Abstract
Methane, a potent greenhouse gas, represents both a challenge and an opportunity in the quest for sustainable energy. This work investigates the biotechnology for converting methane into clean, renewable hydrogen. The co-culture of Chlorella sacchrarophila FACHB 4 and Methylomonas sp. HYX-M1 was demonstrated to completely convert 1 mmol of methane to biomass within 96 h. After acid digestion of such biomass, up to 45.05 μmol of glucose, 4.07 μmol of xylose, and 26.5 μmol of lactic acid were obtained. Both Clostridium pasteurianum DSM525 and Clostridium sp. BZ-1 can utilize those sugars to produce hydrogen without any additional organic carbon sources. The higher light intensity in methane oxidation co-culture systems resulted in higher hydrogen production, with the BZ-1 strain producing up to 14.00 μmol of hydrogen, 8.19 μmol of lactate, and 6.09 μmol of butyrate from the co-culture biomass obtained at 12,000 lux. The results demonstrate that the co-culture biomass of microalgae and methanotroph has the potential to serve as a feedstock for dark fermentative hydrogen production. Our study highlights the complexities inherent in achieving efficient and complete methane-to-hydrogen conversion, positioning this biological approach as a pivotal yet demanding area of research for combating climate change and propelling the global energy transition.
1. Introduction
Methane (CH4) is the second major greenhouse gas, and its global warming potential is about 28–34 times higher than that of carbon dioxide, contributing 20% of the global warming effect [1,2,3]. Methanotrophs are demonstrated to consider methane as the sole carbon and generate carbon dioxide as final product [1,4]. Microalgae are widely distributed in terrestrial and aquatic ecosystems and are capable of fixing carbon dioxide and producing oxygen through photosynthesis [5]. The established co-cultures of aerobic methanotrophs and microalgae show potential for rapid methane utilization without external oxygen supply [6,7,8]. David successfully established co-cultures of aerobic methanotroph enrichment and microalgae with the supply of methane and carbon dioxide in a continuous reactor [8,9]. These co-cultures not only continuously consumed methane but also accumulated biomass of 641 mg/L [8,9]. Li constructed a co-culture of Methylocystis bryophila and Scenedesmus obliquus with biogas feeding. Compared with the single culture of S. obliquus of 3 × 107 cells/mL, higher microalgal biomass of 5 × 107 cells/mL was observed [10]. The co-culture of alkaliphilic methanotrophs and Scenedesmus obtusiusculus reached a biomass of 3.3 g/L, while alkaliphilic methanotrophs cultured alone only reached a biomass of 0.86 g/L [11]. Furthermore, the co-cultures of Methylomicrobium alcaliphilum 20Z and Chlorella sp. HS2 led to the final dry cell weight value of 2.4 g/L, whereas the final cell weight values of HS2 and 20Z monocultures were 0.3–0.5 g/L and 0.2–0.3 g/L, respectively [12]. The synergistic cultivation of microalgae with methanotrophic bacteria holds promise in converting methane into biomass; yet, the exploration of subsequent applications for this co-generated biomass remains limited.
Hydrogen is considered as an ideal energy carrier and produces clean combustion with a high-energy density of 122 kJ/g [13,14]. Fermentative hydrogen production from biomass is energy-saving and environmentally friendly, thus providing great potential for industrial application [15,16]. Microalgae and methane-oxidizing bacteria are both high-quality biomass. Some researchers have previously investigated hydrogen production from reducing sugar in microalgal biomass by hydrogen-producing bacteria through batch fermentation [17,18,19]. For instance, Clostridium butyricum CGS5 utilized 9 g /L of reducing sugars from C. vulgaris ESP6 biomass, and the cumulative hydrogen production finally reached 1476 mL/L [15]. In a batch experiment, C. butyricum, as the dominant species, efficiently utilized 4.9 g/L of reducing sugars to produce hydrogen of 32.0 mL/g-TVS. Furthermore, aerobic methanotrophs such as Methylomicrobium alcaliphilum 20Z and Methylomonas methanica are able to provide sugar and organic acids [20,21]; therefore, they also have the potential to serve as substrate for hydrogen production.
In this work, a co-culture of Chlorella sacchrarophila FACHB 4 and Methylomonas sp. HYX-M1 was established, and the capacity of methane oxidation was assessed at the same time. Subsequently, the co-culture biomass was digested with 3% H2SO4 and was analyzed by the HPLC system and excitation–emission matrix spectrometry. Furthermore, two Clostridium sp. were induced to produce hydrogen from co-culture biomass, without any additional organic carbon sources, under various conditions. The influence of the carbon source composition was evaluated in order to select the best production profile according to the characteristics of the microalgal biomass. Our experimental outcomes aim to bridge this gap by presenting a novel approach to valorize the combined biomass output from methanotrophic bacteria and microalgae, thereby charting a path towards sustainable resource utilization and circular bioeconomy.
2. Materials and Methods
2.1. Precultures of the Microorganism
The microalgae used in this work was Chlorella sacchrarophila FACHB 4 purchased from the Freshwater Algae Culture Collection at the Institute of Hydrobiology (Wuhan, China). C. sacchrarophila was grown in BG-11 medium [22] and was cultivated under continuous illumination at 6000 lux and at the temperature of 30 °C.
The methanotroph strain Methylomonas sp. HYX-M1 was isolated from the Pearl River Estuary (Guangzhou, China). Methylomonas sp. HYX-M1 was grown in modified NMS medium under aerobic conditions with approximately 25% methane and was incubated in darkness at a temperature of 30 °C [23].
Clostridium pasteurianum DSM525 purchased from the DSMZ and Clostridium sp. BZ-1 isolated from The Pearl River Estuary were cultured in modified mineral salt glucose (MSG) medium [24]. The two microorganisms were both incubated at the temperature of 37 °C in the dark. The pH value was adjusted to 6.5 ± 0.1.
2.2. Co-Culture Experiments of Microalgae and Methanotroph
The culture solution containing C. sacchrarophila. FACHB 4 and Methylomonas sp. HYX-M1 was concentrated by centrifugation (8000 rpm, 5 min) and resuspended in 50 mL of NMS, respectively. In the following co-culture experiments, cells were grown in 125 mL serum bottles of 25 mL of NMS sealed with a septum and aluminum cap. Cells were inoculated at a 4:1 (Methylomonas sp. HYX-M1: C. sacchrarophila. FACHB 4) ratio based on volume. In each vial, the initial OD680 of the co-cultures were 0.1. 25 mL air was extracted, and the 25 mL of methane was added as the only carbon substrate. All experiments were incubated in a light incubator at 30 °C under a light intensity of 6000 lux, 8000, and 12,000 lux (144 μmol/s/m2). Single culture of C. sacchrarophila. FACHB 4 and Methylomonas sp. HYX-M1 were set as control. Three parallel controls were set for each group. An amount of 200 μL of gas sample was taken from the headspace by a sterilized syringe for gas detection. CH4 and CO2 were analyzed by gas chromatography (GC; Agilent 7820A, Santa Clara, CA, USA) equipped with a flame ionization detector and a thermal conductivity detector, as previously described [23].
2.3. Hydrolysis of the Biomass of Co-Cultures
The co-cultures were centrifuged at 8000 rpm for 5 min and subsequently resuspended with 10 mL distilled water. The harvested microalgal biomass was first treated with 3% H2SO4 under autoclave condition (120 °C for 30 min). After acidification, the solution was centrifuged at 8000 rpm for 5 min and filtrated through a 0.22 filter membrane. The supernatants under different light intensities were stored in −20 °C for the following experiments.
2.4. Fermentation Experiments
Experiment 1. To identify the hydrogen production of Clostridium sp. from co-culture biomass, 10 mL of acid solution was added into 10 mL of prepared MSG medium without glucose. The pH value of mixed culture medium was adjusted to 7 and then transferred into 25 mL serum bottles with a working capacity of 5 mL. These bottles were sealed with butyl rubber plugs and aluminum caps. By using equipment furnished with vacuum pumps and nitrogen cylinders, the serum bottles were deoxygenated by evacuating and flushing them with high pure N2 to create an anoxic environment over five cycles. Then, 5% Clostridium pasteurianum DSM 525 (0.25 mL) and 5% Clostridium sp. BZ-1 (0.25 mL) were injected into the sterilized medium, respectively. The MSG medium, without a carbon source and with 5 mM glucose, 5 mM xylose, or 5 mM lactic acid, was set as control. All batch tests were kept stable at 37 °C in the dark. Hydrogen was analyzed by a gas chromatograph (GC, Agilent 7820A, USA) equipped with a thermal conductivity detector.
Experiment 2. To determine the hydrogen production of Clostridium sp. with co-culture biomass under various light intensities, 10 mL of acid solution cultured under various light intensities was added into 10 mL of prepared MSG medium without glucose. The pH value of mixed culture medium was adjusted to 7 and then transferred into 25 mL serum bottles with a working capacity of 5 mL. The headspace of serum bottles was replaced with nitrogen gas. Then, 5% Clostridium sp. (0.25 mL) was injected into the sterilized medium. All batch tests were kept stable at 37 °C in the dark.
2.5. Analysis of Soluble Metabolites
Organic acids and solubilized sugars were quantified in the cell-free supernatants by HPLC (LaChrom, Merck, Darmstadt, Germany) equipped with a guard, an analytical column (Aminex HPX-87H, Bio-Rad Laboratories, Hercules, CA, USA), and a refraction index (RI) detector (LaChrom L-7490, Merck, Germany). The temperature of the column and the RI detector were maintained constant at 50 °C and 45 °C, respectively. The samples were eluted with diluted H2SO4 at a flow rate of 0.6 mL/min. The solutions of the analyzed organic acids and sugars served as external standards.
2.6. Three-Dimensional Fluorescence Spectrum
All the samples were characterized by using three-dimensional fluorescence spectrum (Hitachi, FP6500, Tokyo, Japan). Three-dimensional excitation–emission matrix spectra were determined with scanning emission spectra from 250 nm to 550 nm at 2 nm increments by varying the excitation wavelength from 220 nm to 500 nm at 5 nm increments, with a speed of 1200 nm/min. The blank spectrum was recorded with deionized water.
2.7. Statistical Analysis
All the batch cultures were conducted in triplicate, and the data were the mean ± standard deviation. Statistical analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) or Origin 2018. Significant differences were determined by one-way ANOVA, and a p value < 0.05 was considered statistically significant. NS presented no significant differences (p > 0.05).
