Health-Promoting Effects of Lactobacillus acidophilus and Its Technological Applications in Fermented Food Products and Beverages
Abstract
1. Introduction
2. Health-Promoting Benefits of L. acidophilus
2.1. Immunity Enhancement
2.2. Gut Wellness
2.3. Antimicrobial Activity
2.4. Antitumor Activity
2.5. Antioxidant Activity
2.6. Bioactive Peptides
2.7. Extracellular Polysaccharides Production
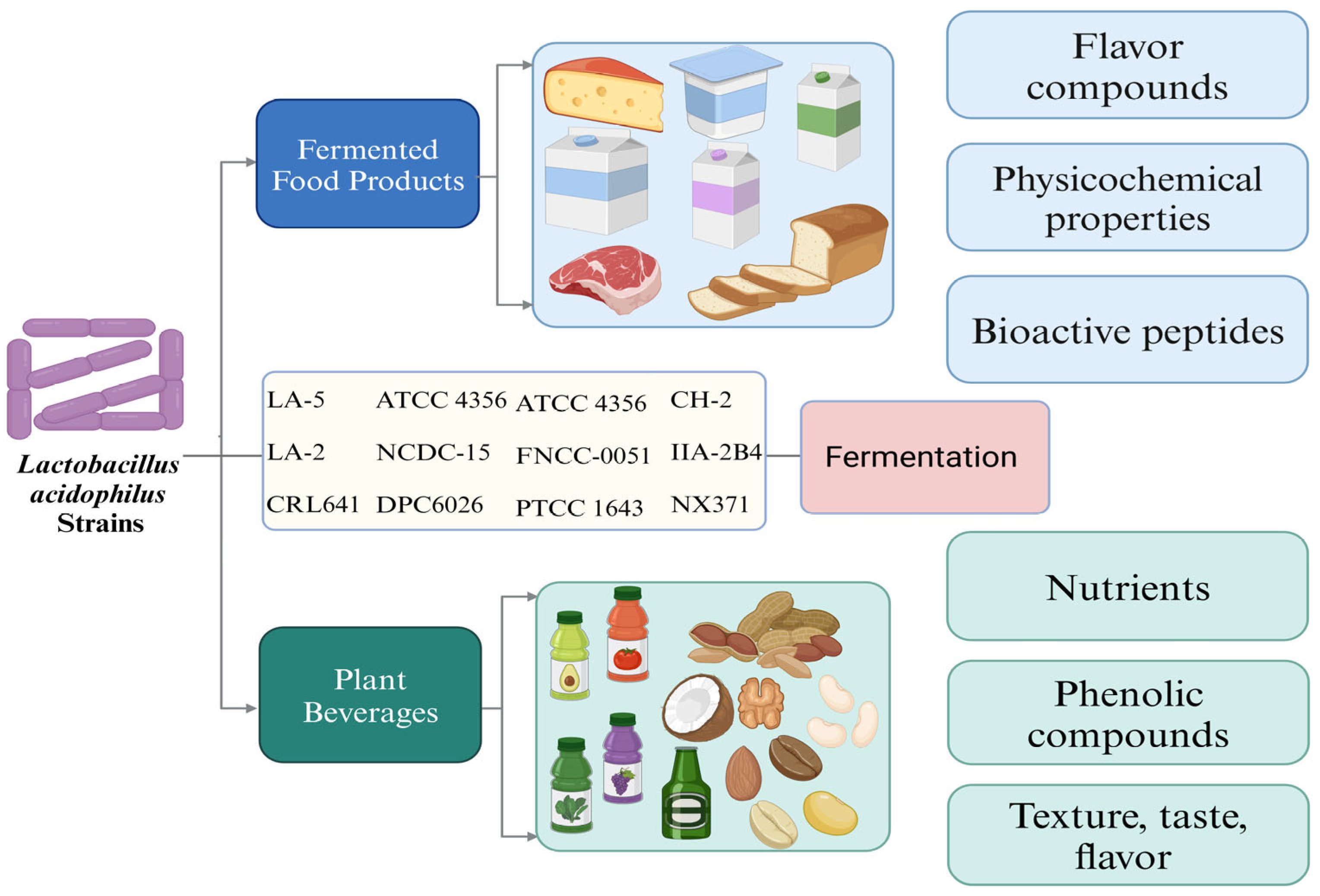
3. Applications of L. acidophilus in Fermented Food Products
3.1. Cheese
3.2. Yogurt
3.3. Fermented Milk
3.4. Fortified Milk Products
3.5. Meat Products
3.6. Baking Products
4. Applications of L. acidophilus in Fermented Plant-Based Beverages
4.1. Fruit Juices
4.2. Vegetable Juices
4.3. Grains and Nuts Beverages
4.4. Bean Milk
5. Safety of Lactobacillus acidophilus
6. Challenges and Opportunities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinev, T.; Beev, G.; Denev, S.; Dermendzhieva, D.; Tzanova, M.; Valkova, E. Antimicrobial activity of Lactobacillus acidophilus against pathogenic and food spoilage microorganisms: A review. Agric. Sci. Technol. 2017, 8, 3–9. [Google Scholar] [CrossRef]
- Anjum, N.; Maqsood, S.; Masud, T.; Ahmad, A.; Sohail, A.; Momin, A. Lactobacillus acidophilus: Characterization of the species and application in food production. Crit. Rev. Food Sci. Nutr. 2014, 54, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Soleymanzadeh Moghadam, S.; Minaeian, S.; Majidpour, A.; Adabi, M.; Hosseini Doust, R. The Lactobacillus acidophilus Supernatant: An Effective and Safe Alternative to Antibiotics. Iran. J. Toxicol. 2024, 18, 52–60. [Google Scholar] [CrossRef]
- Diniz-Silva, H.T.; Brandao, L.R.; de Sousa Galvao, M.; Madruga, M.S.; Maciel, J.F.; Leite de Souza, E.; Magnani, M. Survival of Lactobacillus acidophilus LA-5 and Escherichia coli O157:H7 in Minas Frescal cheese made with oregano and rosemary essential oils. Food Microbiol. 2020, 86, 103348. [Google Scholar] [CrossRef] [PubMed]
- CoŞAnsu AkdemİR, S.; Toupal, S.; Aslan, Ö. Growth Kinetics and Survival of Lactobacillus acidophilus in Black Rice Milk. Gıda 2021, 46, 1440–1449. [Google Scholar] [CrossRef]
- Meng, L.; Li, S.; Liu, G.; Fan, X.; Qiao, Y.; Zhang, A.; Lin, Y.; Zhao, X.; Huang, K.; Feng, Z. The nutrient requirements of Lactobacillus acidophilus LA-5 and their application to fermented milk. J. Dairy Sci. 2021, 104, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Rianingsih, L.; Sumardianto. Antioxidant activity in seaweed (Sargassum sp.) extract fermented with Lactobacillus plantarum and Lactobacillus acidophilus. IOP Conf. Ser. Earth Environ. Sci. 2020, 530, 012011. [Google Scholar] [CrossRef]
- Chan, C.-L.; Gan, R.-Y.; Shah, N.P.; Corke, H. Enhancing antioxidant capacity of Lactobacillus acidophilus-fermented milk fortified with pomegranate peel extracts. Food Biosci. 2018, 26, 185–192. [Google Scholar] [CrossRef]
- Aween, M.M.; Hassan, Z.; Muhialdin, B.J.; Eljamel, Y.A.; Al-Mabrok, A.S.; Lani, M.N. Antibacterial activity of Lactobacillus acidophilus strains isolated from honey marketed in Malaysia against selected multiple antibiotic resistant (MAR) Gram-positive bacteria. J. Food Sci. 2012, 77, M364–M371. [Google Scholar] [CrossRef]
- Gürmeriç, H.E.; Şengül, M.; Erkaya-Kotan, T. Assessment of the antioxidant and ACE-inhibitory activities and some quality characteristics of Kaşar cheese produced by probiotic Lactobacillus acidophilus. Food Biosci. 2024, 59, 103812. [Google Scholar] [CrossRef]
- Padghan, P.V.; Mann, B.; Sharma, R.; Bajaj, R.; Saini, P. Production of Angiotensin-I-Converting-Enzyme-Inhibitory Peptides in Fermented Milks (Lassi) Fermented by Lactobacillus acidophillus with Consideration of Incubation Period and Simmering Treatment. Int. J. Pept. Res. Ther. 2016, 23, 69–79. [Google Scholar] [CrossRef]
- Wen, J.; Ma, X.; Liu, Y. The Latest Research Progress on Application of Lactobacillus acidophilus. Adv. Biosci. Biotechnol. 2023, 14, 298–307. [Google Scholar] [CrossRef]
- Aung, T.; Park, S.-S.; Kim, M.-J. Influence of Lactobacillus (LAB) Fermentation on the Enhancement of Branched Chain Amino Acids and Antioxidant Properties in Bran among Wheat By-Products. Fermentation 2022, 8, 732. [Google Scholar] [CrossRef]
- Enujiugha, V.N.; Badejo, A.A. Probiotic potentials of cereal-based beverages. Crit. Rev. Food Sci. Nutr. 2017, 57, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.; Khaliq, A.; Chughtai, M.F.J.; Nadeem, M.; Tahir, A.B.; Din, A.A.; Ntsefong, G.N.; Shariati, M.A.; Rebezov, M.; Yessimbekov, Z.; et al. Technofunctional quality assessment of soymilk fermented with Lactobacillus acidophilus and Lactobacillus casei. Biotechnol. Appl. Biochem. 2022, 69, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Guney, D.; Gungormusler, M. Development and Comparative Evaluation of a Novel Fermented Juice Mixture with Probiotic Strains of Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2021, 13, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and Storage Characteristics of “Fuji” Apple Juice Using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial Growth, Metabolism of Bioactives and in vitro Bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef]
- Chen, K.; Xin, J.; Zhang, G.; Xie, H.; Luo, L.; Yuan, S.; Bu, Y.; Yang, X.; Ge, Y.; Liu, C. A combination of three probiotic strains for treatment of acute diarrhoea in hospitalised children: An open label, randomised controlled trial. Benef. Microbes 2020, 11, 339–346. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Meta-analysis of randomized controlled trials of the effects of probiotics on functional constipation in adults. Clin. Nutr. 2020, 39, 2960–2969. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Huang, Y.Y.; Tsai, S.Y.; Kuo, Y.W.; Lin, J.H.; Ho, H.H.; Chen, J.F.; Hsia, K.C.; Sun, Y. Efficacy of Probiotic Supplements on Brain-Derived Neurotrophic Factor, Inflammatory Biomarkers, Oxidative Stress and Cognitive Function in Patients with Alzheimer’s Dementia: A 12-Week Randomized, Double-Blind Active-Controlled Study. Nutrients 2023, 16, 16. [Google Scholar] [CrossRef]
- Farag, M.A.; El Hawary, E.A.; Elmassry, M.M. Rediscovering acidophilus milk, its quality characteristics, manufacturing methods, flavor chemistry and nutritional value. Crit. Rev. Food Sci. Nutr. 2020, 60, 3024–3041. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xing, H.Y.; Liu, H.J.; Chen, Z.F.; Tang, B.B. Efficacy of probiotics in the treatment of acute diarrhea in children: A systematic review and meta-analysis of clinical trials. Transl. Pediatr. 2021, 10, 3248–3260. [Google Scholar] [CrossRef]
- Liong, M.T.; Easa, A.M.; Lim, P.T.; Kang, J.Y. Survival, growth characteristics and bioactive potential of Lactobacillus acidophilus in a soy-based cream cheese. J. Sci. Food Agric. 2009, 89, 1382–1391. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of Bioactive Peptides by Lactobacillus Species: From Gene to Application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef]
- Alaqil, A.A.; Abbas, A.O.; El-Beltagi, H.S.; El-Atty, H.K.A.; Mehaisen, G.M.K.; Moustafa, E.S. Dietary Supplementation of Probiotic Lactobacillus acidophilus Modulates Cholesterol Levels, Immune Response, and Productive Performance of Laying Hens. Animals 2020, 10, 1588. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Kwok, L.-Y.; Cai, T.; Zhang, W. The immune regulatory role of Lactobacillus acidophilus: An updated meta-analysis of randomized controlled trials. Food Biosci. 2020, 36, 100656. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, K.; Zhang, A.; Chang, W.; Zheng, A.; Chen, Z.; Cai, H.; Liu, G. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult. Sci. 2021, 100, 101323. [Google Scholar] [CrossRef]
- Nouri Gharajalar, S.; Mirzai, P.; Nofouzi, K.; Madadi, M.S. Immune enhancing effects of Lactobacillus acidophilus on Newcastle disease vaccination in chickens. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101520. [Google Scholar] [CrossRef] [PubMed]
- Maria Remes Troche, J.; Coss Adame, E.; Angel Valdovinos Diaz, M.; Gomez Escudero, O.; Eugenia Icaza Chavez, M.; Antonio Chavez-Barrera, J.; Zarate Mondragon, F.; Antonio Ruiz Velarde Velasco, J.; Rafael Aceves Tavares, G.; Antonio Lira Pedrin, M.; et al. Lactobacillus acidophilus LB: A useful pharmabiotic for the treatment of digestive disorders. Ther. Adv. Gastroenterol. 2020, 13, 1756284820971201. [Google Scholar] [CrossRef]
- Farid, W.; Masud, T.; Sohail, A.; Ahmad, N.; Naqvi, S.M.S.; Khan, S.; Ali, A.; Khalifa, S.A.; Hussain, A.; Ali, S.; et al. Gastrointestinal transit tolerance, cell surface hydrophobicity, and functional attributes of Lactobacillus acidophilus strains isolated from Indigenous Dahi. Food Sci. Nutr. 2021, 9, 5092–5102. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, Y.; Liu, X.; Tian, C.; Zhong, X.; Zhao, L. A Systematic Review and Meta-Analysis: Lactobacillus acidophilus for Treating Acute Gastroenteritis in Children. Nutrients 2022, 14, 682. [Google Scholar] [CrossRef]
- Abd Elmacksood, A.; Basha, O.; Talat, D.; Ahmed, H.; Ibrahim, M. In Vitro evaluation of antimicrobial activity of Lactobacillus acidophilus against some pathogens. Damanhour J. Vet. Sci. 2021, 6, 21–28. [Google Scholar] [CrossRef]
- Karska-Wysocki, B.; Bazo, M.; Smoragiewicz, W. Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA). Microbiol. Res. 2010, 165, 674–686. [Google Scholar] [CrossRef]
- Abd-Allah, D.A.; Sotohy, S.; Hassan, E.A.; Mwafy, A. Antagonistic effect of Lactobacillus acidophilus against some pathogenic bacteria. Assiut Vet. Med. J. 2024, 70, 146–155. [Google Scholar] [CrossRef]
- Celebi, O.; Taghizadehghalehjoughi, A.; Celebi, D.; Mesnage, R.; Golokhvast, K.S.; Arsene, A.L.; Spandidos, D.A.; Tsatsakis, A. Effect of the combination of Lactobacillus acidophilus (probiotic) with vitamin K3 and vitamin E on Escherichia coli and Staphylococcus aureus: An in vitro pathogen model. Mol. Med. Rep. 2023, 27, 119. [Google Scholar] [CrossRef]
- Onur, E.; Gokmen, G.G.; Nalbantsoy, A.; Kisla, D. Investigation of the supportive therapy potential of propolis extract and Lactobacillus acidophilus LA-5 milk combination against breast cancer in mice. Cytokine 2022, 149, 155743. [Google Scholar] [CrossRef]
- Khedr, O.M.S.; El-Sonbaty, S.M.; Moawed, F.S.M.; Kandil, E.I.; Abdel-Maksoud, B.E. Lactobacillus acidophilus ATCC 4356 Exopolysaccharides Suppresses Mediators of Inflammation through the Inhibition of TLR2/STAT-3/P38-MAPK Pathway in DEN-Induced Hepatocarcinogenesis in Rats. Nutr. Cancer 2022, 74, 1037–1047. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, S.; Shi, J.; Xie, Q.; Li, N.; Guan, J.; Evivie, S.E.; Liu, F.; Li, B.; Huo, G. Effects of Lactobacillus acidophilus KLDS1.0901 on Proliferation and Apoptosis of Colon Cancer Cells. Front. Microbiol. 2022, 12, 788040. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Gao, J.; Jiang, X.; Tao, M.; Zeng, X.; Wu, Z.; Pan, D. Lactobacillus acidophilus CICC 6074 inhibits growth and induces apoptosis in colorectal cancer cells in vitro and in HT-29 cells induced-mouse model. J. Funct. Foods 2020, 75, 104290. [Google Scholar] [CrossRef]
- Al-Asfour, A.; Bhardwaj, R.G.; Karched, M. Growth Suppression of Oral Squamous Cell Carcinoma Cells by Lactobacillus acidophilus. Int. Dent. J. 2024, in press. [Google Scholar] [CrossRef]
- Baghbani-Arani, F.; Asgary, V.; Hashemi, A. Cell-free extracts of Lactobacillus acidophilus and Lactobacillus delbrueckii display antiproliferative and antioxidant activities against HT-29 cell line. Nutr. Cancer 2020, 72, 1390–1399. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Bahadori, M.B.; Pimentel, T.C. Effect of Microencapsulation on the Development of Antioxidant Activity and Viability of Lactobacillus acidophilus LA5 in Whey Drink During Fermentation. Biointerface Res. Appl. Chem. 2020, 11, 9762–9771. [Google Scholar]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Esmerino, E.A.; Margalho, L.P.; Santos-Junior, V.A.; Coimbra, P.T.; Cappato, L.P.; Silva, M.C.; Garcia-Gomes, A.S.; et al. Assessment of antioxidant activity, lipid profile, general biochemical and immune system responses of Wistar rats fed with dairy dessert containing Lactobacillus acidophilus La-5. Food Res. Int. 2016, 90, 275–280. [Google Scholar] [CrossRef]
- Nada, H.G.; Sudha, T.; Darwish, N.H.E.; Mousa, S.A. Lactobacillus acidophilus and Bifidobacterium longum exhibit antiproliferation, anti-angiogenesis of gastric and bladder cancer: Impact of COX2 inhibition. PharmaNutrition 2020, 14, 100219. [Google Scholar] [CrossRef]
- Taha, S.; El Abd, M.; De Gobba, C.; Abdel-Hamid, M.; Khalil, E.; Hassan, D. Antioxidant and antibacterial activities of bioactive peptides in buffalo’s yoghurt fermented with different starter cultures. Food Sci. Biotechnol. 2017, 26, 1325–1332. [Google Scholar] [CrossRef]
- Okon, A.; Stadnik, J.; Dolatowski, Z.J. Effect of Lactobacillus acidophilus Bauer and Bifidobacterium animalis ssp. lactis BB12 on proteolytic changes in dry-cured loins. Food Sci. Biotechnol. 2017, 26, 633–641. [Google Scholar] [CrossRef]
- da Silva, B.S.; Díaz-Roa, A.; Yamane, E.S.; Hayashi, M.A.F.; Silva Junior, P.I. Doderlin: Isolation and characterization of a broad-spectrum antimicrobial peptide from Lactobacillus acidophilus. Res. Microbiol. 2023, 174, 103995. [Google Scholar] [CrossRef]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef]
- Ha, G.E.; Chang, O.K.; Han, G.S.; Ham, J.S.; Park, B.Y.; Jeong, S.G. Comparison of Antioxidant Activities of Hydrolysates of Domestic and Imported Skim Milk Powders Treated with Papain. Korean J. Food Sci. Anim. Resour. 2015, 35, 360–369. [Google Scholar] [CrossRef]
- Qureshi, T.M.; Vegarud, G.E.; Abrahamsen, R.K.; Skeie, S. Characterization of the Norwegian autochthonous cheese Gamalost and its angiotensin I-converting enzyme (ACE) inhibitory activity during ripening. Dairy Sci. Technol. 2012, 92, 613–625. [Google Scholar] [CrossRef]
- Sedaghati, M.; Ezzatpanah, H.; Mashhadiakbar Boojar, M.; Tajabadi Ebrahimi, M.; Aminafshar, M. Plasmin-digest of β-lactoglobulin with antibacterial properties. Food Agric. Immunol. 2014, 26, 218–230. [Google Scholar] [CrossRef]
- Modiri, S.; Kasra Kermanshahi, R.; Reza Soudi, M.; Dad, N.; Ebadi, M.; Shahbani Zahiri, H.; Akbari Noghabi, K. Growth Optimization of Lactobacillus acidophilus for Production of Antimicrobial Peptide Acidocin 4356: Scale up from Flask to Lab-Scale Fermenter. Iran. J. Biotechnol. 2021, 19, e2686. [Google Scholar]
- Wang, H.; Niu, Y.; Pan, J.; Li, Q.; Lu, R. Antibacterial effects of Lactobacillus acidophilus surface-layer protein in combination with nisin against Staphylococcus aureus. LWT 2020, 124, 109208. [Google Scholar] [CrossRef]
- Meng, F.; Zhu, X.; Zhao, H.; Nie, T.; Lu, F.; Lu, Z.; Lu, Y. A class III bacteriocin with broad-spectrum antibacterial activity from Lactobacillus acidophilus NX2-6 and its preservation in milk and cheese. Food Control 2021, 121, 107597. [Google Scholar] [CrossRef]
- Huang, J.-J.; Yang, L.-C.; Liu, Y.-C. Production, purification, and structural characteristics of extracellular polysaccharides derived from Lactobacillus acidophilus. J. Taiwan Inst. Chem. Eng. 2022, 137, 104189. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Guo, H.; Cheng, Q.; Abbas, Z.; Tong, Y.; Yang, T.; Zhou, Y.; Zhang, H.; Wei, X.; et al. Optimization of Exopolysaccharide Produced by Lactobacillus plantarum R301 and Its Antioxidant and Anti-Inflammatory Activities. Foods 2023, 12, 2481. [Google Scholar] [CrossRef]
- Deepak, V.; Ram Kumar Pandian, S.; Sivasubramaniam, S.D.; Nellaiah, H.; Sundar, K. Optimization of anticancer exopolysaccharide production from probiotic Lactobacillus acidophilus by response surface methodology. Prep. Biochem. Biotechnol. 2016, 46, 288–297. [Google Scholar] [CrossRef]
- Abedfar, A.; Abbaszadeh, S.; Hosseininezhad, M.; Taghdir, M. Physicochemical and biological characterization of the EPS produced by L. acidophilus isolated from rice bran sourdough. LWT-Food Sci. Technol. 2020, 127, 9. [Google Scholar] [CrossRef]
- Lopes, L.A.A.; Pimentel, T.C.; Carvalho, R.S.F.; Madruga, M.S.; Galvao, M.S.; Bezerra, T.K.A.; Barao, C.E.; Magnani, M.; Stamford, T.C.M. Spreadable goat Ricotta cheese added with Lactobacillus acidophilus La-05: Can microencapsulation improve the probiotic survival and the quality parameters? Food Chem. 2021, 346, 128769. [Google Scholar] [CrossRef]
- Mojaddar Langroodi, A.; Mehdizadeh, T.; Majidi, L.; Neyriz-Naghadehi, M. Lactobacillus acidophilus and Anethum graveolens essential oil in Iranian cheese against Escherichia coli O157:H7. Flavour Fragr. J. 2020, 36, 190–196. [Google Scholar] [CrossRef]
- Lopes Neto, J.H.P.; Santos, M.C.G.d.; Leite, K.S.; Silva, L.A.d.; Campos, M.I.F.; Silveira, E.S.d.; Amaral, J.B.S.; Madruga, M.S.; Braga, A.L.M.; Cardarelli, H.R. Development and characterization of Lactobacillus acidophilus (LA-3) microparticles with reducing substances and its addition to Reino cheese. LWT 2021, 143, 111083. [Google Scholar] [CrossRef]
- Mosallaie, F.; Jooyandeh, H.; Hojjati, M.; Fazlara, A. Biological reduction of aflatoxin B1 in yogurt by probiotic strains of Lactobacillus acidophilus and Lactobacillus rhamnosus. Food Sci. Biotechnol. 2019, 29, 793–803. [Google Scholar] [CrossRef]
- Özhamamci, İ.; Cheraghi, K.S.; Ilgaz, Ş.; Gülçin, İ.; Polat, A.; Çakiroğlu, K.; Öz, E.; Çakmakçi, S. Lactobacillus acidophilus ve Yeşil Çay Pudrası ile Üretilen Yoğurtların Probiyotik Raf Ömrü, Antioksidan, Duyusal, Fiziksel ve Kimyasal Özellikleri. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 673–682. [Google Scholar]
- Masoumi, S.J.; Mehrabani, D.; Saberifiroozi, M.; Fattahi, M.R.; Moradi, F.; Najafi, M. The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food Sci. Nutr. 2021, 9, 1704–1711. [Google Scholar] [CrossRef]
- Hussien, H.; Abd-Rabou, H.S.; Saad, M.A. The impact of incorporating Lactobacillus acidophilus bacteriocin with inulin and FOS on yogurt quality. Sci. Rep. 2022, 12, 13401. [Google Scholar] [CrossRef]
- Mousavi, M.; Heshmati, A.; Garmakhany, A.D.; Vahidinia, A.; Taheri, M. Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed-enriched stirred probiotic yogurt by using response surface methodology. LWT 2019, 102, 80–88. [Google Scholar] [CrossRef]
- Hasani, S.; Sari, A.A.; Heshmati, A.; Karami, M. Physicochemical and sensory attributes assessment of functional low-fat yogurt produced by incorporation of barley bran and Lactobacillus acidophilus. Food Sci. Nutr. 2017, 5, 875–880. [Google Scholar] [CrossRef]
- Ertem, H.; Çakmakçı, S. Shelf life and quality of probiotic yogurt produced with Lactobacillus acidophilus and Gobdin. Int. J. Food Sci. Technol. 2017, 53, 776–783. [Google Scholar] [CrossRef]
- Dabaj, F.K.; Lasekan, O.; Manap, M.Y.A.; Ling, F.H. Evaluation of the volatilomic potentials of the Lactobacillus casei 431 and Lactobacillus acidophilus La-5 in fermented milk. CyTA-J. Food 2020, 18, 291–300. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Abd El-Maksoud, A.A.; Alharbi, S.A.; Alfarraj, S.; Mohamed, M.S.M. Fortification of acidophilus-bifidus-thermophilus (ABT) Fermented Milk with Heat-Treated Industrial Yeast Enhances Its Selected Properties. Molecules 2021, 26, 3876. [Google Scholar] [CrossRef]
- Li, S.; Ma, C.; Gong, G.; Liu, Z.; Chang, C.; Xu, Z. The impact of onion juice on milk fermentation by Lactobacillus acidophilus. LWT-Food Sci. Technol. 2016, 65, 543–548. [Google Scholar] [CrossRef]
- Abdollahzadeh, S.M.; Zahedani, M.R.; Rahmdel, S.; Hemmati, F.; Mazloomi, S.M. Development of Lactobacillus acidophilus-fermented milk fortified with date extract. LWT 2018, 98, 577–582. [Google Scholar] [CrossRef]
- Islam, M.Z.; Akhter, S.; Liza, R.I.; Sojib, M.S.I.; Hasan, M.S.; Habib, R.; Harun-ur-Rashid, M. Using β-galactosidase and Novel Probiotic Strains to Formulate Lactose-Free Acidophilus Milk Fortified with Cocoa Powder. Res. Sq. 2022, accepted. [Google Scholar]
- Elkot, W.F.; Ateteallah, A.H.; Al-Moalem, M.H.; Shahein, M.R.; Alblihed, M.A.; Abdo, W.; Elmahallawy, E.K. Functional, Physicochemical, Rheological, Microbiological, and Organoleptic Properties of Synbiotic Ice Cream Produced from Camel Milk Using Black Rice Powder and Lactobacillus acidophilus LA-5. Fermentation 2022, 8, 187. [Google Scholar] [CrossRef]
- Arief, I.I.; Afiyah, D.N.; Wulandari, Z.; Budiman, C. Physicochemical Properties, Fatty Acid Profiles, and Sensory Characteristics of Fermented Beef Sausage by Probiotics Lactobacillus plantarum IIA-2C12 or Lactobacillus acidophilus IIA-2B4. J. Food Sci. 2016, 81, M2761–M2769. [Google Scholar] [CrossRef]
- Segli, F.; Melian, C.; Vignolo, G.; Castellano, P. Inhibition of a spoilage exopolysaccharide producer by bioprotective extracts from Lactobacillus acidophilus CRL641 and Latilactobacillus curvatus CRL705 in vacuum-packaged refrigerated meat discs. Meat Sci. 2021, 178, 108509. [Google Scholar] [CrossRef]
- Agrawal, N.; Singh, P.K.; Yadav, S.; Gupta, S.; Garg, A.; Singh, R.; Gupta, B.; Shukla, S. Sensory characteristics of developed probiotic chicken meat spread fermented with Lactobacillus acidophilus. Int. J. Adv. Biochem. Res. 2024, 8, 271–278. [Google Scholar] [CrossRef]
- Razavizadeh, S.; Alencikiene, G.; Salaseviciene, A.; Vaiciulyte-Funk, L.; Ertbjerg, P.; Zabulione, A. Impact of fermentation of okara on physicochemical, techno-functional, and sensory properties of meat analogues. Eur. Food Res. Technol. 2021, 247, 2379–2389. [Google Scholar] [CrossRef]
- Segli, F.; Melian, C.; Munoz, V.; Vignolo, G.; Castellano, P. Bioprotective extracts from Lactobacillus acidophilus CRL641 and Latilactobacillus curvatus CRL705 inhibit a spoilage exopolysaccharide producer in a refrigerated meat system. Food Microbiol. 2021, 97, 103739. [Google Scholar] [CrossRef]
- Hadidi, M.; Majidiyan, N.; Jelyani, A.Z.; Moreno, A.; Hadian, Z.; Mousavi Khanegah, A. Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage. Foods 2021, 10, 2215. [Google Scholar] [CrossRef]
- Duc Thang, T.; Hanh Quyen, L.T.; Thuy Hang, H.T.; Thien Luan, N.; KimThuy, D.T.; Lieu, D.M. Survival Survey of Lactobacillus acidophilus In Additional Probiotic Bread. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 588–592. [Google Scholar] [CrossRef]
- Mirzamani, S.S.; Bassiri, A.R.; Tavakolipour, H.; Azizi, M.H.; Kargozari, M. Survival of fluidized bed encapsulated Lactobacillus acidophilus under simulated gastro-intestinal conditions and heat treatment during bread baking. J. Food Meas. Charact. 2021, 15, 5477–5484. [Google Scholar] [CrossRef]
- Maciel, G.M.; Chaves, K.S.; Grosso, C.R.; Gigante, M.L. Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J. Dairy Sci. 2014, 97, 1991–1998. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, Z.; Zhang, T.; Hendricks, G.; Guo, M. Microencapsulation of Lactobacillus acidophilus NCFM using polymerized whey proteins as wall material. Int. J. Food Sci. Nutr. 2016, 67, 670–677. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Rai, P.; Duke, M. Cheese whey to biohydrogen and useful organic acids: A non-pathogenic microbial treatment by L. acidophilus. Sci. Rep. 2019, 9, 8320. [Google Scholar] [CrossRef]
- Ribeiro, M.C.E.; Chaves, K.S.; Gebara, C.; Infante, F.N.S.; Grosso, C.R.F.; Gigante, M.L. Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res. Int. 2014, 66, 424–431. [Google Scholar] [CrossRef]
- Šiler, B.T.; Ribeiro, E.S.S.; Damasceno, K.S.F.S.C.; Dantas, L.M.d.C.; Azevedo, W.M.d.; Leite, P.I.P.; Assis, C.F.d.; Sousa Junior, F.C.d. Fermented yellow mombin juice using Lactobacillus acidophilus NRRL B-4495: Chemical composition, bioactive properties and survival in simulated gastrointestinal conditions. PLoS ONE 2020, 15, e0239392. [Google Scholar]
- Mashitoa, F.M.; Akinola, S.A.; Manhevi, V.E.; Garcia, C.; Remize, F.; Slabbert, R.M.; Sivakumar, D. Influence of Fermentation of Pasteurised Papaya Puree with Different Lactic Acid Bacterial Strains on Quality and Bioaccessibility of Phenolic Compounds during In Vitro Digestion. Foods 2021, 10, 962. [Google Scholar] [CrossRef]
- Li, X.; Gao, J.; Simal-Gandara, J.; Wang, X.; Caprioli, G.; Mi, S.; Sang, Y. Effect of fermentation by Lactobacillus acidophilus CH-2 on the enzymatic browning of pear juice. LWT 2021, 147, 111489. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef]
- Karbasi, M.; Yarmand, M.S.; Mousavi, M. Fermentation Potential of Lactobacillus rhamnosus and Lactobacillus acidophilus in Date Syrup to Develop a Functional Fermented Beverage: A Comparative Study. J. Food Process. Preserv. 2015, 39, 863–870. [Google Scholar] [CrossRef]
- Sheng, J.; Shan, C.; Liu, Y.; Zhang, P.; Li, J.; Cai, W.; Tang, F. Comparative evaluation of the quality of red globe grape juice fermented by Lactobacillus acidophilus and Lactobacillus plantarum. Int. J. Food Sci. Technol. 2022, 57, 2235–2248. [Google Scholar] [CrossRef]
- Xu, H.; Feng, L.; Deng, Y.; Chen, L.; Li, Y.; Lin, L.; Liang, M.; Jia, X.; Wang, F.; Zhang, X.; et al. Change of phytochemicals and bioactive substances in Lactobacillus fermented Citrus juice during the fermentation process. LWT 2023, 180, 114715. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Rosend, J.; Kaleda, A.; Kuldjarv, R.; Arju, G.; Nisamedtinov, I. The Effect of Apple Juice Concentration on Cider Fermentation and Properties of the Final Product. Foods 2020, 9, 1401. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Song, X.; Hu, X.; He, Y.; Yin, J.; Nie, S.; Xie, M. Changes in volatile and nutrient components of mango juice by different Lactic acid bacteria fermentation. Food Biosci. 2023, 56, 103141. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, H.; Zeng, H.; Prinyawiwatukul, W.; Xu, W.; Xu, Z. Increases in Phenolic, Fatty Acid, and Phytosterol Contents and Anticancer Activities of Sweet Potato after Fermentation by Lactobacillus acidophilus. J. Agric. Food Chem. 2018, 66, 2735–2741. [Google Scholar] [CrossRef]
- Lavinia, B.C.; Manea, I.; Bratu, M.G.; Avram, D.; Nicolescu, C.L. Evaluation of the cabbage and cucumber juices as substrate for Lactobacillus acidophilus LA-5. Rom. Biotechnol. Lett. 2012, 17, 7418–7429. [Google Scholar]
- Tenguria, M.; Golhani, P.; Joshi, P.D.; Bais, R.T.; Chansoria, A. In vitro exploration on deviation in certain physico-chemical properties of daucus carota juice supplemented with probiotic Lactobacillus acidophilus. Asian J. Pharm. Educ. Res. 2019, 8, 57–63. [Google Scholar] [CrossRef]
- Srisuk, N.; Nopharatana, M.; Jirasatid, S. Co-encapsulation of Dictyophora indusiata to improve Lactobacillus acidophilus survival and its effect on quality of sweet fermented rice (Khoa-Mak) sap beverage. J. Food Sci. Technol. 2021, 58, 3598–3610. [Google Scholar] [CrossRef]
- Igwebuike, J.I.; Barber, L.I.; Obinna-Echem, P.C. Quality Characteristics of Probiotic (Lactobacillus acidophilus) Beverage from Hydrolyzed Tigernut Milk Supplemented with Beetroot Juice. Am. J. Food Sci. Technol. 2022, 10, 95–102. [Google Scholar]
- Boudjou, S.; Zaidi, F.; Hosseinian, F.; Oomah, B.D. Effects of Faba Bean (Vicia faba L.) Flour on Viability of Probiotic Bacteria During Kefir Storage. J. Food Res. 2014, 3, 13–22. [Google Scholar] [CrossRef][Green Version]
- Ziarno, M.; Brys, J.; Parzyszek, M.; Veber, A. Effect of Lactic Acid Bacteria on the Lipid Profile of Bean-Based Plant Substitute of Fermented Milk. Microorganisms 2020, 8, 1348. [Google Scholar] [CrossRef]
- Cichonska, P.; Ziarno, M. Legumes and Legume-Based Beverages Fermented with Lactic Acid Bacteria as a Potential Carrier of Probiotics and Prebiotics. Microorganisms 2021, 10, 91. [Google Scholar] [CrossRef]
- Martineau-Cote, D.; Achouri, A.; Karboune, S.; L’Hocine, L. Faba Bean: An Untapped Source of Quality Plant Proteins and Bioactives. Nutrients 2022, 14, 1541. [Google Scholar] [CrossRef]
- Tangyu, M.; Fritz, M.; Tan, J.P.; Ye, L.; Bolten, C.J.; Bogicevic, B.; Wittmann, C. Flavour by design: Food-grade lactic acid bacteria improve the volatile aroma spectrum of oat milk, sunflower seed milk, pea milk, and faba milk towards improved flavour and sensory perception. Microb. Cell Fact. 2023, 22, 133. [Google Scholar] [CrossRef]
- Aduol, K.O.; Onyango, A.N.; Imathiu, S.M. Proximate, microbial and sensory characteristics of cowpea milk fermented with probiotic starter cultures. Eur. J. Agric. Food Sci. 2020, 2, 2–6. [Google Scholar] [CrossRef]
- Parra, K.; Ferrer, M.; Pinero, M.; Barboza, Y.; Medina, L.M. Use of Lactobacillus acidophilus and Lactobacillus casei for a potential probiotic legume-based fermented product using pigeon pea (Cajanus cajan). J. Food Prot. 2013, 76, 265–271. [Google Scholar] [CrossRef]
- Angelica Andrade Lopes, L.; de Siqueira Ferraz Carvalho, R.; Stela Santos Magalhaes, N.; Suely Madruga, M.; Julia Alves Aguiar Athayde, A.; Araujo Portela, I.; Eduardo Barao, C.; Colombo Pimentel, T.; Magnani, M.; Christina Montenegro Stamford, T. Microencapsulation of Lactobacillus acidophilus La-05 and incorporation in vegan milks: Physicochemical characteristics and survival during storage, exposure to stress conditions, and simulated gastrointestinal digestion. Food Res. Int. 2020, 135, 109295. [Google Scholar] [CrossRef]
- Marques da Silva, T.; Sonza Pinto, V.; Ramires Fonseca Soares, V.; Marotz, D.; Cichoski, A.J.; Queiroz Zepka, L.; Jacob Lopes, E.; de Bona da Silva, C.; de Menezes, C.R. Viability of microencapsulated Lactobacillus acidophilus by complex coacervation associated with enzymatic crosslinking under application in different fruit juices. Food Res. Int. 2021, 141, 110190. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D.; Jouki, M. Improving bioactive properties of peach juice using Lactobacillus strains fermentation: Antagonistic and anti-adhesion effects, anti-inflammatory and antioxidant properties, and Maillard reaction inhibition. Food Chem. 2021, 365, 130501. [Google Scholar] [CrossRef]
- Meng, F.-B.; Lei, Y.-T.; Li, Q.-Z.; Li, Y.-C.; Deng, Y.; Liu, D.-Y. Effect of Lactobacillus plantarum and Lactobacillus acidophilus fermentation on antioxidant activity and metabolomic profiles of loquat juice. LWT 2022, 171, 114104. [Google Scholar] [CrossRef]
- Bull, M.J.; Jolley, K.A.; Bray, J.E.; Aerts, M.; Vandamme, P.; Maiden, M.C.; Marchesi, J.R.; Mahenthiralingam, E. The domestication of the probiotic bacterium Lactobacillus acidophilus. Sci. Rep. 2014, 4, 7202. [Google Scholar] [CrossRef]
- Wasteson, Y.; Vold, L.; Skjerve, E.; Skjerdal, O.T.; Rosnes, J.T.; Robertson, L.; Nesbakken, T.; Narvhus, J.; Lassen, J.; Kapperud, G.; et al. Risk Assessment of Specific Strains of Lactobacillus acidophilus Used as “Other Substances”. Eur. J. Nutr. Food Saf. 2019, 16, 110–111. [Google Scholar]
- Hutt, P.; Koll, P.; Stsepetova, J.; Alvarez, B.; Mandar, R.; Krogh-Andersen, K.; Marcotte, H.; Hammarstrom, L.; Mikelsaar, M. Safety and persistence of orally administered human Lactobacillus sp. strains in healthy adults. Benef. Microbes 2011, 2, 79–90. [Google Scholar] [CrossRef]
- Chen, J.F.; Hsia, K.C.; Kuo, Y.W.; Chen, S.H.; Huang, Y.Y.; Li, C.M.; Hsu, Y.C.; Tsai, S.Y.; Ho, H.H. Safety Assessment and Probiotic Potential Comparison of Bifidobacterium longum subsp. infantis BLI-02, Lactobacillus plantarum LPL28, Lactobacillus acidophilus TYCA06, and Lactobacillus paracasei ET-66. Nutrients 2023, 16, 126. [Google Scholar] [CrossRef]
- Malilay, J.K.; Oliveros, M.C.; Bautista, J.A.; Castillo-Israel, K.A. Functional, safety and technological properties of Lactobacillus acidophilus BIOTECH 1900. Philipp. J. Vet. Anim. Sci. 2019, 45, 11–21. [Google Scholar]
- Bernardeau, M.; Guguen, M.; Vernoux, J.P. Beneficial lactobacilli in food and feed: Long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol. Rev. 2006, 30, 487–513. [Google Scholar] [CrossRef]
- Bang, W.Y.; Chae, S.A.; Ban, O.H.; Oh, S.; Park, C.; Lee, M.; Shin, M.; Yang, J.; Jung, Y.H. The in vitro and in vivo Safety Evaluation of Lactobacillus acidophilus IDCC 3302. Microbiol. Biotechnol. Lett. 2021, 49, 39–44. [Google Scholar] [CrossRef]
- Su, X.; Menghe, B.; Zhang, H.; Liu, W. In Vitro Evaluation of Intestinal Transport and High-Density Fermentation of Lactobacillus acidophilus. Metabolites 2023, 13, 1077. [Google Scholar] [CrossRef] [PubMed]
- Mrvcic, J.; Butorac, A.; Solic, E.; Stanzer, D.; Bacun-Druzina, V.; Cindric, M.; Stehlik-Tomas, V. Characterization of Lactobacillus brevis L62 strain, highly tolerant to copper ions. World J. Microbiol. Biotechnol. 2013, 29, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rachwał, K.; Gustaw, K. Lactic Acid Bacteria in Sustainable Food Production. Sustainability 2024, 16, 3362. [Google Scholar] [CrossRef]
- Waoo, A.A.; Dixit, S. Production and Immobilization of Lactobacillus acidophilus on Fruit Wastes Containing Media as Probiotics. Biol. Sci. 2018, 2, 9–18. [Google Scholar]
- Morya, S.; Awuchi, C.G.; Neumann, A.; Napoles, J.; Kumar, D. Advancement in Acidophilus Milk Production Technology; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Kajikawa, A.; Zhang, L.; LaVoy, A.; Bumgardner, S.; Klaenhammer, T.R.; Dean, G.A. Mucosal Immunogenicity of Genetically Modified Lactobacillus acidophilus Expressing an HIV-1 Epitope within the Surface Layer Protein. PLoS ONE 2015, 10, e0141713. [Google Scholar] [CrossRef]
- Gibson, G.R.; McCartney, A.L.; Rastall, R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005, 93 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak-Siedlecka, K.; Roviello, G.; Catalano, M.; Polom, K. Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus spp. and Bifidobacterium spp. in Gastrointestinal Cancers. Nutrients 2021, 13, 2674. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Zhang, Z.; Yin, X.; Wang, B.; Yang, X. Recent progress in adaptive laboratory evolution of industrial microorganisms. J. Ind. Microbiol. Biotechnol. 2023, 50, kuac023. [Google Scholar] [CrossRef]

| Strain | Properties | Subjects | Findings | References |
|---|---|---|---|---|
| ATCC 43121 | Antiproliferative, proapoptotic, and antioxidant effects | HT-29 cell line | Intrinsic pathway-dependent apoptosis was induced. Cell viability was significantly reduced to 42.2 ± 0.01% and 19.40 ± 0.01% by 5 and 8 mg ml−1 | [42] |
| LA-5 | Antioxidant activity | Wistar rats | Improve the antioxidant defenses | [44] |
| LA-5 | Antitumor effects | Mouse xenograft breast cancer model | Inhibited the tumor volumes by 59.16%, 28.29%, and 63.39%. Acidophilus milk and PE combination significantly enhanced the ConA-, LPS-, and PHA-induced splenocyte proliferation | [37] |
| LA | Antiproliferation and anti-angiogenesis properties | Gastric (AGS) and bladder (J253) cancer cell lines | Downregulated COX2 expression in AGS by 70 % and 95 %; antiproliferation and anti-angiogenesis of LA against gastric cancer by downregulating COX2 expression | [45] |
| ATCC 4356 | Immunomodulatory and antitumor activities | Rats | ATCC 4356 exopolysaccharides suppress mediators of inflammation through the inhibition of TLR2/STAT-3/P38-MAPK pathway | [38] |
| CICC 6074 | Anti-cancer effect | HT-29 cells induced-mouse model | CICC 6074 induced colon cancer apoptosis by up-regulating Bax, down-regulating Bcl-2, releasing Cyt c from the mitochondria into the cytoplasm, and activating Caspase-3 and Caspase-9 | [40] |
| KLDS1.0901 | Anti-cancer effect | Colon cancer cells (HT-29, Caco-2, and IEC-6 cells) | Inhibited the proliferation of HT-29 and Caco-2 cells, reduced the mitochondrial membrane potential of HT-29 cells | [39] |
| ATCC 4356 | Anti-cancer effect | Oral squamous cell carcinoma cells (HNO97 cell line) | Exhibits antiproliferative activity against OSCC cells possibly partially via a TRAIL-induced mechanism of apoptosis | [41] |
| Strain | Bioactive Peptides | Food System | Bioactivities | References |
|---|---|---|---|---|
| NCDC-15 | KVLPVPQK (β-CN f169–176) YQEPVLGPVRGPFPIIV (β-CN f193–209) | Fermented milks | Angiotensin-I-converting-enzyme (ACE)-inhibitory peptides | [11] |
| DPC6026 | IKHQGLPQE, VLNENLLR, and SDIPNPIGSENSE | Bovine αs1-casein | Antibacterial activity against pathogenic strains Enterobacter sakazakii ATCC 12,868 and Escherichia coli DPC5063 | [49] |
| LA-5 | GVSKVKEAMAPK | Bovine β-CN | Antioxidant | [50] |
| LA-5 | DVENLHLPLPL | Bovine β-CN | ACE-inhibitory activity | [51] |
| LA-5 | GLDIQKVAGT, GLDIQKVAGTW | Bovine β-LG | ACE-inhibitory activity, antibacterial activity | [52] |
| LA | NEPTHLLKAFSKAGFQ | Milk, yogurt, cheeses | Antimicrobial activity | [48] |
| LA | Acidocin 4356 | Whey | Antimicrobial activity | [53] |
| CGMCC1.1878 | SLPS | Yogurt | Inhibited Staphylococcus aureus growth | [54] |
| ATCC 4356 | SLA | Kasar cheese | Increased the bioactivity of the cheese | [10] |
| NX371 | CAAATCAGTAATATGGAAAATC | Milk and cheese | Damaged the cell wall, and disrupted the membrane structure, resulting in leakage of intracellular ATP. | [55] |
| Strains | Fermentation Temperature and Time | Food Products | Findings | References |
|---|---|---|---|---|
| LA-5 | At 22 °C for 18 h | Milk products | Used as a bacterial supplement in fermented products | [84] |
| LA-5 | At 37 °C for 15 h | Sweet whey and skim milk | Skim milk microparticles allowed an increase in the viability of the probiotic | [84] |
| LA-5 | At 37 °C for 8 h | Fermented milk | Regulated the growth of probiotics in fermented milk prepared by a single probiotic strain | [6] |
| LA-5 | At 37 °C for 48 h | Yogurt | The addition of microencapsulated Lactobacillus acidophilus LA-5 improved the physiochemical properties of the yogurts | [85] |
| ATCC 4356 | At 42 °C for 4 h | Low-fat yogurt | High levels of barley bran (1.2%) decreased sensory prosperity scores and led to a viscosity increment; the amount of L. acidophilus and viscosity in samples containing barley bran was significantly higher than the control group | [68] |
| ATCC 4356 | At 37 °C for 18 h | Cheese whey | The production of valuable organic acids including pyruvate, propionate, acetate, lactate, formate, and butyrate | [86] |
| LA-3 | At 37 ◦C for 18 h | Reino cheese | Showed the highest lactobacilli viability (8.49 ± 0.08 Log CFU/g), provided additional protection to the L. acidophilus microorganism, benefiting microbial cell survival, and therefore resulted in a ripened Reino cheese | [62] |
| LA-308 | At 37 °C for 24 h | Meat analogs | Improved water-holding capacity and sensory properties of meat analogs, as well as reducing hardness and protein oxidation levels | [79] |
| CRL641 | At 37 °C for 24 h | Refrigerated meat | Inhibited Latilactobacillus sakei CRL1407; improved sensorial effects of both extracts | [87] |
| LA-5 | At 37 °C for 24 h | Chicken meat spread | Showed positive effects on its sensory attributes, resulting in an appreciable-quality probiotic chicken meat spread | [78] |
| IIA-2B4 | At room temperature for 24 h | Fermented beef sausage | The development of unique flavor compounds including acid, alcohols, aldehydes, aromatics, ketones, sulfur, hydrocarbons, and terpenes | [76] |
| ATCC 4356 | At 37 ◦C for 24 h | Probiotic bread | The combination of maltodextrin and xanthan gum in the alginate matrix provides the best survivability during storage | [82] |
| LA-5 | At 37 ◦C for 24 h | Bread | The application of alginate and chitosan in the microcapsules can protect the L. acidophillus and it is considered as an effective method in probiotic bread production | [83] |
| Strains | Fermentation Temperature and Time | Plant Beverage | Findings | References |
|---|---|---|---|---|
| ATCC 314 | At 37 °C for 12 h | Pigeon pea (Cajanus cajan) product | Higher viability and good sensory attributes; it should be considered suitable for a pigeon pea-based fermented probiotic product | [109] |
| La-05 | At 37 °C for 24 h | Incorporation in vegan milks | The utilization of chitosan coating in the alginate microparticle is recommended only for increasing the survival of the probiotic cultures in vegan milks | [110] |
| LA-02 | At 37 °C for 17 h | Different fruit juices | In apple juice, there is an increase in probiotic viability. In orange juice, microencapsulation also showed satisfactory results, as only microencapsulated probiotics were able to survive for 63 days, showing high viability | [111] |
| PTCC 1643 | At 37 °C for 24 h | Peach juice | The biological activities of peach juice including Maillard reaction inhibition, superoxide anion radical-scavenging activity, Fe-reducing power, and anti-inflammatory activity were markedly increased during the fermentation period | [112] |
| CH-2 | At 37 °C for 24 h | Pear juice | Metabolites were produced with strong antioxidant activity via the fermentation by L. acidophilus CH-2 for the degradation of browning products by fermentation | [90] |
| CICC®20709 | At 36 °C for 24 h | Loquat juice | Significantly enhanced the antioxidant activity of loquat juice | [113] |
| LA-26 | At 36 °C for 24 h | Grape juice | Better tastes, such as sourness, and aromas but also decreased the amount of bitterness and levels of sulfur compounds | [93] |
| LA-20079 | At 37 °C for 48 h | Fermented beverage | Highest pH decreases and sugar consumption showed a much better growth rate | [92] |
| TISTR 2365 | At 36 °C for 24 h | Fermented rice (khoa mak) sap beverage | Significant increase in total phenolic contents and DPPH radical scavenging activities | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Nawazish, H.; Farid, M.S.; Abdul Qadoos, K.; Habiba, U.E.; Muzamil, M.; Tanveer, M.; Sienkiewicz, M.; Lichota, A.; Łopusiewicz, Ł. Health-Promoting Effects of Lactobacillus acidophilus and Its Technological Applications in Fermented Food Products and Beverages. Fermentation 2024, 10, 380. https://doi.org/10.3390/fermentation10080380
Liu Y, Nawazish H, Farid MS, Abdul Qadoos K, Habiba UE, Muzamil M, Tanveer M, Sienkiewicz M, Lichota A, Łopusiewicz Ł. Health-Promoting Effects of Lactobacillus acidophilus and Its Technological Applications in Fermented Food Products and Beverages. Fermentation. 2024; 10(8):380. https://doi.org/10.3390/fermentation10080380
Chicago/Turabian StyleLiu, Yanyan, Hira Nawazish, Muhammad Salman Farid, Khansa Abdul Qadoos, Umm E. Habiba, Muhammad Muzamil, Mahwish Tanveer, Monika Sienkiewicz, Anna Lichota, and Łukasz Łopusiewicz. 2024. "Health-Promoting Effects of Lactobacillus acidophilus and Its Technological Applications in Fermented Food Products and Beverages" Fermentation 10, no. 8: 380. https://doi.org/10.3390/fermentation10080380
APA StyleLiu, Y., Nawazish, H., Farid, M. S., Abdul Qadoos, K., Habiba, U. E., Muzamil, M., Tanveer, M., