A Preliminary Study of the Effects of Gaseous Ozone on the Microbiological and Chemical Characteristics of Whole-Plant Corn Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Site, and Condition
2.2. Experimental Design and Silage Preparation
- 0% was the control treatment.
- 1.25% was achieved by applying 0.5 g of O3 for three minutes.
- 3.12% was achieved by applying 1.25 g of O3 for seven minutes and thirty seconds.
- 4.15% was achieved by applying 1.66 g of O3 for ten minutes.
- 6.25% was achieved by applying 2.5 g of O3 for fifteen minutes.
2.3. Sampling Operations and Analysis
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zubairova, L.; Tagirov, H.; Mironova, I.; Iskhakov, R.; Vagapov, I. Biotechnology Techniques in Animal Nutrition for Improving Quality Indicators of Beef and Dairy Products. Biocatal. Agric. Biotechnol. 2022, 40, 102294. [Google Scholar] [CrossRef]
- Cavallini, D.; Raspa, F.; Marliani, G.; Nannoni, E.; Martelli, G.; Sardi, L.; Valle, E.; Pollesel, M.; Tassinari, M.; Buonaiuto, G. Growth Performance and Feed Intake Assessment of Italian Holstein Calves Fed a Hay-Based Total Mixed Ration: Preliminary Steps towards a Prediction Model. Vet. Sci. 2023, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Kokko, C.; Ballard, C.S.; Dann, H.M.; Fustini, M.; Palmonari, A.; Formigoni, A.; Cotanch, K.W.; Grant, R.J. Influence of Fiber Degradability of Corn Silage in Diets with Lower and Higher Fiber Content on Lactational Performance, Nutrient Digestibility, and Ruminal Characteristics in Lactating Holstein Cows. J. Dairy Sci. 2021, 104, 1728–1743. [Google Scholar] [CrossRef] [PubMed]
- Hisadomi, S.; Oba, M. Evaluation of Dehydrated Corn Silage as the Primary Forage for Lactating Dairy Cows. JDS Commun. 2022, 3, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; Mello, A.; Guim, A.; Cunha, M.; Silva, P.; Atroch, T.; Neto, D.; Neto, P.; Medeiros, A.; Clemente, J. Grass Size and Butterfly Pea Inclusion Modify the Nutritional Value of Elephant Grass Silage. Pesqui. Agropecuária Bras. 2021, 56, e02409. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.L.S.; Carvalho, B.F. Silage Fermentation—Updates Focusing on the Performance of Micro-organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef]
- Ferraretto, L.F.; Shaver, R.D.; Luck, B.D. Silage Review: Recent Advances and Future Technologies for Whole-Plant and Fractionated Corn Silage Harvesting. J. Dairy Sci. 2018, 101, 3937–3951. [Google Scholar] [CrossRef]
- Diogénes, L.V.; Pereira Filho, J.M.; Edvan, R.L.; de Oliveira, J.P.F.; Nascimento, R.R.D.; Santos, E.M.; Alencar, E.J.S.; Mazza, P.H.S.; Oliveira, R.L.; Bezerra, L.R. Effect of Different Additives on the Quality of Rehydrated Corn Grain Silage: A Systematic Review. Ruminants 2023, 3, 425–444. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage Review: Recent Advances and Future Uses of Silage Additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Stokes, M.R.; Lin, C.J. Silage Additives. In Silage Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 305–360. ISBN 978-0-89118-234-4. [Google Scholar]
- Kung, L.; Sheperd, A.C.; Smagala, A.M.; Endres, K.M.; Bessett, C.A.; Ranjit, N.K.; Glancey, J.L. The Effect of Preservatives Based on Propionic Acid on the Fermentation and Aerobic Stability of Corn Silage and a Total Mixed Ration1. J. Dairy Sci. 1998, 81, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Mitloehner, F.; Cohen, M. Impacts and Mitigation of Emissions from Dairy Feeds on Air Quality. In Large Dairy Herd Management, 3rd ed.; American Dairy Science Association: Champaign, IL, USA, 2017; pp. 47–60. ISBN 978-0-9634491-2-2. [Google Scholar]
- Rotz, C.A.; Shinners, K.J.; Digman, M. Hay Harvest and Storage. In Forages, Volume II: The Science of Grassland Agriculutre, 7th ed.; Moore, K.J., Collins, M., Nelson, C.J., Redfearn, D.D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 749–765. ISBN 9781119436577. [Google Scholar]
