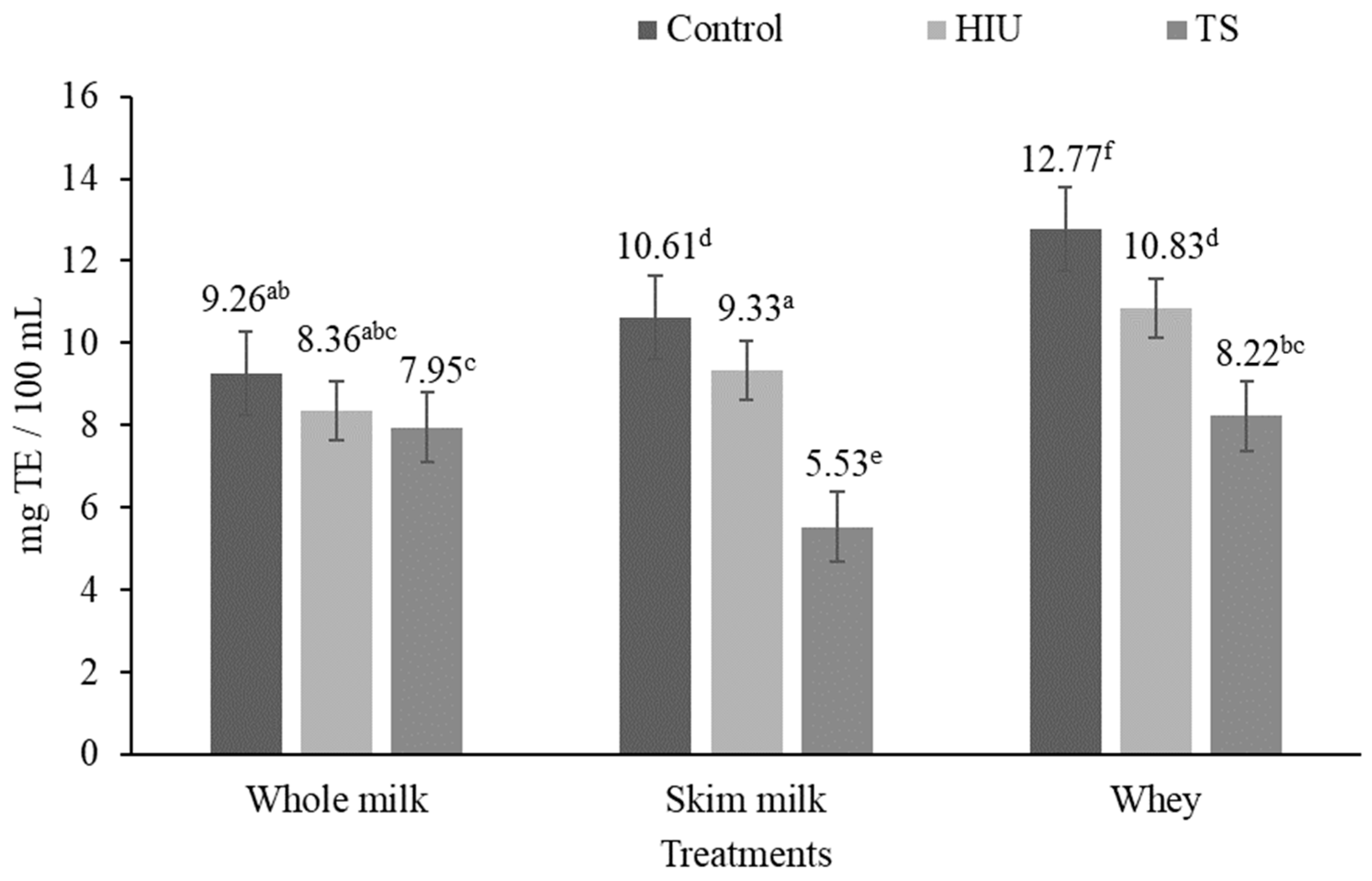
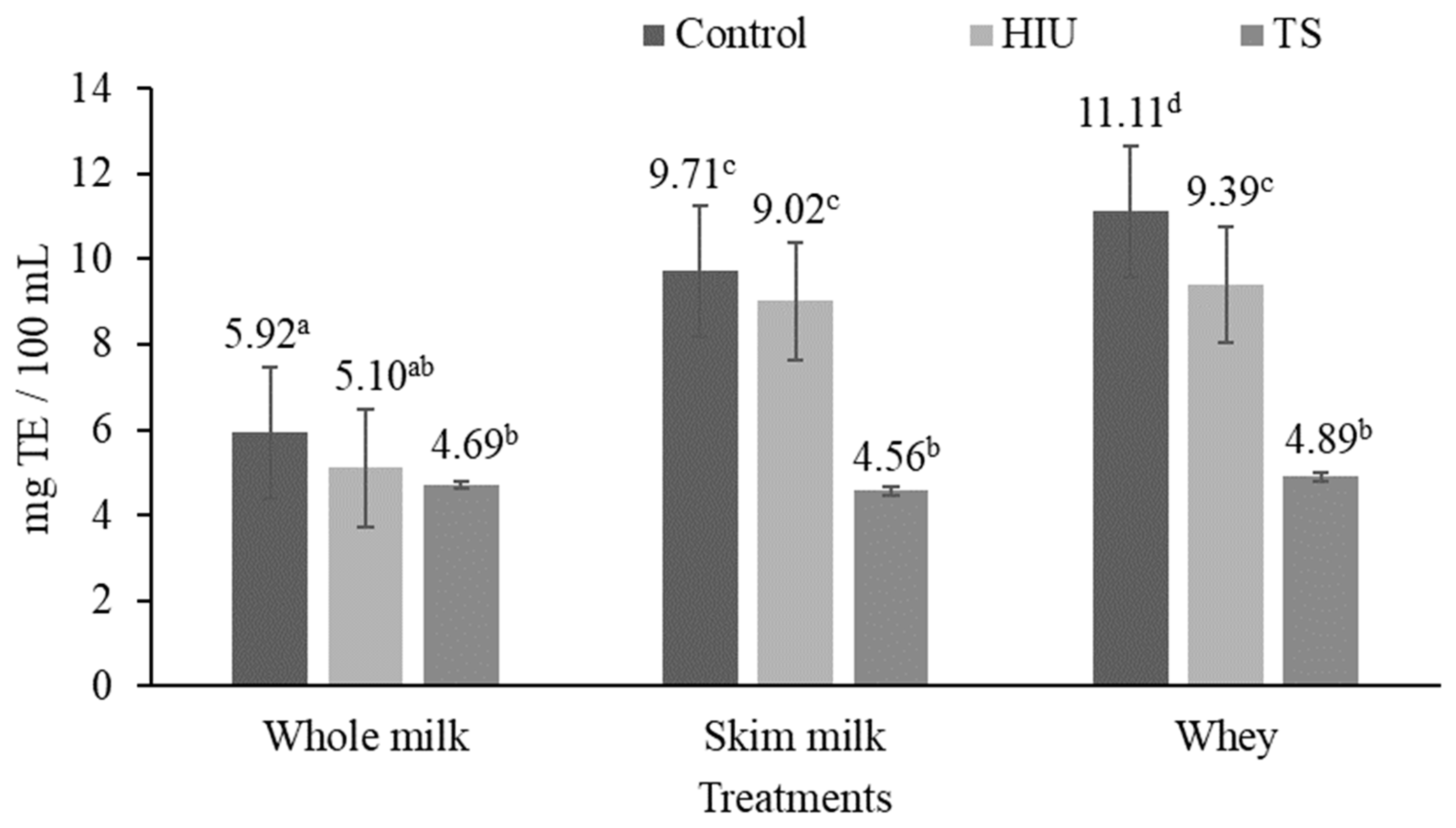
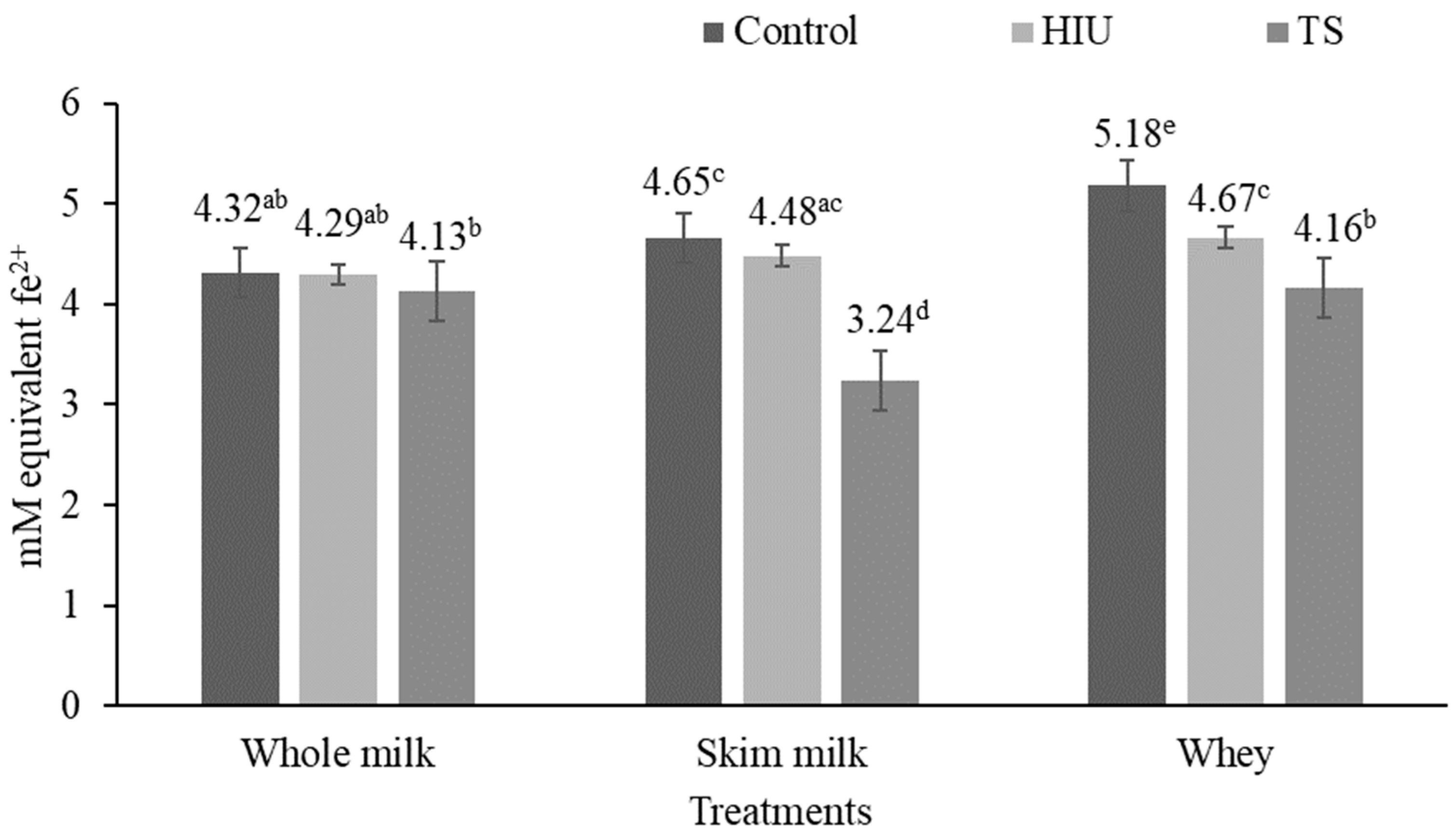
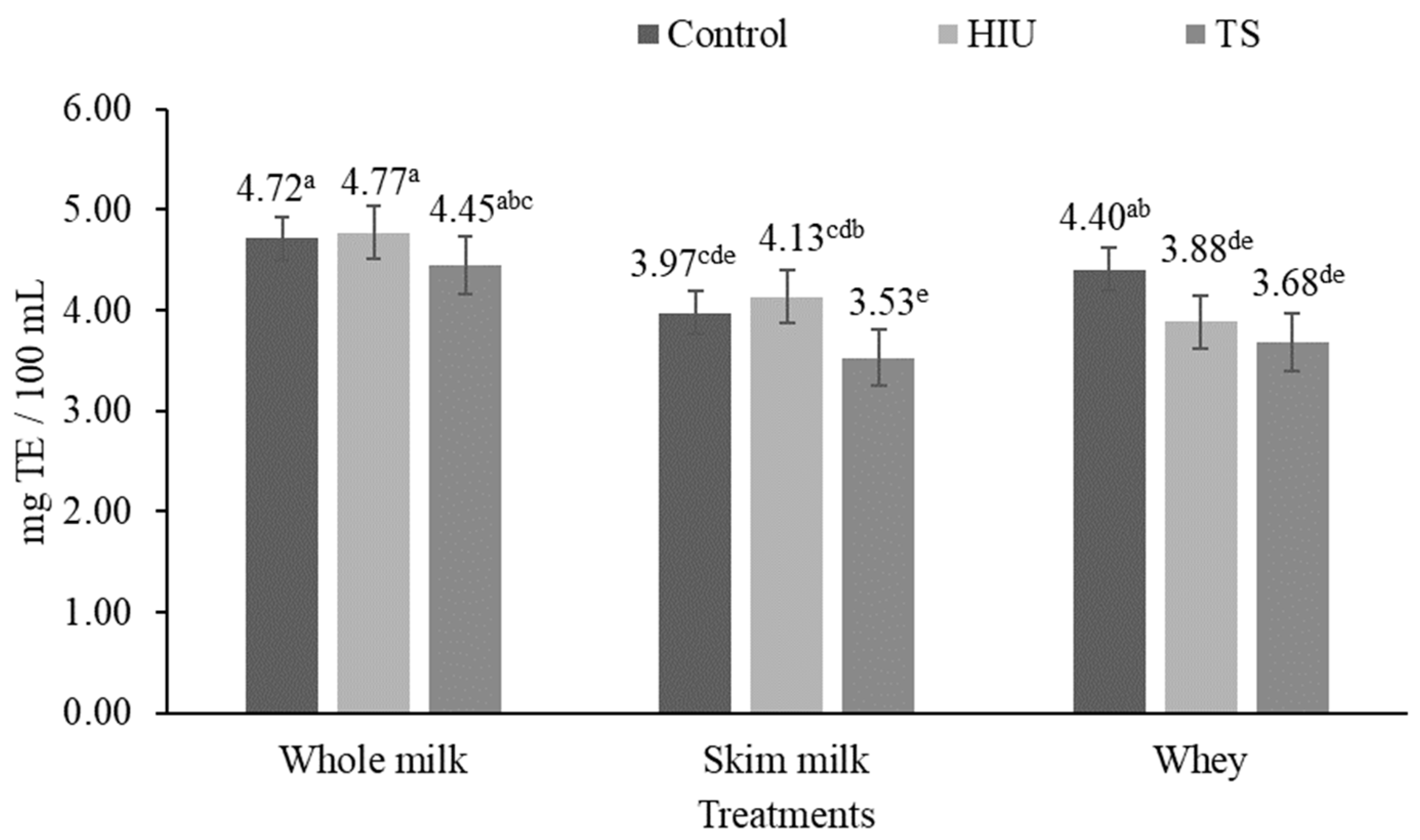
3.2. Antioxidant Activity