3. Results and Discussion
3.1. Methane Consumption of Co-Cultures
The methane consumption and growth of microalgae, methanotrophs, and co-cultures were investigated, respectively. Under pure culture conditions, C. sacchrarophila. FACHB 4 utilized a small amount of CO2 to grow and failed to consume methane, whereas the growth of Methylomonas sp. HYX-M1 was determined by the content of headspace oxygen, which could only consume 59.32% of the total methane (Figure 1a,b). In co-cultures, microalgae grew with carbon dioxide produced by methanotrophs, while microalgae produce oxygen through photosynthesis to supply the methanotroph in return [8,10]. Thus, the co-culture system was able to utilize more methane for biomass production compared to methanotroph culture alone. The highest methane utilization of the co-culture system was 1.00 mmol, which was twice as much as that of HYX-M1 cultured alone; meanwhile, the maximum OD680 value of the culture solution was 2.5, which was five times as much as that of HYX-M1 cultured alone (Figure 1). Similar to our study, Wang found that methane assimilation of the combined system (Chlorella vulgaris and aerobic methanotrophs) was 12.50% higher than that of aerobic methanotrophs cultured solely and the dry biomass was as high as 250 mg/L [25]. The highest methane consumption rate of alkaliphilic methanotrophs and Scenedesmus obtusiusculus co-culture in batch assays ranged from 2.5 to 19.8 mmol CH4/gbiomass/d [11]. The methane oxidation rate in a co-culture of methane oxidizing communities and microalgae was 10.68 mmol/L/d w, and the average methane oxidation rate of Scenedesmus sp. and Methylocystis parvus co-culture was 20.56–36.8 mmol/L/d [8,9]. The methane assimilation rate of Chlorella sorokiniana–Methylococcus capsulatus co-culture was approximately 8 mmol/L/d [6]. In our study, the co-culture’s average methane oxidation rate was 10 mmol/L/d (9.52 mmol CH4/gbiomass/d), suggesting that the methane oxidation rate of constructive co-culture was at a moderate level.
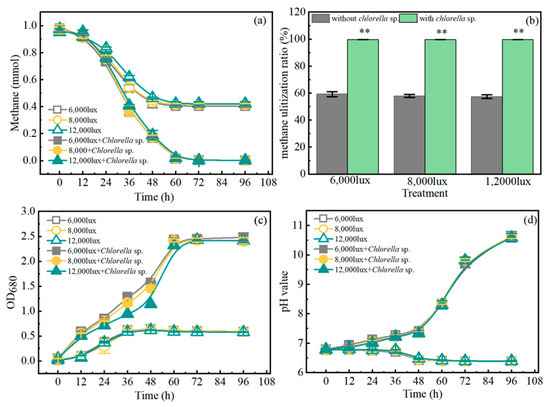
Figure 1.
The dynamics of methane consumption (a), methane oxidation rate (b), and OD680 (c) and pH value (d) for Methylomonas sp. HYX-M1when cultured alone or co-cultivated with C. sacchrarophila. FACHB 4 under various light intensities. ** p < 0.01.
The pH value has always been a key factor affecting the growth of microorganisms; meanwhile, the growth of microorganisms will also cause a change in a system’s pH value. By monitoring the change in the pH value of the system, the state of microbial growth can be revealed. In general, the growth of methanotrophs brings about a decrease in the pH value of the medium (Figure 1d), whereas the growth of microalgae could lead to an increase in pH value. This allowed the co-culture system to maintain a stable pH value between 7.0 and 8.0 for the first 64 h (Figure 1d). After 64 h, with the depletion of methane in the culture system, the growth of methanotrophic bacteria stopped, and the continued increase of the medium indicated that microalgae could still continue to grow at this time. However, the OD680 value of the system did not continue to increase after 60 h, indicating that a part of methanotroph suffered death, thus offsetting the increase in OD680 caused by the growth of microalgae.
3.2. Metabolite Production in Co-Cultures
In this work, the co-culture biomass of microalgae and methanotroph was pretreated with 3% H2SO4, and then most of the glucose was released from the raw biomass into the pretreated hydrolysates. The maximum glucose production of 45.05 μmol was obtained after 4 days of batch cultivation at 12,000 lux light intensity. As the light intensity decreased from 12,000 lux to 6000 lux, the glucose yield of the co-culture hydrolysate gradually decreased, and the xylose yield gradually increased (Figure 2). The biomass obtained from 12,000 lux cultivation reached a maximum reducing sugar ratio of 13.68% (Table 1). Similarly, carbohydrate productivity from Nannochloropsis gaditana increased with light intensity from 6000 lux to 12,000 lux, and the carbohydrate content of Nannochloropsis gaditana was 21.3 ± 0.9% under 12,000 lux [26]. Sriram and Seenivasan reported that maximum carbohydrate content (25.4%) was obtained in Desmodesmus sp. VIT after 15 days of batch cultivation at 16,000 lux light intensity [27]. The results of the present study concluded that 12,000 lux light intensity is the optimum condition for maximum carbohydrate accumulation in co-culture. Lactic acid also accumulated in all experimental systems, and the accumulation of lactic acid gradually decreased with the increase in light intensity. Under 6000 lux light conditions, the lactic acid production of co-culture biomass was as high as 26.45 μmol (Figure 2). Some microalgae isolated from seawater, such as Nannochlorum sp. 26A4, had a starch content of 40% (dry weight) and a conversion rate for converting consumed starch into lactic acid of 70% [28]. Furthermore, some of the methanotrophs, such as Methylomonas sp. DH-1 and Methylomicrobium alcaliphilum 20z, were able to produce lactic acid from methane [29,30]. A dark environment is more conducive to the lactic acid fermentation of algae and the growth of methane-oxidizing bacteria, which may account for the higher lactic acid content observed under low light intensities.