- Moriel, P.; Piccolo, M.B.; Artioli, L.F.A.; Santos, G.S.; Poore, M.H.; Ferraretto, L.F. Method of Propionic Acid—Based Preservative Addition and Its Effects on Nutritive Value and Fermentation Characteristics of Wet Brewers Grains Ensiled in the Summertime. Prof. Anim. Sci. 2016, 32, 591–597. [Google Scholar] [CrossRef]
- Sarron, E.; Gadonna-Widehem, P.; Aussenac, T. Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review. Foods 2021, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Macleod, J.; Blaxland, J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods 2023, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.P.; Kothakota, A.; Ramesh, S.V.; Shahir, S. Ozone Based Food Preserva-tion: A Promising Green Technology for Enhanced Food Safety. Ozone Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of Ozone for Degradation of Mycotoxins in Food: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Fontanelli, M.; Galli, F.; Cotrozzi, L.; Pagni, L.; Pellegrini, E. Mycotoxins in Feed and Food and the Role of Ozone in Their Detoxification and Degradation: An Update. Toxins 2020, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, S.; Prasath, V.A.; Pandiselvam, R.; Kothakota, A.; Mousavi Khaneghah, A. Recent Advances in Applications of Ozone in the Cereal Industry. LWT 2021, 146, 111412. [Google Scholar] [CrossRef]
- Luiz, D.D.B.; E Silva, C.D.F.; Campelo, S.R.; Dos Santos, V.R.V.; De Lima, L.K.F.; Chicrala, P.C.M.S.; Iwashita, M.K.P. Evaluation of the Effectiveness of Ozone as a Sanitizer for Fish Experimentally Contaminated with Salmonella Sp. Braz. J. Food Technol. 2017, 20, e2016150. [Google Scholar] [CrossRef]
- Gonçalves, A.A. Elimination and Control of Pathogens by Novel and Hurdle Technologies. In Handbook of Seafood: Quality and Safety Maintenance and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 175–189. ISBN 978-1-63485-823-6. [Google Scholar]
- Kaur, K.; Pandiselvam, R.; Kothakota, A.; Padma Ishwarya, S.; Zalpouri, R.; Mahanti, N.K. Impact of Ozone Treatment on Food Polyphenols—A Comprehensive Review. Food Control. 2022, 142, 109207. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Felini, R.; Cavallini, D.; Buonaiuto, G.; Bordin, T. Assessing the Impact of Thermoregulatory Mineral Supplementation on Thermal Comfort in Lactating Holstein Cows. Vet. Anim. Sci. 2024, 24, 100363. [Google Scholar] [CrossRef] [PubMed]
- Bolsen, K.K.; Lin, C.; Brent, B.E.; Feyerherm, A.M.; Urban, J.E.; Aimutis, W.R. Effect of Silage Additives on the Microbial Succession and Fermentation Process of Alfalfa and Corn Silages. J. Dairy Sci. 1992, 75, 3066–3083. [Google Scholar] [CrossRef]
- Ferlizza, E.; Fasoli, S.; Cavallini, D.; Bolcato, M.; Andreani, G.; Isani, G. Preliminary study on urine chemistry and protein profile in cows and heifers. Pak. Vet. J. 2020, 40, 413–418. [Google Scholar] [CrossRef]
- Cavallini, D.; Mammi, L.M.E.; Biagi, G.; Fusaro, I.; Giammarco, M.; Formigoni, A.; Palmonari, A. Effects of 00-rapeseed meal inclusion in Parmigiano Reggiano hay-based ration on dairy cows’ production, reticular pH and fibre digestibility. Ital. J. Anim. Sci. 2021, 20, 295–303. [Google Scholar] [CrossRef]
- da Silva, M.S.J.; Jobim, C.C.; Poppi, E.C.; Tres, T.T.; Osmari, M.P. Production Technology and Quality of Corn Silage for Feeding Dairy Cattle in Southern Brazil. Rev. Bras. Zootec. 2015, 44, 303–313. [Google Scholar] [CrossRef]
- Johnson, L.; Harrison, J.H.; Hunt, C.; Shinners, K.; Doggett, C.G.; Sapienza, D. Nutritive Value of Corn Silage as Affected by Maturity and Mechanical Processing: A Contemporary Review. J. Dairy Sci. 1999, 82, 2813–2825. [Google Scholar] [CrossRef]
- Der Bedrosian, M.C.; Nestor, K.E.; Kung, L. The Effects of Hybrid, Maturity, and Length of Storage on the Composition and Nutritive Value of Corn Silage. J. Dairy Sci. 2012, 95, 5115–5126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.M.; Harrison, J.H.; Davidson, D.; Mahanna, W.C.; Shinners, K.; Linder, D. Corn Silage Management: Effects of Maturity, Inoculation, and Mechanical Processing on Pack Density and Aerobic Stability. J. Dairy Sci. 2002, 85, 434–444. [Google Scholar] [CrossRef]
- Windle, M.C.; Walker, N.; Kung, L. Effects of an Exogenous Protease on the Fermentation and Nutritive Value of Corn Silage Harvested at Different Dry Matter Contents and Ensiled for Various Lengths of Time. J. Dairy Sci. 2014, 97, 3053–3060. [Google Scholar] [CrossRef]
- Yan, J.; Gao, Y.P.; Wang, W.J.; Mu, S.Q. Variation law of whole-plant corn silage nutritional quality under different storage periods. J. Northwest A F Univ. Nat. Sci. Ed. 2009, 37, 75–80. [Google Scholar]
- Velarde-Guillén, J.; Sainz-Ramírez, A.; Celis-Álvarez, M.D.; Arriaga-Jordán, C.M.; Martínez-García, C.G. Characterisation of Landrace ‘Criollo’ Maize Silage from the Highlands of Mexico in Terms of Starch Content. Trop. Anim. Health Prod. 2022, 54, 283. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.L.P.; Bernardes, T.F.; Jobim, C.C.; Schmidt, P.; Nussio, L.G. Production and Utilization of Silages in Tropical Areas with Focus on Brazil. Grass Forage Sci. 2019, 74, 188–200. [Google Scholar] [CrossRef]
- Horst, E.H.; Neumann, M. Assessing Crop and Corn Silage Profile in Beef Cattle Farms in Southern Brazil: Ten Years’ Results. Agriculture 2022, 12, 1200. [Google Scholar] [CrossRef]
- Aksu, T.; Baytok, E.; Karslı, M.A.; Muruz, H. Effects of Formic Acid, Molasses and Inoculant Additives on Corn Silage Composition, Organic Matter Digestibility and Microbial Protein Synthesis in Sheep. Small Rumin. Res. 2006, 61, 29–33. [Google Scholar] [CrossRef]
- Jobim, C.C.; de Oliveira, F.C.L.; Junior, V.H.B.; da Silva, M.S. Productive Characteristics of Maize Hybrids at Different Cutting Heights for Silage and Organic Matter and Mineral Rates in Post-Harvest Residues. Acta Sci. Anim. Sci. 2013, 35, 133–138. [Google Scholar] [CrossRef][Green Version]
- Von Pinho, R.G.; de Vasconcelos, R.C.; Borges, I.D.; de Resende, A.V. Produtividade e qualidade da silagem de milho e sorgo em função da época de semeadura. Bragantia 2007, 66, 235–245. [Google Scholar] [CrossRef]
- Terler, G.; Gruber, L.; Knaus, W.F. Nutritive Value of Ensiled Maize Stover from Nine Different Varieties Harvested at Three Different Stages of Maturity. Grass Forage Sci. 2019, 74, 53–64. [Google Scholar] [CrossRef]
- Bernardes, T.F.; Daniel, J.L.P.; Adesogan, A.T.; McAllister, T.A.; Drouin, P.; Nussio, L.G.; Huhtanen, P.; Tremblay, G.F.; Bélanger, G.; Cai, Y. Silage Review: Unique Challenges of Silages Made in Hot and Cold Regions. J. Dairy Sci. 2018, 101, 4001–4019. [Google Scholar] [CrossRef]
- Costa, E.R.; Mello, A.C.L.; Guim, A.; Costa, S.B.M.; Abreu, B.S.; Silva, P.H.F.; Silva, V.J.; Neto, D.E.S. Adding Corn Meal in-to Mixed Elephant Grass—Butterfly Pea Legume Silages Improves Nutritive Value and Dry Matter Recovery. J. Agric. Sci. 2022, 160, 185–193. [Google Scholar] [CrossRef]
- dos Anjos, A.N.A.; Almeida, J.C.D.C.; Viegas, C.R.; da Silva, P.H.F.; de Morais, L.F.; Nepomuceno, D.d.D.; de Carvalho, C.A.B.; Soares, F.A. Protein and Carbohydrate Profiles of “Massai” Grass Silage with Pelleted Citrus Pulp and Micro-bial Inoculant. Pesq. Agropec. Bras. 2022, 57, e02732. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Ashbell, G. Engineering Aspects of Ensiling. Biochem. Eng. J. 2003, 13, 181–188. [Google Scholar] [CrossRef]
- Basso, F.C.; Bernardes, T.F.; Roth, A.P.D.T.P.; Lodo, B.N.; Berchielli, T.T.; Reis, R.A. Fermentation and Aerobic Stability of Corn Silage Inoculated with Lactobacillus Buchneri. Rev. Bras. Zootec. 2012, 41, 1789–1794. [Google Scholar] [CrossRef]
- Ren, X.; Tian, H.; Zhao, K.; Li, D.; Xiao, Z.; Yu, Y.; Liu, F. Research on pH Value Detection Method during Maize Silage Secondary Fermentation Based on Computer Vision. Agriculture 2022, 12, 1623. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The Performance of Lactic Acid Bacteria in Silage Production: A Review of Modern Biotechnology for Silage Improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef]
- Gotlieb, A. Mycotoxins in Silage: A Silent Loss in Profits. The Vermont Crops Soils Home Page. Available online: http://pss.uvm.edu/vtcrops/articles/Mycotoxins.html (accessed on 10 April 2024).
- Carvalho, B.F.; Ávila, C.L.S.; Krempser, P.M.; Batista, L.R.; Pereira, M.N.; Schwan, R.F. Occurrence of Mycotoxins and Yeasts and Moulds Identification in Corn Silages in Tropical Climate. J. Appl. Microbiol. 2016, 120, 1181–1192. [Google Scholar] [CrossRef]
- Lemos, M.F.; Andrade, A.P.; de Quadros, D.G.; da Silva, P.H.F.; Santos, C.O.; Souza, C.F.B.; Silva, M.A.V.; Medeiros, A.S.; Neto, P.M.D.O. Nutritional value, fermentation losses and aerobic stability of elephant grass (Pennisetum purpureum Schum.) silage treated with exogenous fibrolytic enzymes. Acta Sci. Anim. Sci. 2020, 42, e48272. [Google Scholar] [CrossRef]
- Muca, E.; Buonaiuto, G.; Lamanna, M.; Silvestrelli, S.; Ghiaccio, F.; Federiconi, A.; de Matos Vettori, J.; Colleluori, R.; Fu-saro, I.; Raspa, F.; et al. Reaching a Wider Audience: Instagram’s Role in Dairy Cow Nutrition Education and Engagement. Educ. Sci. 2023, 13, 3503. [Google Scholar] [CrossRef]
- Muca, E.; Cavallini, D.; Raspa, F.; Bordin, C.; Bergero, D.; Valle, E. Integrating New Learning Methods into Equine Nutrition Classrooms: The Importance of Students’ Perceptions. J. Equine Vet. Sci. 2023, 126, 104537. [Google Scholar] [CrossRef] [PubMed]
| Before | After | |
|---|---|---|
| Dry matter | 257 | 256 |
| Organic matter | 966 | 951 |
| Crude protein | 84 | 79 |
| Starch | 340 | 297 |
| Ether extract | 13 | 16 |
| Neutral detergent fiber | 445 | 444 |
| Acid detergent fiber | 219 | 291 |
| Non-fiber carbohydrates | 424 | 414 |
| Total digestible nutrients | 703 | 693 |
| Ash | 34 | 49 |
| Calcium | 1.3 | 2.1 |
| Total phosphorus | 1.0 | 1.6 |
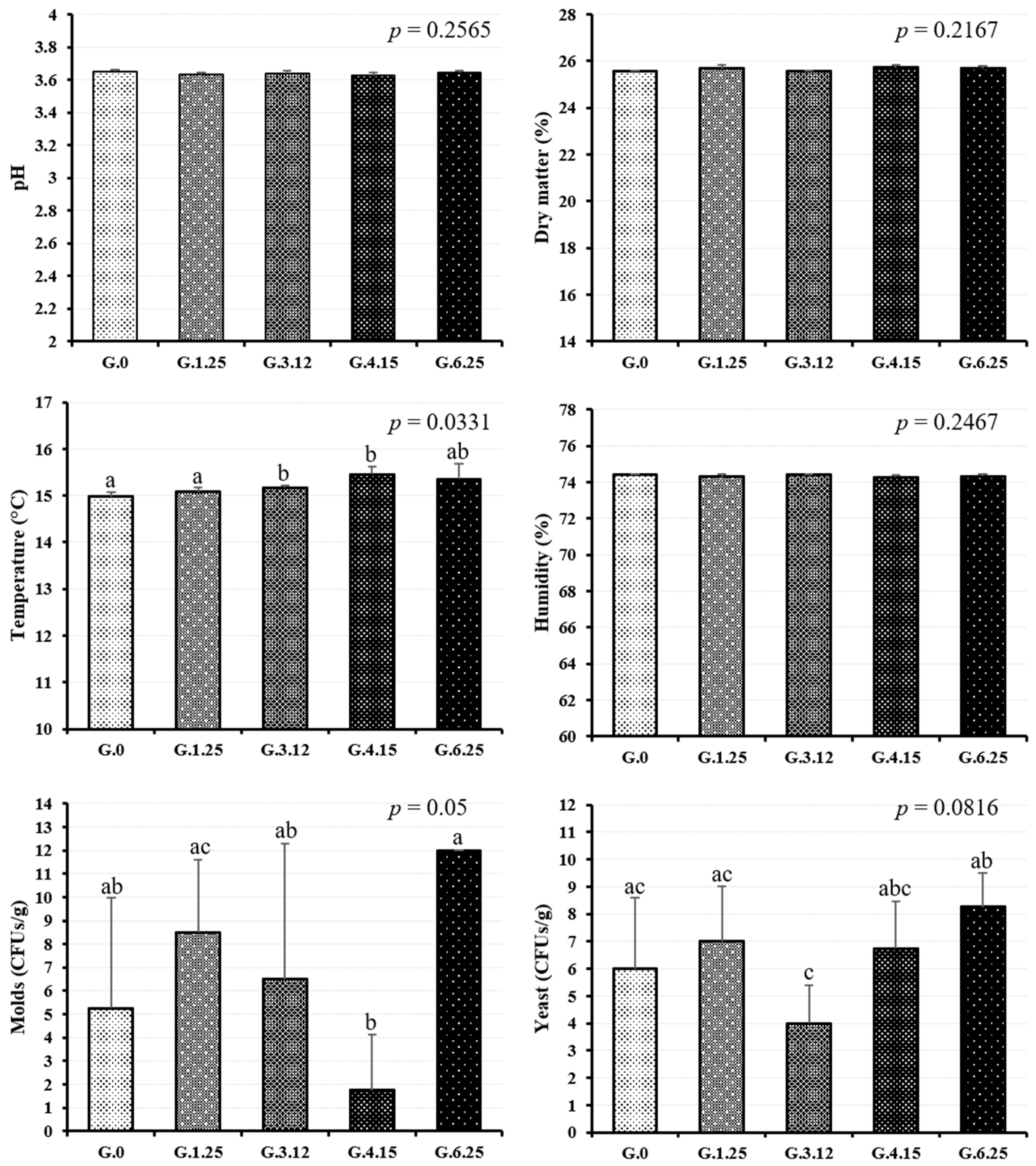
| Variable | Ozone Level (% as Feed) | SEM | p-Value 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 1.25 | 3.12 | 4.15 | 6.25 | L | Q | C | Qr | ||
| pH | 3.64 | 3.65 | 3.63 | 3.64 | 3.63 | 0.01 | 0.189 | 0.263 | 0.267 | 0.140 |
| DM, g/kg | 257 | 256 | 257 | 256 | 257 | 0.02 | 0.674 | 0.396 | 0.064 | 0.244 |
| Temperature, °C | 15.3 | 15.0 | 15.1 | 15.2 | 15.4 | 0.05 | 0.392 | 0.770 | 0.206 | 0.001 |
| Molds CFUs/g | 12.0 | 3.0 | 7.0 | 3.3 | 1.7 | 0.87 | <0.001 | <0.001 | <0.001 | <0.001 |
| Yeasts CFUs/g | 8.5 | 6.7 | 6.2 | 5.1 | 3.7 | 0.42 | <0.001 | 0.215 | 0.094 | 0.705 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koakoski, D.L.; Bordin, T.; Cavallini, D.; Buonaiuto, G. A Preliminary Study of the Effects of Gaseous Ozone on the Microbiological and Chemical Characteristics of Whole-Plant Corn Silage. Fermentation 2024, 10, 398. https://doi.org/10.3390/fermentation10080398
Koakoski DL, Bordin T, Cavallini D, Buonaiuto G. A Preliminary Study of the Effects of Gaseous Ozone on the Microbiological and Chemical Characteristics of Whole-Plant Corn Silage. Fermentation. 2024; 10(8):398. https://doi.org/10.3390/fermentation10080398
Chicago/Turabian StyleKoakoski, Douglas Luiz, Tiago Bordin, Damiano Cavallini, and Giovanni Buonaiuto. 2024. "A Preliminary Study of the Effects of Gaseous Ozone on the Microbiological and Chemical Characteristics of Whole-Plant Corn Silage" Fermentation 10, no. 8: 398. https://doi.org/10.3390/fermentation10080398
APA StyleKoakoski, D. L., Bordin, T., Cavallini, D., & Buonaiuto, G. (2024). A Preliminary Study of the Effects of Gaseous Ozone on the Microbiological and Chemical Characteristics of Whole-Plant Corn Silage. Fermentation, 10(8), 398. https://doi.org/10.3390/fermentation10080398







