Analysis of Composition, Antioxidation, and Immunoregulation for Exopolysaccharide Produced by Dellaglioa algida
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Extraction and Purification of the EPS
2.3. Composition Analysis of EPS
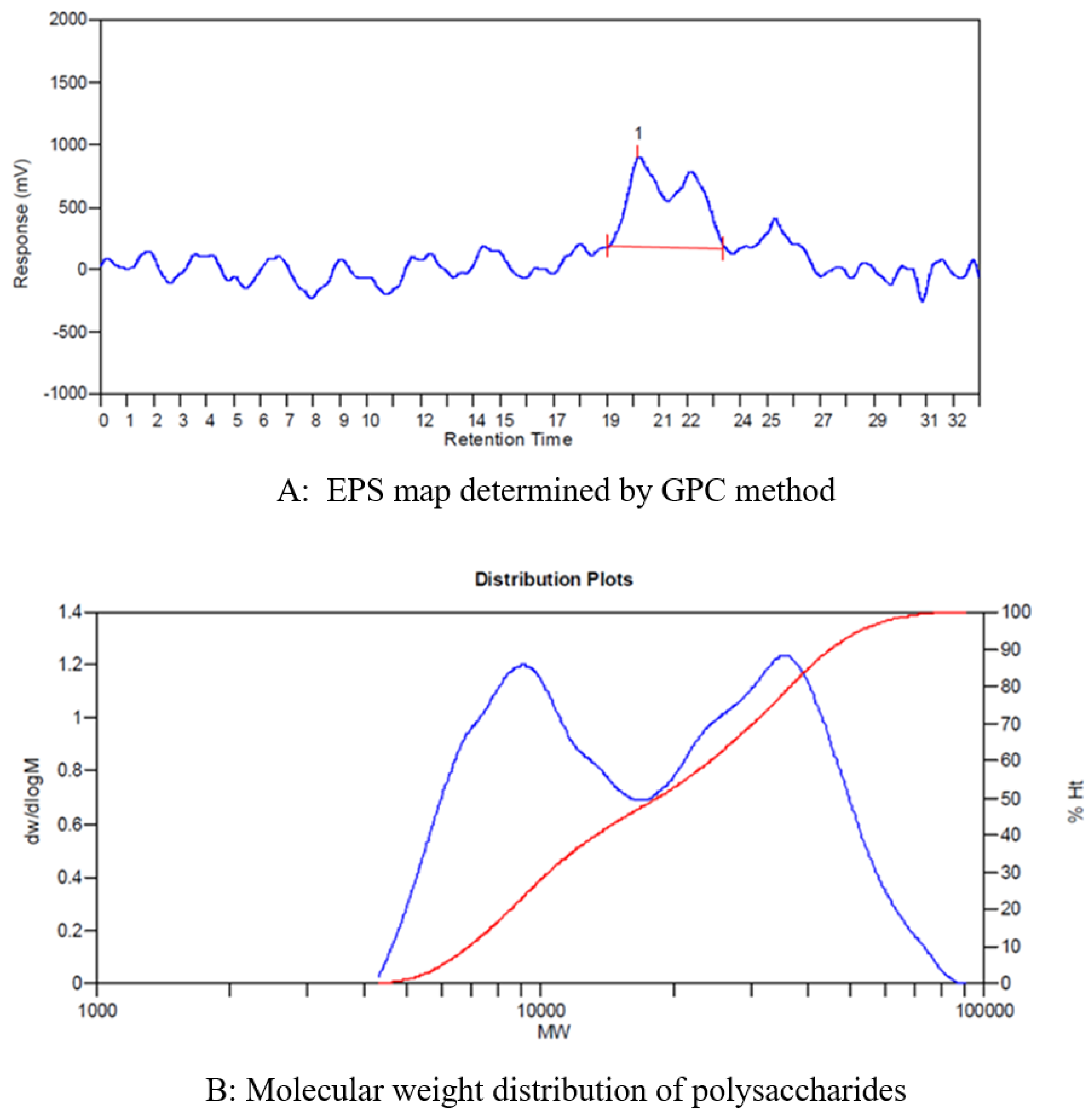
2.3.1. Average Molecular Weight Measurements
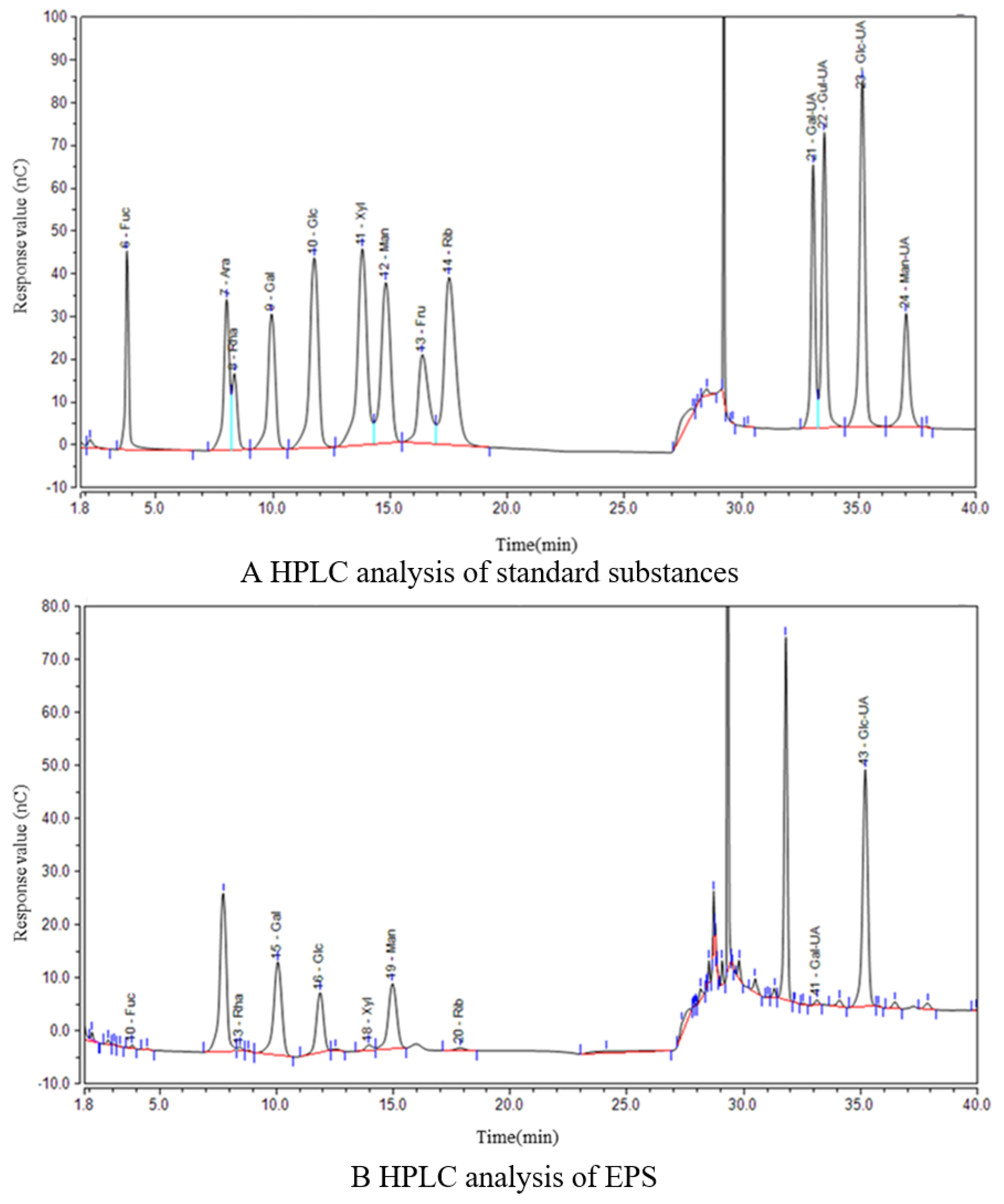
2.3.2. Monosaccharide Components’ Analysis
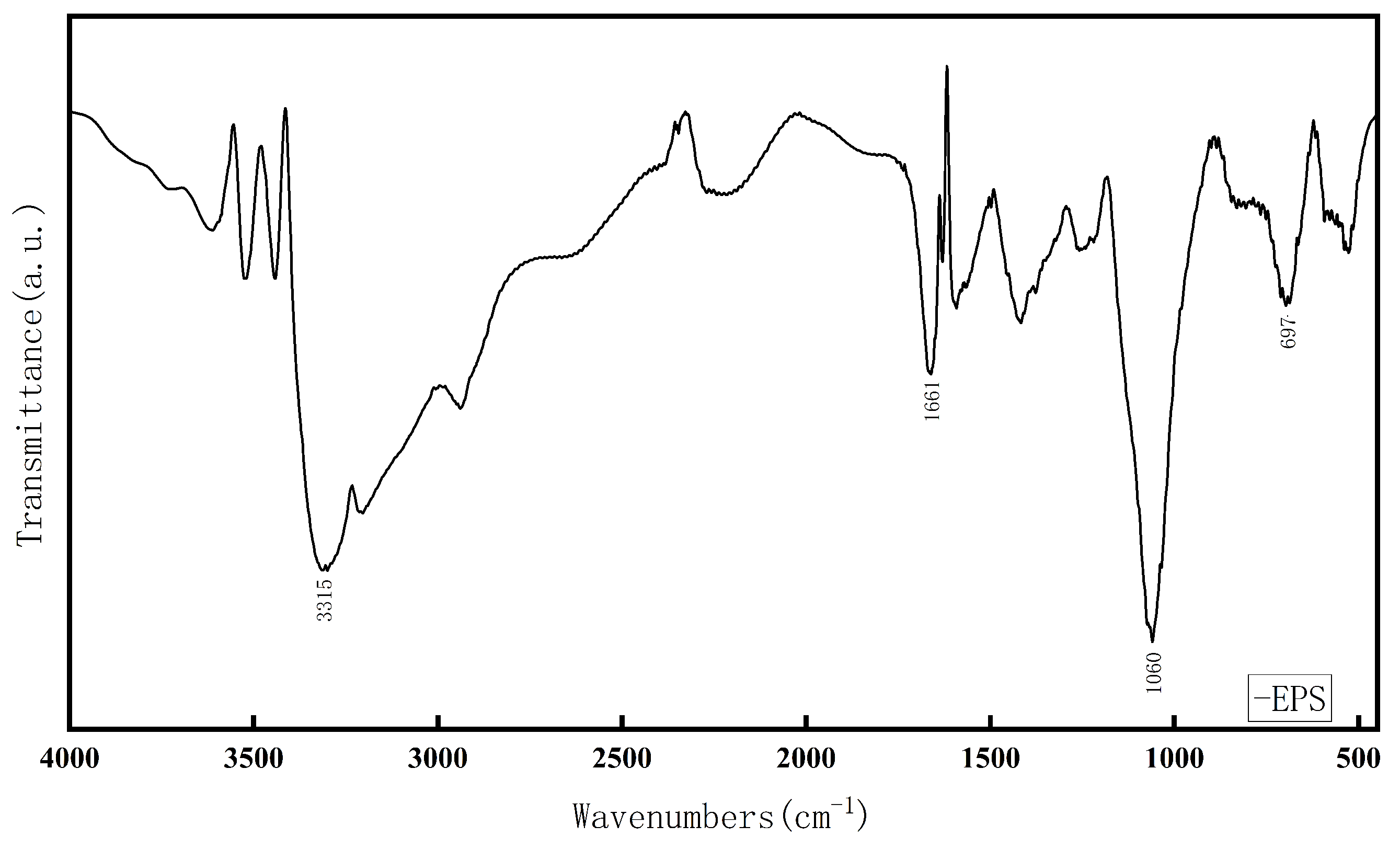
2.3.3. UV Full-Wavelength Scanning and FTIR Analysis
2.3.4. Scanning Electron Microscope (SEM) Analysis
2.3.5. NMR Analysis
2.4. Antioxidation Activities of EPS
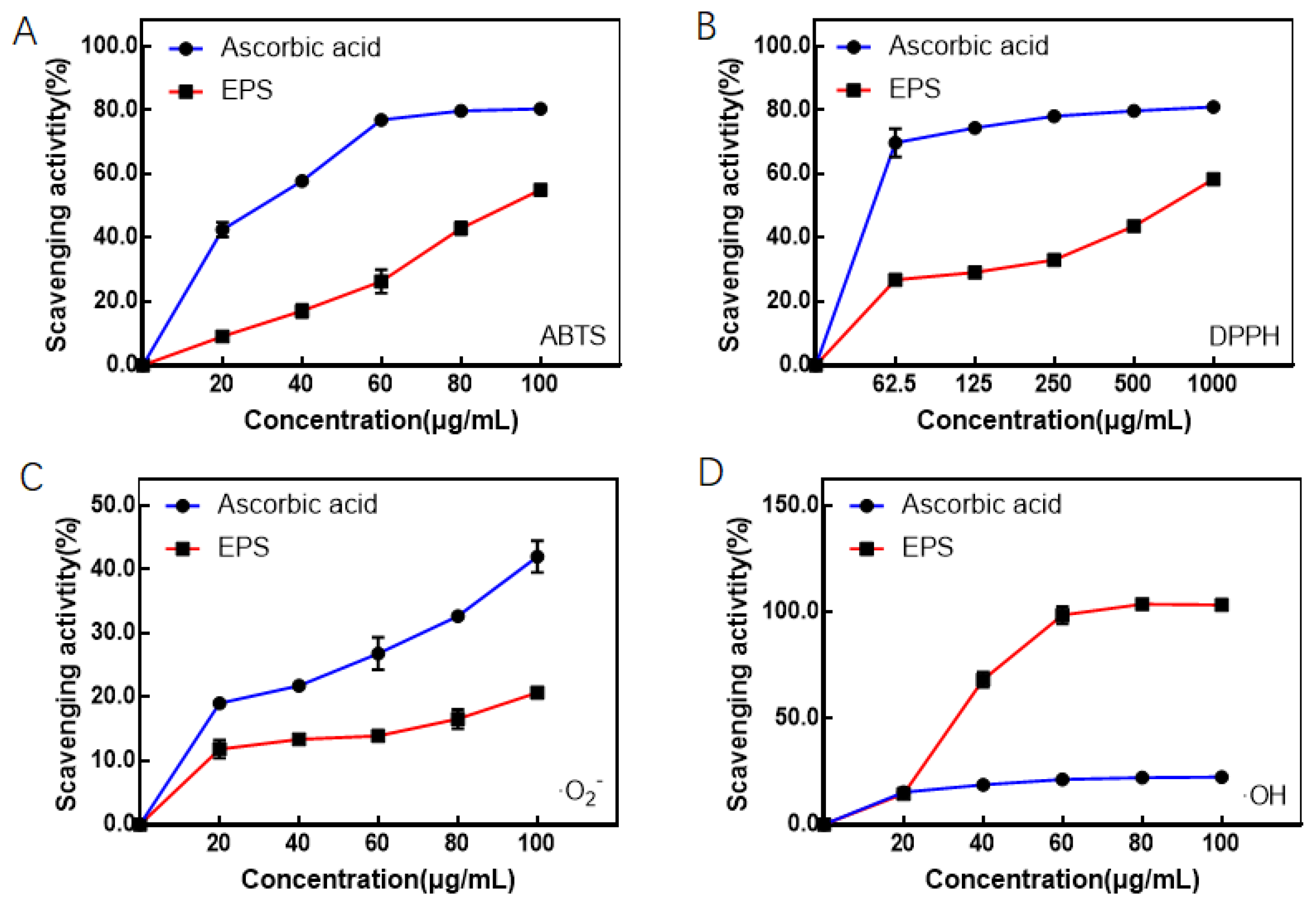
2.4.1. ABTS+ Free Radical Scavenging Assay
2.4.2. DPPH Free Radical Scavenging Assay
2.4.3. Hydroxyl (HO·) Radical Scavenging Assay
2.4.4. Superoxide Anion () Scavenging Assay
2.5. Immunoregulation Activities of EPS
2.5.1. Inflammation Model of RAW264.7 Cells
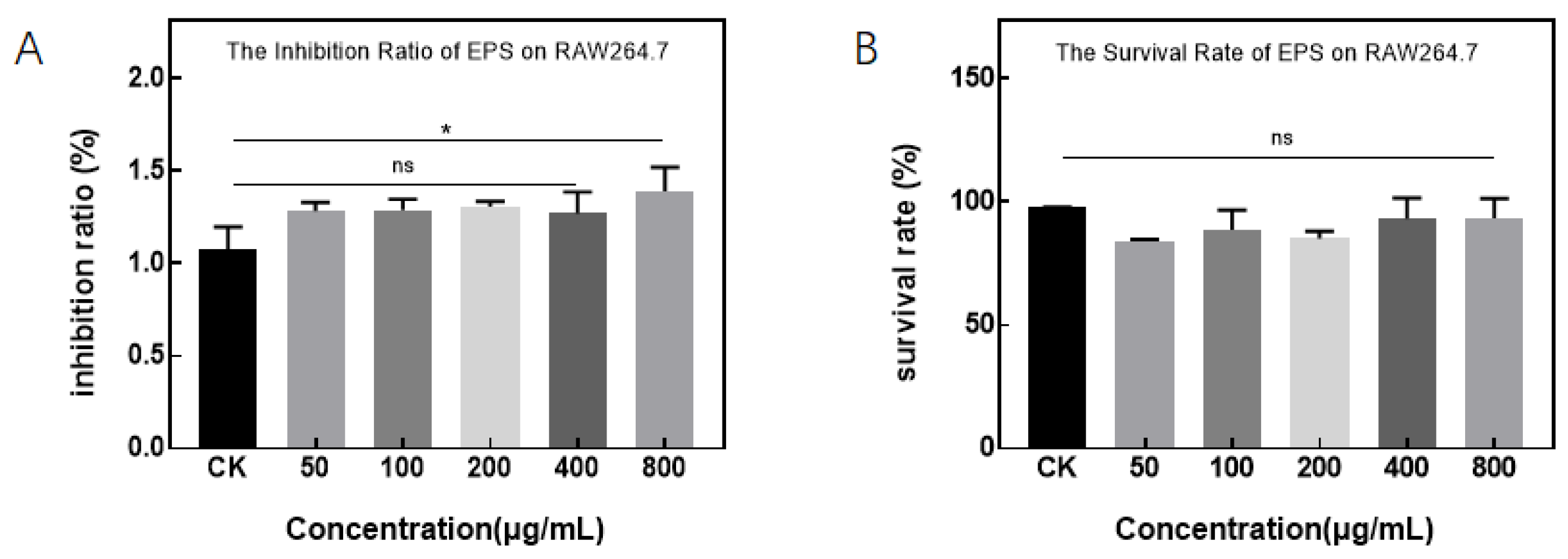
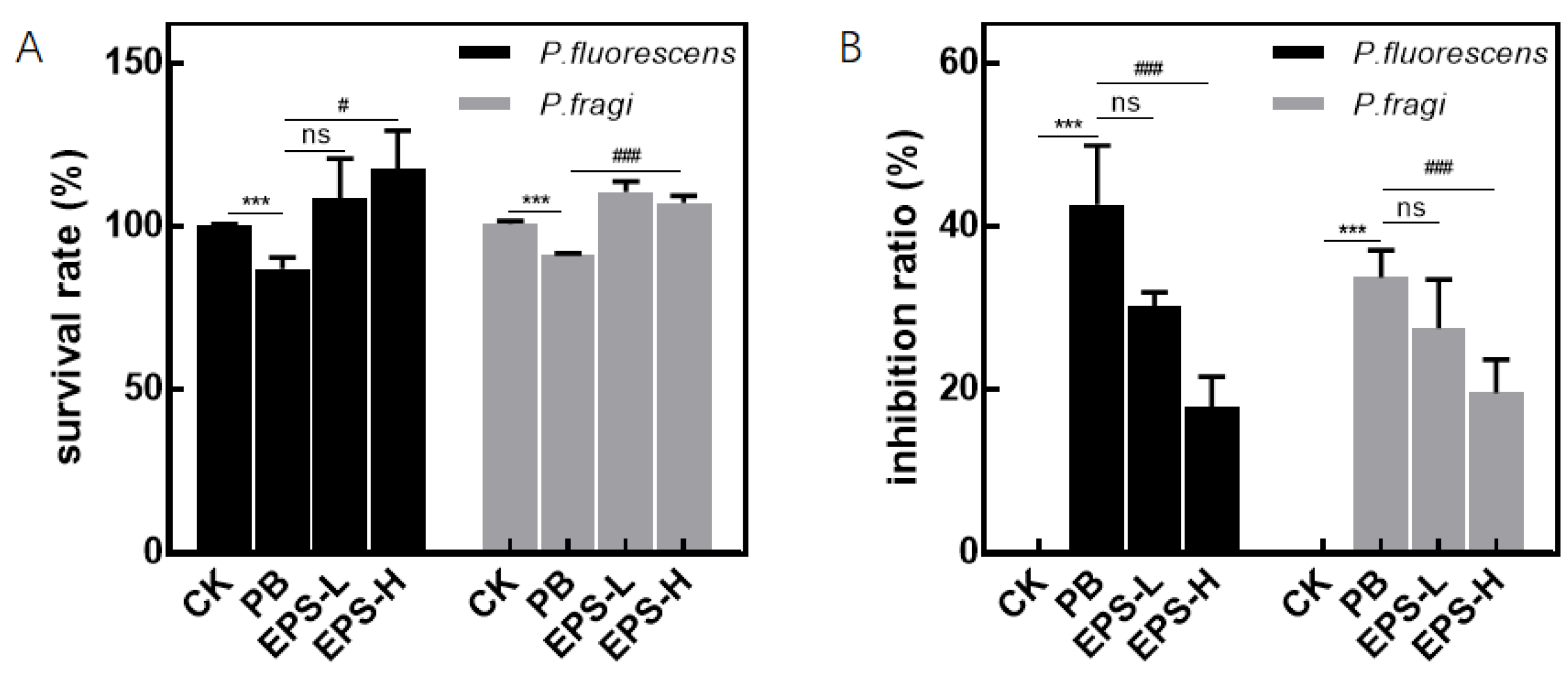
2.5.2. The Effect of EPS on Cell Activity
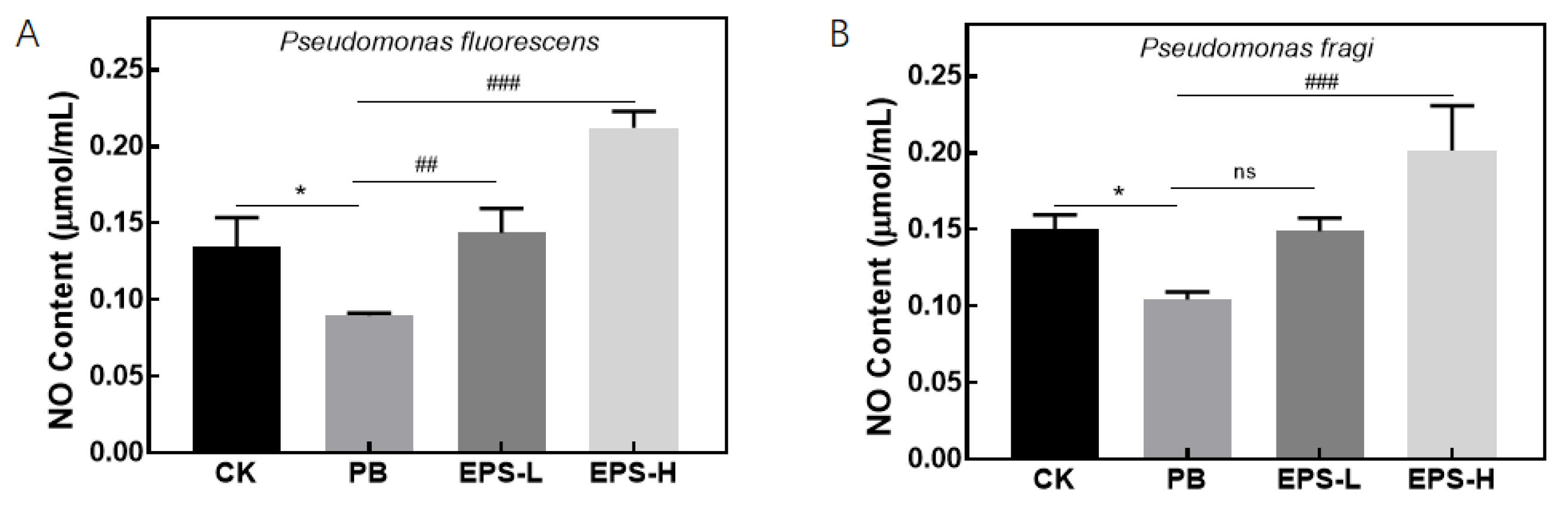
2.5.3. Effects of EPS on NO Secretion of Inflammatory RAW264.7 Cells
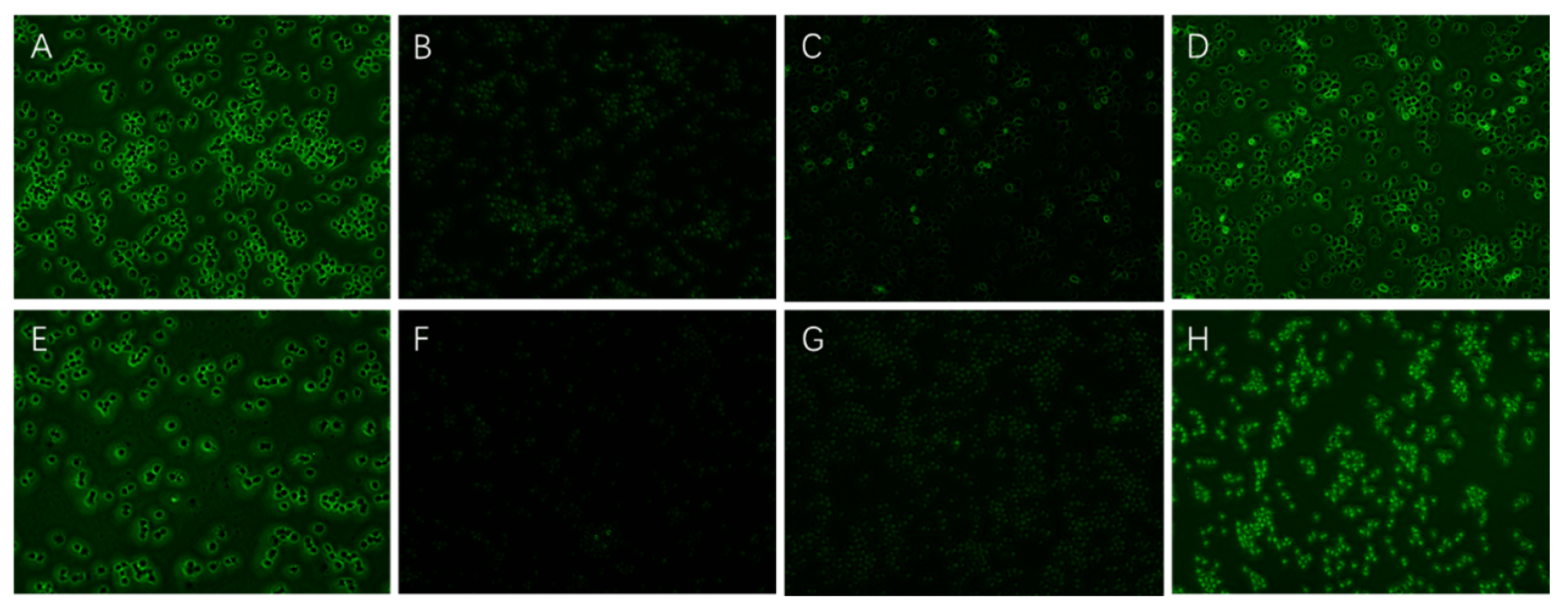
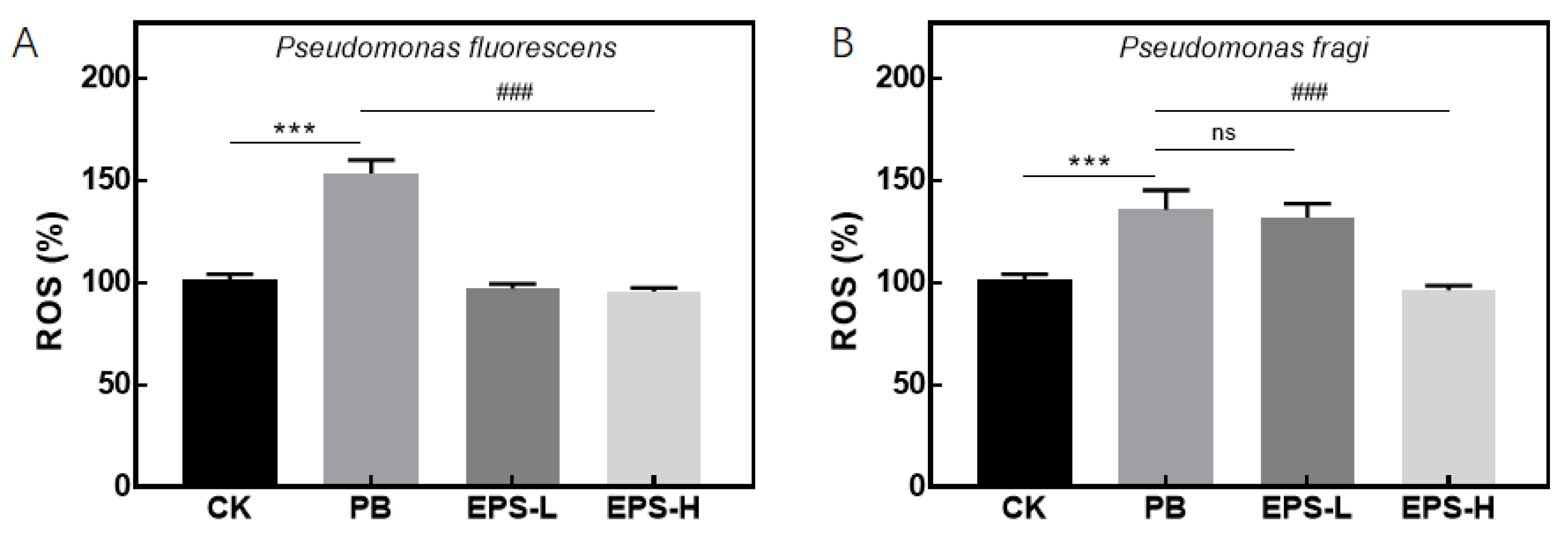
2.5.4. Effects of EPS on ROS Secretion of Inflammatory RAW264.7 Cells
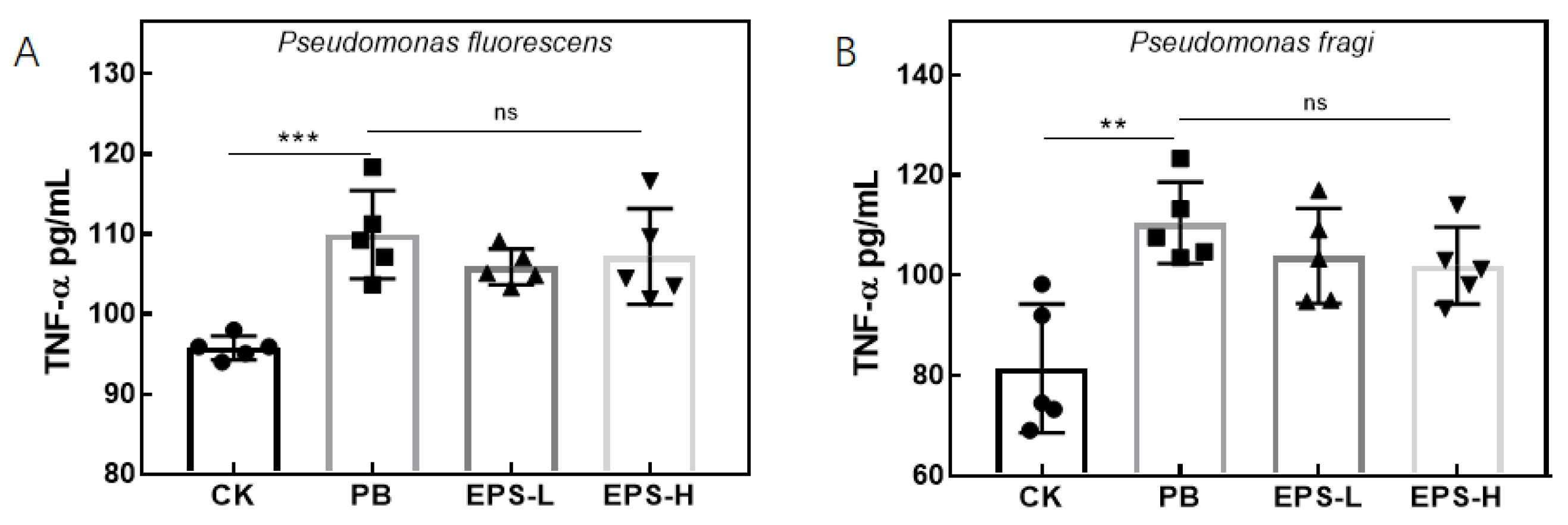
2.5.5. Effects of EPS on Cytokines Secretion of Inflammatory RAW264.7 Cells
2.5.6. Effects of EPS on mRNA Expression of Inflammatory RAW264.7 Cells
2.6. Data Analysis
3. Results
3.1. Composition Analysis of EPS Produced by Del. algida
3.1.1. Average Molecular Weight of EPS
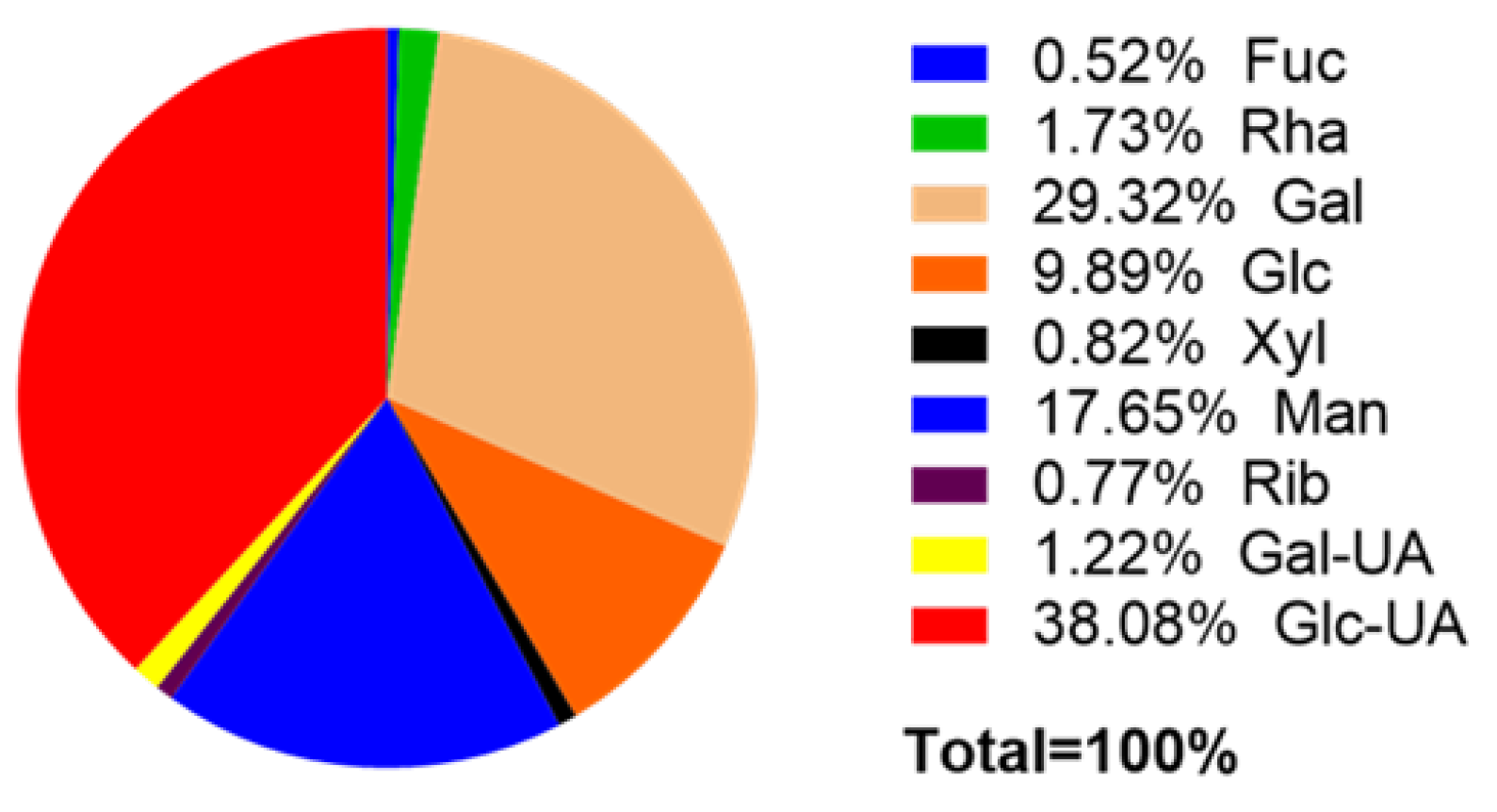
3.1.2. Monosaccharide Components of EPS
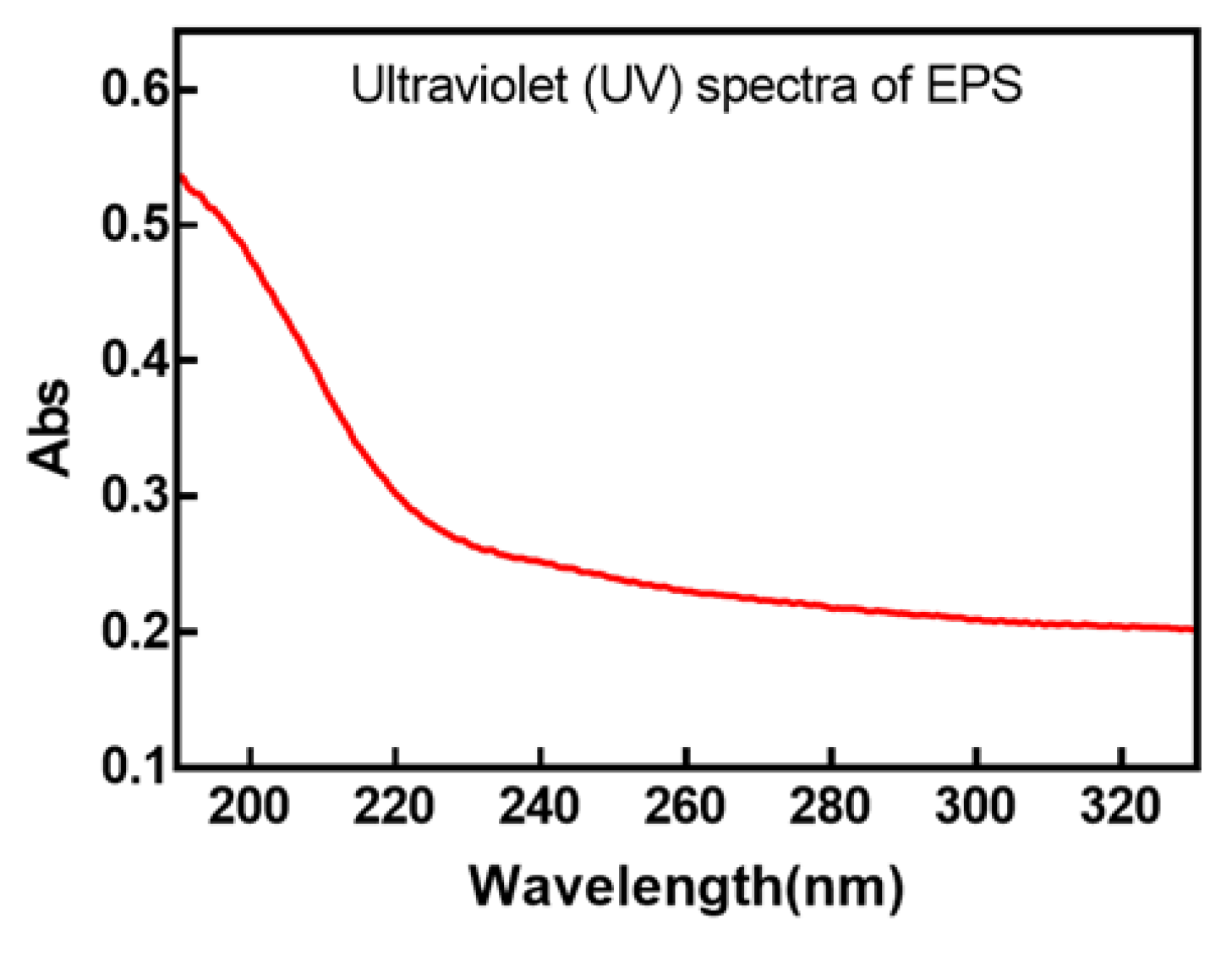
3.1.3. FTIR and UV Analysis of EPS
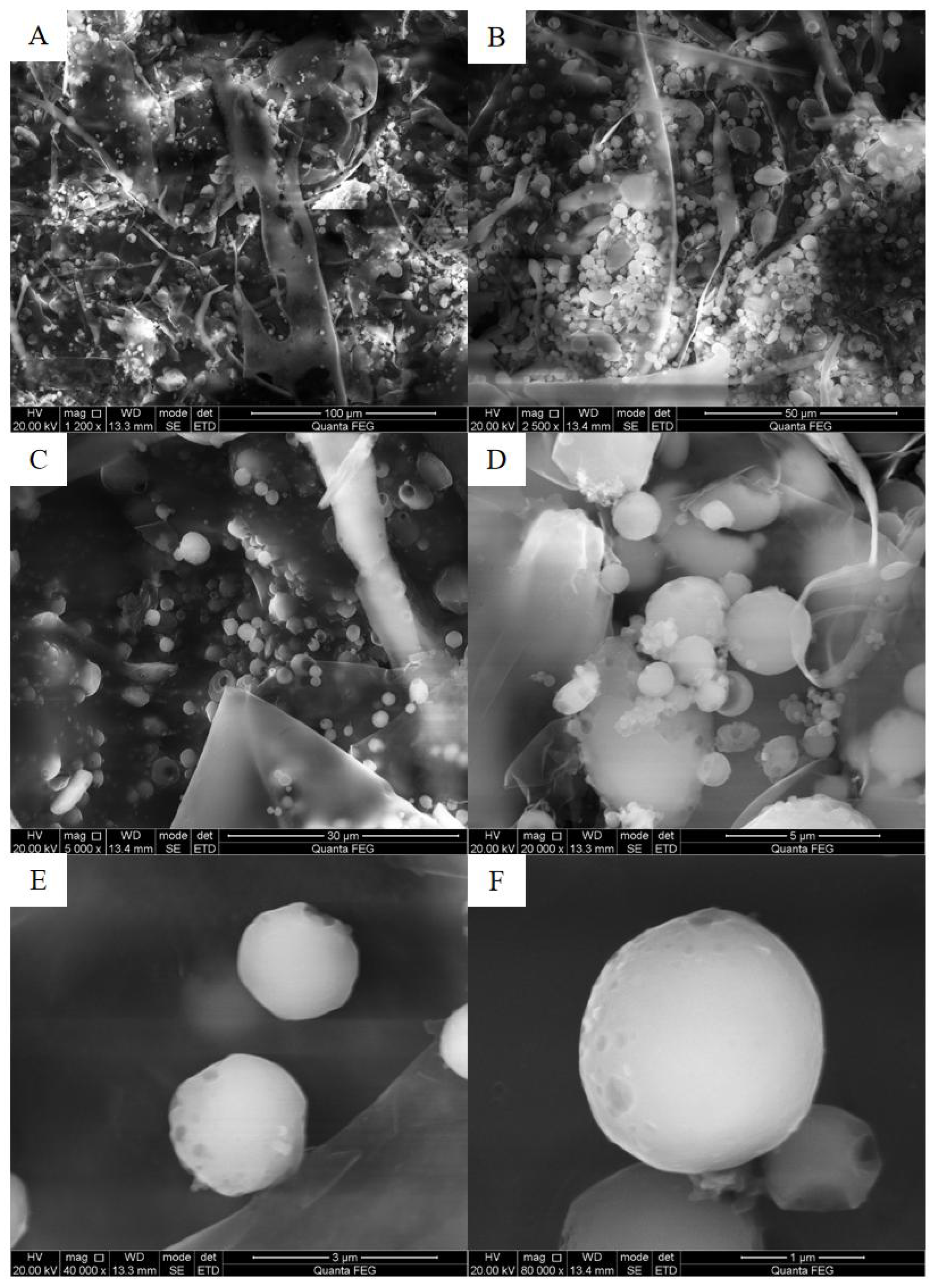
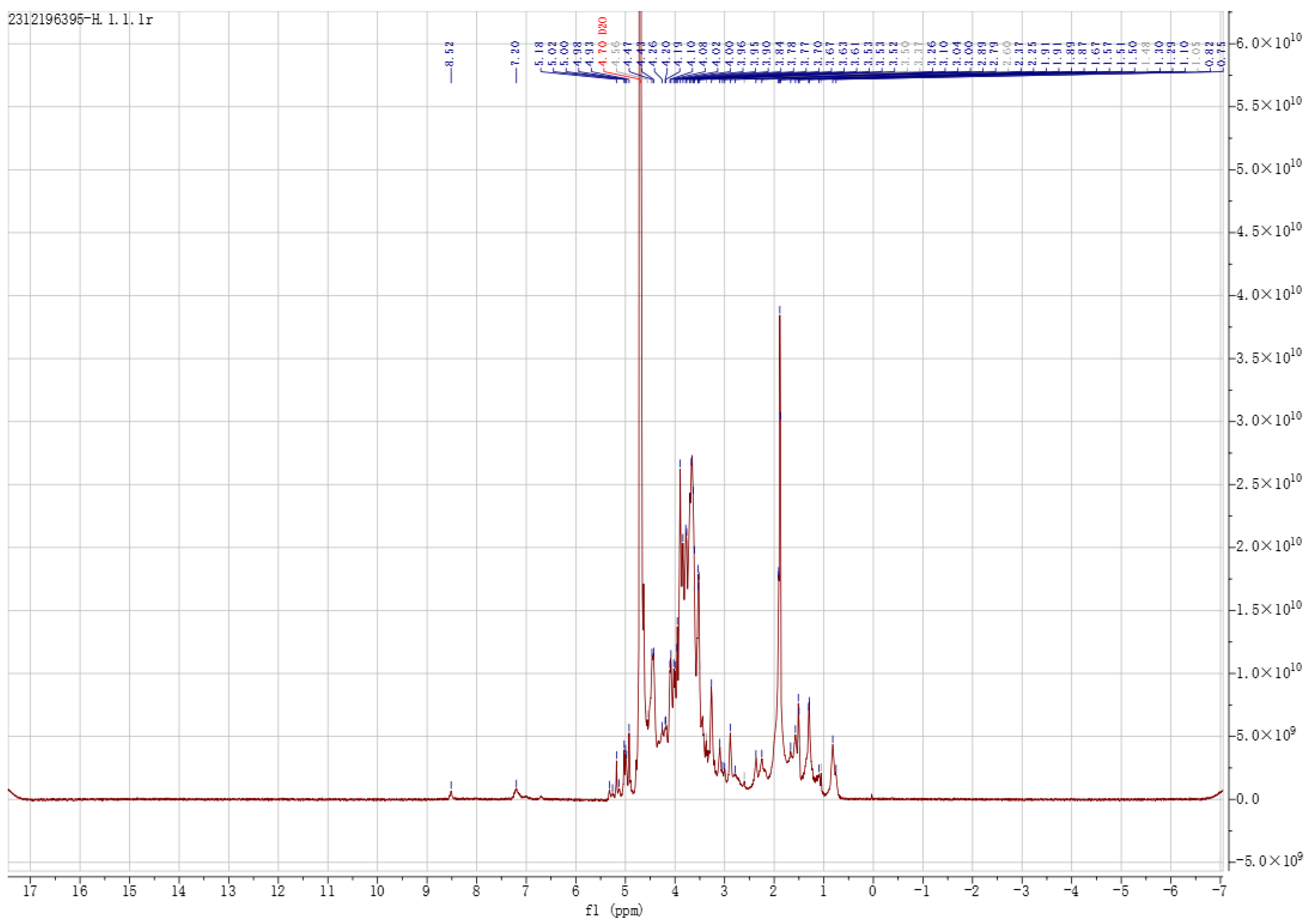
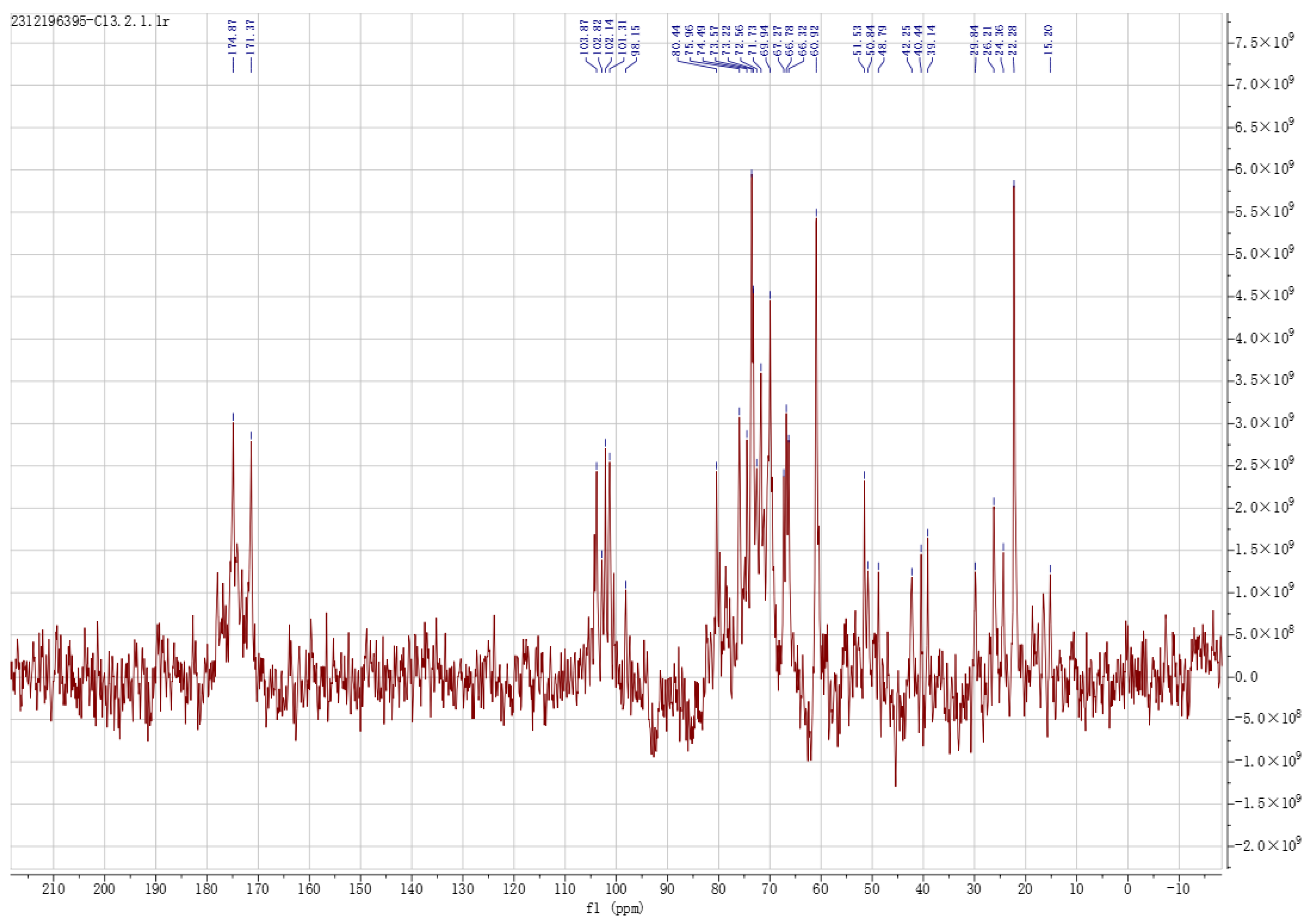
3.1.4. SEM and NMR Analysis of EPS
3.2. Antioxidation Activities of EPS In Vitro
3.3. Immunoregulation Activities of EPS Produced by Del. algida
3.3.1. Effects of Pseudomonas and EPS on Cell Activity
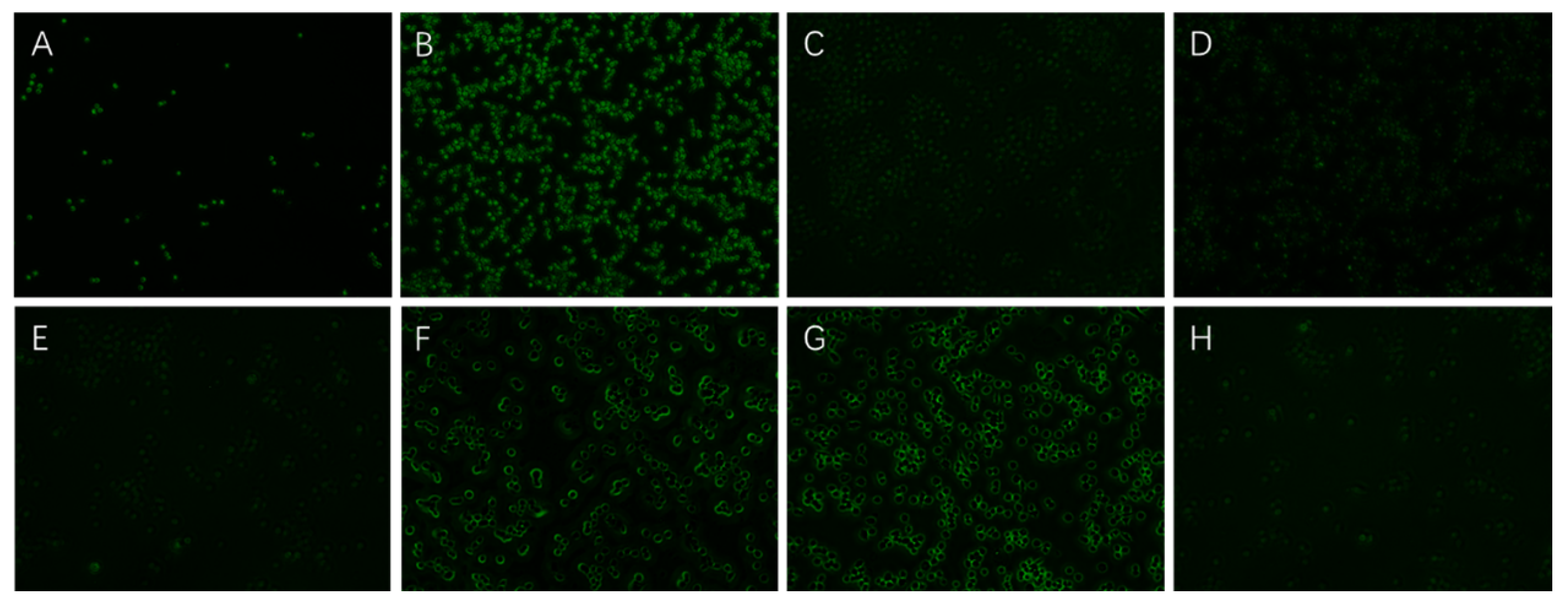
3.3.2. Effect of EPS on Inflammatory RAW264.7 Cells
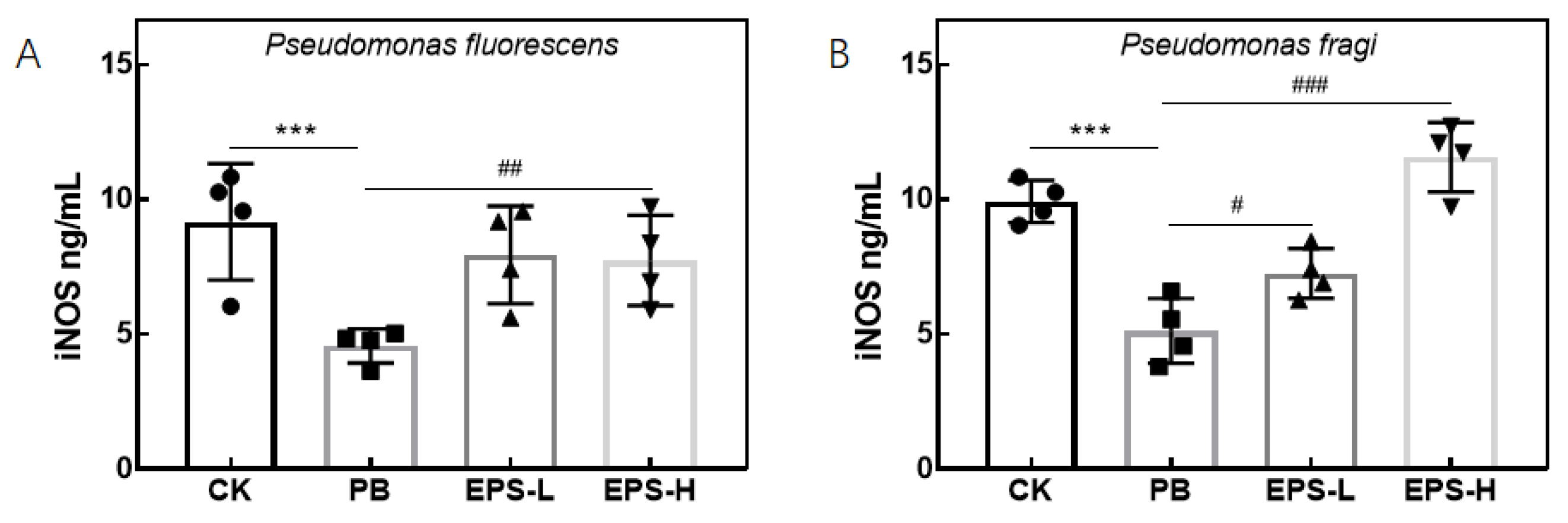
3.3.3. Effects of EPS on NO Secretion of Inflammatory RAW264.7 Cells
3.3.4. Effects of EPS on ROS Secretion of Inflammatory RAW264.7 Cells
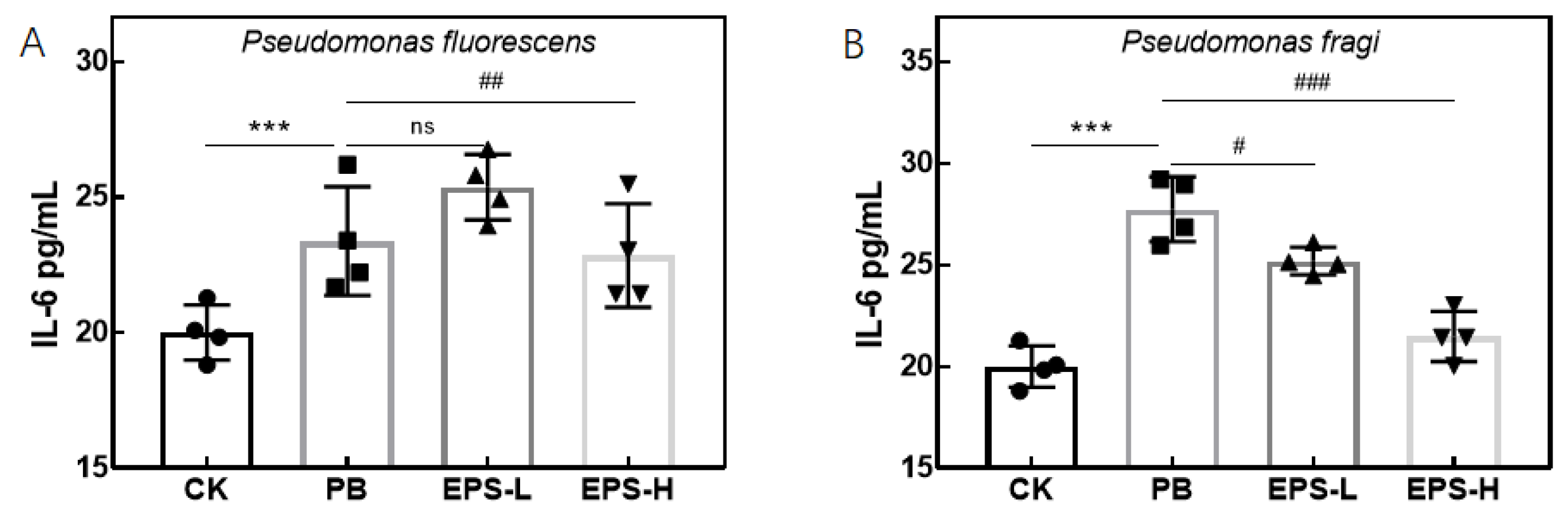
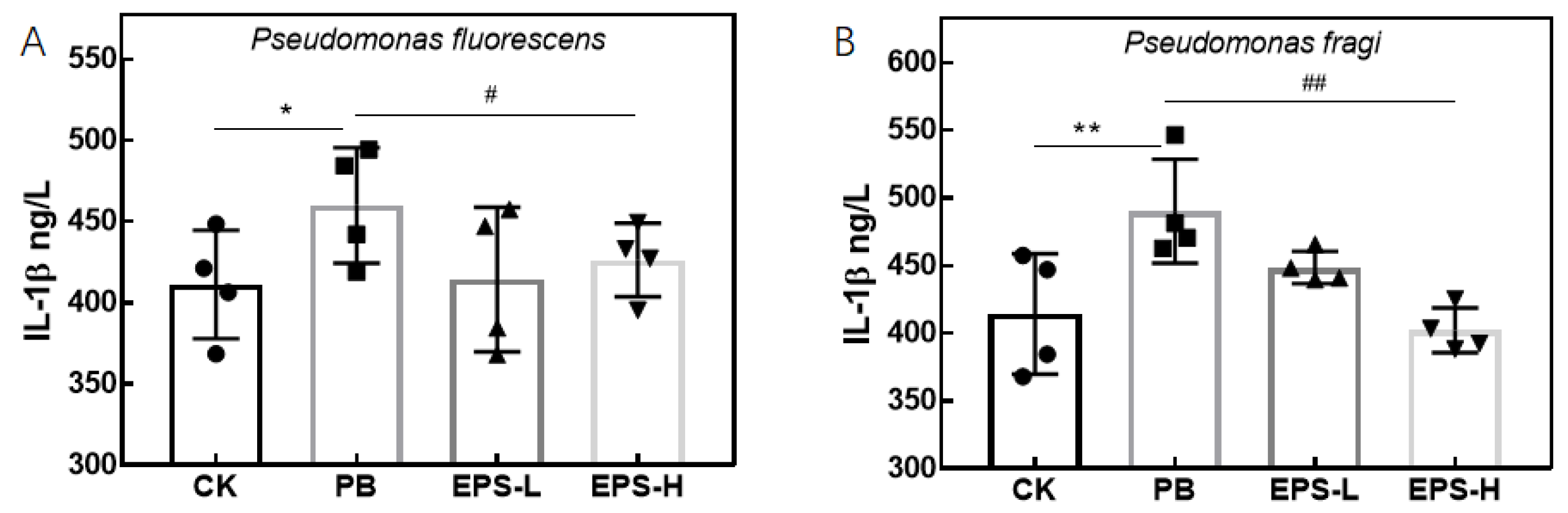
3.3.5. Effects of EPS on Cytokines Secretion of Inflammatory RAW264.7 Cells
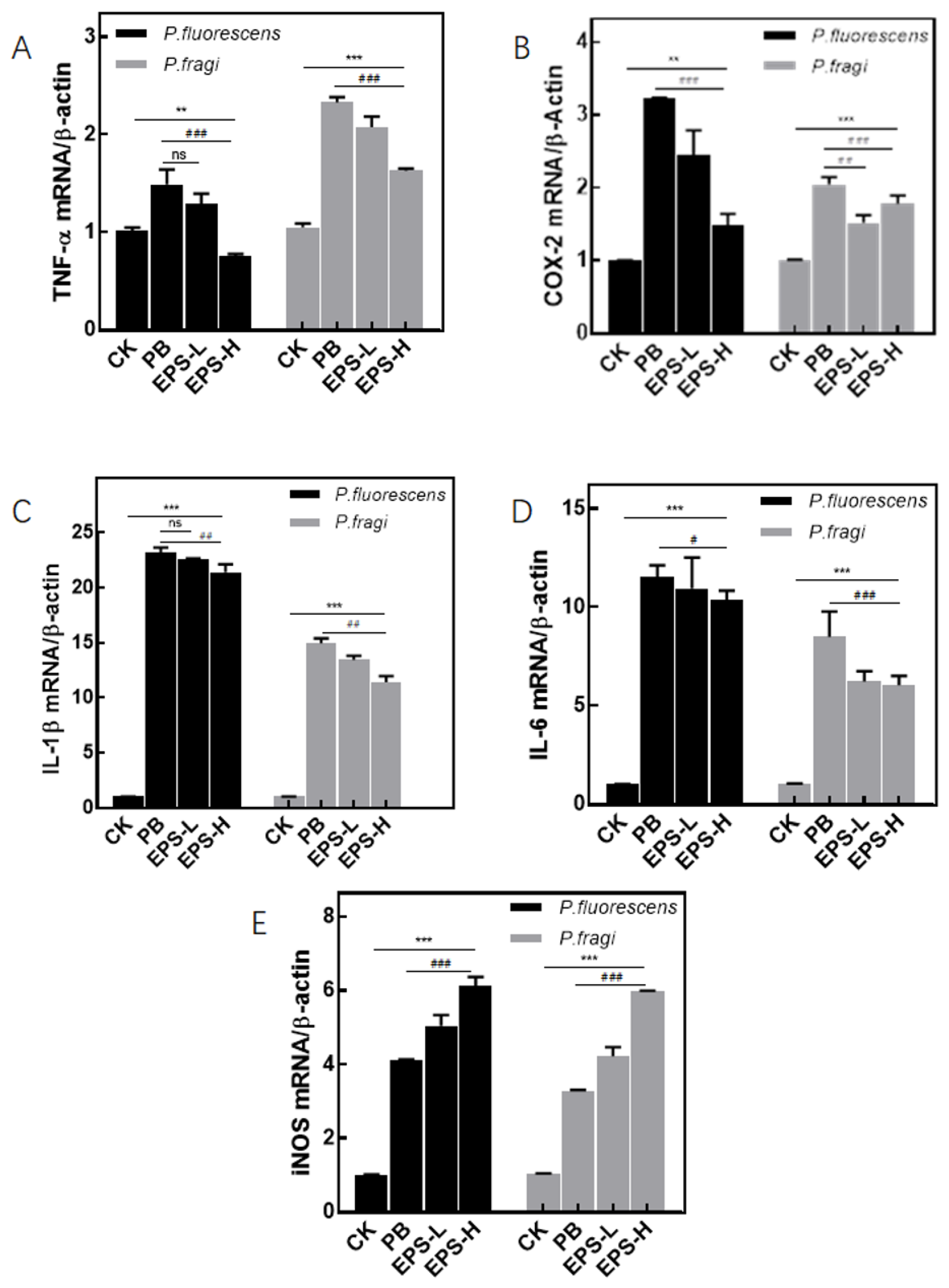
3.3.6. Effects of EPS on mRNA Expression of Inflammatory RAW264.7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mouafo, H.T.; Sokamte, A.T.; Manet, L.; Mbarga, A.J.M.; Nadezdha, S.; Devappa, S.; Mbawala, A. Biofilm inhibition, antibacterial and antiadhesive properties of a novel biosurfactant from Lactobacillus paracasei N2 against multi-antibiotics-resistant pathogens isolated from braised fish. Fermentation 2023, 9, 646. [Google Scholar] [CrossRef]
- Yang, R.; Li, J.; Jiang, C.; Shi, J. Preventive and therapeutic effects of an exopolysaccharide produced by Lacticaseibacillus rhamnosus on alcoholic gastric ulcers. Int. J. Biol. Macromol. 2023, 235, 123845. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Hwang, J.W.; Lee, S.G. Bacillus subtilis Fermentation Augments the Anti-Inflammatory and Skin Moisture Improvement Activities of Tetragonia tetragonoides through the Upregulation of Antioxidant Components. Fermentation 2023, 9, 800. [Google Scholar] [CrossRef]
- Kwon, M.; Lee, J.; Park, S.; Kwon, O.H.; Seo, J.; Roh, S. Exopolysaccharide isolated from Lactobacillus plantarum L-14 has anti-inflammatory effects via the Toll-like receptor 4 pathway in LPS-induced RAW 264.7 cells. Int. J. Mol. Sci. 2020, 21, 9283. [Google Scholar] [CrossRef]
- Noda, M.; Danshiitsoodol, N.; Sakaguchi, T.; Kanno, K.; Sugiyama, M. Exopolysaccharide produced by plant-derived Lactobacillus plantarum SN35N exhibits antiviral activity. Biol. Pharm. Bull. 2021, 44, 1886–1890. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Delattre, C.; Reddy, G.B.; Shetty, P.H. Exopolysaccharides produced by Enterococcus genus—An overview. Int. J. Biol. Macromol. 2023, 226, 111–120. [Google Scholar] [CrossRef]
- Kanmani, P.; Albarracin, L.; Kobayashi, H.; Iida, H.; Komatsu, R.; Kober, A.H.; Ikeda-Ohtsubo, W.; Suda, Y.; Aso, H.; Makino, S.; et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol. Immunol. 2018, 93, 253–265. [Google Scholar] [CrossRef]
- Kim, K.; Lee, G.; Thanh, H.D.; Kim, J.H.; Konkit, M.; Yoon, S.; Park, M.; Yang, S.; Park, E.; Kim, W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. J. Dairy Sci. 2018, 101, 5702–5712. [Google Scholar] [CrossRef]
- Zaghloul, E.H.; Ibrahim, M.I. Production and characterization of exopolysaccharide from newly isolated marine probiotic Lactiplantibacillus plantarum EI6 with in vitro wound healing activity. Front. Microbiol. 2022, 13, 903363. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wu, J.M.; Wu, W.T.; Lin, J.W.; Liang, Y.T.; Hong, Z.Z.; Jia, X.Z.; Liu, D.M. Structural, antioxidant, and immunomodulatory activities of an acidic exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010. Front. Nutr. 2022, 9, 1073071. [Google Scholar] [CrossRef]
- Tyutkov, N.; Zhernyakova, A.; Birchenko, A.; Eminova, E.; Nadtochii, L.; Baranenko, D. Probiotics viability in frozen food products. Food Biosci. 2022, 50, 101996. [Google Scholar] [CrossRef]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del Rocío López-Cuellar, M.; Rodríguez-Hernández, A.I. A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. From the adverse to diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef]
- Kumar, A.; Mukhia, S.; Kumar, R. Production, characterisation, and application of exopolysaccharide extracted from a glacier bacterium Mucilaginibacter sp. ERMR7: 07. Process Biochem. 2022, 113, 27–36. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Kato, Y.; Sakala, R.; Hayashidani, H.; Kiuchi, A.; Kaneuchi, C.; Ogawa, M. Lactobacillus algidus sp. nov., a psychrophilic lactic acid bacterium isolated from vacuum-packaged refrigerated beef. Int. J. Syst. Evol. Microbiol. 2000, 50, 1143–1149. [Google Scholar] [CrossRef]
- Säde, E.; Johansson, P.; Heinonen, T.; Hultman, J.; Björkroth, J. Growth and metabolic characteristics of fastidious meat-derived Lactobacillus algidus strains. Int. J. Food Microbiol. 2020, 313, 108379. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, S.; Zhan, Z.; Wei, T.; Ma, T.; Sun, J.; Song, J. Antibacterial Mechanism of Dellaglioa algida against Pseudomonas fluorescens and Pseudomonas fragi. Fermentation 2022, 8, 298. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhang, J.; Wang, X.; Hao, L.; Jia, L. Purification, in vitro antioxidant and in vivo anti-aging activities of exopolysaccharides by Agrocybe cylindracea. Int. J. Biol. Macromol. 2017, 102, 351–357. [Google Scholar] [CrossRef]
- Khalid, N.; Asgher, M.; Hussain, F.; Iqbal, J. Exopolysaccharides production from marine Bacillus strains and their antioxidant and bio-flocculant capacities. Arch. Microbiol. 2022, 204, 250. [Google Scholar] [CrossRef]
- McSharry, S.; Koolman, L.; Whyte, P.; Bolton, D. The microbiology of beef from carcass chilling through primal storage to retail steaks. Curr. Res. Food Sci. 2021, 4, 150–162. [Google Scholar] [CrossRef]
- Prado Acosta, M.; Valdman, E.; Leite, S.; Battaglini, F.; Ruzal, S.M. Biosorption of copper by Paenibacillus polymyxa cells and their exopolysaccharide. World J. Microbiol. Biotechnol. 2005, 21, 1157–1163. [Google Scholar] [CrossRef]
- Krishnamurthy, M.; Uthaya, C.J.; Thangavel, M.; Annadurai, V.; Rajendran, R.; Gurusamy, A. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohydr. Polym. 2020, 227, 115369. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.D.; Suganuma, T.; Kitahara, K.; Tanoue, H.; Ichiki, M. Monosaccharide composition of sweetpotato fiber and cell wall polysaccharides from sweetpotato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. J. Agric. Food Chem. 2000, 48, 3448–3454. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Qiao, Y.; Shi, B. A novel exopolysaccharide produced by Lactobacillus coryniformis NA-3 exhibits antioxidant and biofilm-inhibiting properties in vitro. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ahmad, W.; Nawaz, S.; Farah, M.A.; Ali, A. Optimized extraction of polyphenols from unconventional edible plants: LC-MS/MS profiling of polyphenols, biological functions, molecular docking, and pharmacokinetics study. Molecules 2023, 28, 6703. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.; Lee, G.J.; Oh, J.M.; Kim, T.i. Polyethylenimine-functionalized cationic barley β-glucan derivatives for macrophage RAW264. 7 cell-targeted gene delivery systems. Carbohydr. Polym. 2019, 226, 115324. [Google Scholar] [CrossRef]
- Lu, Y.; Han, S.; Zhang, S.; Wang, K.; Lv, L.; McClements, D.J.; Xiao, H.; Berglund, B.; Yao, M.; Li, L. The role of probiotic exopolysaccharides in adhesion to mucin in different gastrointestinal conditions. Curr. Res. Food Sci. 2022, 5, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Puxeu, M.; Andorra, I.; De Lamo-Castellví, S.; Ferrer-Gallego, R. Determination of nutrient supplementation by means of ATR-FTIR spectroscopy during wine fermentation. Fermentation 2019, 5, 58. [Google Scholar] [CrossRef]
- Zhou, K.; Zeng, Y.; Yang, M.; Chen, S.; He, L.; Ao, X.; Zou, L.; Liu, S. Production, purification and structural study of an exopolysaccharide from Lactobacillus plantarum BC-25. Carbohydr. Polym. 2016, 144, 205–214. [Google Scholar] [CrossRef]
- Liao, Y.; Gao, M.; Wang, Y.; Liu, X.; Zhong, C.; Jia, S. Structural characterization and immunomodulatory activity of exopolysaccharide from Aureobasidium pullulans CGMCC 23063. Carbohydr. Polym. 2022, 288, 119366. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, C.; Waite, T.D. Hydroxyl radicals in anodic oxidation systems: Generation, identification and quantification. Water Res. 2022, 217, 118425. [Google Scholar] [CrossRef]
- Chang, Z.Q.; Lee, J.S.; Hwang, M.H.; Hong, J.H.; Jung, H.K.; Lee, S.P.; Park, S.C. A novel β-glucan produced by Paenibacillus polymyxa JB115 induces nitric oxide production in RAW264.7 macrophages. J. Vet. Sci. 2009, 10, 165–167. [Google Scholar] [CrossRef]
- Saito, Y.; Kuramitsu, Y.; Arai, H.; Kato, Y.; Fujimoto, M.; Ita, M.; Hayatsu, Y.; Shinozaki, F.; Nakamura, K. A signaling pathway by a new synthetic lipid A analog, ONO-4007, in RAW264. 7 cells. Anti-Cancer Drugs 2002, 13, 1069–1075. [Google Scholar] [CrossRef]
- Rohman, A.; Riyanto, S.; Sasi, A.M.; Yusof, F.M. The use of FTIR spectroscopy in combination with chemometrics for the authentication of red fruit (Pandanus conoideus Lam) oil from sunflower and palm oils. Food Biosci. 2014, 7, 64–70. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, J.; Wu, W.; Wen, Y.; Lu, S.; El-Seedi, H.R.; Zhao, C. Structure–immunomodulatory activity relationships of dietary polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wu, Z.; Zhang, H.; Chen, W.; Ai, L.; Guo, B. Partial characterization and immunostimulatory activity of exopolysaccharides from Lactobacillus rhamnosus KF5. Carbohydr. Polym. 2014, 107, 51–56. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Tan, C.; Zhao, Y.; Zhu, Y.; Bai, J.; Xiao, X.; Zhang, L.; Teng, D.; Tian, J.; et al. Effects of L. plantarum dy-1 fermentation time on the characteristic structure and antioxidant activity of barley β-glucan in vitro. Curr. Res. Food Sci. 2022, 5, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wu, Y.J.; Hu, C.Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Du, R.; Guo, S.; Ping, W.; Ling, H.; Ge, J. Purification and characterization of an exopolysaccharide from Leuconostoc lactis L2. Int. J. Biol. Macromol. 2019, 139, 1224–1231. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Takei, T.; Kato, K.; Meguro, A.; Chikazawa, M. Infrared spectra of geminal and novel triple hydroxyl groups on silica surface. Colloids Surf. A Physicochem. Eng. Asp. 1999, 150, 77–84. [Google Scholar] [CrossRef]
- Khatri, S.; Sazinas, P.; Strube, M.; Ding, L.; Dubey, S.; Shivay, Y.; Sharma, S.; Jelsbak, L. Pseudomonas is a key player in conferring disease suppressiveness in organic farming. Plant Soil 2023, 1–20. [Google Scholar] [CrossRef]
- Housden, N.G.; Webby, M.N.; Lowe, E.D.; El-Baba, T.J.; Kaminska, R.; Redfield, C.; Robinson, C.V.; Kleanthous, C. Toxin import through the antibiotic efflux channel TolC. Nat. Commun. 2021, 12, 4625. [Google Scholar] [CrossRef]
- Ahmed, N.; Khalid, H.; Mushtaq, M.; Basha, S.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Al Mutair, A.; Alhumaid, S.; Al Alawi, Z.; et al. The molecular characterization of virulence determinants and antibiotic resistance patterns in human bacterial uropathogens. Antibiotics 2022, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Sheng Du, R.; Li, J.; Cai, Z.J.; Han, L.; Mao, Y.; Zhou, Y.Y.; Yu, Q.L.; Chen, L.H. The potential mediation of nitric oxide in the activation of mitochondrion-dependent apoptosis and yak meat tenderness during postmortem aging. Food Biosci. 2021, 42, 101131. [Google Scholar] [CrossRef]
- Xiu, L.; Sheng, S.; Hu, Z.; Liu, Y.; Li, J.; Zhang, H.; Liang, Y.; Du, R.; Wang, X. Exopolysaccharides from Lactobacillus kiferi as adjuvant enhanced the immuno-protective against Staphylococcus aureus infection. Int. J. Biol. Macromol. 2020, 161, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, X.; Wu, C.; Chen, J.; Yuan, J.; Pang, Q.; Wang, Z. Heme oxygenase-1 induction by methylene blue protects RAW264.7 cells from hydrogen peroxide-induced injury. Biochem. Pharmacol. 2018, 148, 265–277. [Google Scholar] [CrossRef]
- Liang, Y.; Zha, S.; Tentaku, M.; Okimura, T.; Jiang, Z.; Ueno, M.; Hirasaka, K.; Yamaguchi, K.; Oda, T. Suppressive effects of sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on the production of NO and ROS in LPS-stimulated RAW264.7 cells. Biosci. Biotechnol. Biochem. 2021, 85, 882–889. [Google Scholar] [CrossRef]
- Yamamoto, N.; Shoji, M.; Hoshigami, H.; Watanabe, K.; Takatsuzu, T.; Yasuda, S.; Igoshi, K.; Kinoshita, H. Antioxidant capacity of soymilk yogurt and exopolysaccharides produced by lactic acid bacteria. Biosci. Microbiota Food Health 2019, 38, 97–104. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Yang, Y.; Ayivi-Tosuh, S.M.; Wang, F.; Li, H.; Wang, G. A polysaccharide isolated from the fruits of Physalis alkekengi L. induces RAW264. 7 macrophages activation via TLR2 and TLR4-mediated MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2019, 140, 895–906. [Google Scholar] [CrossRef]
- Yamamoto, M.; Handa, N.; Nakamura, A.; Takahashi, H.; Kuda, T. In vitro antioxidant, anti-glycation, and bile acid-lowering capacity of peanut milk fermented with Lactiplantibacillus plantarum Kinko-SU4. Curr. Res. Food Sci. 2022, 5, 992–997. [Google Scholar] [CrossRef]
- Lee, H.A.; Song, B.R.; Kim, H.R.; Kim, J.E.; Yun, W.B.; Park, J.J.; Lee, M.L.; Choi, J.Y.; Lee, H.S.; Hwang, D.Y. Butanol extracts of Asparagus cochinchinensis fermented with Weissella cibaria inhibit iNOS-mediated COX-2 induction pathway and inflammatory cytokines in LPS-stimulated RAW264. 7 macrophage cells. Exp. Ther. Med. 2017, 14, 4986–4994. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, H.; Liu, Z.; Lin, L.; Wang, L.; Xie, M.; Li, D.; Zhang, J.; Zhang, R. Endoplasmic reticulum stress-dependent activation of iNOS/NO-NF-κB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity. FASEB J. 2020, 34, 10835–10849. [Google Scholar] [CrossRef]
- Deng, X.; Liu, Q.; Fu, Y.; Luo, X.; Hu, M.; Ma, F.; Wang, Q.; Lai, X.; Zhou, L. Effects of Lycium barbarum polysaccharides with different molecular weights on function of RAW264. 7 macrophages. Food Agric. Immunol. 2018, 29, 808–820. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.W.; Ahn, C.B.; Oh, Y.; Je, J.Y. Lotus (Nelumbo nucifera) seed protein isolate exerts anti-inflammatory and antioxidant effects in LPS-stimulated RAW264. 7 macrophages via inhibiting NF-κB and MAPK pathways, and upregulating catalase activity. Int. J. Biol. Macromol. 2019, 134, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, L.; Du, J.; Zhang, C.; Sun, H. The polysaccharide from the roots of Actinidia eriantha activates RAW264. 7 macrophages via regulating microRNA expression. Int. J. Biol. Macromol. 2019, 132, 203–212. [Google Scholar] [CrossRef]
- Ma, H.; Mueed, A.; Liu, D.; Ali, A.; Wang, T.; Ibrahim, M.; Su, L.; Wang, Q. Polysaccharides of Floccularia luteovirens regulate intestinal immune response, and oxidative stress activity through MAPK/Nrf2/Keap1 signaling pathway in immunosuppressive mice. Int. J. Biol. Macromol. 2024, 277, 134140. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5’ to 3’) |
|---|---|
| COX-2-F | TGAGTACCGCAAACGCTTCT |
| COX-2-R | CAGCCATTTCCTTCTCTCCTGTT |
| iNOS-F | TCTAGTGAAGCAAAGCCCAACA |
| iNOS-R | CCTCACATACTGTGGACGGG |
| TNF--F | GATCGGTCCCCAAAGGGATG |
| TNF--R | CCACTTGGTGGTTTGTGAGTG |
| IL-6-F | TGGTCTTCTGGAGTACCATAGC |
| IL-6-R | TGTGACTCCAGCTTATCTCTTGG |
| IL-1-F | TGCCACCTTTTGACAGTGATG |
| IL-1-R | TGATGTGCTGCTGCGAGATT |
| -actin-F | CACTGTCGAGTCGCGTCC |
| -actin-R | TCATCCATGGCGAACTGGTG |
| Monosaccharide | Content (μg/mg) | Monosaccharide | Content (μg/mg) |
|---|---|---|---|
| Fuc | 1.0316 | Fru | 0 |
| Ara | 0 | Rib | 1.5462 |
| Rha | 3.4620 | Gal-UA | 2.4371 |
| Gal | 58.6263 | Gul-UA | 0 |
| Glc | 19.7670 | Glc-UA | 76.1418 |
| Xyl | 1.6417 | Man-UA | 0 |
| Man | 35.2810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wei, T.; Ma, T.; Guan, J.; Wang, Z.; Fan, Z.; Song, J. Analysis of Composition, Antioxidation, and Immunoregulation for Exopolysaccharide Produced by Dellaglioa algida. Fermentation 2024, 10, 491. https://doi.org/10.3390/fermentation10090491
Sun Y, Wei T, Ma T, Guan J, Wang Z, Fan Z, Song J. Analysis of Composition, Antioxidation, and Immunoregulation for Exopolysaccharide Produced by Dellaglioa algida. Fermentation. 2024; 10(9):491. https://doi.org/10.3390/fermentation10090491
Chicago/Turabian StyleSun, Yao, Tianhui Wei, Tongqing Ma, Jiaqi Guan, Zhiwei Wang, Zhiying Fan, and Jinzhu Song. 2024. "Analysis of Composition, Antioxidation, and Immunoregulation for Exopolysaccharide Produced by Dellaglioa algida" Fermentation 10, no. 9: 491. https://doi.org/10.3390/fermentation10090491
APA StyleSun, Y., Wei, T., Ma, T., Guan, J., Wang, Z., Fan, Z., & Song, J. (2024). Analysis of Composition, Antioxidation, and Immunoregulation for Exopolysaccharide Produced by Dellaglioa algida. Fermentation, 10(9), 491. https://doi.org/10.3390/fermentation10090491