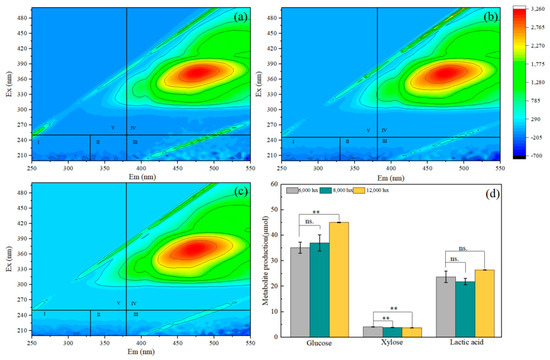
Figure 2.
The three-dimensional fluorescence spectrum of co-culture biomass under different light conditions with acid treatment; (a) 6000 lux, (b) 8000 lux, and (c) 12,000 lux. (d) Metabolite production of co-culture biomass. ** p < 0.01; n.s. indicates that these differences are not significant. Region I and II: aromatic protein; region III: fulvic acid-like; region IV: soluble microbial by-product-like; region V: humic acid-like.

Table 1.
The content of carbohydrates under various light intensities. All values were compared to the biomass cultured in 6000 lux. * p < 0.05, ** p < 0.01; n.s. indicates that these differences are not significant.
In order to explore other metabolites during the co-culture of microalgae and methanotroph, the 3DEEM fluorescence spectrum of the pretreatment sample was analyzed. Amino acids such as arginine, serine and tyrosine were demonstrated to be produced by microalgae biomass after microwave pretreatment with diluted acid and aspartic acid [16]. However, no peak in the Type I and II region indicative of a protein-like peak was observed. One peak at Ex/Em of 375 nm/475 nm was observed in the 3DEEM spectra in the Type IV region. These were humic-like peaks (Figure 2). The dehydration of polysaccharide fractions in the presence of acid catalysts is a chemical process that produces secondary humic matter. Consistent with our study, the humic acid mixture was extracted from microalgae biomass rich in polysaccharides using a standard alkali treatment [31].
3.3. Biohydrogen of Clostridium sp. from Co-Culture Biomass Hydrolysate
The pretreated biomass under 8000 lux light intensity was used as feedstock to produce hydrogen by Clostridium sp. during dark fermentation. Glucose, xylose, and lactic acid are the primary components of biomass [32,33], and thus, the ability of Clostridium sp. to utilize these substances was also tested. Strain DSM 525 failed to produce hydrogen from xylose, while strain DSM525 produced a low amount of hydrogen (0.56 μmol) from lactic acid (Figure 3). Strain DSM525 successfully generated hydrogen gas by utilizing pretreated biomass and glucose, and its maximum hydrogen production was 10.94 μmol and 27.09 μmol, respectively (Figure 3). The ability of BZ-1 to utilize glucose, xylose, lactic acid, and pretreated biomass for hydrogen production was similar to that of DSM525. Amounts of 12.26 μmol and 35.07 μmol of hydrogen were obtained by BZ-1 from pretreated biomass and glucose (Figure 3). The biomass utilization of two Clostridium sp. were 10.84 μmol/mL and 14.03 μmol/mL. These results suggested that selected Clostridium sp. were able to produce hydrogen from biomass hydrolysate, with BZ-1 exhibiting superior hydrogen production capability. The pH value decreased to 5.5 and 6.5, respectively, in the treatment of glucose and pretreated biomass (Figure 3). The decreased pH value was attributed to the increasing accumulation of organic acids and the dissolution of CO2 [24,34]. The Clostridium sp. adapted to a pH value range from 5.0 to 7.0, and, therefore, a decrease in pH value within the system was not likely to inhibit hydrogen production by Clostridium. Acid hydrolysis is a simple and crude hydrolysis method that may produce many toxic substances [35]. Clostridium sp. can directly grow and produce hydrogen by using the acid hydrolysis solution, indicating that Clostridium sp. have the ability to adapt to sufficient environmental resistance. This result has positive significance for industrial hydrogen production with biomass, greatly contributing to simplifying the steps and reducing costs.
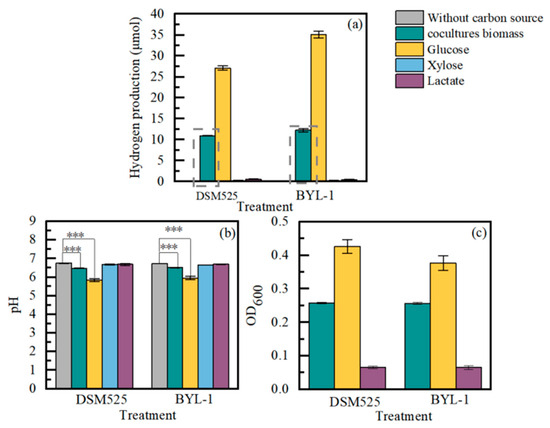
Figure 3.
The dynamics of hydrogen production (a), pH value (b), and OD680 (c) of Clostridium pasteurianum DSM525 and Clostridium sp. BZ-1 with various carbon resources. The gray box represents the amount of hydrogen obtained from biomass utilization. *** p < 0.001.
3.4. Hydrogen Production from Co-Cultured Biomass under Different Light Intensities by Strain BZ-1
The measurement of the content of reducing sugar in the acid solution demonstrated that co-cultured biomass under 12,000 lux had the highest content of reducing sugar, and theoretically, the highest hydrogen production could be achieved by using pretreated biomass under this light intensity. However, microalgae biomass contains certain other components which were able to be utilized by Clostridium sp. for hydrogen production. Therefore, hydrogen production experiments with biomass acid hydrolysates under different light conditions were carried out to further verify the optimal acid hydrolysates. The cumulative hydrogen production and maximum hydrogen yield was enhanced when the biomass cultured light intensity increased. Both the highest cumulative hydrogen production (14.00 μmol) and biomass utilization (5.6 μmol/mL) occurred at the biomass cultured under 12,000 lux (Figure 4a). Similarly, C. vulgaris ESP6 obtained the highest hydrogen production of 1424 mL/L when the reducing sugar in biomass increased from 3 g/L to 9 g/L [15]. Thus, the hydrogen production from biomass under higher light intensity seemed to have a positive consistency with the reducing sugars in biomass. However, the lower light intensity in methane oxidation co-culture systems resulted in higher hydrogen production, and the maximum methane yield of 0.71 was detected from co-culture biomass obtained at 6000 lux. Some components in biomass would be degraded into 5-hydroxymethylfurfural, formic acid, and levulinic acid through acid digestion [36], which are known as potential inhibitors in the following hydrogen production, leading to lower methane yield. The conversion rate of methane to hydrogen was relatively low: only 0.47%. The loss in biomass production from methane, biomass hydrolysis, and hydrogen production from hydrolysis resulted in low methane-to-hydrogen conversion. To further improve methane-to-hydrogen conversion, it is necessary to optimize the conditions for each step.
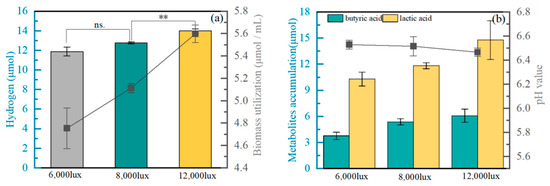
Figure 4.
The hydrogen production and biomass utilization (a), metabolites accumulation and pH value (b), of Clostridium sp. BZ-1 with pretreated co-culture biomass incubated under different light intensities. Light intensity on the horizontal axis represents the cultivation conditions of the co-culture biomass. ** p < 0.01; n.s. indicates that these differences are not significant.
In the process of producing hydrogen, Clostridium sp. also produces organic acids, such as acetic acid, butyric acid, and lactic acid, which significantly decrease the pH value of the fermentation liquid phase, resulting in serious inhibition of hydrogen fermentation. Thus, pH value and various organic acids were detected in the experiment. The accumulation of butyric acid was enhanced when the BZ-1 strain with pretreated co-culture biomass was cultured in 12,000 lux, and the maximum production was 6.09 μmol (Figure 4b). The metabolic pathways for the production of butyric acid from glucose involved the breakdown of hexose to pyruvate and its conversion into acetyl-CoA via the Embden–Meyerhof–Parnas pathway [37,38]. Ultimately, butyric acid is produced from acetyl-CoA-derived acetoacetyl CoA via phosphotrans butyrylase and butyrate kinase. Several anaerobic Clostridium species, including Clostridium tyrobutyricum, Clostridium sp. S1, and C. thermobutyricum, are known to produce butyric acid as the main metabolite from glucose [38,39,40]. Moreover, accumulated lactic acid reached levels of 10.31, 11.84, and 14.80 μmol, respectively, with various pretreated co-culture biomasses. Compared to the initial system, the final lactic acid obtained in the system was slightly increased (Table 2). Therefore, the initial lactic acid in the system seemed to not be utilized as a carbon source but, rather, as a product of carbohydrate metabolism. Glucose is oxidized to pyruvate and then converted to lactate by lactate dehydrogenase. Similar to our study, researchers previously had produced approximately 8 g/L lactic acid from a PY medium with glycerol under different pH value [41]. The pH value decreased faster with increased accumulation of organic acid. Cultured with various pretreated co-culture biomasses, the pH values reached 6.52, 6.51, and 6.47, respectively (Figure 4b). Although the pH value decreased, the system remained in the range suitable for the growth of Clostridium sp., thereby having a limited inhibition on the hydrogen production of the BZ-1 strain.

Table 2.
The consumption or production of glucose by strain BZ-1with pretreated biomass cultured under various light intensities.
The influence of the biomass cultured under different light intensities on the metabolism of strain BZ-1 was comprehensively illustrated based on the carbon in different cultures. Most of the carbon in the culture was retained in the biomass of the BZ-1 strain (17.07–21.03%) (Figure 5). The highest carbon (18.15%) fluxes flowing to butyrate were observed when the BZ-1 strain grew with the co-culture biomass obtained at 12,000 lux. Meanwhile, the higher light intensity in methane oxidation co-culture systems resulted in higher carbon flows to lactate, with maximum values of 18.15%. These results indicate co-culture biomass obtained from different light intensities leads to various carbon fluxes of strain BZ-1. Overall, this study provides a new method for obtaining hydrogen from biomass with methane, but the optimization of culture conditions remains to be further explored.
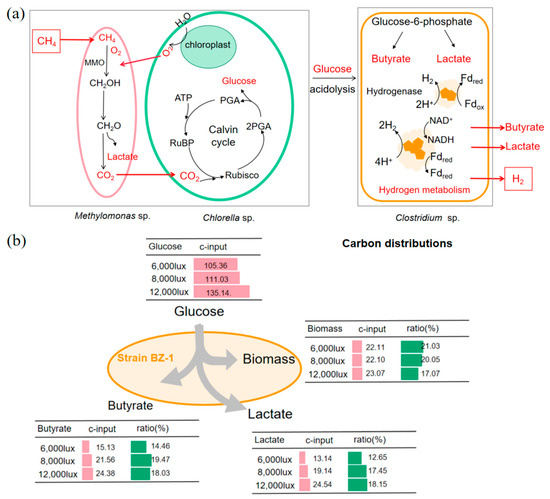
Figure 5.
Mechanism of synergistic metabolism of aerobic methanotroph, microalgae, and Clostridium sp. to produce methane removal and hydrogen production (a). Aerobic methanotroph first utilizes methane to produce carbon dioxide as a substrate for microalgae growth, while microalgae provide oxygen for further methane oxidation. Clostridium sp. use the reducing sugar in co-culture biomass to accumulate hydrogen and organic acid. Average carbon (b) of the products from glucose fermentation with pretreated co-culture biomass incubated under different light intensities.
4. Conclusions
The co-culture of Chlorella sacchrarophila FACHB 4 and Methylomonas sp. HYX-M1 produced biomass from 1 mmol of methane in 96 h. Furthermore, up to 45.05 μmol of glucose, 4.07 μmol of xylose and 26.5 μmol of lactic acid were obtained from co-cultured biomass after acid digestion. Clostridium pasteurianum DSM525 and Clostridium sp. BZ-1 produce hydrogen from soluble sugars in pretreated co-culture biomass. The hydrogen production of strain BZ-1 increased to 14.00 μmol with 8.19 μmol lactate and 6.09 μmol butyrate from the co-culture biomass obtained at 12,000 lux. These findings introduce an innovative pathway for valorizing the co-produced biomass from methanotrophic microorganisms and microalgae, thereby offering a dual strategy that concurrently promotes environmental remediation and bioenergy generation.
Author Contributions
Conceptualization, Z.X. and F.L.; Methodology, Y.S. and L.L.; Formal analysis, L.L.; Investigation, Y.S., L.L. and S.Z.; Data curation, O.W.; Writing—original draft, Y.S.; Writing—review & editing, Z.X. and F.L.; Visualization, S.Z. and F.L.; Supervision, O.W.; Funding acquisition, Z.X., O.W., S.Z. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (Grant No. 2020YFA0907300), the National Natural Science Foundation of China (Grant Nos. 42321005, U20A20109, 92251301 and 42077044), the Guangdong Foundation for Program of Science and Technology Research (Grant Nos. 2020B1111530002 and 2023B1212060044), the GDAS’ Project of Science and Technology Development (Grant Nos. 2019GDASYL-0102003 and 2019GDASYL-0102005), the Pearl River Talent Recruitment Program of Guangdong Province (Grant No. 2019QN01L735) and the Taishan Scholar Program of Shandong Province (tsqn202306293).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank Yuechao Zhang for helpful discussion and valuable suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cai, Y.; Zheng, Y.; Bodelier, P.L.E.; Conrad, R.; Jia, Z. Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat. Commun. 2016, 7, 11728. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wik, M.; Varner, R.K.; Anthony, K.W.; MacIntyre, S.; Bastviken, D. Climate-sensitive northern lakes and ponds are critical components of methane release. Nat. Geosci. 2016, 9, 99–105. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, S.; Ma, C.; Xia, Y.; Qiao, H.; Zhao, J.; Gao, W.; Tu, Q.; Zhang, Y.; Rui, Y.; et al. Type I dominated methane oxidation and assimilation in rice paddy fields by the consequence of niche differentiation. Biol. Fert. Soils 2024, 60, 153–165. [Google Scholar] [CrossRef]
- Burlacot, A.; Peltier, G. Energy crosstalk between photosynthesis and the algal CO2-concentrating mechanisms. Trends Plant Sci. 2023, 28, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.; Hilliard, M.; He, Q.P.; Wang, J. A Microalgae-Methanotroph Coculture is a Promising Platform for Fuels and Chemical Production From Wastewater. Front. Energy Res. 2020, 8, 563352. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, P.; Gómez-Borraz, T.L.; Revah, S.; Morales, M. Methanotroph-microalgae co-culture for greenhouse gas mitigation: Effect of initial biomass ratio and methane concentration. Chemosphere 2020, 259, 127418. [Google Scholar] [CrossRef] [PubMed]
- Van der Ha, D.; Bundervoet, B.; Verstraete, W.; Boon, N. A sustainable, carbon neutral methane oxidation by a partnership of methane oxidizing communities and microalgae. Water Res. 2011, 45, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Van der Ha, D.; Nachtergaele, L.; Kerckhof, F.M.; Rameiyanti, D.; Bossier, P.; Verstraete, W.; Boon, N. Conversion of Biogas to Bioproducts by Algae and Methane Oxidizing Bacteria. Environ. Sci. Technol. 2012, 46, 13425–13431. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Li, N.; Wang, Y.; Yu, R.; Zhu, G.; Zeng, R.J. Mixotrophic Cultivation of Microalgae Using Biogas as the Substrate. Environ. Sci. Technol. 2022, 56, 3669–3677. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, P.; Gómez-Borraz, T.L.; Saldivar, A.; Hernández, S.; Morales-Ibarría, M.; Revah, S. Diluted methane mitigation by a co-culture of alkaliphilic methanotrophs and the microalgae Scenedesmus obtusiusculus towards carbon neutrality. Biochem. Eng. J. 2024, 203, 109211. [Google Scholar] [CrossRef]
- Yun, J.H.; Lee, H.; Nam, J.W.; Ko, M.; Park, J.; Lee, D.H.; Lee, S.G.; Kim, H.S. Unlocking synergies: Harnessing the potential of biological methane sequestration through metabolic coupling between Methylomicrobium alcaliphilum 20Z and Chlorella sp. HS2. Bioresour. Technol. 2024, 399, 130607. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F. Bio-hydrogen production by different operational modes of dark and photo-fermentation: An overview. Int. J. Hydrogen Energy 2011, 36, 7443–7459. [Google Scholar] [CrossRef]
- Saray, J.A.; Gharehghani, A.; Hosseinzadeh, D. Towards sustainable energy Carriers: A solar and Wind-Based systems for green liquid hydrogen and ammonia production. Energy Convers. Manag. 2024, 304, 118215. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, C.Y.; Cheng, C.L.; Lee, D.J.; Chang, J.S. Fermentative hydrogen production by Clostridium butyricum CGS5 using carbohydrate-rich microalgal biomass as feedstock. Int. J. Hydrogen Energy 2012, 37, 15458–15464. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Lin, R.; Lu, H.; Zhou, J.; Cen, K. Comparison in dark hydrogen fermentation followed by photo hydrogen fermentation and methanogenesis between protein and carbohydrate compositions in Nannochloropsis oceanica biomass. Bioresour. Technol. 2013, 138, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chang, H.Y.; Chang, J.S. Producing carbohydrate-rich microalgal biomass grown under mixotrophic conditions as feedstock for biohydrogen production. Int. J. Hydrogen Energy 2016, 41, 4413–4420. [Google Scholar] [CrossRef]
- Cheng, J.; Xia, A.; Liu, Y.; Lin, R.; Zhou, J.; Cen, K. Combination of dark- and photo-fermentation to improve hydrogen production from Arthrospira platensis wet biomass with ammonium removal by zeolite. Int. J. Hydrogen Energy 2012, 37, 13330–13337. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Elshobary, M.; Abdullah, E.; Abdel-Basset, R.; Metwally, M. Application of a novel biological-nanoparticle pretreatment to Oscillatoria acuminata biomass and coculture dark fermentation for improving hydrogen production. Microb. Cell Factories 2023, 22, 34. [Google Scholar] [CrossRef]
- But, S.Y.; Rozova, O.N.; Khmelenina, V.N.; Reshetnikov, A.S.; Trotsenko, Y.A. Properties of Recombinant ATP-Dependent Fructokinase from the Halotolerant Methanotroph Methylomicrobium alcaliphilum 20Z. Biochemistry 2012, 77, 372–377. [Google Scholar] [CrossRef]
- Rozova, O.N.; Khmelenina, V.N.; Vuilleumier, S.; Trotsenko, Y.A. Characterization of recombinant pyrophosphate-dependent 6-phosphofructokinase from halotolerant methanotroph Methylomicrobium alcaliphilum 20Z. Res. Microbiol. 2010, 161, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, Z.; Valverde-Pérez, B.; D’Este, M.; De Francisci, D.; Angelidaki, I. Nutrient recovery from industrial wastewater as single cell protein by a co-culture of green microalgae and methanotrophs. Biochem. Eng. J. 2018, 134, 129–135. [Google Scholar] [CrossRef]
- Hao, Q.; Liu, F.; Zhang, Y.; Wang, O.; Xiao, L. Methylobacter accounts for strong aerobic methane oxidation in the Yellow River Delta with characteristics of a methane sink during the dry season. Sci. Total. Environ. 2020, 704, 135383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, L.; Wang, S.; Liu, F. Stimulation of ferrihydrite nanorods on fermentative hydrogen production by Clostridium pasteurianum. Bioresour. Technol. 2019, 283, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-H.; Zhu, M.Y.; Lian, S.J.; Zou, H.; Fu, S.F.; Guo, R.B. Conversion of Renewable Biogas into Single-Cell Protein Using a Combined Microalga- and Methane-Oxidizing Bacterial System. ACS EST Eng. 2022, 2, 2317–2325. [Google Scholar] [CrossRef]
- Onay, M. Enhancing carbohydrate productivity from Nannochloropsis gaditana for bio-butanol production. Energy Rep. 2020, 6, 63–67. [Google Scholar] [CrossRef]
- Sriram, S.; Seenivasan, R. Biophotonic perception on Desmodesmus sp. VIT growth, lipid and carbohydrate content. Bioresour. Technol. 2015, 198, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Ueda, R. Production of optically pure D-Lactic acid by Nannochlorum sp. 26A4. Appl. Biochem. and Biotech. 2004, 119, 71–77. [Google Scholar] [CrossRef]
- Henard, C.A.; Franklin, T.G.; Youhenna, B.; But, S.; Alexander, D.; Kalyuzhnaya, M.G.; Guarneri, M.T. Biogas Biocatalysis: Methanotrophic Bacterial Cultivation, Metabolite Profiling, and Bioconversion to Lactic Acid. Front. Microbiol. 2018, 9, 2610. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, S.; Kim, W.; Kim, S.; Cha, S.; Moon, H.; Hur, D.H.; Kim, S.Y.; Na, J.G.; Lee, J.W.; et al. Efficient production of d-lactate from methane in a lactate-tolerant strain of Methylomonas sp. DH-1 generated by adaptive laboratory evolution. Biotechnol. Biofuels 2019, 12, 234. [Google Scholar] [CrossRef]
- Radu, E.; Oprescu, E.E.; Enascuta, C.E.; Calin, C.; Stoica, R.; Scaeteanu, G.V.; Vasilievici, G.; Capra, L.; Ivan, G.; Ion, A.C. Kinetic Adsorption of Humic Acids Mixture Obtained from Microalgae on Exfoliated Graphite Nanoplatelets. Rev. Chim. 2018, 69, 191–195. [Google Scholar] [CrossRef]
- Dos Santos Vieira, C.F.; Codogno, M.C.; Maugeri Filho, F.; Maciel Filho, R.; Mariano, A.P. Sugarcane bagasse hydrolysates as feedstock to produce the isopropanol-butanol-ethanol fuel mixture: Effect of lactic acid derived from microbial contamination on Clostridium beijerinckii DSM 6423. Bioresour. Technol. 2021, 319, 124140. [Google Scholar]
- Kim, S.H.; Yi, Y.D.; Kim, H.J.; Bhatia, S.K.; Gurav, R.; Jeon, J.M.; Yoon, J.J.; Kim, S.H.; Park, J.H.; Yang, Y.H. Hyper biohydrogen production from xylose and xylose-based hemicellulose biomass by the novel strain Clostridium sp. YD09. Biochem. Eng. J. 2022, 187, 108624. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Hao, Q.; Li, X.; Liu, F. Ferrihydrite Reduction Exclusively Stimulated Hydrogen Production by Clostridium with Community Metabolic Pathway Bifurcation. ACS Sustain. Chem. Eng. 2020, 8, 7574–7580. [Google Scholar] [CrossRef]
- Chng, L.M.; Lee, K.T.; Chan, D.J.C. Synergistic effect of pretreatment and fermentation process on carbohydrate-rich Scenedesmus dimorphus for bioethanol production. Energy Convers. Manag. 2017, 141, 410–419. [Google Scholar] [CrossRef]
- Park, J.H.; Cheon, H.C.; Yoon, J.J.; Park, H.D.; Kim, S.H. Optimization of batch dilute-acid hydrolysis for biohydrogen production from red algal biomass. Int. J. Hydrogen Energy 2013, 38, 6130–6136. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Yang, S.T. Construction and characterization of ack deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid and hydrogen production. Biotechnol. Progress. 2006, 22, 1265–1275. [Google Scholar] [CrossRef]
- Liu, S.; Bischoff, K.M.; Leathers, T.D.; Qureshi, N.; Rich, J.O.; Hughes, S.R. Butyric acid from anaerobic fermentation of lignocellulosic biomass hydrolysates by Clostridium tyrobutyricum strain RPT-4213. Bioresour. Technol. 2013, 143, 322–329. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.Y.; Lee, K.M.; Youn, S.H.; Lee, S.-M.; Woo, H.M.; Oh, M.-K.; Um, Y. Butyric acid production from softwood hydrolysate by acetate-consuming Clostridium sp. S1 with high butyric acid yield and selectivity. Bioresour. Technol. 2016, 218, 1208–1214. [Google Scholar] [CrossRef]
- Wang, L.; Ou, M.S.; Nieves, I.; Erickson, J.E.; Vermerris, W.; Ingram, L.O.; Shanmugam, K.T. Fermentation of sweet sorghum derived sugars to butyric acid at high titer and productivity by a moderate thermophile Clostridium thermobutyricum at 50 °C. Bioresour. Technol. 2015, 198, 533–539. [Google Scholar] [CrossRef]
- Leja, K.; Myszka, K.; Czaczyk, K. The ability of Clostridium bifermentans strains to lactic acid biosynthesis in various environmental conditions. SpringerPlus 2013, 2, 44. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).