3.2.1. ABTS Assay
3.2.2. DPPH Assay
3.2.3. FRAP Assay
3.2.4. ORAC Assay
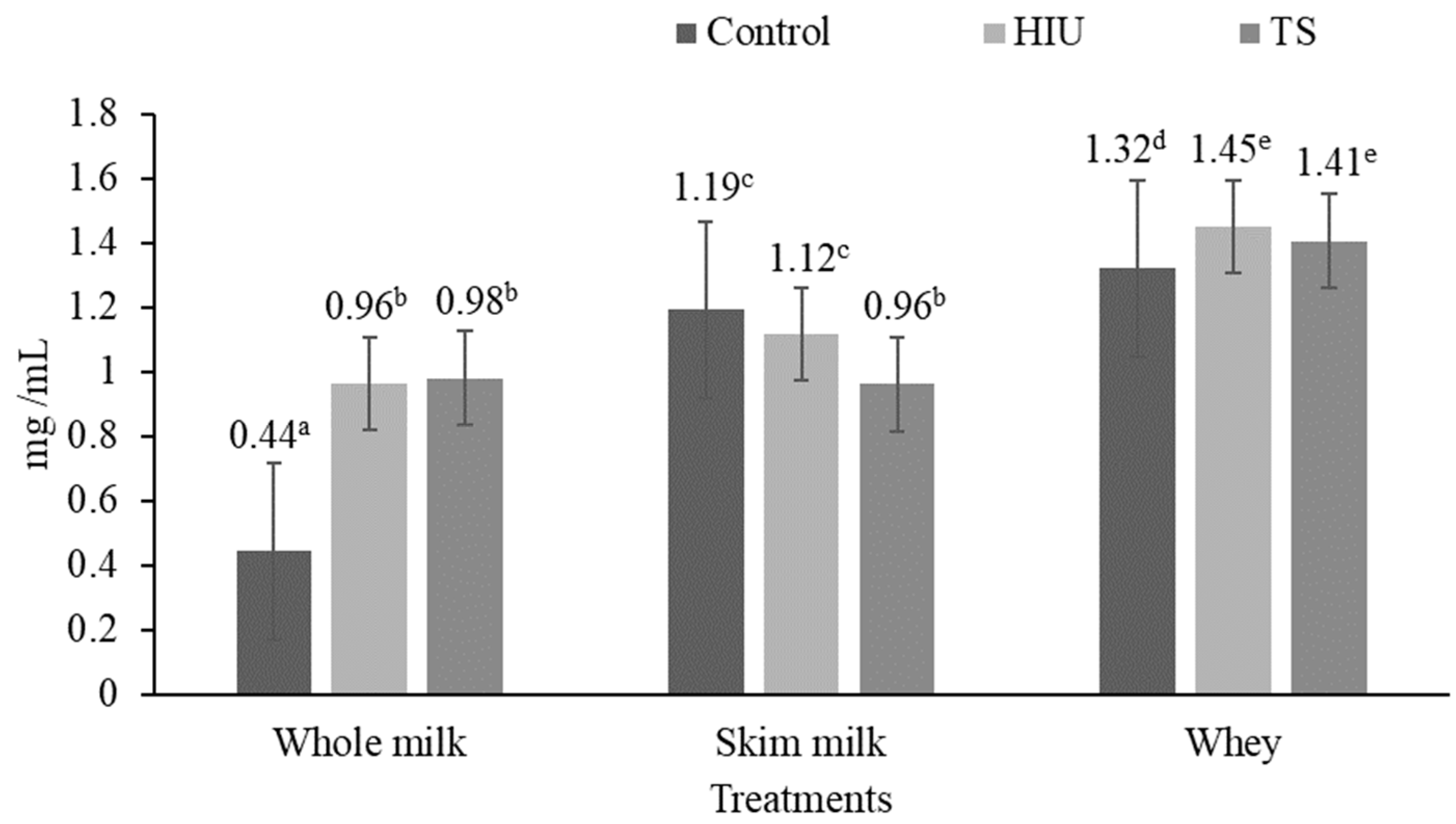
3.3. Total Water-Soluble Protein Content
3.4. Proteolysis
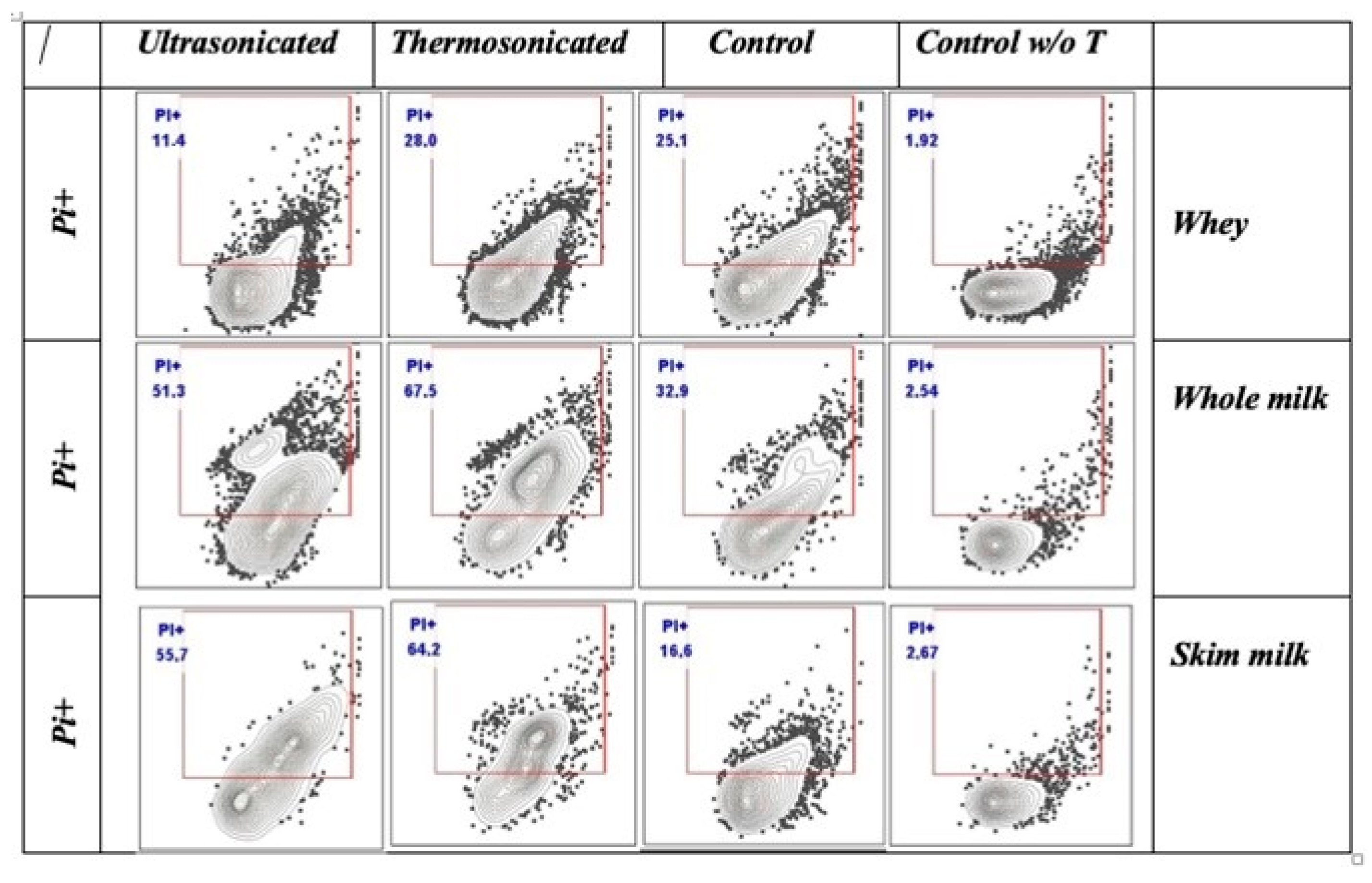
3.5. Cell Membrane Permeability
4. Discussion
4.1. Potential of Hydrogen (pH)
4.2. Antioxidant Activity
4.3. Total Water-Soluble Protein Content
4.4. Proteolysis
4.5. Cell Membrane Permeability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO/WHO: Cordoba, Argentina, 2001. [Google Scholar]
- Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; García-Cayuela, T. Probióticos y prebióticos en productos lácteos y su efecto sobre la diabetes tipo 2. Alimentaria 2021, 526, 98–105. Available online: http://hdl.handle.net/10261/263844 (accessed on 15 May 2024).
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics: A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Vinderola, G. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Bolivar-Jacobo, N.A.; Reyes-Villagrana, R.A.; Chávez-Martínez, A. Relación entre probióticos-postbióticos y sus principales efectos bioactivos. Tecnociencia Chihuah. 2021, 15, 124–139. [Google Scholar] [CrossRef]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Malashree, L.; Angadi, V.; Yadav, K.S.; Prabha, R. “Postbiotics”: One Step Ahead of Probiotics. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2049–2053. [Google Scholar] [CrossRef]
- Feng, H.; Luo, N.; Xiong, X.; Wu, Y. Prevalence of food allergy in the Chinese population: A systematic review and meta-analysis of population-based studies. Allergy Asthma Proc. 2023, 44, 315–325. [Google Scholar] [CrossRef]
- Hayes, E.; Wallace, D.; O’Donnell, C.; Greene, D.; Hennessy, D.; O’Shea, N.; Tobin, J.T.; Fenelon, M.A. Trend analysis and prediction of seasonal changes in milk composition from a pasture-based dairy research herd. JDS 2023, 106, 2326–2337. [Google Scholar] [CrossRef]
- Ait-Lhaj, F.; Elhamri, H.; Ait-Lhaj, Z.; Malisch, R.; Kypke, K.; Kabriti, M.; El Hajjaji, S.; Bellaouchou, A. First WHO/UNEP survey of the current concentrations of persistent organic pollutants in human milk in Morocco. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2023, 40, 282–293. [Google Scholar] [CrossRef]
- Müller, A.C.; Herter, J.; Huber, R.; Storz, M.A. Potential renal acid load of non-dairy plant-based milk alternatives. Int. J. Food Prop. 2023, 26, 2128–2136. [Google Scholar] [CrossRef]
- Çakir-Biçer, N.; Baş, D.; Seçkiner, S.; Kahriman, M.; Baş, M. Evaluation of Consumers’ Perceptions and Purchase Decisions Regarding Plant-Based Milk Alternatives in Turkey. IDUHeS 2023, 6, 82–102. [Google Scholar] [CrossRef]
- Pointke, M.; Albrecht, E.; Geburt, K.; Gerken, M.; Traulsen, I.; Pawelzik, E. A Comparative Analysis of Plant-Based Milk Alternatives Part 1: Composition, Sensory, and Nutritional Value. Sustainability 2022, 14, 7996. [Google Scholar] [CrossRef]
- Antunes, I.C.; Bexiga, R.; Pinto, C.; Roseiro, L.C.; Quaresma, M.A.G. Cow’s Milk in Human Nutrition and the Emergence of Plant-Based Milk Alternatives. Foods 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Vale, A.; de Melo-Pereira, G.V.; de Oliveira, A.C.; de Carvalho-Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, C.R. Production, Formulation, and Application of Postbiotics in the Treatment of Skin Conditions. Fermentation 2023, 9, 264. [Google Scholar] [CrossRef]
- Dunand, E.; Burns, P.; Binetti, A.; Bergamini, C.; Peralta, G.H.; Forzani, L.; Reinheimer, J.; Vinderola, G. Postbiotics produced at laboratory and industrial level as potential functional food ingredients with the capacity to protect mice against Salmonella infection. J. Appl. Microbiol. 2019, 127, 282–295. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Cruz, A.G.; Pereira, E.A.; Costa, W.K.A.; Rocha, R.T.S.; Pedrosa, G.R.; Alves, C.S.; Alvarenga, J.M.; Ortiz, V.; Sant’Ana, A.S.; et al. Postbiotics: An overview of concepts, inactivation technologies, health effects, and driver trends. Trends Food Sci. Technol. 2023, 132, 103–116. [Google Scholar] [CrossRef]
- Encinas-Vazquez, I.A.; Carrillo-Pérez, E.; Mártin-García, A.R.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; Bastarrachea, L.J.; Rodríguez-Figueroa, J.C. Effects of High-Intensity Ultrasound Pretreatment on the Exopolysaccharide Concentration and Biomass Increase in Cheese Whey Kefir. Processes 2023, 11, 1905. [Google Scholar] [CrossRef]
- Abdullah, N.; Chin, N.L. Application of Thermosonication Treatment in Processing and Production of High Quality and Safe-to-Drink Fruit Juices. Agric. Agric. Sci. Procedia 2014, 2, 320–327. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Lee, Y.K.; Zhou, W. Stimulating fermentative activities of bifidobacteria in milk by high-intensity ultrasound. Int. Dairy J. 2009, 19, 410–416. [Google Scholar] [CrossRef]
- Dahroud, B.D.; Mokarram, R.R.; Khiabani, M.S.; Hamishehkar, H.; Bialvaei, A.Z.; Yousefi, M.; Kafil, H.S. Low intensity ultrasound increases the fermentation efficiency of Lactobacillus casei subsp. casei ATTC 39392. Int. J. Biol. Macromol. 2016, 86, 462–467. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Y.; Chen, M.; Chen, L.; Lin, C. Antioxidative Activities of Kefir. Anim. Biosci. 2005, 18, 567–573. [Google Scholar] [CrossRef]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Sahin, S. Comparison of antioxidant capacity of cow and ewe milk kefirs. JDS 2018, 101, 3788–3798. [Google Scholar] [CrossRef]
- Kesenkaş, H.; Dinkci, N.; Seçkin, A.K.; Kinik, Ö.; Gönç, S. Antioxidant Properties of Kefir Produced from Different Cow and Soymilk Mixtures. JAS 2011, 17, 253–259. [Google Scholar] [CrossRef]
- Satir, G.; Guzel-Seydim, Z. Influence of Kefir fermentation on the bioactive substances of different breed goat milks. LWT Food Sci. Technol. 2015, 63, 852–858. [Google Scholar] [CrossRef]
- Bolívar-Jacobo, N.A.; Reyes-Villagrana, R.A.; Espino-Solís, G.P.; Rentería-Monterrubio, A.L.; Arévalos-Sánchez, M.M.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Flores, D.; Chávez-Martínez, A. The Effects of a High-Intensity Ultrasound on the Fermentative Activity and Kinetic Growth of Lactobacillus acidophilus and Lactobacillus helveticus. Fermentation 2023, 9, 356. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ong, L.; Shah, N.P. Probiotic cheddar cheese: Influence of ripening temperatures on proteolysis and sensory characteristics of cheddar cheeses. J. Food Sci. 2009, 74, 182–191. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Lee, Y.K.; Zhou, W. Effect of high-intensity ultrasound on carbohydrate metabolism of bifidobacteria in milk fermentation. Food Chem. 2012, 130, 866–874. [Google Scholar] [CrossRef]
- Wang, D.; Sakakibara, M. Lactose hydrolysis and β-galactosidase activity in sonicated fermentation with Lactobacillus strains. Ultrason. Sonochem. 1997, 4, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.; Viorica, B.; Rodica, S. Effect of Temperature, pH and Amount of Enzyme Used in the Lactose Hydrolysis of Milk. FNS J. 2021, 12, 1243–1254. [Google Scholar] [CrossRef]
- Bosso, A.; Morioka, L.R.; Santos, L.F.; Suguimoto, H.H. Lactose Hydrolysis Potential and Thermal Stability of Commercial β-Galactosidase in UHT and Skimmed Milk. FS&T J. 2016, 36, 159–165. [Google Scholar] [CrossRef]
- Carvalho, J.R.B.; Meireles, A.N.; Marques, S.S.; Gregório, B.J.R.; Ramos, I.I.; Silva, E.M.P.; Barreiros, L.; Segundo, M.A. Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity. Antioxidants 2023, 12, 505. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Grażyna, C.; Hanna, C.; Adam, A.; Magdalena, B.M. Natural antioxidants in milk and dairy products. Int. J. Dairy Technol. 2017, 70, 165–178. [Google Scholar] [CrossRef]
- Bielecka, M.; Cichosz, G.; Czeczot, H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates-a review. Int. Dairy J. 2022, 127, 105208. [Google Scholar] [CrossRef]
- Gagnon, M.; Savard, P.; Rivière, A.; LaPointe, G.; Roy, D. Bioaccessible antioxidants in milk fermented by Bifidobacterium longum subsp. longum strains. Biomed Res. Int. 2015, 2015, 169381. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Dhiny, A.D.; Jariyah, J.; Wicaksono, L.A.; Priyanto, A.D.; Safitri, S.; Esfandiar, W.N. Effect of frequency and duration of thermosonication on the physical, chemical and microbiological quality of cow’s milk. J. Pangan Agroindustri 2023, 11, 136–146. [Google Scholar] [CrossRef]
- Seung-Yong, L.; Benner, L.C.; Clark, S. Neither thermosonication nor cold sonication is better than pasteurization for milk shelf life. J. Dairy Sci. 2019, 102, 3965–3977. [Google Scholar] [CrossRef]
- Ragab, E.S.; Lu, J.; Pang, X.Y.; Nassar, K.S.; Yang, B.Y.; Zhang, S.W.; Lv, J.P. Effect of thermosonication process on physicochemical properties and microbial load of goat’s milk. J. Food Sci. Technol. 2019, 56, 5309–5316. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Bos, C.; Léonil, J.; Airinei, G.; Luengo, C.; Daré, S.; Benamouzig, R.; Fouillet, H.; Fauquant, J.; Tomé, D.; et al. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. AJCN 2006, 84, 1070–1079. [Google Scholar] [CrossRef]
- García-Risco, M.R.; Ramos, M.; López-Fandiño, R. Proteolysis, protein distribution and stability of UHT milk during storage at room temperature. J. Sci. Food Agric. 1999, 79, 1171–1178. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, S.; Pang, X. Separation of serum proteins and micellar casein from skim goat milk by pilot-scale 0.05-μm pore-sized ceramic membrane at 50 °C. J. Food Process Eng. 2020, 43, 13334. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, X.X.; Shao, X.Q.; Cheng, M.; Wang, C.F.; Jiang, H.; Zhang, X.N.; Yuan, C.Z. Modifying the physicochemical properties, solubility and foaming capacity of milk proteins by ultrasound-assisted alkaline pH-shifting treatment. Ultrason. Sonochem. 2022, 88, 106089. [Google Scholar] [CrossRef]
- Zhao, R.; Fu, W.; Li, D.; Dong, C.; Bao, Z.; Wang, C. Structure and functionality of whey protein, pea protein, and mixed whey and pea proteins treated by pH shift or high-intensity ultrasound. JDS 2024, 107, 726–741. [Google Scholar] [CrossRef]
- Jovanovic, S.; Barac, M.; Macej, O.; Vucic, T.; Lacnjevac, C. SDS-PAGE Analysis of Soluble Proteins in Reconstituted Milk Exposed to Different Heat Treatments. Sensors 2007, 7, 371–383. [Google Scholar] [CrossRef]
- Hong, Z.H.; Xiao, N.; Li, L.; Li, Y.; Xie, X.A. Glycation of whey protein isolate and emulsions prepared by conjugates. J. Food Eng. 2022, 316, 110852. [Google Scholar] [CrossRef]
- Pellegrino, L. Influence of fat content on some heat-induced changes in milk and cream. Neth. Milk Dairy J. 1994, 48, 71–80. Available online: https://api.core.ac.uk/oai/oai:air.unimi.it:2434/197487 (accessed on 1 May 2024).
- Yukalo, V.; Krupa, O. Proteolytic systems of lactic acid microorganisms: A review. Ukr. Food J. 2017, 6, 417–432. [Google Scholar] [CrossRef]
- Ardö, Y.; McSweeney, P.L.H.; Magboul, A.A.A.; Upadhyay, V.K.; Fox, P.F. Chapter 18-Biochemistry of Cheese Ripening: Proteolysis. In Cheese (Fourth Edition): Chemistry, Physics and Microbiology; Academic Press: Cambridge, MA, USA, 2017; pp. 445–482. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Liang, Z.Q.; Zhang, C.Y.; Hao, S.Q.; Han, H.Y.; Du, P.; Li, A.L.; Shao, H.; Li, C.; Liu, L.B. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. Lebensm. Wiss. Technol. 2021, 152, 112272. [Google Scholar] [CrossRef]
- Hsieh, H.H.; Wang, S.Y.; Chen, T.L.; Huang, Y.L.; Chen, M.J. Effects of cow’s and goat’s milk as fermentation media on the microbial ecology of sugary kefir grains. Int. J. Food Microbiol. 2012, 157, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Fiorda, F.A.; Pereira, G.V.; Thomaz-Soccol, V.; Medeiros, A.B.; Rakshit, S.K.; Soccol, C.R. Development of kefir-based probiotic beverages with DNA protection and antioxidant activities using soybean hydrolyzed extract, colostrum and honey. Lwt-Food Sci. Technol. 2016, 68, 690–697. [Google Scholar] [CrossRef]
- Khadem, H.; Tirtouil, A.M.; Drabo, M.S.; Boubakeur, B. Ultrasound conditioning of Streptococcus thermophilus CNRZ 447: Growth, biofilm formation, exopolysaccharide production, and cell membrane permeability. Biotechnologia 2020, 101, 159–165. [Google Scholar] [CrossRef]
- Tabatabaie, F.; Mortazavi, A. Studying the Effects of Ultrasound Shock on Cell Wall Permeability and Survival of Some LAB in Milk. World Appl. Sci. J. 2008, 3, 119–121. [Google Scholar] [CrossRef]
- Ramteke, S.P.; Desale, R.J.; Kankhare, D.H.; Fulpagare, Y.G. Thermosonication Technology in the Dairy Industry: A Review. IJARBS 2020, 7, 82–89. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Alzaga, G.; Reyes-Villagrana, R.A.; Espino-Solis, G.P.; Arévalos-Sánchez, M.M.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Santellano-Estrada, E.; Bolivar-Jacobo, N.A.; Tirado-Gallegos, J.M.; Chávez-Martínez, A. The Effects of Substrates and Sonication Methods on the Antioxidant Activity of Kefir Postbiotics. Fermentation 2024, 10, 492. https://doi.org/10.3390/fermentation10090492
Chávez-Alzaga G, Reyes-Villagrana RA, Espino-Solis GP, Arévalos-Sánchez MM, Rentería-Monterrubio AL, Sánchez-Vega R, Santellano-Estrada E, Bolivar-Jacobo NA, Tirado-Gallegos JM, Chávez-Martínez A. The Effects of Substrates and Sonication Methods on the Antioxidant Activity of Kefir Postbiotics. Fermentation. 2024; 10(9):492. https://doi.org/10.3390/fermentation10090492
Chicago/Turabian StyleChávez-Alzaga, Gerardo, Raúl Alberto Reyes-Villagrana, Gerardo Pavel Espino-Solis, Martha María Arévalos-Sánchez, Ana Luisa Rentería-Monterrubio, Rogelio Sánchez-Vega, Eduardo Santellano-Estrada, Norma Angélica Bolivar-Jacobo, Juan Manuel Tirado-Gallegos, and América Chávez-Martínez. 2024. "The Effects of Substrates and Sonication Methods on the Antioxidant Activity of Kefir Postbiotics" Fermentation 10, no. 9: 492. https://doi.org/10.3390/fermentation10090492
APA StyleChávez-Alzaga, G., Reyes-Villagrana, R. A., Espino-Solis, G. P., Arévalos-Sánchez, M. M., Rentería-Monterrubio, A. L., Sánchez-Vega, R., Santellano-Estrada, E., Bolivar-Jacobo, N. A., Tirado-Gallegos, J. M., & Chávez-Martínez, A. (2024). The Effects of Substrates and Sonication Methods on the Antioxidant Activity of Kefir Postbiotics. Fermentation, 10(9), 492. https://doi.org/10.3390/fermentation10090492






