Soy Molasses as Culture Medium for Bacillus Species Aiming at Plant Growth Promotion
Abstract
1. Introduction
2. Material and Methods
2.1. Physico-Chemical Characteristics of Soy Molasses
2.2. Microorganisms
2.3. Culture Media
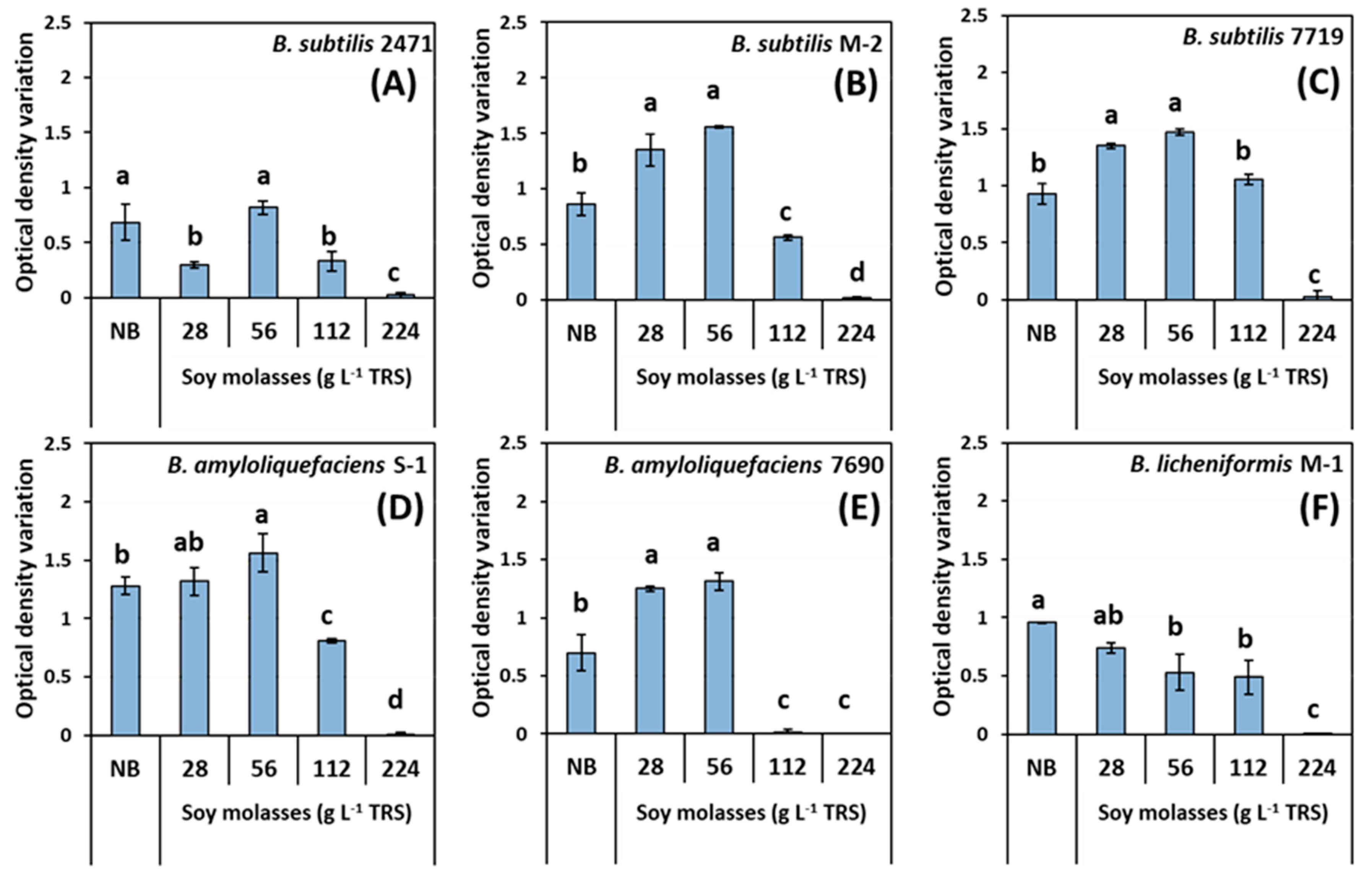
2.4. Preliminary Evaluation of Bacillus Species Growth in Soy Molasses with Increasing Concentrations of Total Reducing Sugars
2.4.1. Inoculum Preparation
2.4.2. Bacterial Growth in Microplates
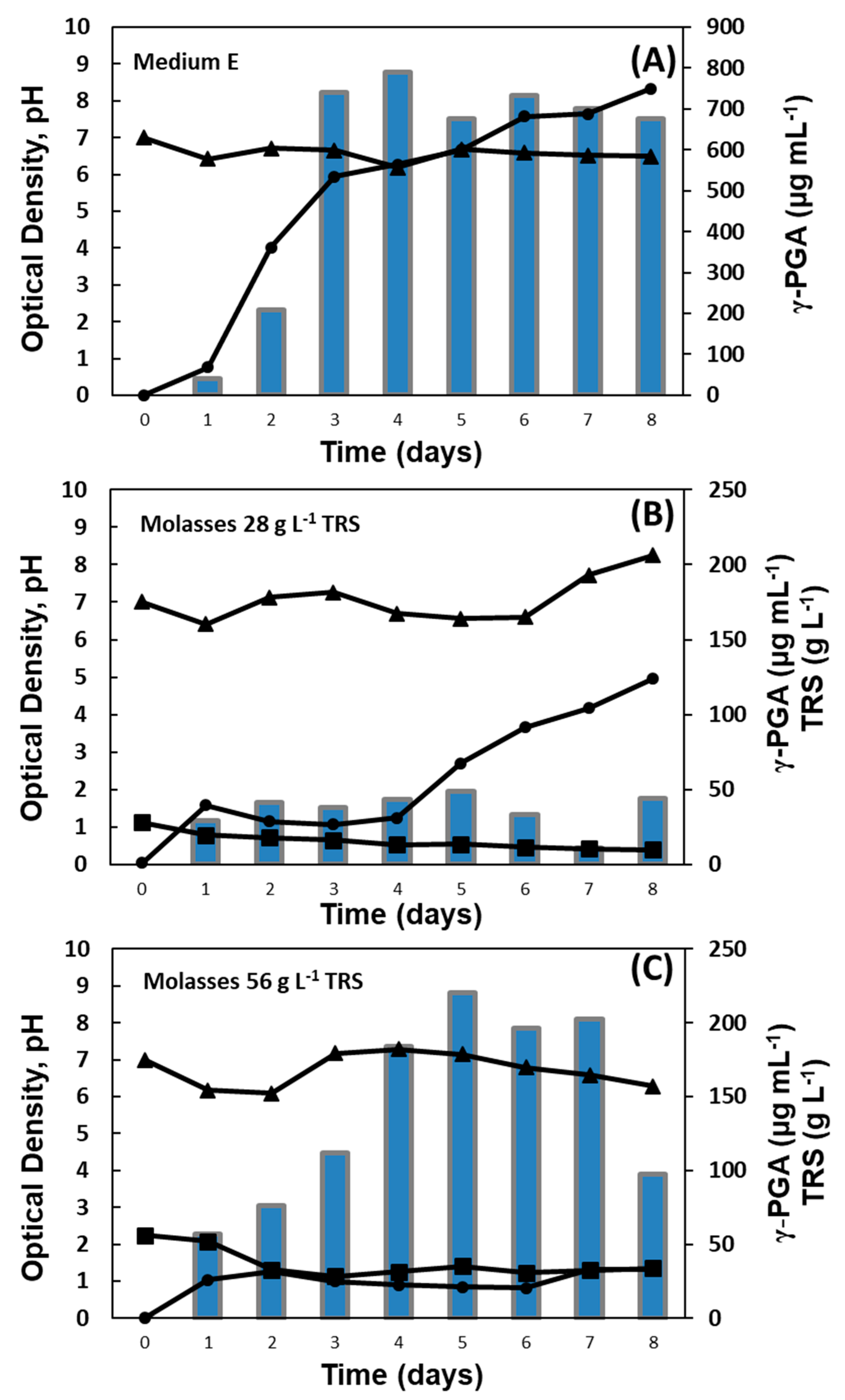
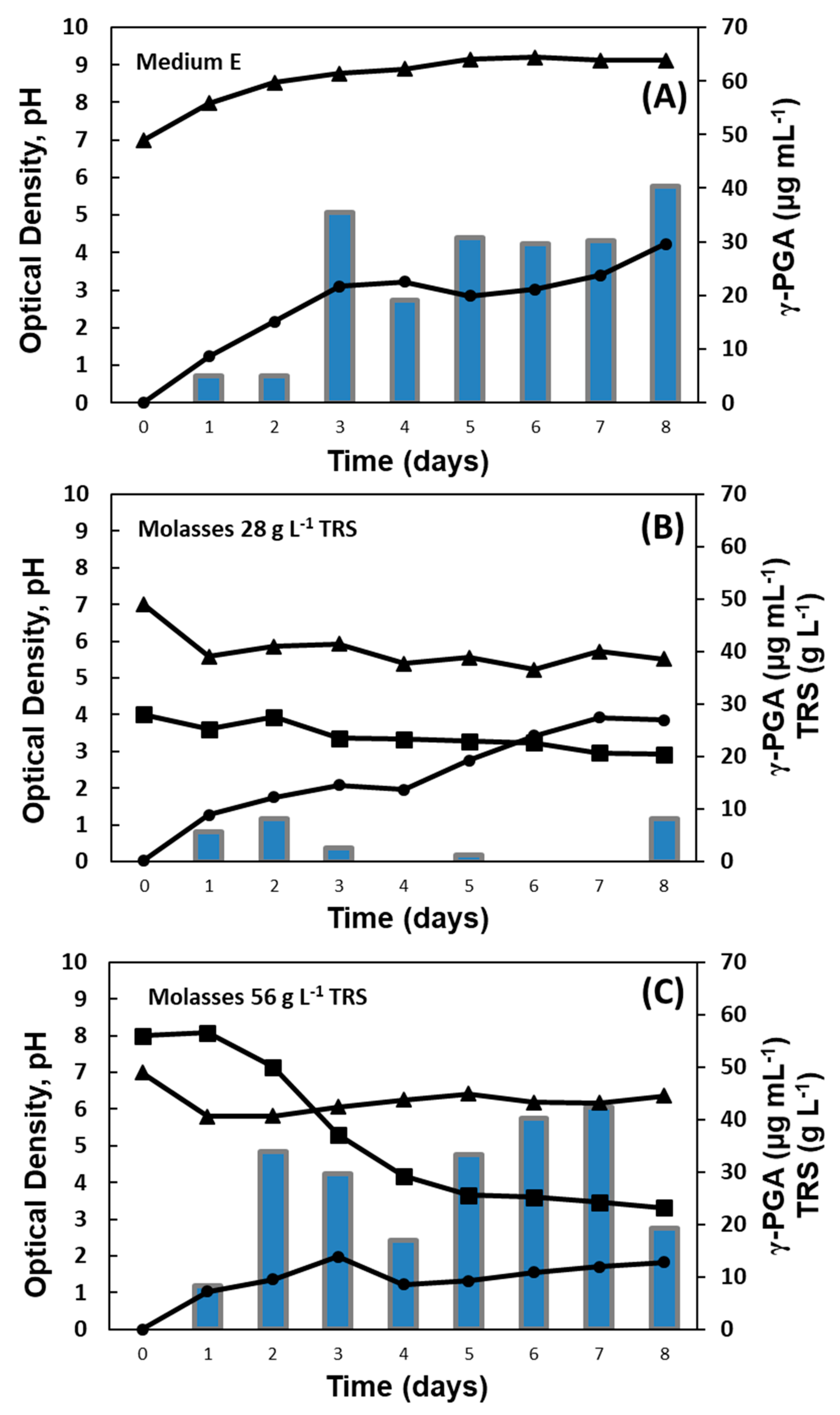
2.5. Shaken-Flask Assays for Growing Bacillus Strains from Soy Molasses
2.5.1. Inoculum and Assay Preparation
2.5.2. Analysis
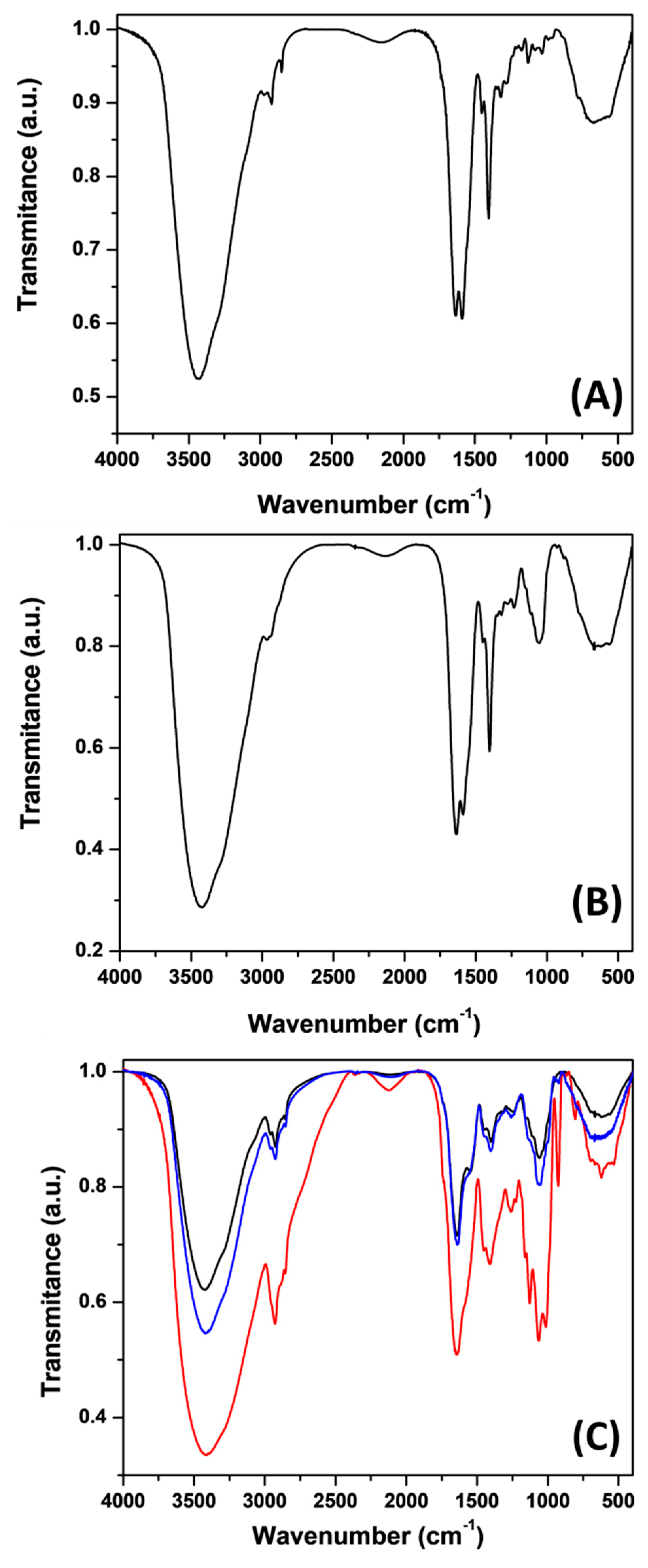
2.6. Structural Characterization of γ-PGA Using FTIR Analysis
2.7. Effect of the Bacterial Fermented Broth on the Germination and Initial Development of Maize
3. Results and Discussion
3.1. Preliminary Evaluation of Bacillus Species Growth in Soy Molasses with Increasing Concentrations of Total Reducing Sugars
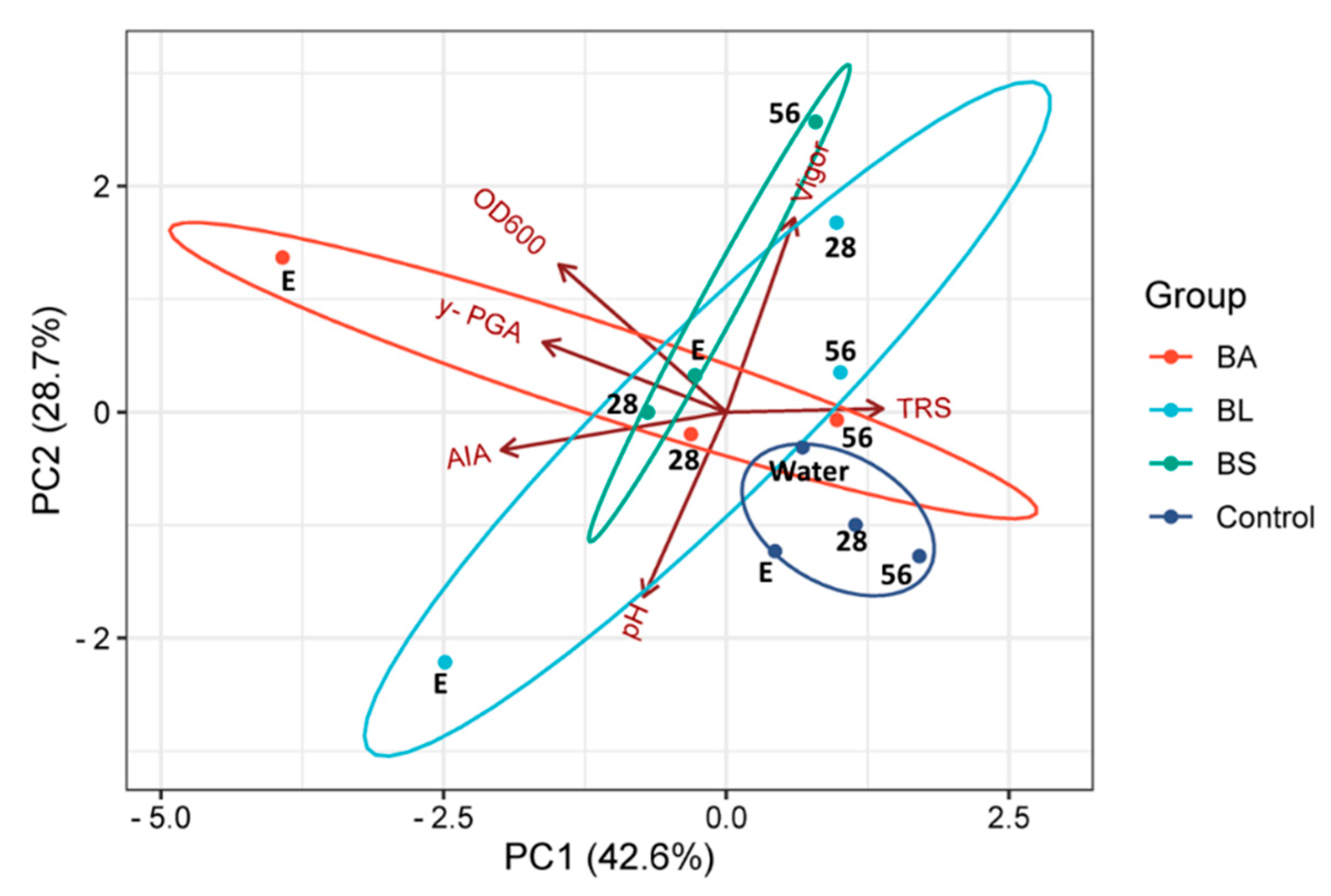
3.2. Shaken-Flask Assays for Growing Bacillus Strains from Soy Molasses
3.3. Structural Characterization of γ-PGA Using FTIR Analysis
3.4. Effect of the Bacterial Fermented Broth on the Germination and Initial Development of Maize
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CONAB. Safra de Grãos 2023/2024 Está Estimada em 294,1 Milhões de Toneladas. 2024. Available online: https://agenciagov.ebc.com.br/noticias/202404/safra-de-graos-2023-2024-esta-estimada-em-294-1-milhoes-de-toneladas (accessed on 11 July 2024).
- Oliveira, M.P.; Malagolli, G.A.; Cella, D. Mercado de fertilizantes: Dependência de importações do Brasil. Rev. Interf. Tecnol. 2019, 16, 489–498. [Google Scholar]
- ONU Brasil. Os Objetivos de Desenvolvimento Sustentável no Brasil. Nações Unidas Brasil. 2024. Available online: https://brasil.un.org/pt-br/sdgs (accessed on 7 June 2024).
- Ngearnpat, N.; Chunhachart, O.; Kotabin, N.; Issakul, K. Comparative assessment of gamma-polyglutamic acid and Bacillus subtilis cells as biostimulants to improve rice growth and soil quality. J. Ecol. Eng. 2023, 24, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Kundan, R.; Pant, G.; Jadon, N.; Agrawal, P.K. Plant growth promoting rhizobacteria: Mechanism and current prospective. J. Fertil. Pestic. 2015, 6, 155. [Google Scholar] [CrossRef]
- Braga Junior, G.M.; Chagas, L.F.B.; Amaral, L.R.O.; Miller, L.O.; Chagas Junior, A.F. Efficiency of inoculation by Bacillus subtilis on soybean biomass and productivity. Rev. Bras. Cienc. Agrar. 2018, 13, e5571. [Google Scholar] [CrossRef]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for plant growth promotion and stress resilience: What have we learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Radhakrishnan, R.; Kang, S.M.; You, Y.H.; Jeong, E.J.; Kim, J.G.; Lee, I.J. Plant growth promoting effect of Bacillus amyloliquefaciens H-2-5 on crop plants and influence on physiological changes in soybean under soil salinity. Physiol. Mol. Biol. Plants. 2017, 23, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moenne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 29, 134. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zhao, J.; Xia, J.Y. γ-PGA Fermentation by Bacillus subtilis PG-001 with glucose feedback control pH-stat strategy. Appl. Biochem. Biotechnol. 2022, 194, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Deol, R.; Louis, A.; Glazer, H.L.; Hosseinion, W.; Bagley, A.; Chandrangsu, P. Poly-gamma-glutamic acid secretion protects Bacillus subtilis from zinc and copper intoxication. Microbiol. Spectr. 2022, 10, e01329-21. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Thapa, A.; Khadka, S.; Sapkota, S.; Panta, O.P.; Sharma, S.; Karki, T.B.; Poudel, P. Screening and characterization of potent poly glutamic acid producing Bacillus sp. isolated from Kinema, water and soil samples. Heliyon 2021, 7, e07715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Gao, D.; Wang, L.; Wei, Z.; Shi, Y. Effects of poly-γ-glutamic acid (γ-PGA) on plant growth and its distribution in a controlled plant-soil system. Sci. Rep. 2017, 7, 6090. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lei, P.; Feng, X.; Xu, X.; Liang, J.; Chi, B.; Xu, H. Calcium involved in the poly (γ-glutamic acid)-mediated promotion of Chinese cabbage nitrogen metabolism. Plant Physiol. Biochem. 2014, 80, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Candela, T.; Fouet, A. Poly-gamma-glutamate in bacteria. Mol. Microbiol. 2006, 60, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, S.; Zhang, J.; Sun, M.; Liu, Z.; Yu, Z. Co-producing lipopeptides and poly-c-glutamic acid by solid-state fermentation of Bacillus subtilis using soybean and sweet potato residues and its biocontrol and fertilizer synergistic effects. Bioresour. Technol. 2008, 99, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, P.F.; Karp, S.G.; Carvalho, J.C.; Sturm, W.; Rodriguez-León, J.A.; Tholozan, J.L.; Singhania, R.R.; Pandey, A.; Soccol, C.R. Production of bio-ethanol from soybean molasses by Saccharomyces cerevisiae at laboratory, pilot and industrial scales. Bioresour. Technol. 2008, 99, 8156–8163. [Google Scholar] [CrossRef]
- Acosta, S.B.P.; Marchioro, M.L.K.; Santos, V.A.Q.; Calegari, G.C.; Lafay, C.B.B.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Cunha, M.A.A. Valorization of soybean molasses as fermentation substrate for the production of microbial exocellular β-glucan. J. Polym. Environ. 2020, 28, 2149–2160. [Google Scholar] [CrossRef]
- Romão, B.B.; Silva, F.S.; Resende, M.M.; Cardoso, V.L. Ethanol production from hydrolyzed soybean molasses. Energy Fuels 2012, 26, 2310–2316. [Google Scholar] [CrossRef]
- Qureshi, N.; Lolas, A.; Blaschek, H.P. Soy molasses as fermentation substrate for production of butanol using Clostridium beijerinckii BA101. J. Ind. Microbiol. Biotechnol. 2001, 26, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.C.; Gomes, R.J.; Mandarino, J.M.G.; Ida, E.I.; Spinosa, W.A. Acetic acid fermentation of soybean molasses and characterisation of the produced vinegar. Food Technol. Biotechnol. 2020, 58, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Michelon, M.; Burkert, C.A.V. Biotechnological potential of soybean molasses for the production of extracellular polymers by diazotrophic bacteria. Biocatal. Agric. Biotechnol. 2020, 25, 101609. [Google Scholar] [CrossRef]
- Gomes, R.J.; Faria-Tischer, P.C.S.; Tischer, C.A.; Constantino, L.V.; Rosa, M.F.; Chideroli, R.T.; Pereira, U.P.; Spinosa, W.A. Komagataeibacter intermedius V-05: An acetic acid bacterium isolated from vinegar industry, with high capacity for bacterial cellulose production in soybean molasses medium. Food Technol. Biotechnol. 2021, 59, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Moreira, F.S.; Cardoso, V.L.; Resende, M.M. Soy molasses as a fermentation substrate for the production of biosurfactant using Pseudomonas aeruginosa ATCC 10145. Environ. Sci. Pollut. Res. 2017, 24, 18699–18709. [Google Scholar] [CrossRef] [PubMed]
- Fabrini, F.F.; Avelino, K.V.; Marim, R.A.; Cardoso, B.K.; Colauto, G.A.L.; Colauto, N.B.; Valle, J.S. Produção de lacase de Pycnoporus sanguineus em meio de cultivo a base de melaço soja. Arq. Ciênc. Vet. Zool. UNIPAR 2016, 19, 159–164. [Google Scholar] [CrossRef][Green Version]
- Lima, F.A.; Rola, J.C.; Freitas, M.M.G.; Afonso, J.M.M.A.; Resende, M.M. Acid phosphatase immobilization and production study by Trichoderma spp. in soybean molasses. Chem. Eng. Technol. 2022, 45, 979–984. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Liu, Y. Production of single cell protein from soy molasses using Candida tropicalis. Ann. Microbiol. 2012, 62, 1165–1172. [Google Scholar] [CrossRef]
- Brasil. MAPA. Manual de Métodos Analíticos Oficiais para Fertilizantes e Corretivos. 2017; 240p. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/manual-de-metodos_2017_isbn-978-85-7991-109-5.pdf (accessed on 12 July 2024).
- Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa: Rio de Janeiro, Brazil, 2009; 627p. [Google Scholar]
- Alcarde, J.C. Manual de Análises de Fertilizantes; Fealq: Piracicaba, Brazil, 2009; 259p. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bezerra, G.A.; Takita, M.A.; Tosta, C.D.; Ceccato-Antonini, S.R.; Rosa-Magri, M.M. Screening of plant growth-promoting bacteria isolated from sugarcane. Semin. Ciênc. Agrár. 2022, 43, 1757–1768. [Google Scholar] [CrossRef]
- Leonard, C.G.; Housewright, R.D.; Thorne, C.B. Effects of some metallic ions on glutamyl polypeptide synthesis by Bacillus subtilis. J. Bacteriol. 1958, 76, 499–503. [Google Scholar] [CrossRef]
- Goto, A.; Kunioka, M. Biosynthesis and hydrolysis of poly (γ-glutamic acid) from Bacillus subtilis IFO3335. Biosci. Biotech. Biochem. 1992, 56, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Chen, G.; Zhang, Y.; Wu, K.; Liang, Z. Studies on the UV spectrum of poly (γ-glutamic acid) based on development of a simple quantitative method. Int. J. Biol. Macromol. 2012, 51, 83–90. [Google Scholar] [CrossRef]
- Kanno, A.; Takamatsu, H. Determination of polyglutamic acid in “Natto” using cetyltrimethylammonium bromide (Studies on “Natto” part V). J. Jpn. Soc. Food Sci. Technol. 1995, 42, 878–886. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Garladinne, M.; Lee, Y.H. Volatile indole produced by Rhizobacterium Proteus vulgaris JBLS202 stimulates growth of Arabidopsis thaliana through auxin, cytokinin, and brassinosteroid pathways. J. Plant Growth Regul. 2015, 34, 158–168. [Google Scholar] [CrossRef]
- Buchelt, A.C.; Metzler, C.R.; Castiglioni, J.L.; Dassoller, T.F.; Lubian, M.S. Aplicação de bioestimulantes e Bacillus subtilis na germinação e desenvolvimento inicial da cultura do milho. Rev. Agric. Neotrop. 2019, 6, 69–74. [Google Scholar] [CrossRef]
- Ministério da Agricultura e Reforma Agrária, Brasil. Regras para Análise de Sementes; SNDA/DND/CLAV: Brasília, Brazil, 2009; 395p. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria 1. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Kongklom, N.; Luo, H.; Shi, Z.; Pechyend, C.; Chisti, Y.; Sirisansaneeyakul, S. Production of poly-γ-glutamic acid by glutamic acid-independent Bacillus licheniformis TISTR 1010 using different feeding strategies. Biochem. Eng. J. 2015, 100, 67–75. [Google Scholar] [CrossRef]
- Song, D.Y.; Reddy, L.V.; Charalampopoulos, D.; Wee, Y.J. Poly-(γ-glutamic acid) production and optimization from agro-industrial bioresources as renewable substrates by Bacillus sp. fbl-2 through response surface methodology. Biomolecules 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Loman, A.A.; Ju, L.K. Soybean carbohydrate as fermentation feedstock for production of biofuels and value-added chemicals. Process Biochem. 2016, 51, 1046–1057. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, Y.; Lin, M.; Wei, P.; Yang, S.T. Polymalic acid fermentation by Aureobasidium pullulans for malic acid production from soybean hull and soy molasses: Fermentation kinetics and economic analysis. Bioresour. Technol. 2017, 223, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Reddy, L.V.; Charalampopoulos, D.; Kim, Y.M.; Wee, Y.J. Optimized production of poly (γ-glutamic acid) by Bacillus sp. fbl-2 through response surface methodology using central composite design. J. Microbiol. Biotechnol. 2019, 29, 1061–1070. [Google Scholar] [CrossRef]
- Sun, J.D.; Tang, C.; Zhou, J.; Wei, P.; Wang, Y.J.; An, W.; Yan, Z.Y.; Yong, X.Y. Production of poly-γ-glutamic acid (γ-PGA) from xylose-glucose mixtures by Bacillus amyloliquefaciens C1. 3 Biotech 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Du, S.; Yan, Y.; Pan, F.; Wang, R.; Li, S.; Xu, H.; Luo, Z. Systematic engineering of Bacillus amyloliquefaciens for efficient production of poly-γ-glutamic acid from crude glycerol. Bioresour. Technol. 2022, 359, 127382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.; Wang, J.; Lin, W.; Chen, Y. Enhancement of bacterial cellulose production in Bacillus amyloliquefaciens. IOP Conf. Ser. Mater. Sci. Eng. 2019, 493, 012036. [Google Scholar] [CrossRef]
- Li, D.; Hou, L.; Gao, Y.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Recent advances in microbial synthesis of poly-γ-glutamic acid: A review. Foods 2022, 11, 739. [Google Scholar] [CrossRef]
- Ko, Y.H.; Gross, R.A. Effects of glucose and glycerol on gamma-poly(glutamic acid) formation by Bacillus licheniformis ATCC 9945a. Biotechnol. Bioeng. 1998, 57, 430–437. [Google Scholar] [CrossRef]
- Kanwugu, O.N.; Shatunova, S.A.; Glukhareva, T.V.; Kovaleva, E.G. Effect of different sugar sources on P. rhodozyma Y1654 growth and astaxanthin production. Agron. Res. 2020, 1, 1700–1716. [Google Scholar]
- Wu, Q.; Xu, H.; Ying, H.; Ouyang, P. Kinetic analysis and pH-shift control strategy for poly (γ-glutamic acid) production with Bacillus subtilis CGMCC 0833. Biochem. Eng. J. 2010, 50, 24–28. [Google Scholar] [CrossRef]
- Nair, P.; Navale, G.R.; Dharne, M.S. Poly-gamma-glutamic acid biopolymer: A sleeping giant with diverse applications and unique opportunities for commercialization. Biomass Convers. Bior. 2023, 13, 4555–4573. [Google Scholar] [CrossRef] [PubMed]
- Manocha, B.; Margaritis, A. A novel method for the selective recovery and purification of c-polyglutamic acid from Bacillus licheniformis fermentation broth. Biotechnol. Prog. 2010, 26, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Solaiman, D.; Ashby, R.D.; Garcia, R.A.; Gordon, S.H.; Harry-O’Kuru, R.E. Properties of starch–polyglutamic acid (PGA) graft copolymer prepared by microwave irradiation—Fourier transform infrared spectroscopy (FTIR) and rheology studies. Starch 2017, 69, 1600021. [Google Scholar] [CrossRef]
- Bai, Y.; Tan, R.; Chen, T.; Feng, Y.; Sun, Q.; Li, J.; Wang, Y.; Liu, F.; Wang, J.; Zhang, Y.; et al. Effect of addition of γ-poly glutamic acid on bacterial nanocellulose production under agitated culture conditions. Biotechnol. Biofuels Bioprod. 2024, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association (ISTA). Handbook of Vigour Test Methods, 3rd ed.; ISTA: Zürich, Switzerland, 2012; 117p. [Google Scholar]
- Zhang, H.; Liu, X.L.; Zhang, R.X.; Yuan, H.Y.; Wang, M.M.; Yang, H.Y.; Ma, H.Y.; Liu, D.; Jiang, C.J.; Liang, Z.W. Root damage under alkaline stress is associated with reactive oxygen species accumulation in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1580. [Google Scholar] [CrossRef]
- Khan, I.; Zafar, H.; Chatha, M.U.; Mahmood, A.; Maqbool, R.; Athar, F.; Alahdal, M.A.; Bibi, F.; Mahmood, F.; Hassan, M.U.; et al. Seed priming with different agents mitigate alkalinity induced oxidative damage and improves maize growth. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12615. [Google Scholar] [CrossRef]
- Mendes, I.C.; Suhet, A.R.; Peres, J.R.R.; Vargas, M.A.T. Eficiência fixadora de estirpes de rizóbio em duas cultivares de feijoeiro. Rev. Bras. Ciência Solo 1994, 18, 421–425. [Google Scholar]
- Brandão Junior, O.; Hungria, M. Efeito de concentrações de solução açucarada na aderência do inoculante turfoso às sementes, na nodulação e no rendimento da soja. Rev. Bras. Ciência Solo 2000, 24, 515–526. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Sandoval-Herrera, I.E.; Zavala-Betancourt, S.A.; Oliveira, H.C.; Ledezma-Pérez, A.S.; Romero, J.; Fraceto, L.F. γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: Characterization and evaluation of biological activity. Carbohydr. Polym. 2017, 157, 1862–1873. [Google Scholar] [CrossRef] [PubMed]

| Analysis | Value |
|---|---|
| pH | 6.00 |
| Density (g L−1) | 1.31 |
| Total reducing sugars—TRS (g L−1) | 432.00 |
| Organic matter (g L−1) | 370.58 |
| Organic carbon (g L−1) | 205.88 |
| Mineral residue + organic (g L−1) | 528.62 |
| Total mineral residue (g L−1) | 158.04 |
| Insoluble mineral residue (g L−1) | 0.04 |
| Soluble mineral residue (g L−1) | 158.00 |
| Nitrogen (g L−1) | 7.98 |
| Phosphorus (g L−1) | 6.07 |
| Potassium (g L−1) | 20.50 |
| Sodium (mg L−1) | 110.00 |
| Calcium (g L−1) | 0.18 |
| Magnesium (g L−1) | 1.51 |
| Sulfur (g L−1) | 1.44 |
| Copper (mg L−1) | 25.50 |
| Iron (mg L−1) | 210.00 |
| Manganese (mg L−1) | 0.50 |
| Zinc (mg L−1) | 24.00 |
| Bacteria | Medium 1 | OD600 2 | γ-PGA (µg mL−1) | pH | TRS 3 (g L−1) | IAA 4 (µg mL−1) |
|---|---|---|---|---|---|---|
| BA | E | 8.33 | 675.71 | 6.48 | 0 | 36.73 |
| 28 | 4.96 | 43.97 | 8.24 | 9.68 | 1.31 | |
| 56 | 1.31 | 97.48 | 6.29 | 33.95 | 1.05 | |
| BL | E | 4.23 | 40.38 | 9.12 | 0 | 40.42 |
| 28 | 3.85 | 8.10 | 5.51 | 20.45 | 0 | |
| 56 | 1.83 | 19.24 | 6.36 | 23.16 | 0 | |
| BS | E | 3.73 | 1.49 | 5.45 | 0 | 11.47 |
| 28 | 4.02 | 52.06 | 7.45 | 4.47 | 14.97 | |
| 56 | 6.68 | 0 | 5.26 | 25.30 | 0 | |
| Control | E | - | - | 7.0 | 0 | - |
| 28 | - | - | 7.0 | 28 | - | |
| 56 | - | - | 7.0 | 56 | - | |
| Water | - | - | 7.5 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa da Silva, A.P.F.; Dorigan, B.S.R.; da Silva-Neto, J.M.; Rosa-Magri, M.M.; Rossi, F.; Francisco, K.R.; Ceccato-Antonini, S.R.; Fontanetti, A. Soy Molasses as Culture Medium for Bacillus Species Aiming at Plant Growth Promotion. Fermentation 2024, 10, 403. https://doi.org/10.3390/fermentation10080403
Correa da Silva APF, Dorigan BSR, da Silva-Neto JM, Rosa-Magri MM, Rossi F, Francisco KR, Ceccato-Antonini SR, Fontanetti A. Soy Molasses as Culture Medium for Bacillus Species Aiming at Plant Growth Promotion. Fermentation. 2024; 10(8):403. https://doi.org/10.3390/fermentation10080403
Chicago/Turabian StyleCorrea da Silva, Ana Paula Fragoso, Bianca Santa Rosa Dorigan, José Machado da Silva-Neto, Marcia Maria Rosa-Magri, Fabricio Rossi, Kelly Roberta Francisco, Sandra Regina Ceccato-Antonini, and Anastácia Fontanetti. 2024. "Soy Molasses as Culture Medium for Bacillus Species Aiming at Plant Growth Promotion" Fermentation 10, no. 8: 403. https://doi.org/10.3390/fermentation10080403
APA StyleCorrea da Silva, A. P. F., Dorigan, B. S. R., da Silva-Neto, J. M., Rosa-Magri, M. M., Rossi, F., Francisco, K. R., Ceccato-Antonini, S. R., & Fontanetti, A. (2024). Soy Molasses as Culture Medium for Bacillus Species Aiming at Plant Growth Promotion. Fermentation, 10(8), 403. https://doi.org/10.3390/fermentation10080403






