Abstract
This study aimed to assess how the bacterial–enzyme co-fermentation of corn straw affects fermentation quality, the digestion rate in Hu sheep, and rumen microorganisms. Orthogonal experiments were utilized to establish the optimal fermentation conditions, which were subsequently applied in bacterial–enzyme fermentation trials involving four groups: group A (control), group B (enzyme added), group C (bacteria added), and group D (bacteria + enzyme). The results show that the optimal fermentation conditions were the addition of 2% corn flour, 2% cottonseed meal, and 60% moisture. In comparison with group A, group D demonstrated the most favorable outcomes, notably reducing the pH and cellulose content while enhancing the lactic acid content. The relative abundances of Pantoea and Weissella reduced, whereas those of Lactiplantibacillus and Limosilactobacillus increased, in the fermented corn straw. In the animal experiments, group D exhibited significantly higher digestibility of NDF and ADF; total VFA, acetic acid, and NH3-N contents; and enzyme activity compared with groups A and B. Additionally, the relative abundances of Prevotella, NK4A214-group, Entodinium, and Polyplastron increased, while those of Dasytricha and Enoploplastron decreased, in group D compared with group A. It can be concluded that Lactobacillus plantarum and cellulase treatments enhance the nutritional value of corn straw by improving ruminal fermentation and regulating the sheep rumen microbiota.
1. Introduction
In China, the total annual output of rice, wheat, and corn straw is about 800 million tons [1]. Corn stalks make up 41.92% of all crop residues, making them the most abundant agricultural byproduct [2,3]. Straw utilization is one of the important components of the “double carbon” strategy, and national and local policies have also been introduced to accelerate the process of straw utilization. The use of corn straw as animal feed is an effective way to improve the utilization of corn straw resources as well as alleviate the problem of feed shortage. Corn straw contains high levels of carbohydrates, specifically, cellulose and hemicellulose, serving as valuable energy sources for ruminants. Nonetheless, its digestibility and nutritional value for ruminants are limited by the presence of lignin, a resilient polymer that encases cellulose and hemicelluloses, thereby impeding their utilization by rumen microbes [3,4]. At present, the commonly used methods for treating straw include silage, alkalization, ammonification, and saccharification. Straw silage is commonly used for the preservation of seasonally harvested energy crops. Alkalization and ammonization have been shown to enhance lignocellulose’s digestibility, although they may also lead to environmental contamination. Preserving straw saccharification poses challenges. In contrast, microbial fermentation and enzyme hydrolysis of straw offer benefits, such as prolonged storage, freedom from seasonal limitations, and minimal environmental impacts, making them favorable options for farmers.
Fermented corn straw involves the treatment of corn straw using microorganisms, enzymes, or a combination of both. Commonly utilized microorganisms include lactic acid bacteria and fungi, while the primary enzyme employed is cellulase. Lactobacillus plantarum is a homofermentative lactic acid bacterium that can completely convert lactose into lactic acid [5], which serves to soften lignin and enhance feed odor. Softening lignin facilitates the susceptibility of cellulose to cellulase activity, resulting in the production of additional small-molecule carbohydrates. Furthermore, these small-molecule carbohydrates can serve as an energy source for L. plantarum, thereby promoting its growth. It was reported that Lactobacillus plantarum and cellulase have a synergistic effect [6].
The stability of the rumen microflora is essential for preserving the host’s health and productivity, as the rumen microbiota is a complex system that is influenced by diet [7]. Fermented corn straw contains beneficial bacteria that can enhance animal health by modulating the gut microflora [8]. Research has demonstrated that Lactobacillus can have a beneficial impact on the host’s intestinal microbiota [9]. Yanti et al. indicated that the introduction of lactic acid bacteria during straw fermentation can alter rumen fermentation and metabolic products in Hu sheep by influencing the gut bacterial community, which is beneficial to the health of ruminants [10]. Liu et al. studied the co-fermentation of total mixed rations (TMRs), including rape straw, by Lactobacillus plantarum and found that the fermentation quality, nutritional characteristics, and digestibility in vitro were improved [11]. Cellulases can degrade cellulose into oligosaccharides, which have demonstrated probiotic properties in animal feed, and cellulosic biomass can be degraded by cellulases into glucose, which can provide energy for microorganisms [12].
Therefore, it was hypothesized that cellulase can decompose cellulose to produce glucose, providing energy for lactic acid bacteria. Both exhibit a synergistic effect that can improve the fermentation quality of corn straw, increase feed digestibility, and enhance the gut microbiota of animals. To test this hypothesis, Lactobacillus plantarum and cellulase corn straw co-fermentation and a Hu sheep feeding experiment were conducted to evaluate the digestibility, rumen fermentation, and microbial community, thereby assessing the value enhancement of corn straw through microbial–enzyme synergistic fermentation.
2. Materials and Methods
2.1. Preparation of the Differently Treated Corn Straw
For the experiment, a three-factor and three-level orthogonal design was used. The three factors were labeled as A, B, and C. A indicated the corn flour; B represented the cottonseed meal; and C represented water. Each factor had three levels, denoted as 1, 2, and 3. Table 1 displays the amounts for each level of the influencing factors. Nine sets of experiments were performed using this orthogonal design, with an additional control group, as shown in Table 2. Each group had 6 replicates, with each replicate weighing approximately 500 g. The control group contained only corn straw, with the moisture content adjusted to 65%. Each experimental group was mixed evenly according to the orthogonal experimental design, and a certain amount of Lactobacillus plantarum was added to achieve a live count of 1 × 106 colony-forming units per gram (CFU)/g. After mixing, the materials were placed into polyethylene vacuum bags, sealed with a vacuum-packaging machine, and then fermented at room temperature (18–26 °C) for 28 days. After fermentation, the pH was measured, and the optimal substrate ratio and water content were determined using the pH as the main index.

Table 1.
Orthogonal experimental design factor table.

Table 2.
Orthogonal experimental design table.
2.2. Composite Fermentation of Corn Straw by Bacteria and Enzymes
Based on the optimal composition of fermentation substrates obtained in Section 2.1, fermentation was performed with the bacterium–enzyme complex. The following four groups were established: Group A, naturally fermented corn straw without feed additives; Group B, fermented corn straw with L. plantarum (1 × 106 CFU/g biomass); Group C, fermented corn straw with cellulase (0.1 filter paper unit (FPU)/g biomass); Group D, fermented corn straw with L. plantarum (1 × 106 CFU/g biomass) and cellulase (0.1 FPU/g biomass). The China General Microbiological Culture Collection Center (CGMCC) provided L. plantarum (CGMCC 1.12934). Cellulase (347 FPU/g) was purchased from Xiasheng Industrial Group Co., Ltd. (Yinchuan, China). Corn straw was packed into vacuum-sealed polyethylene plastic bags and fermented at room temperature (18–26 °C) for 28 days. Subsequently, the pH, viable count of Lactobacillus, and cellulose degradation rate were measured. Microbial sequencing was then conducted to analyze the microbial community.
2.3. Fermentation Parameter Determination
The homogenized microstorage sample (20 g) was extracted with 100 mL of distilled water at 4 °C for 24 h. Next, the solution was filtered through four layers of gauze and qualitative filter paper. The pH of the extracted solution was immediately measured using a digital pH meter. The remaining filtrate was then transferred to a centrifuge tube and stored at −20 °C for further analysis. The concentration of ammoniacal nitrogen (NH3-N) was determined using the phenol–sodium hypochlorite colorimetric method [13]. Meanwhile, the concentrations of lactic acid and volatile fatty acids (VFAs) (acetic acid, propionic acid, and butyric acid) were determined using a gas chromatograph (GC-2010 Pro; Shimadzu, Kyoto, Japan), following a previously reported protocol [14]. The filtrate (10 mL) was appropriately diluted and incubated in De Man, Rogosa, and Sharpe medium at 37 °C for 48 h. The viable count of lactic acid bacteria was determined using a plate-counting method. The microbial counts were indicated as the natural logarithm of the numbers of CFU per gram of the sample.
2.4. Nutrient Analysis
The feed samples were dried at 65 °C in an oven, pulverized, and passed through a 40-mesh screen to ensure consistency. Neutral detergent fiber (NDF), acid detergent fiber (ADF), cellulose, and hemicellulose were quantified, following the methods of Van Soest et al. [15].
2.5. Animals Trial Design
All animal experimental procedures were approved by the Animal Care and Use Committee of Henan Agricultural University (Approval number: HENAU-2021–025). Male Hu sheep (n = 24) aged 3 months with a body weight of 22.05 ± 0.77 kg were randomly classified into three groups (8 sheep/group). Each sheep was raised in an individual cage. The sheep were fed diets with a forage-to-concentrate ratio of 50:50 (dry matter basis) comprising the same concentrate and alfalfa and different treatment groups of fermented corn straw (Table 3). The concentration of different treatment groups of fermented corn straw in the diet was 40% (dry matter basis). The roughage was evenly mixed with the concentrate feed before feeding. Diet and water were provided ad libitum. The feeding experiment duration was 50 days, which included a 10-day adaptation period.

Table 3.
Feed composition and nutrient levels of diets (%, dry matter basis).
2.6. Sample Collection
At the end of the feeding experiment, the fecal samples were collected for three consecutive days (days 47–49). Nylon nets with a mesh size of 5 mm × 7 mm were positioned beneath the cages to facilitate the separation of feces from urine. At the end of each day, fecal matter from each cage was collected, and cecotrophs were discarded. The fecal samples were then dried in a draft oven at 60 °C until a constant weight was obtained. The dried fecal samples were subsequently pooled, and approximately 50 g of the samples were allocated for further nutrient analyses.
At the end of the feeding experiment, the ruminal fluid was collected using a rumen catheter with sterile handling in the morning before feeding. The fluid was filtered through a four-layer gauze. A sample of the ruminal fluid was immediately used to measure pH with a portable pH meter (PHB-4; Shanghai Leici Co. Ltd., Shanghai, China). Additionally, the remaining sample was transferred to centrifuge tubes and stored in a −80 °C refrigerator to analyze ruminal fermentation parameters and microbial flora.
2.7. Apparent Nutrient Digestibility Measurements
The acid-insoluble ash was used as an indicator to calculate apparent nutritional digestibility. The nutrient contents in the diets and feces were identified based on the following Chinese Standards: GB 5009.4–2016 for ash, GB/T 6432–2018 for crude protein (CP), GB/T 6433–2006 for ether extract (EE), GB/T 6436–2018 for calcium (Ca), and GB/T 6437–2018 for total phosphorus (P). NDF and ADF were determined as described in Section 2.4.
2.8. Rumen Fermentation Parameters and Enzyme Activity Determinations
The concentrations of NH3-N and VFAs (acetic acid, propionic acid, isobutyric acid, and butyric acid) were determined as described in Section 2.3. Cellulase, amylase, and protease activities were determined following the methods of Elolimy et al. [16].
2.9. Microbial 16S and 18S rRNA Sequencing
Majorbio Bio-Pharm Co. Ltd. (Shanghai, China) performed RNA sequencing. Total DNA was extracted from each sample as described previously [17]. To determine ruminal bacterial diversity, the V3–V4 region of 16S rRNA was amplified using specific primers (338F: 5′-ACTCCTACGGGAGGCAGCAG-3′; 806R: 5′-GGAC-TACHVGGGTWTCTAAT-3′). Ruminal ciliate diversity was determined using 18S rRNA sequencing. The V4–V5 hypervariable region of 18S rRNA was amplified with eukaryotic primers (547F: 5′-CCAGCASCYGCGG-TAATTCC); 4R: 5′-ACTTTCGTTCTTGATYRA-3′). Amplicon sequencing was performed using the Illumina MiSeq system (Illumina MiSeq, San Diego, CA, USA). The raw sequencing data were spliced, filtered, and dechimerized to obtain an optimized sequence. Based on the optimized sequences, operational taxonomic unit (OTU) abundance tables were generated for further bioinformatics analyses.
The data from this study have been deposited in the National Center for Biotechnology Information Sequence Read Archive (accession number: PRJNA1095653).
2.10. Statistical Analysis
The data are represented as mean ± standard deviation. The mean digestibility and fermentation parameters of nutrients and enzyme activity of the rumen were analyzed using one-way analysis of variance, followed by Duncan’s multiple comparison test. Statistical analyses were performed using IBM SPSS Statistics 26. Differences were considered significant at p < 0.05.
A 97% similarity cutoff was used to cluster the OTUs, and chimeras were removed with the UPARSE software (v 7.1, http://drive5.com/uparse/, accessed on 25 July 2024). The Bayesian algorithm of the RDP classifier was used to perform the taxonomic analysis of OTU representative sequences with 97% similarity. Community species composition was determined for each sample. In the final step, the identified classifications were matched against the Silva 16S rRNA database (Release138, http://www.arb-silva.de, accessed on 25 July 2024) with a 70% confidence threshold. The correlation between environmental factors and rumen bacteria or ciliates was analyzed using the Spearman correlation analysis (Spearman coefficient). All correlation heat maps and hierarchical clusters were prepared with the CRAN “pheatmap” package of R studio (version 3.3.1, http://www.r-project.org, accessed on 25 July 2024).
3. Results
3.1. Orthogonal Experimental Analysis Results
The results of an orthogonal experimental analysis performed using pH as the reference index are presented in Table 4. The order of factors with the primary and secondary influences on the pH of corn straw microstorage samples was as follows: C > A > B. The orthogonal test identified the optimal combination as A2B2C1 (group 5), which exhibited a significantly lower pH when compared with the other groups (p < 0.05). Consequently, the optimal composition for dry matter in the corn straw microstorage samples was determined to be 96% corn straw, 2% cornmeal, 2% cottonseed meal, and 60% moisture content. Subsequent experiments were conducted using this optimal composition.

Table 4.
The orthogonal test results.
3.2. Effect of Bacterium–Enzyme Compound Fermentation on the Quality of Corn Straw
Table 5 shows the pH values, the number of lactic acid bacteria, and the levels of cellulose, hemicellulose, NDF, and ADF. After 28 days of fermentation, the pH values of fermented corn straw in group D were lower than those in groups A, B, and C. Additionally, group D exhibited the highest concentration of viable bacteria. Furthermore, the levels of cellulose, hemicellulose, NDF, and ADF in groups C and D were markedly lower than those in groups A and B. The cellulose, hemicellulose, NDF, and ADF levels were not significantly different between groups C and D (p > 0.05).

Table 5.
Effect of bacteria–enzyme compound fermentation on the quality of corn straw.
3.3. Effect of Bacterium–Enzyme Complex-Mediated Fermentation on Fermentation Parameters and Bacterial Composition of Corn Straw
The results presented in Section 3.2 demonstrated that the cellulose and hemicellulose levels in group C were lower than those in group B. However, group C exhibited a significantly high pH value, which is not ideal for storing fermented straw. Thus, groups A, B, and D were selected for further analyses.
Table 6 shows the fermentation parameters. The NH3-N concentration in group D was markedly lower than that in groups A and B. The lactic acid levels in group D were significantly higher than those in groups A and B (p < 0.05), but were not significantly different between groups A and B (p > 0.05).

Table 6.
Effect of bacteria–enzyme compound fermentation on fermentation parameters of corn straw.
Figure 1 shows the abundances of the top 10 bacterial genera in the fermented corn straw. The dominant genera were Pantoea, Lactiplantibacillus, unclassified_o__Enterobacterales, Levilactobacillus, Weissella, and Limosilactobacillus. Compared with those in groups A and B, the relative abundances of Lactiplantibacillus and Limosilactobacillus were markedly higher, and the abundances of Pantoea and Weissella were lower in group D.
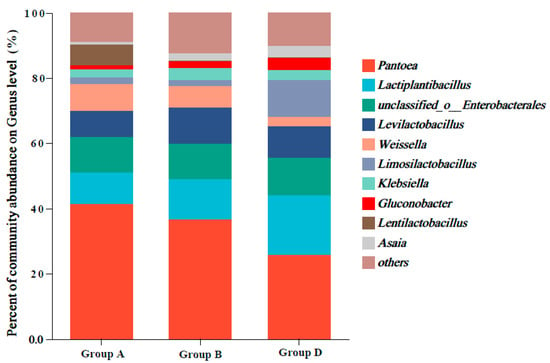
Figure 1.
Genus-level bacterial compositions and abundances in the fermented corn straw.
3.4. Effect of Fermented Corn Straw on Apparent Digestibility of Hu Sheep
As shown in Table 7, the apparent digestibility of EE in group D was significantly higher than that in group A (p < 0.05). Additionally, the apparent digestibility of P, NDF, and ADF in group D was markedly higher than that in groups A and B (p < 0.05), but was not significantly different between groups A and B. Furthermore, the apparent digestibility of CP and Ca was not significantly different between groups A, B, and D (p > 0.05).

Table 7.
Effects of fermented corn straw on apparent digestibility of Hu Sheep.
3.5. Effects of Fermented Corn Straw on Rumen Fermentation Parameters of Hu Sheep
As shown in Table 8, the ruminal pH value in group D was significantly lower than that in group A (p < 0.05). The ruminal NH3-N, total VFA, and acetic acid contents in group D were significantly higher than those in groups A and B (p < 0.05). The levels of ruminal propionic acid, isobutyric acid, and butyric acid were not significantly different between groups A, B, and D (p > 0.05). The protease activity in group D was significantly higher than that in groups A and B (p < 0.05) and was not significantly different between groups A and B. Amylase activity in group D was significantly higher than that in group A (p < 0.05) and was similar to that in group B.

Table 8.
Effects of fermented corn straw on rumen fermentation parameters and enzyme activities of Hu Sheep.
3.6. Effects of Fermented Corn Straw on Ruminal Bacterial Community of Hu Sheep
Figure 2A shows the ruminal abundances of the top 15 bacterial phyla in Hu sheep. The predominant phyla were Firmicutes and Bacteroidetes, followed by Actinobacteriota and Proteobacteria. The abundances of Proteobacteria in group B were higher than those in groups A and D. Compared with those in groups A and B, the abundances of Bacteroidetes and Actinobacteriota were significantly higher in group D.
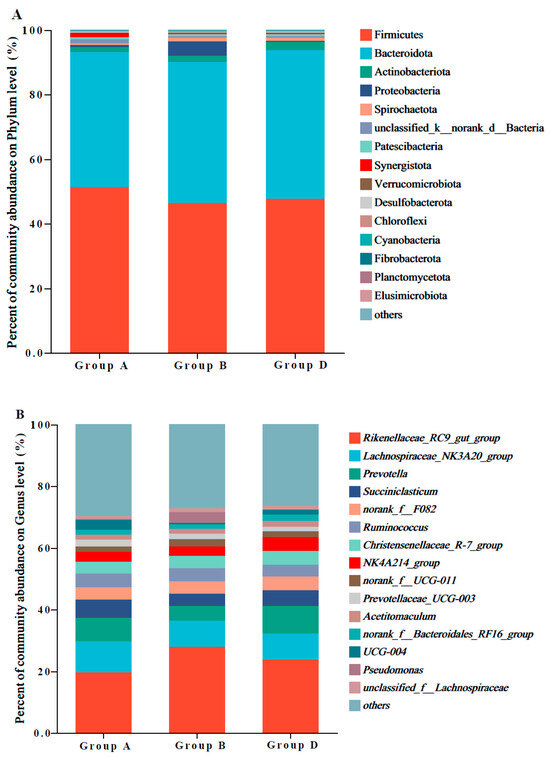
Figure 2.
Rumen bacterial composition and abundances at the phylum and genus levels in Hu sheep. (A) the top 15 ruminal bacterial phyla and their relative abundance; (B) the top 15 ruminal bacterial genera and their relative abundance.
Figure 2B shows the ruminal abundances of the top 15 bacterial genera in Hu sheep. The predominant genera were Rikenellaceae_RC9_gut_group, Lachnospiraceae_NK3A20_group, Prevotella, and Succiniclasticum. The abundances of Prevotella and NK4A214_group in group D were significantly higher than those in groups A and B. Compared with those in groups A and C, the abundances of Rikenellaceae_RC9_gut_group were significantly higher in group B.
3.7. Correlation of Rumen Bacterial Abundance with Nutrient Digestibility, Rumen Fermentation Parameters, and Enzyme Activity
Figure 3 shows the correlation of ruminal bacterial abundances with environmental factors, such as nutrient digestibility, rumen fermentation parameters, and enzyme activity. The ruminal NH3-N content was significantly and positively correlated with the abundances of Lachnospiraceae_NK3A20_group and Ruminococcus. Meanwhile, the abundances of NK4A214_group were positively correlated with ruminal proteinase activity. Furthermore, the abundances of Norank_f__F082 and NK4A214_group were positively correlated with EE digestibility.
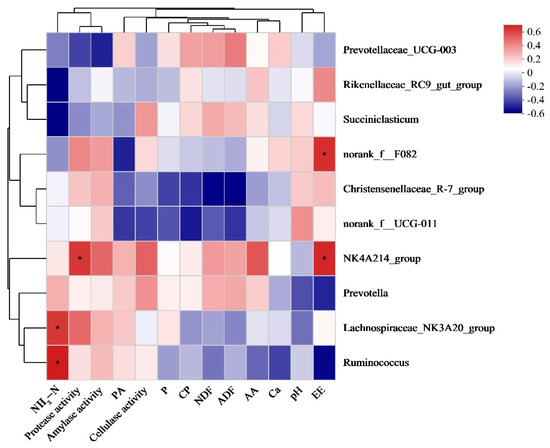
Figure 3.
Correlation of ruminal bacterial abundances with environmental factors. Correlations in red and blue suggest positive and negative correlations, respectively. * p < 0.05. NH3-N, ammoniacal nitrogen; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; AA, acetic acid; PA, propionic acid; Ca, calcium; P, phosphorus.
3.8. Effects of Fermented Corn Straw on Ruminal Ciliate Community in Hu Sheep
As shown in Figure 4, nine genera of ciliates were identified. The top five major ciliate genera were Dasytricha, Epidinium, Entodinium, Polyplastron, and Enoploplastron. The abundance of Dasytricha in group A was significantly higher than that in groups B and D. Compared with that in groups A and B, the abundance of Entodinium was significantly higher in group D. The abundance of Polyplastron in group D was markedly higher than that in group A. Compared with that in groups A and D, the abundance of Enoploplastron was significantly higher in group B.
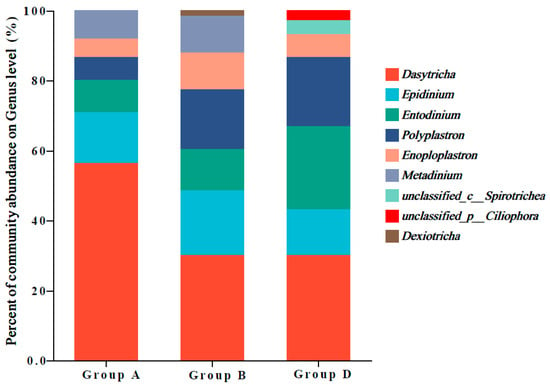
Figure 4.
Abundances of ruminal ciliate genera in Hu sheep. The ordinate represents the percentage of relative bacterial abundances at the genus level (%).
3.9. Correlation of Ruminal Ciliate Abundances with Nutrient Digestibility, Rumen Fermentation Parameters, and Enzyme Activity
Figure 5 shows the correlation of ruminal ciliate abundances with environmental factors, such as nutrient digestibility, rumen fermentation parameters, and enzyme activity. The ruminal NH3-N concentration was significantly and positively correlated with Enoploplastron abundance (p < 0.05). Polyplastron abundance was positively correlated with ruminal amylase activity (p < 0.05).
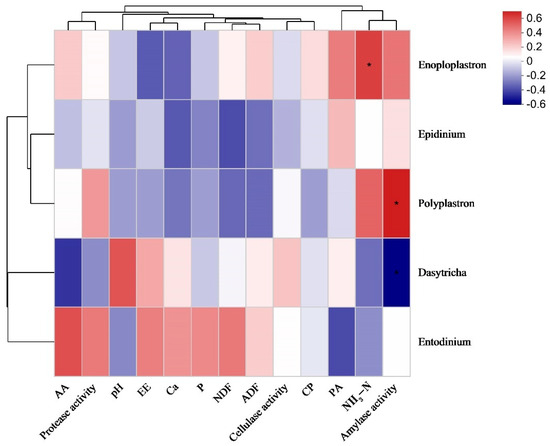
Figure 5.
Correlation of ruminal ciliate abundances with environmental factors. Correlations in red and blue suggest positive and negative correlations, respectively. * p < 0.05. NH3-N, ammoniacal nitrogen; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; AA, acetic acid; PA, propionic acid; Ca, calcium; P, phosphorus.
4. Discussion
The nutrients in fermentation media significantly impact microbial growth and fermentation quality [18]. Straw cannot support optimal microbial growth owing to limited nutrient contents. Therefore, the supplementation of sufficient carbon and nitrogen sources can promote microbial growth. Corn flour, which contains a high level of soluble carbohydrates, is a valuable carbon source for lactic acid bacterial growth when added to fermentation substrates [19]. This results in increased lactic acid production, reducing the pH of fermented straw and enhancing the storage stability of corn stalks [20]. Cottonseed meal is a valuable nutrient for fermentation processes due to its high protein and mineral content. Moisture content is a crucial abiotic factor influencing microbial growth. Excessive moisture can cause spoilage during microstorage, whereas insufficient moisture inhibits microbial growth and straw fermentation [21]. In this study, the supplementation of appropriate amounts of corn meal and cottonseed meal significantly reduced the pH of the fermentation substrate of corn straw after microstorage. This is because corn meal and cottonseed meal provide lactic acid bacteria with sufficient carbon and nitrogen sources to grow and reproduce.
Lactic acid bacteria can affect fermentation, fermenting carbohydrates to produce lactic acid and enhancing the quality of straw fermentation [22]. Cellulase provides the fermentation substrates for lactic acid bacteria by degrading cellulose into simple sugars or oligosaccharides [23,24]. To determine the quality of fermentation, pH is an important indicator [25]. The production of lactic acid by lactic acid bacteria decreases the pH during fermentation. In this study, group D exhibited the lowest pH, indicating synergistic effects of cellulase and lactic acid bacteria. This study demonstrated that the supplementation of cellulase reduces the contents of NDF, ADF, cellulose, and hemicellulose in corn straw, which was consistent with the findings of previous studies [24,26].
The NH3-N content in fermented feed indicates the degree of protein degradation during microstorage. Decreased NH3-N content indicates less decomposition of protein and amino acids, enhancing fermentation quality [27]. Mu et al. reported that the combination of cellulase and L. plantarum decreased NH3-N content in high-moisture amaranth and rice straw by inhibiting protease [28]. In this study, the lactic acid content was correlated with the pH and the relative abundance of Lactobacillus. Additionally, Lactobacillus can produce acetic acid during fermentation [29]. The acetic acid content in the Lactobacillus and bacterial–enzyme groups was markedly higher than that in the control group.
The bacterial genera in 28-day fermented corn straw are shown in Figure 1. Pantoea, which was the most abundant genus, ferments sugars into acids under anaerobic conditions and produces acetic acid, propionic acid, and succinate [30]. However, Pantoea is considered undesirable as it competes with lactic acid bacteria for substrates during the ensiling process. The downregulation of these genera can be attributed to the rapid acidification induced by L. plantarum [31]. After fermentation, the abundance of Lactobacillus in group D was higher than that in groups A and B even though Lactobacillus was the predominant bacterium in corn straw. Homofermentative Lactiplantibacillus species ferment hexose to produce lactic acid. Meanwhile, heterofermentative Lactiplantibacillus species ferment hexose and pentose to produce equal amounts of lactic acid and acetic acid [32]. Compared with those in group A, the lactic acid and acetic acid levels were higher and the pH was lower in groups B and D. This can be attributed to the predominance of Lactiplantibacillus and suggests that enhanced fermentation quality was achieved in this study. Previous studies have demonstrated the predominance of Lactiplantibacillus in ensiled silages, including alfalfa [33], guinea grass [34], and corn [35]. Consistently, this study demonstrated that Lactiplantibacillus was the dominant bacterium in fermented or silage corn straw. After fermentation, compared with those in groups A and B, the relative abundances of Weissella were lower in group D. After ensiling, Weissella is reported to be gradually replaced by Lactobacillus, which becomes the dominant genus [36]. Weissella abundance is negatively correlated with pH [37]. The increased Limosilactobacillus abundance in group D may be related to its increased acid resistance and metabolic adaptability [38].
Nutrient digestibility is a crucial indicator of the extent to which nutrients are digested and absorbed by animals. High nutrient digestibility indicates efficient nutrient digestion and absorption. This study demonstrated that the fermentation of corn straw with bacteria and enzymes significantly improved the apparent digestibility of CP, EE, NDF, and ADF in Hu sheep. This can be attributed to bacterial fermentation and the cellulase-mediated hydrolysis of corn straw in vitro. Previous studies have indicated that feeding fermented paddy straw improved nutrient digestibility in goats [39], which is consistent with the findings of this study. Additionally, nutrient digestibility is positively correlated with digestive enzyme activity [40]. The increased nutrient digestibility observed in this study may also be due to elevated protease and cellulase activities in the rumen. Rumen digestive enzyme activity can also be influenced by the rumen microbiome [41]. Therefore, nutrient digestibility is closely related to dietary composition and intestinal enzyme activity.
Rumen fermentation parameters (pH, NH3-N, and VFA) are crucial indicators for evaluating rumen health. Changes in rumen VFA concentrations can reflect alterations in rumen fermentation patterns. The increased release of readily fermentable carbohydrates from corn straw after bacteria/enzyme-mediated fermentation promotes the production of VFAs (especially propionic acid and butyric acid) [42] and reduces rumen pH. Additionally, providing readily fermentable carbohydrates to sheep increases the transfer of urea into the rumen, leading to the upregulation of NH3-N content [43]. A high-fiber diet is reported to increase acetic acid production [44], which is consistent with the findings of this study.
The rumen microbiota composition is correlated with animal feed composition. Animal feed determines the composition of functional microbiota [45]. Firmicutes and Bacteroidetes were the predominant phyla in this study, which was consistent with the findings of previous studies [46,47,48]. Carbohydrate-active enzyme annotation indicated that Firmicutes and Bacteroidetes encode a diverse set of cellulose and hemicellulose degradation enzymes [49]. The main functions of Bacteroidetes are fermenting carbohydrates and degrading plant-derived materials to convert them into VFAs, providing energy for animal metabolic activities [50]. The abundance of Bacteroidetes was significantly high in group D, suggesting that the fermentation of corn straw with bacteria and enzymes regulates rumen microbiota and promotes cellulose degradation in corn straw and the digestibility of NDF and ADF. Actinobacteriota is involved in the degradation of plant lignin, cellulose, and pectin in soil [51], as well as in nitrogen transformation [52]. The increased abundance of Actinobacteriota can enhance nutrient digestibility.
Some studies have reported that the rumen contains a high number of Rikenellaceae_RC9_gut_group, which promotes carbohydrate transport and metabolism [53] and ferments structural carbohydrates [54]. Rikenellaceae_RC9_gut_group is also involved in butyrate production [55,56]. However, the increased abundance of Rikenellaceae_RC9_gut_group in group B did not lead to increased nutrient digestibility and butyrate production in this study, which can be due to different diet compositions. Prevotella can promote the production of short-chain fatty acids, which are beneficial to both hosts and microbes, by utilizing starch and non-cellulosic polysaccharides [57]. A recent study demonstrated that NK4A214_group abundance increases with a high dietary concentrate-to-forage ratio, exhibiting a strong positive correlation with metabolites [58]. The high abundances of Prevotella and NK4A214_group, along with a high total VFA content in the rumen of group D, indicated that corn straw treated with L. plantarum and cellulase can increase corn straw digestibility by regulating the ruminal microbiota of Hu sheep. NH3-N in the rumen is the sole nitrogen source for Ruminococcus growth [59]. The positive correlation between ruminal Ruminococcus abundance and NH3-N content observed in this study confirms their close relationship.
Ciliates play crucial roles in the rumen environment, contributing to nutrient digestion and absorption efficiency. This study demonstrated that Dasytricha, Epidinium, Entodinium, Polyplastron, and Enoploplastron were the predominant ciliate genera in all groups, which was consistent with the findings of previous studies [60,61]. Dasytricha, a saccharolytic protozoan, exhibits glucosidase and cellobiosidase activities [62]. Entodinium, Epidinium, and Polyplastron exhibit fibrolytic activities. Coleman et al. (1983) isolated enzymes from Entodinium that can degrade cellulose, hemicellulose, and pectin [63]. The increased abundances of Entodinium and Polyplastron in group D were associated with high nutrient digestibility, amylase activity, and ruminal fermentation, which was consistent with the results of previous studies. Ciliates can directly degrade soluble or insoluble proteins in the rumen and participate in protein metabolism. Proteolysis also affects the metabolism of nitrogen-containing substances [64]. The significant positive correlation between ruminal Enoploplastron abundance and NH3-N concentrations indicated the mechanisms involved in the Enoploplastron-mediated regulation of ruminal NH3-N metabolism.
5. Conclusions
The supplementation of 2% corn flour, 2% cottonseed meal, and 60% moisture content to corn straw significantly improves the fermentation quality of the corn straw, decreases the abundances of Pantoea and Weissella, and increases the abundances of Lactiplantibacillus and Limosilactobacillus. Fermented corn straw enhances nutrient digestibility, ruminal fermentation, enzyme activity, and total VFA and NH3-N contents in sheep by increasing the abundances of Prevotella, NK4A214_group, Entodinium, and Polyplastron in the rumen. The in vivo treatment of corn straw with Lactobacillus and cellulase is an effective method to enhance its nutritive value and subsequently improve ruminant production.
Author Contributions
Conceptualization, L.W.; Methodology, J.W.; Software, J.W.; Validation, S.J. and Q.Z.; Formal analysis, S.J. and P.W.; Investigation, C.L.; Resources, X.L.; Data curation, X.L.; Writing—original draft, L.W.; Writing—review and editing, Q.Y.; Visualization, J.C.; Supervision, Q.Z.; Project administration, J.C.; Funding acquisition, Q.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Project of Science and Technology of Henan Province (232102110103); Nature Science Foundation of Henan (242300421573); Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2023JD18), and Xinxiang Key Scientific and Technological Projects (22ZD011).
Institutional Review Board Statement
All of the experimental procedures and operations conducted on animals during the present research followed the ethical guidelines and regulatory authority of the Animal Care Committee (SKLAB-B-2010–003-01) of Henan Agricultural University.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Acknowledgments
We acknowledge Fushan Lu and Yayu Liu for their assistance with this work.
Conflicts of Interest
Qun Zhu is employed by Henan Delin Biological Product Co., Ltd. The remaining authors of the paper declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Cheng, F.; Dehghanizadeh, M.; Audu, M.A.; Jarvis, J.M.; Holguin, F.O.; Brewer, C.E. Characterization and evaluation of guayule processing residues as potential feedstock for biofuel and chemical production. Ind. Crop. Prod. 2020, 150, 112311. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, H.; Liu, J.; Wang, X.; Li, J.; Shi, E.; Wang, C.; Yang, J.; Zhang, Z. A study on and adsorption mechanism of ammonium nitrogen by modified corn straw biochar. R. Soc. Open Sci. 2023, 10, 221535. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Wang, Z.; Xu, Z.; Zhuang, W.; Liu, D.; Lv, Y.; Wang, S.; Xu, J.; Ying, H. Solid-state fermentation of corn straw using synthetic microbiome to produce fermented feed: The feed quality and conversion mechanism. Sci. Total Environ. 2024, 920, 171034. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, J.; Liu, Z.; Zhang, G.; Zhang, Y. Fermentation quality and microbial community of corn stover or rice straw silage mixed with soybean curd residue. Animals 2022, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Derabli, B.; Nancib, A.; Nancib, N.; Aníbal, J.; Raposo, S.; Rodrigues, B.; Boudrant, J. Opuntia ficus indica waste as a cost effective carbon source for lactic acid production by Lactobacillus plantarum. Food Chem. 2022, 370, 131005. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Wang, C.; Dong, W.; Zhang, Z.; Zhao, L.; Zhang, X. Effects of cellulase and Lactobacillus plantarum on fermentation quality, chemical composition, and microbial community of mixed silage of whole-plant corn and peanut vines. Appl. Biochem. Biotechnol. 2022, 194, 2465–2480. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zeng, H.; Wang, C.; Han, Z. Effect of methionine hydroxy analog on Hu sheep digestibility, rumen fermentation, and rumen microbial community in vitro. Metabolites 2023, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska-Turak, E.; Walczak, M.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Witrowa-Rajchert, D. Influence of fermentation beetroot juice process on the physico-chemical properties of spray dried powder. Molecules 2022, 27, 1008. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhai, Q.; Zhang, H.; Chen, W.; Hill, C. Gut colonization mechanisms of Lactobacillus and Bifidobacterium: An argument for personalized designs. Annu. Rev. Food Sci. Technol. 2021, 12, 213–233. [Google Scholar] [CrossRef]
- Yanti, Y.; Kawai, S.; Yayota, M. Effect of total mixed ration silage containing agricultural by-products with the fermented juice of epiphytic lactic acid bacteria on rumen fermentation and nitrogen balance in ewes. Trop Anim. Health Prod. 2019, 51, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Xiang-Yu, L.I.; Desta, S.T.; Zhang, J.G.; Shao, T. Effects of Lactobacillus plantarum and fibrolytic enzyme on the fermentation quality and in vitro digestibility of total mixed rations silage including rape straw. J. Integr. Agric. 2016, 15, 2087–2096. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Qin, Y.; Li, W.; Li, Y.; Cao, H.; Cao, P.; Ding, K.; He, W. Screening, characterization, and optimization of the fermentation conditions of a novel cellulase-producing microorganism from soil of Qinghai-Tibet Plateau. Biotechnol. Appl. Biochem. 2024, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.J.; Li, S.L.; Xing, J.J.; Ma, M.; Wang, L.L. Effects of maize grain and lucerne particle size on ruminal fermentation, digestibility and performance of cows in midlactation. J. Anim. Physiol. Anim. Nutr. 2008, 92, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Elolimy, A.A.; Arroyo, J.M.; Batistel, F.; Iakiviak, M.A.; Loor, J.J. Association of residual feed intake with abundance of ruminal bacteria and biopolymer hydrolyzing enzyme activities during the peripartal period and early lactation in Holstein dairy cows. J. Anim. Sci. Biotechnol. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of high forage/concentrate diet on volatile fatty acid production and the microorganisms involved in VFA production in cow rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Ferdouse, J.; Kusaba, Y.; Fujimaru, Y.; Yamamoto, Y.; Kitagaki, H. Methionine and glycine stabilize mitochondrial activity in sake yeast during ethanol fermentation. Food Technol. Biotechnol. 2019, 57, 535–543. [Google Scholar] [PubMed]
- Deepak, T.S.; Jayadeep, P.A. Prospects of maize (corn) wet milling by-products as a source of functional food ingredients and nutraceuticals. Food Technol. Biotechnol. 2022, 60, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, S.; Lu, J.; Zhang, C.; Pang, X.; Lv, J. Screening for cholesterol-lowering probiotics from lactic acid bacteria isolated from corn silage based on three hypothesized pathways. Int. J. Mol. Sci. 2019, 20, 2073. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.L.M.; Bueno, A.V.I.; Jacovaci, F.A.; Donadel, G.; Ferraretto, L.F.; Nussio, L.G.; Jobim, C.C.; Daniel, J.L.P. Effects of processing, moisture, and storage length on the fermentation profile, particle size, and ruminal disappearance of reconstituted corn grain. J. Anim. Sci. 2020, 98, skaa332. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Talaia, G.; Sá-Pessoa, J.; Bessa, D.; Gonçalves, M.J.; Moreira, R.; Paiva, S.; Casal, M.; Queirós, O. Lactic acid production in Saccharomyces cerevisiae is modulated by expression of the monocarboxylate transporters Jen1 and Ady. FEMS Yeast Res. 2012, 12, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Y.; Hu, B.; Qv, M.; Ma, C.; Wei, M.; Zhang, H. Insight into the potentiality of big biochar particle as an amendment in aerobic composting of sewage sludge. Bioresour. Technol. 2019, 288, 121469. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Niu, H.; Tong, Q.; Chang, J.; Yu, J.; Li, S.; Zhang, S.; Ma, D. The microbiota dynamics of alfalfa silage during ensiling and after air exposure, and the metabolomics after air exposure are affected by Lactobacillus casei and cellulase addition. Front. Microbiol. 2020, 11, 519121. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Guo, X.; Wu, S.; Chen, D.; Ge, L.; Zhou, W.; Zhang, Q.; Pian, R. Tannin tolerance lactic acid bacteria screening and their effects on fermentation quality of stylo and soybean silages. Front. Microbiol. 2022, 13, 991387. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Cherdthong, A.; Wanapat, M. Improving sugarcane bagasse quality as ruminant feed with Lactobacillus, cellulase, and molasses. J. Anim. Sci. Technol. 2020, 62, 648–658. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, W.; Wang, C.; Yang, F.; Chen, X.; Zhang, Q. Effect of cellulase and Lactobacillus casei on ensiling characteristics, chemical composition, antioxidant activity, and digestibility of mulberry leaf silage. J. Dairy Sci. 2019, 102, 9919–9931. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.H.; Hsieh, C.W.; Chen, K.L. Screening lactic acid bacteria to manufacture two-stage fermented feed and pelleting to investigate the feeding effect on broilers. Poult. Sci. 2018, 97, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, J.; Dong, Z.; Shao, T. The reconstitution mechanism of napier grass microiota during the ensiling of alfalfa and their contributions to fermentation quality of silage. Bioresour. Technol. 2020, 297, 122391. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhou, W.; Chen, X.; Zhang, Q. Chemical and bacterial composition of Broussonetia papyrifera leaves ensiled at two ensiling densities with or without Lactobacillus plantarum. J. Clean. Prod. 2021, 329, 129792. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.L.; Niu, D.Z.; Jiang, D.; Zuo, S.S.; Xu, C.C. Dynamics of microbial community during ensiling direct-cut alfalfa with and without LAB inoculant and sugar. J. Appl. Microbiol. 2017, 122, 1456–1470. [Google Scholar] [CrossRef] [PubMed]
- Parvin, S.; Nishino, N. Bacterial community associated with ensilage process of wilted guinea grass. J. Appl. Microbiol. 2009, 107, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Jiang, Y.; Kim, D.H.; Cervantes, A.A.P.; Arriola, K.G.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Fate of Escherichia coli O157:H7 and bacterial diversity in corn silage contaminated with the pathogen and treated with chemical or microbial additives. J. Dairy Sci. 2017, 100, 1780–1794. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Ding, Z.; Su, R.; Wang, M.; Cheng, M.; Xie, D.; Guo, X. Storage temperature is more effective than lactic acid bacteria inoculations in manipulating fermentation and bacterial community diversity, co-occurrence and functionality of the whole-plant corn silage. Microbiol. Spectr. 2022, 10, e0010122. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liao, L.; Xiao, Y.; Zhai, J.; Su, H.; Chen, Y.; Guo, Y. Epicuticular wax of sweet sorghum influenced the microbial community and fermentation quality of silage. Front. Microbiol. 2022, 13, 960857. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, W.; Zhang, A.; Li, P.; Liu, J. Dynamics and functionalities of bacterial community during foxtail millet dough fermentation by metagenomic analysis. J. Future Foods 2024, 4, 343–352. [Google Scholar] [CrossRef]
- Singh, G.P.; Gupta, B.N.; Singh, K. Effect of microbial treatment of paddy straw on chemical composition and nutrient utilization in crossbred goats. Indian J. Anim. Nutr. 1990, 7, 251–256. [Google Scholar]
- Guzmán-Pino, S.A.; Solà-Oriol, D.; Figueroa, J.; Pérez, J.F. Influence of the protein status of piglets on their ability to select and prefer protein sources. Physiol. Behav. 2014, 129, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, L.D.; Dohme-Meier, F.; Münger, A.; Bruckmaier, R.M.; van Dorland, H.A. Metabolism of grazed vs. zero-grazed dairy cows throughout the vegetation period: Hepatic and blood plasma parameters. J. Anim. Physiol. Anim. Nutr. 2012, 96, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.A.; Titgemeyer, E.C.; Olson, K.C.; Brake, D.W.; Jones, M.L.; Anderson, D.E. Effects of ruminal casein and glucose on forage digestion and urea kinetics in beef cattle. J. Anim. Sci. 2012, 90, 3505–3514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, Z.Z.; Cheng, Y.Y.; Wang, S.Q.; Ge, J.Z.; Shi, H.P.; Kou, J.C. Positive effects of dietary supplementation of three probiotics on milk yield, milk composition and intestinal flora in Sannan dairy goats varied in kind of probiotics. J. Anim. Physiol. Anim. Nutr. 2020, 104, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Teather, R.; Forster, R. Composition, spatial distribution, and diversity of the bacterial communities in the rumen of cows fed different forages. FEMS Microbiol. Ecol. 2010, 74, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.M.; Ahir, V.B.; Tripathi, A.K.; Ramani, U.V.; Sajnani, M.; Koringa, P.G.; Jakhesara, S.; Pandya, P.R.; Rank, D.N.; Murty, D.S.; et al. Metagenomic analysis of Surti buffalo (Bubalus bubalis) rumen: A preliminary study. Mol. Biol. Rep. 2012, 39, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.N.; Jewell, K.A.; Freitas, F.S.; Benjamin, L.A.; Tótola, M.R.; Borges, A.C.; Moraes, C.A.; Suen, G. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet. Microbiol. 2013, 164, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Auffret, M.D.; Warr, A.; Walker, A.W.; Roehe, R.; Watson, M. Compendium of 4,941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat. Biotechnol. 2019, 37, 953–961. [Google Scholar] [CrossRef]
- Wongfaed, N.; O-Thong, S.; Sittijunda, S.; Reungsang, A. Taxonomic and enzymatic basis of the cellulolytic microbial consortium KKU-MC1 and its application in enhancing biomethane production. Sci. Rep. 2023, 13, 2968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, P.; Wang, L.; Zhao, Z.; Chen, Y.; Yang, Y. Bacterial community diversity associated with different levels of dietary nutrition in the rumen of sheep. Appl. Microbiol. Biotechnol. 2017, 101, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Kirby, R. Actinomycetes and lignin degradation. Adv. Appl. Microbiol. 2006, 58, 125–168. [Google Scholar] [PubMed]
- Zeng, T.; Wang, L.; Zhang, X.; Song, X.; Li, J.; Yang, J.; Chen, S.; Zhang, J. Characterization of microbial communities in wastewater treatment plants containing heavy metals located in chemical industrial zones. Int. J. Environ. Res. Public Health 2022, 19, 6529. [Google Scholar] [CrossRef] [PubMed]
- Iudina, T.G.; Briukhanov, A.L.; Netrusov, A.I. Susceptibility of archaea to the antibiotic effect of the parasporal inclusion proteins from Bacillus thuringiensis subspecies. Mikrobiologiia 2004, 73, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Kong, F.; Wang, W.; Wang, Y.; Yang, H.; Cao, Z.; Li, S. In vitro and in vivo studies of soybean peptides on milk production, rumen fermentation, ruminal bacterial community, and blood parameters in lactating dairy cows. Front. Vet. Sci. 2022, 9, 911958. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, L.; Mei, M.; Guo, J.; Yang, X.; Liu, S. Electroacupuncture repairs intestinal barrier by upregulating CB1 through gut microbiota in DSS-induced acute colitis. Chin. Med. 2023, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, Z.; Li, K.; Bai, G.; Liu, L.; Zhong, R.; Chen, L.; Zhang, H. Time-course effects of different fiber-rich ingredients on energy values, microbiota composition and SCFA profile in growing pigs. Anim. Nutr. 2023, 12, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Xie, P.; Li, Z.; Yin, Y.; Blachier, F.; Kong, X. Dietary supplementation with fermented Mao-tai lees beneficially affects gut microbiota structure and function in pigs. AMB Express 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Dai, D.; Wu, H.; Chai, S.; Liu, S.; Meng, Q.; Zhou, Z. Dietary concentrate-to-forage ratio affects rumen bacterial community composition and metabolome of Yaks. Front. Nutr. 2022, 9, 927206. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tsedan, G.; Liu, Y.; Hou, F. Shrub coverage alters the rumen bacterial community of yaks (Bos grunniens) grazing in alpine meadows. J. Anim. Sci. Technol. 2020, 62, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Veira, D.M.; Ivan, M.; Jui, P.Y. Rumen ciliate protozoa: Effects on digestion in the stomach of sheep. J. Dairy Sci. 1983, 66, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, A. Rumen ciliates: Their metabolism and relationships with bacteria and their hosts. Anim. Feed Sci. Technol. 1990, 30, 203–266. [Google Scholar] [CrossRef]
- Ivan, M.; Neill, L.; Forster, R.; Alimon, R.; Rode, L.M.; Entz, T. Effects of Isotricha, Dasytricha, Entodinium, and total fauna on ruminal fermentation and duodenal flow in wethers fed different diets. J. Dairy Sci. 2000, 83, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Coleman, G.S. Hydrolysis of fraction 1 leaf protein and casein by rumen entodiniomorphid protozoa. J. Appl. Bacteriol. 1983, 55, 1365–2672. [Google Scholar] [CrossRef]
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Muñoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M.; et al. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future. Front. Microbiol. 2018, 9, 2161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
