Black Tea Kombucha Consumption: Effect on Cardiometabolic Parameters and Diet Quality of Individuals with and without Obesity
Abstract
1. Introduction
2. Materials and Methods
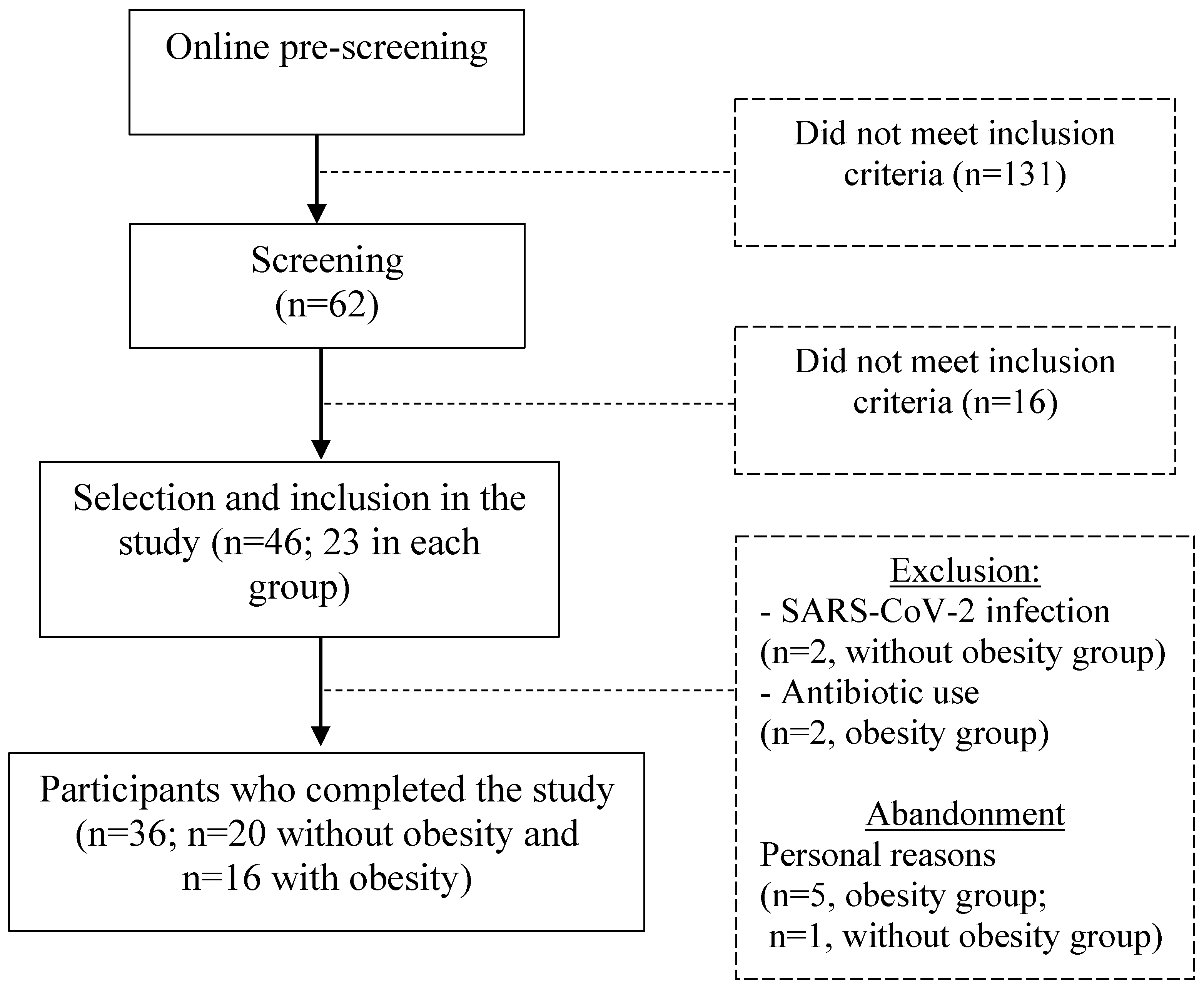
2.1. Subjects
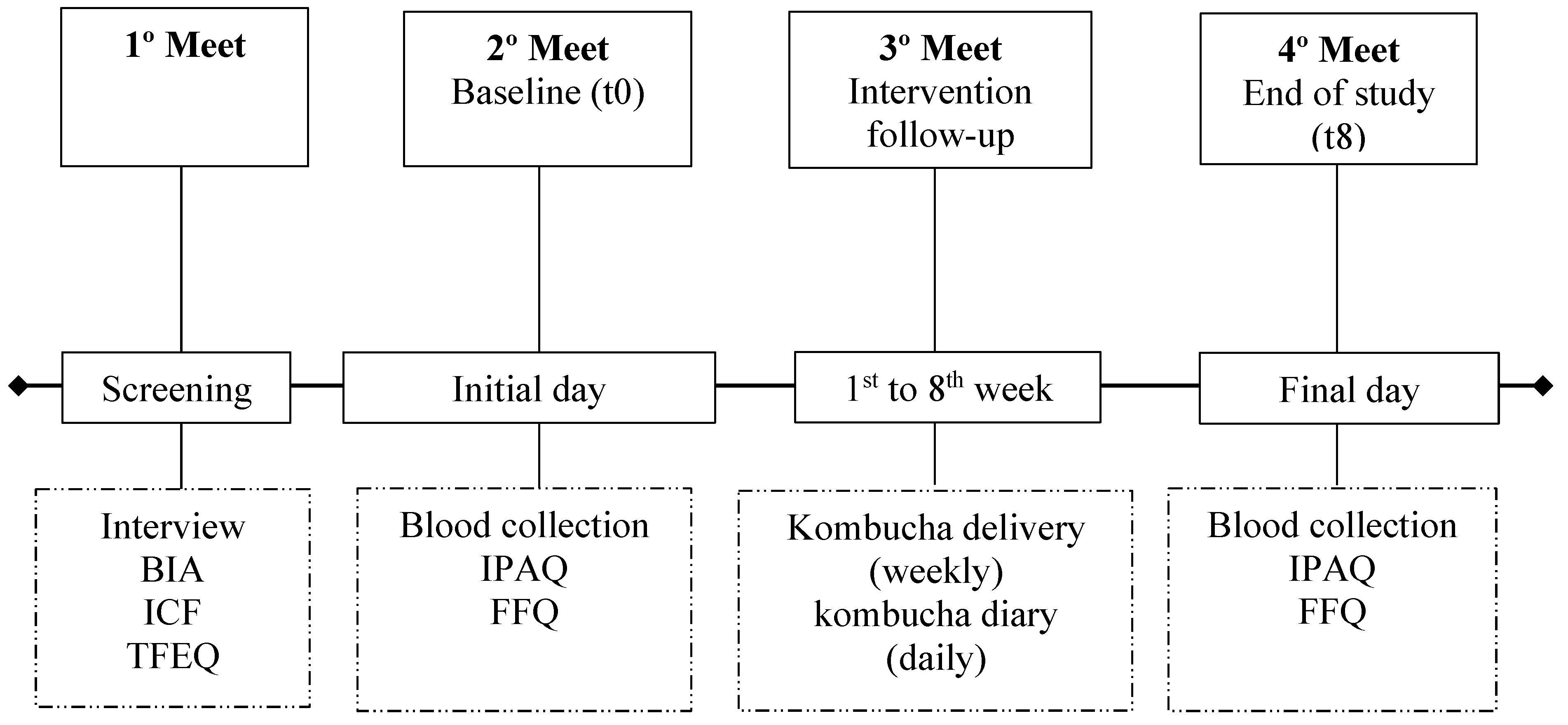
2.2. Study Design
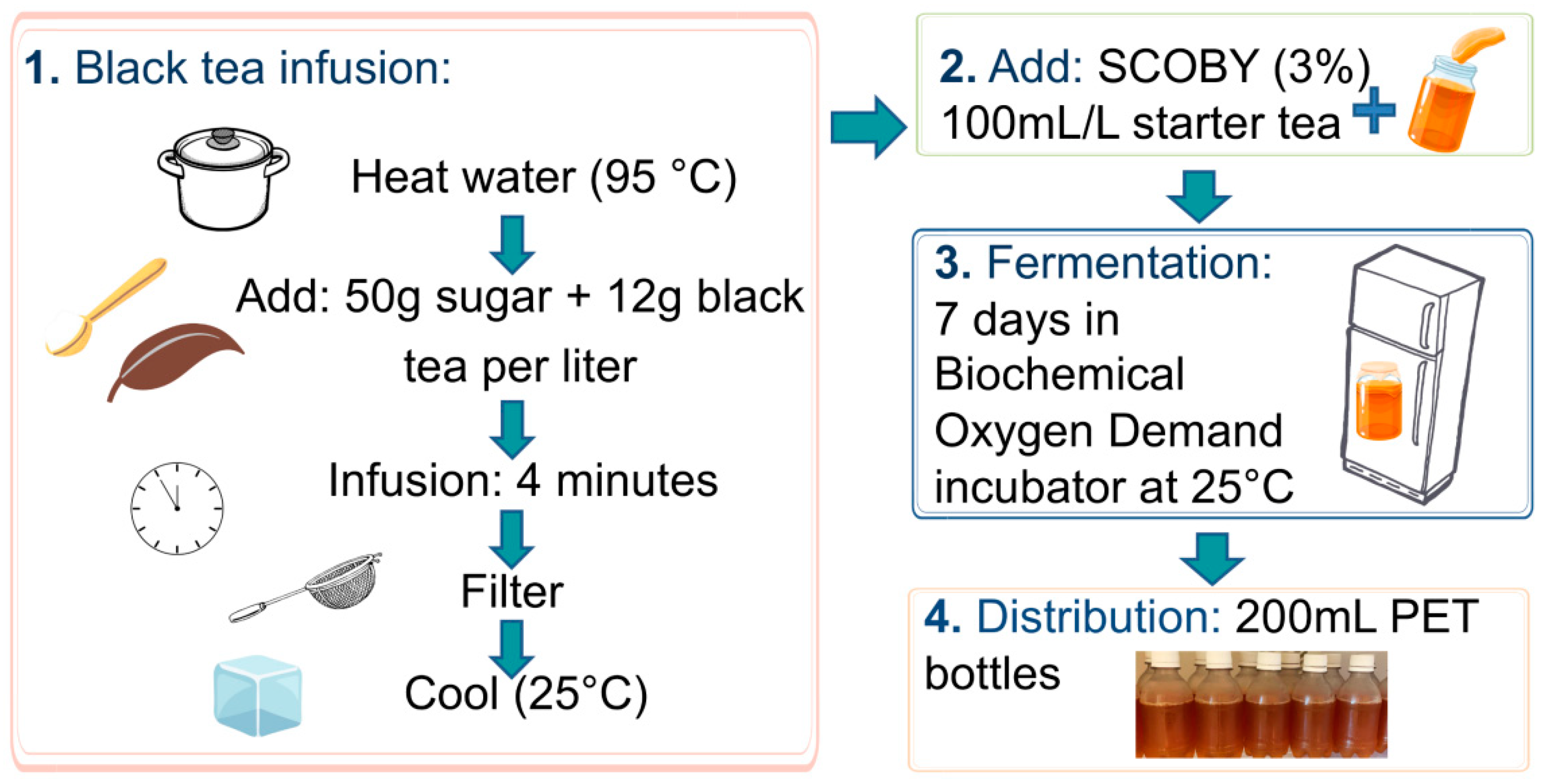
2.3. Black Tea Kombucha Production and Consumption
2.4. Assessment of Cardiometabolic Markers
2.5. Assessment of Physical Activity Pattern and Food Intake
2.6. Diet Quality Indices
2.7. Statistics
3. Results
3.1. Subjects
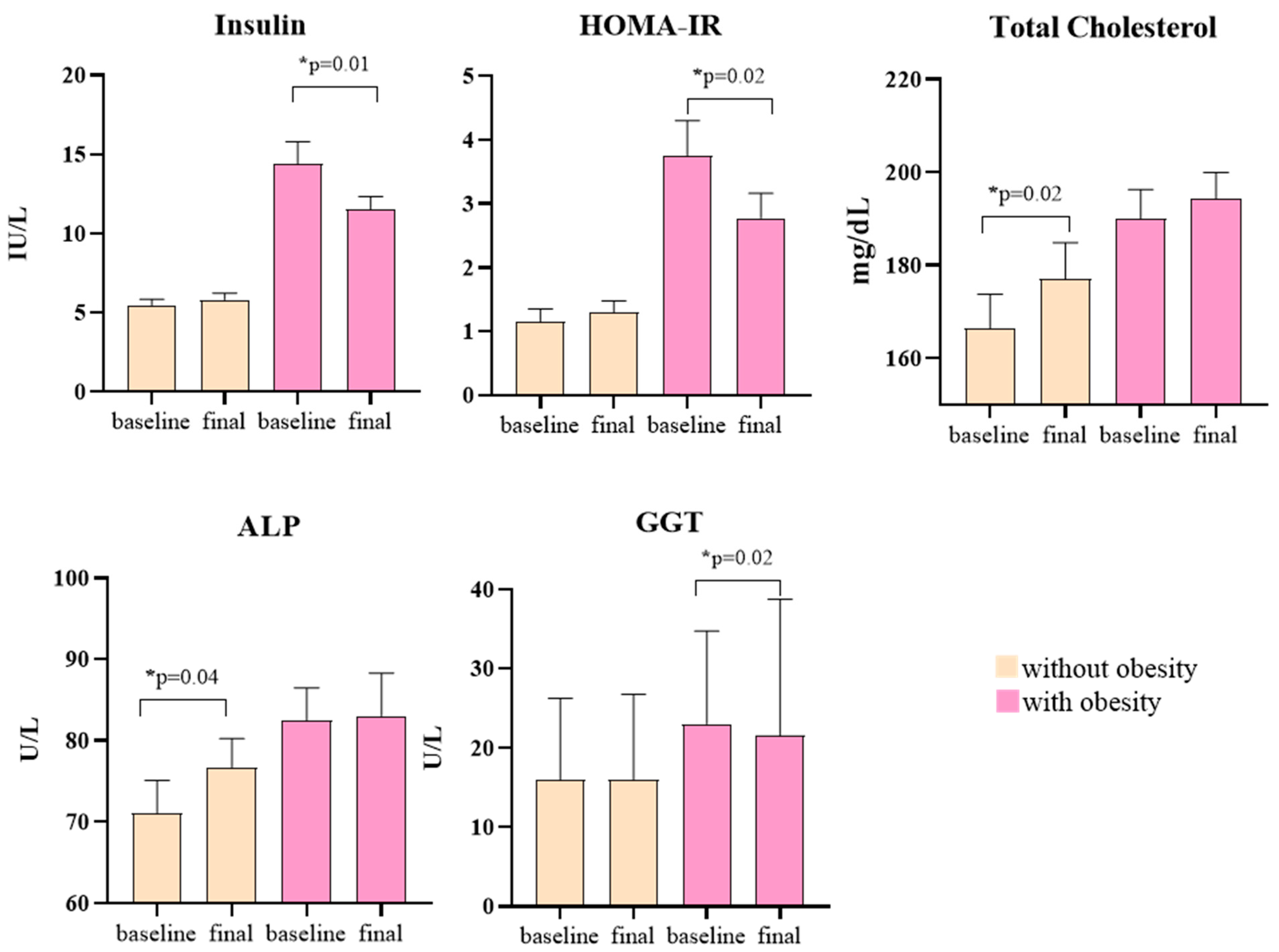
3.2. Cardiometabolic Parameters and Physical Activity
3.3. Stratified Analysis by Group, Considering the Increase or Decrease in the Parameters of Diet Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Total and Volatile Acidity and pH
Appendix A.2. Acetic Acid, Sugar, and Ethanol
Appendix A.3. Total Phenolics, Theaflavin and Thearubigin
Appendix A.4. Microbiological Characterization
References
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hébert, J.R. Diet and acute and chronic, systemic, low-grade inflammation. In Diet, Inflammation, and Health; Academic Press: Cambridge, MA, USA, 2022; pp. 85–111. [Google Scholar]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity Federation. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—WHO. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 October 2022).
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Federation, World Obesity Atlas 2023. 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 1 October 2022).
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- World Health Organization—WHO. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Dreher, M.L.; Ford, N.A. A Comprehensive critical assessment of increased fruit and vegetable intake on weight loss in women. Nutrients 2020, 12, 1919. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Arora, K.; Prakash, S. Microbial medicine: Prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Brazil Ministry of Agriculture, Livestock and Supply. Normative Instruction No. 41, of September 17, 2019. In Official Federal Gazette; Section 1; 2019; pp. 13–15. Available online: https://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?data=18/09/2019&jornal=515&pagina=13&totalArquivos=76 (accessed on 1 October 2022).
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical profile and antioxidant activity of the kombucha beverage derived from white, green, black and red tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea—Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.-L. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef]
- De Noronha, M.C.; Cardoso, R.R.; Dos Santos D’Almeida, C.T.; Vieira do Carmo, M.A.; Azevedo, L.; Maltarollo, V.G.; Júnior, J.I.R.; Eller, M.R.; Cameron, L.C.; Ferreira, M.S.L.; et al. Black tea kombucha: Physicochemical, microbiological and comprehensive phenolic profile changes during fermentation, and antimalarial activity. Food Chem. 2022, 384, 132515. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.R.; Moreira, L.d.P.D.; Costa, M.A.d.C.; Toledo, R.C.L.; Grancieri, M.; Nascimento, T.P.D.; Ferreira, M.S.L.; da Matta, S.L.P.; Eller, M.R.; Martino, H.S.D.; et al. Kombuchas from green and black teas reduce oxidative stress, liver steatosis and inflammation, and improve glucose metabolism in Wistar rats fed a high-fat high-fructose diet. Food Funct. 2021, 12, 10813–10827. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, I.; Mannaa, M.; Kim, J.; Wang, S.; Park, I.; Kim, J.; Seo, Y.-S. Effect of Kombucha on gut-microbiota in mouse having non-alcoholic fatty liver disease. Food Sci. Biotechnol. 2019, 28, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.d.C.; Dias Moreira, L.d.P.; Duarte, V.d.S.; Cardoso, R.R.; São José, V.P.B.d.; Silva, B.P.d.; Grancieri, M.; Corich, V.; Giacomini, A.; Bressan, J.; et al. Kombuchas from Green and Black Tea Modulate the Gut Microbiota and Improve the Intestinal Health of Wistar Rats Fed a High-Fat High-Fructose Diet. Nutrients 2022, 14, 5234. [Google Scholar] [CrossRef]
- Moreira, G.V.; Araujo, L.C.; Murata, G.M.; Matos, S.L.; Carvalho, C.R. Kombucha tea improves glucose tolerance and reduces hepatic steatosis in obese mice. Biomed. Pharmacother. 2022, 155, 113660. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, J.; Wang, S.; Sung, S.; Kim, N.; Lee, H.-H.; Seo, Y.-S.; Jung, Y. Hepatoprotective effect of kombucha tea in rodent model of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2019, 20, 2369. [Google Scholar] [CrossRef]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit Kombucha, black tea Kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Mendelson, C.; Sparkes, S.; Merenstein, D.J.; Christensen, C.; Sharma, V.; Desale, S.; Auchtung, J.M.; Kok, C.R.; Hallen-Adams, H.E.; Hutkins, R. Kombucha tea as an anti-hyperglycemic agent in humans with diabetes—A randomized controlled pilot investigation. Front. Nutr. 2023, 10, 1190248. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Cohen, M.; Lau, K.; Brand-Miller, J.C. Glycemic index and insulin index after a standard carbohydrate meal consumed with live kombucha: A randomised, placebo-controlled, crossover trial. Front. Nutr. 2023, 10, 1036717. [Google Scholar] [CrossRef]
- Costa, M.A.d.C.; Vilela, D.L.d.S.; Fraiz, G.M.; Lopes, I.L.; Coelho, A.I.M.; Castro, L.C.V.; Martin, J.G.P. Effect of kombucha intake on the gut microbiota and obesity-related comorbidities: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 63, 3851–3866. [Google Scholar] [CrossRef]
- Morales, D. Biological activities of kombucha beverages: The need of clinical evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; The Trend Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Neergheen-Bhujun, V.S.; Gunness, T.K.; Googoolye, K.; Auger, C.; Crozier, A.; Aruoma, O.I. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev. Med. 2012, 54, S98–S102. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention—CDC. Unexplained severe illness possibly associated with consumption of Kombucha tea—Iowa, 1995. Morb. Mortal. Wkly. Rep. 1995, 44, 892–900. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Villalobos-Molina, R.; Jiménez-Flores, J.R.; Simental-Mendia, L.E.; Méndez-Cruz, R.; Murguía-Romero, M.; Rodríguez-Morán, M. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch. Med. Res. 2016, 47, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C. Questionário internacional de atividade física (IPAQ): Estudo de validade e reprodutibilidade no Brasil. Ativ. Fís Saúde 2001, 6, 5–18. [Google Scholar]
- Natacci, L.C.; Ferreira Júnior, M. The three factor eating questionnaire-R21: Tradução para o português e aplicação em mulheres brasileiras. Revista de Nutrição 2011, 24, 383–394. [Google Scholar] [CrossRef]
- Tabela Brasileira de Composição de Alimentos (TBCA); Universidade de São Paulo (USP); Food Research Center (FoRC). Versão 7.1. São Paulo. 2020. Available online: http://www.fcf.usp.br/tbca (accessed on 13 August 2022).
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)—Lessons learned, improvements made, and future directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Syddall, H.; Phillips, D.; Sayer, A.; Dennison, E.; Cooper, C.; Robinson, S. Dietary total antioxidant capacity is related to glucose tolerance in older people: The Hertfordshire Cohort Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Pérez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Medina-Remón, A.; Pérez-Jiménez, J.; Neveu, V.; Knaze, V.; Slimani, N.; Scalbert, A. Effects of food processing on polyphenol contents: A systematic analysis using Phenol-Explorer data. Mol. Nutr. Food Res. 2015, 59, 160–170. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Effect of Kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem. Toxicol. 2013, 60, 328–340. [Google Scholar] [CrossRef]
- Anantachoke, N.; Duangrat, R.; Sutthiphatkul, T.; Ochaikul, D.; Mangmool, S. Kombucha Beverages Produced from Fruits, Vegetables, and Plants: A Review on Their Pharmacological Activities and Health Benefits. Foods 2023, 12, 1818. [Google Scholar] [CrossRef]
- Bellassoued, K.; Ghrab, F.; Makni-Ayadi, F.; Van Pelt, J.; Elfeki, A.; Ammar, E. Protective effect of kombucha on rats fed a hypercholesterolemic diet is mediated by its antioxidant activity. Pharm. Biol. 2015, 53, 1699–1709. [Google Scholar] [CrossRef]
- Martínez-Leal, J.; Ponce-García, N.; Escalante-Aburto, A. Recent evidence of the beneficial effects associated with glucuronic acid contained in kombucha beverages. Curr. Nutr. Rep. 2020, 9, 163–170. [Google Scholar] [CrossRef]
- Vīna, I.; Linde, R.; Patetko, A.; Semjonovs, P. Glucuronic acid from fermented beverages: Biochemical functions in humans and its role in health protection. Ijrras 2013, 14, 217–230. [Google Scholar]
- Yang, Z.; Zhou, F.; Ji, B.; Li, B.; Luo, Y.; Yang, L.; Li, T. Symbiosis between microorganisms from kombucha and kefir: Potential significance to the enhancement of kombucha function. Appl. Biochem. Biotechnol. 2010, 160, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Chatterjee, S.; Manna, P.; Das, J.; Ghosh, J.; Gachhui, R.; Sil, P.C. Prophylactic role of d-saccharic acid-1, 4-lactone in tertiary butyl hydroperoxide induced cytotoxicity and cell death of murine hepatocytes via mitochondria-dependent pathways. J. Biochem. Mol. Toxicol. 2011, 25, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.; Rogero, M.M. Polyphenols regulating microRNAs and inflammation biomarkers in obesity. Nutrition 2019, 59, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.; Ribeiro, M.H. Polyphenols in health and disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Riboldi, B.P.; Luft, V.C.; Bracco, P.A.; Cardoso, L.d.O.; Molina, M.d.C.; Alvim, S.; Giatti, L.; Schmidt, M.I.; Duncan, B.B. The inflammatory food index and its association with weight gain and incidence of diabetes: Longitudinal Study of Adult Health (ELSA-Brasil). Nutr. Metab. Cardiovasc. Dis. 2022, 32, 675–683. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality—A Meta-Analysis. Nutrients 2018, 10, 200. [Google Scholar] [CrossRef]
- Zahedi, H.; Djalalinia, S.; Asayesh, H.; Mansourian, M.; Esmaeili Abdar, Z.; Mahdavi Gorabi, A.; Ansari, H.; Noroozi, M.; Qorbani, M. A higher dietary inflammatory index score is associated with a higher risk of incidence and mortality of cancer: A comprehensive systematic review and meta-analysis. Int. J. Prev. Med. 2020, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, M.; Hamzeh, B.; Ayenepour, A.; Rezaeian, S.; Najafi, F.; Shakiba, E.; Pasdar, Y. Anti-inflammatory diet consumption reduced fatty liver indices. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Shu, Y.; Wu, X.; Wang, J.; Ma, X.; Li, H.; Xiang, Y. Associations of dietary inflammatory index with prediabetes and insulin resistance. Front. Endocrinol. 2022, 13, 820932. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Waśkiewicz, A.; Witkowska, A.M.; Cicha-Mikołajczyk, A.; Zujko, K.; Drygas, W. Dietary Total Antioxidant Capacity—A New Indicator of Healthy Diet Quality in Cardiovascular Diseases: A Polish Cross-Sectional Study. Nutrients 2022, 14, 3219. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.; Caldas, A.P.S.; Pinto, S.L.; Hermsdorff, H.H.M.; Marcadenti, A.; Bersch-Ferreira, Â.C.; Torreglosa, C.R.; Weber, B.; Bressan, J. Dietary total antioxidant capacity is inversely associated with cardiovascular events and cardiometabolic risk factors: A cross-sectional study. Nutrition 2021, 89, 111140. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Escalante, M.L.; Coop-Gamas, F.; Cervantes-Rodríguez, M.; Méndez-Iturbide, D.; Aranda-González, I.I. The effect of diet on oxidative stress and metabolic diseases—Clinically controlled trials. J. Food Biochem. 2020, 44, e13191. [Google Scholar] [CrossRef] [PubMed]
- El Feky, A.; Gillies, K.; Gardner, H.; Fraser, C.; Treweek, S. A protocol for a systematic review of non-randomised evaluations of strategies to increase participant retention to randomised controlled trials. Syst. Rev. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Schweizer, M.L.; Braun, B.I.; Milstone, A.M. Research methods in healthcare epidemiology and antimicrobial stewardship—quasi-experimental designs. Infect. Control. Hosp. Epidemiol. 2016, 37, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Zenebon, O.; Pascuet, N.S.; Tiglea, P. Physical-Chemical Methods for Food Analysis; Adolfo Lutz Institute: São Paulo, Brazil, 2008; pp. 1–1020. [Google Scholar]
- Brazil Ministry of Agriculture. Ordinance No. 76 of November 26, 1986. Provides Analytical Methods for Beverages and Vinegar; Official Bulletin of the Federative Republic of Brazil: Brasília, Brazil, 1986; Section 1, pt. 2. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]

| Analysis | Mean (SD) |
|---|---|
| pH | 3.48 (0.05) |
| Volatile acidity (meq/L) | 30.0 (3.75) |
| Total acidity (%, acetic acid-g/100 mL) | 0.31 (0.0095) |
| Acetic acid (g/L) | 0.99 (0.015) |
| Total phenolics (mg/mL) | 0.69 (0.02) |
| Theaflavin (g/100 mL) | 0.12 (0.003) |
| Thearubigin (g/100 mL) | 1.88 (0.07) |
| Yeast (log UFC/mL) | 6.10 (0.21) |
| Acetic bacteria (log UFC/mL) | 5.80 (0.28) |
| Lactic bacteria (log UFC/mL) | 6.20 (0.14) |
| Ethanol (g/L) | 4.53 (0.066) |
| Sucrose (g/L) | 13.22 (0.221) |
| Glucose (g/L) | 4.24 (0.079) |
| Fructose (g/L) | 8.54 (0.188) |
| Variables | Without Obesity (n = 20) | With Obesity (n = 16) | ||||
|---|---|---|---|---|---|---|
| Baseline | After 8 Weeks | p-Value | Baseline | After 8 Weeks | p-Value | |
| Cardiometabolic markers | ||||||
| FBG, mg/dL | 88 (79–99) | 87 (79–101) | 0.2 | 91 (83–123) | 92 (82–115) | 0.89 |
| Insulin, IU/L | 5.3 (2.2–7.6) a | 5.9 (2.7–8.7) | 0.50 | 15 (6.3–23.6) a | 12 (6–18) | 0.01 |
| TC, mg/dL | 166.35 (32.92) a | 177.05 (34.83) | 0.02 | 189.94 (25.4) a | 194.4 (22.1) | 0.20 |
| LDL-C, mg/dL | 86.45 (26.56) a | 90.80 (24.43) | 0.25 | 104.19 (18.2) a | 106.3 (17.2) | 0.64 |
| HDL-C, mg/dL | 60.35 (13.81) | 61.65 (12.65) | 0.59 | 52.81 (16.2) | 52.25 (16.5) | 0.76 |
| TG, mg/dL | 67 (39–136) a | 74 (36–195) | 0.30 | 106 (62–298) a | 97 (56–380) | 0.30 |
| ALT, U/L | 17 (7–30) | 16 (6–51) | 0.23 | 21 (9–130) | 20 (9–70) | 0.23 |
| AST, U/L | 15 (9–22) | 14 (10–25) | 0.06 | 17 (9–57) | 14 (7–32) | 0.06 |
| ALP, U/L | 71.10 (18.0) | 76.7 (15.8) | 0.04 | 82.44 (16.0) | 83.0 (21.1) | 0.89 |
| GGT, U/L | 16 (8–58) | 16 (8–61) | 0.65 | 23 (9–142) | 22 (8–108) | 0.02 |
| Creatinine, mg/dL | 0.94 (0.09) | 0.92 (0.12) | 0.49 | 0.93 (0.14) | 0.94 (0.16) | 0.72 |
| Urea, mg/dL | 22 (16–44) | 23 (15–36) | 0.84 | 23 (15–39) | 25 (12–48) | 0.84 |
| HOMA-IR | 1.15 (0.48–1.67) a | 1.30 (0.59–1.84) | 0.52 | 3.75 (1.89–4.89) a | 2.78 (2.34–3.60) | 0.02 |
| TyG | 4.32 (4.07–4.76) a | 4.37 (4.01–4.89) | 0.41 | 4.64 (4.30–5.17) a | 4.55 (4.27–5.41) | 0.41 |
| FLI | 0.1 (0.02–0.7) a | 0.9 (0.02–1.0) | 0.10 | 11.3 (0.9–77.9) a | 12.9 (0.4–84.8) | 0.33 |
| Diet quality | ||||||
| DII | 1.82 (1.28) | 2.25 (1.02) | 0.02 | 1.23 (0.96) | 1.75 (1.31) | 0.02 |
| DTAC | 5.84 (2.18–9.36) | 4.77 (2.31–9.99) | 0.07 | 5.64 (4.12–11.96) | 5.57 (1.52–17.09) | 0.80 |
| DTP | 1165.9 (449.4) | 1031.1 (420.8) | 0.03 | 1234.6 (414.9) | 1219.9 (516.3) | 0.91 |
| DTP+KTP | 1165.9 (449.4) | 1168.3 (420.8) | 0.97 | 1234.6 (414.9) | 1357.1 (516.3) | 0.34 |
| Variables | Without Obesity (n = 20) | With Obesity (n = 16) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↑ DII | ↓ DII | ↑ DII | ↓ DII | |||||||||
| Baseline | Post 8 Weeks | p-Value | Baseline | Post 8 Weeks | p-Value | Baseline | Post 8 Weeks | p-Value | Baseline | Post 8 Weeks | p-Value | |
| FBG, mg/dL | 85 (79–99) | 86 (79–94) | 0.96 | 88 (88–99) | 87 (82–101) | 0.07 | 91 (83–123) | 92 (86–115) | 0.96 | 85 (83–123) | 92 (82–112) | 0.90 |
| Insulin, IU/L | 5.2 (3.9–9.2) | 6.3 (3.4–9.3) | 0.21 | 6.3 (2.2–7.6) | 4.9 (2.7–7.4) | 0.67 | 10.6 (6–21) | 10.5 (6–14) | 0.07 | 17 (12–24) | 13 (11–18) | 0.08 |
| TC mg/dL | 163 (33) | 179 (42) | 0.004 | 172 (34) | 174 (21) | 0.84 | 193 (24) | 195 (23) | 0.69 | 185 (29) | 194 (23) | 0.14 |
| LDL, mg/dL | 87 (31) | 91 (29) | 0.39 | 86 (21) | 91 (18) | 0.47 | 109 (14) | 111 (20) | 0.82 | 96 (22) | 99 (8) | 0.68 |
| HDL, mg/dL | 59 (15) | 60 (13) | 0.56 | 63 (12) | 64 (12) | 0.80 | 54 (16) | 52 (13) | 0.32 | 52 (18) | 54 (22) | 0.63 |
| TG, mg/dL | 67 (39–136) | 84 (45–195) | 0.02 | 66 (42–126) | 67 (36–116) | 0.58 | 101 (62–298) | 95 (56–270) | 0.09 | 123 (74–269) | 127 (79–380) | 0.75 |
| ALT, U/L | 17 (9–30) | 18 (11–51) | 0.21 | 16 (7–27) | 13 (6–21) | 0.17 | 20 (9–44) | 18 (11–32) | 0.39 | 26 (10–130) | 28 (9–70) | 0.42 |
| AST, U/L | 15 (9–20) | 16 (10–25) | 0.13 | 14 (12–22) | 14 (10–23) | 0.73 | 17 (9–23) | 13 (8–19) | 0.07 | 18 (10–57) | 20 (7–32) | 0.28 |
| ALP, U/L | 76 (20) | 83 (14) | 0.11 | 64 (12) | 67 (14) | 0.25 | 87 (16) | 90 (21) | 0.61 | 75 (14) | 72 (18) | 0.72 |
| GGT, U/L | 19 (8–58) | 21 (10–61) | 0.65 | 12 (8–45) | 13 (8–43) | 1.00 | 22 (9–49) | 21 (8–60) | 0.11 | 28 (15–142) | 29 (11–108) | 0.10 |
| Cr, mg/dL | 0.9 (0.1) | 0.9 (0.1) | 0.37 | 0.1 (0.1) | 0.9 (0.1) | 0.22 | 0.9 (0.1) | 0.9 (0.1) | 0.49 | 1.0 (0.2) | 0.9 (0.2) | 0.76 |
| Urea, mg/dL | 21 (16–33) | 24 (15–36) | 0.59 | 23 (18–44) | 22 (17–28) | 0.23 | 24 (19–33) | 26 (12–34) | 0.74 | 21.5 (15–39) | 23 (18–48) | 0.27 |
| HOMA-IR | 1.12 (0.84–1.24) | 1.38 (0.68–1.85) | 0.20 | 1.40 (0.48–1.67) | 1.15 (0.59–1.53) | 0.61 | 3.56 (1.89–4.89) | 2.61 (2.34–3.21) | 0.13 | 3.76 (2.68–4.89) | 2.92 (2.68–3.56) | 0.04 |
| TyG Index | 4.3 (4.1–4.8) | 4.4 (4.1–4.9) | 0.04 | 4.3 (4.1–4.7) | 4.3 (4.0–4.6) | 0.33 | 4.6 (4.3–5.2) | 4.5 (4.3–5.0) | 0.11 | 4.6 (4.4–5.1) | 4.7 (4.5–5.4) | 0.60 |
| FLI | 0.10 (0.04–0.71) | 0.11 (0.06–1.00) | 0.03 | 0.06 (0.02–0.19) | 0.08 (0.02–0.12) | 0.67 | 10 (0.93–52.87) | 7.5 (0.38–43.07) | 0.29 | 27.83 (9.05–77.93) | 23.0 (8.87–84.81) | 0.75 |
| DTAC | 6.26 (3.10–9.36) | 4.56 (2.33–9.99) | 0.02 | 4.51 (2.18–7.16) | 5.06 (2.31–8.46) | 0.58 | 5.15 (4.12–11.96) | 4.44 (1.52–10.73) | 0.39 | 6.51 (4.91–9.68) | 8.03 (4.89–17.09) | 0.12 |
| DTP | 1150.3 (340.8) | 980.6 (312.3) | 0.06 | 1189.2 (603.9) | 1106.9 (562.5) | 0.29 | 1364.9 (429.3) | 1170.7 (537.5) | 0.19 | 1017.5 (307) | 1302 (516.3) | 0.22 |
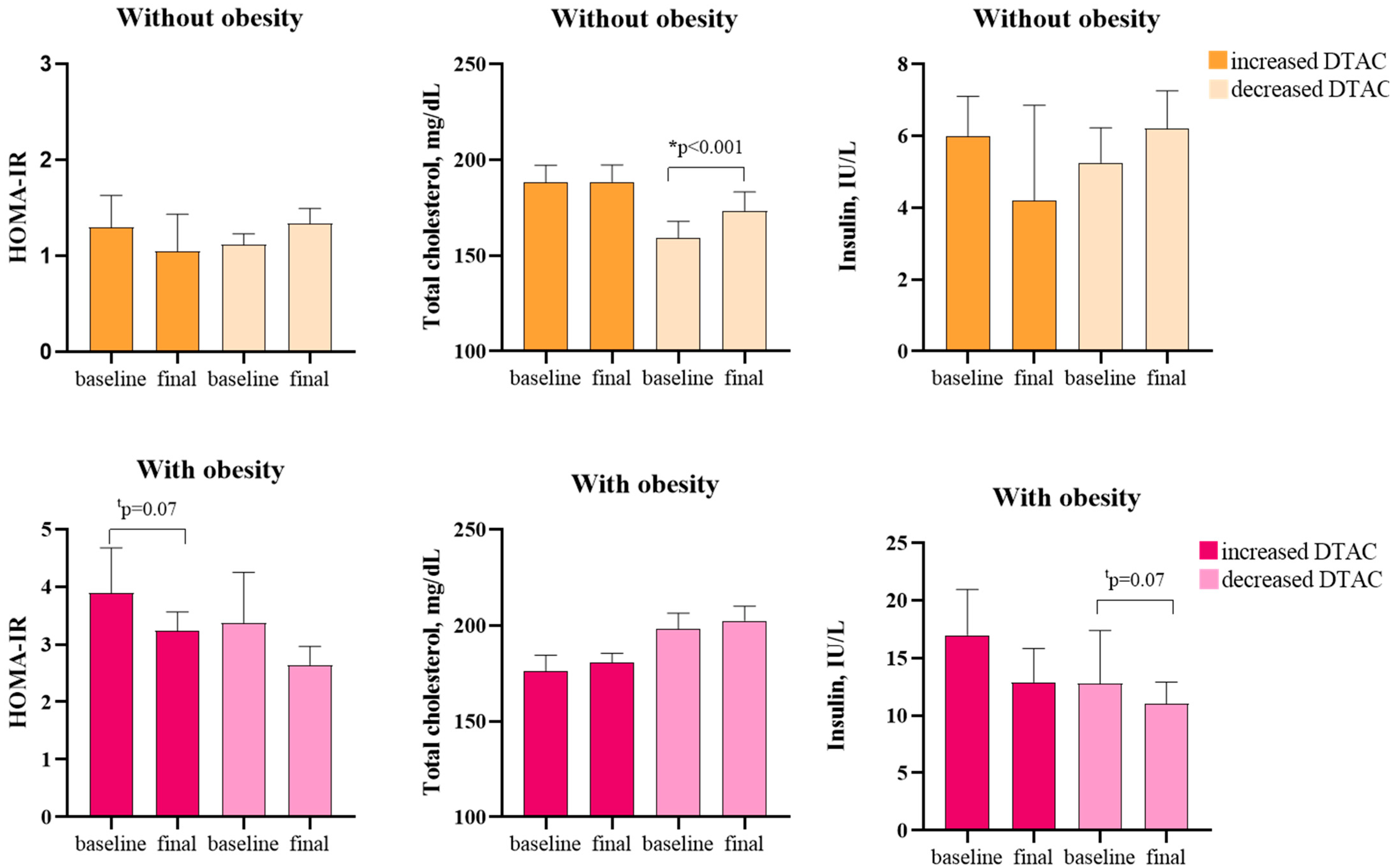
| Variables | Without Obesity (n = 20) | With Obesity (n = 16) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↑ DTAC | ↓ DTAC | ↑ DTAC | ↓DTAC | |||||||||
| Baseline | After 8 Weeks | p-Value | Baseline | After 8 Weeks | p-Value | Baseline | After 8 Weeks | p-Value | Baseline | After 8 Weeks | p-Value | |
| Gl, mg/dL | 88 (83–99) | 88 (82–101) | 0.72 | 88 (79–99) | 85 (79–94) | 0.44 | 85 (83–123) | 90 (82–112) | 0.60 | 93 (83–123) | 92 (86–115) | 0.95 |
| Insulin, IU/L | 6.0 (2.7–7.6) | 4.2 (2.7–7.1) | 0.27 | 5.3 (2.2–9.2) | 6.2 (3.4–9.3) | 0.16 | 17 (6.3–23.6) | 13 (6–18) | 0.14 | 13 (8.4–21.3) | 11 (6.4–14.3) | 0.07 |
| TC mg/dL | 188 (20) | 188 (19) | 0.97 | 159 (34) | 173 (38) | <0.001 | 176 (21) | 181 (12) | 0.50 | 198 (25) | 203 (23) | 0.31 |
| LDL-C, mg/dL | 102 (11) | 102 (15) | 0.98 | 81 (28) | 87 (26 | 0.17 | 95 (17) | 97 (8) | 0.83 | 110 (17) | 112 (19) | 0.68 |
| HDL, mg/dL | 66 (12) | 67 (10) | 0.86 | 58 (14) | 60 (13) | 0.54 | 53 (19) | 52 (14) | 0.75 | 53 (15) | 53 (19) | 0.93 |
| TG, mg/dL | 75 (50–98) | 62 (42–94) | 0.35 | 62 (39–136) | 79 (36–195) | 0.04 | 118 (72–167) | 92 (56–224) | 0.46 | 106 (62–298) | 100 (58–380) | 0.44 |
| ALT, U/L | 18 (7–19) | 13 (6–19) | 0.34 | 16 (8–30) | 17 (8–51) | 0.38 | 29 (10–130) | 26 (12–142) | 0.23 | 20 (9–44) | 18 (9–32) | 0.68 |
| AST, U/L | 15 (13–20) | 14 (12–22) | 1.00 | 15 (9–22) | 15 (10–25) | 0.22 | 17 (13–57) | 16 (7–32) | 0.08 | 17 (9–23) | 14 (8–24) | 0.40 |
| ALP, U/L | 70 (7) | 73 (13) | 0.53 | 71 (21) | 78 (17) | 0.06 | 79 (18) | 67 (13) | 0.20 | 85 (15) | 92 (20) | 0.04 |
| GGT, U/L | 12 (10–24) | 14 (8–26) | 0.48 | 17 (8–58) | 18 (10–61) | 0.89 | 23 (12–142) | 22 (9–108) | 0.04 | 23 (9–49) | 22 (8–60) | 0.17 |
| Cr, mg/dL | 0.9 (0.1) | 0.8 (0.1) | 0.21 | 0.9 (0.1) | 0.9 (0.1) | 0.90 | 0.9 (0.1) | 0.8 (0.1) | 0.41 | 0.9 (0.2) | 1.0 (0.2) | 0.10 |
| Urea, mg/dL | 23 (21–44) | 21 (18–27) | 0.14 | 20 (16–34) | 25 (15–36) | 0.50 | 22 (15–27) | 23 (15–29) | 0.75 | 25 (18–39) | 26 (12–48) | 0.95 |
| HOMA-IR | 1.30 (0.59–1.65) | 1.05 (0.59–1.54) | 0.27 | 1.12 (0.48–1.66) | 1.34 (0.68–1.85) | 0.17 | 3.89 (3.75–4.89) | 3.24 (2.76–3.56) | 0.07 | 3.37 (1.89–4.89) | 2.64 (2.33–3.21) | 0.12 |
| TyG Index | 4.37 (4.19–4.54) | 4.29 (4.07–4.51) | 0.13 | 4.26 (4.07–4.76) | 4.44 (4.01–4.89) | 0.13 | 4.59 (4.36–4.97) | 4.49 (4.27–4.91) | 0.46 | 4.65 (4.30–5.17) | 4.61 (4.35–5.41) | 0.72 |
| FLI | 0.07 (0.03–0.13) | 0.05 (0.02–0.13) | 0.35 | 0.07 (0.02–0.71) | 0.10 (0.02–1.00) | 0.05 | 22.1 (0.9–52.9) | 19.6 (0.4–43.1) | 0.35 | 10.72 (4.86–77.93) | 10.39 (3.76–84.81) | 0.72 |
| DII | 2.34 (1.32) | 2.31 (0.79) | 0.92 | 1.43 (1.36) | 2.21 (1.09) | 0.001 | 0.77 (0.88) | 0.38 (1.34) | 0.20 | 1.27 (1.19) | 2.34 (1.10) | 0.002 |
| DTP | 1036.4 (502.3) | 1123.8 (549.9) | 0.18 | 1209 (440.5) | 1000 (387) | 0.005 | 1218.2 (332.9) | 1628.2 (359.2) | 0.12 | 1244.5 (474.4) | 975 (441.3) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraiz, G.M.; Costa, M.A.C.; Cardoso, R.R.; Hébert, J.R.; Zhao, L.; Corich, V.; Giacomini, A.; Milagro, F.I.; Barros, F.A.R.; Bressan, J. Black Tea Kombucha Consumption: Effect on Cardiometabolic Parameters and Diet Quality of Individuals with and without Obesity. Fermentation 2024, 10, 384. https://doi.org/10.3390/fermentation10080384
Fraiz GM, Costa MAC, Cardoso RR, Hébert JR, Zhao L, Corich V, Giacomini A, Milagro FI, Barros FAR, Bressan J. Black Tea Kombucha Consumption: Effect on Cardiometabolic Parameters and Diet Quality of Individuals with and without Obesity. Fermentation. 2024; 10(8):384. https://doi.org/10.3390/fermentation10080384
Chicago/Turabian StyleFraiz, Gabriela Macedo, Mirian A. C. Costa, Rodrigo R. Cardoso, James R. Hébert, Longgang Zhao, Viviana Corich, Alessio Giacomini, Fermín I. Milagro, Frederico A. R. Barros, and Josefina Bressan. 2024. "Black Tea Kombucha Consumption: Effect on Cardiometabolic Parameters and Diet Quality of Individuals with and without Obesity" Fermentation 10, no. 8: 384. https://doi.org/10.3390/fermentation10080384
APA StyleFraiz, G. M., Costa, M. A. C., Cardoso, R. R., Hébert, J. R., Zhao, L., Corich, V., Giacomini, A., Milagro, F. I., Barros, F. A. R., & Bressan, J. (2024). Black Tea Kombucha Consumption: Effect on Cardiometabolic Parameters and Diet Quality of Individuals with and without Obesity. Fermentation, 10(8), 384. https://doi.org/10.3390/fermentation10080384









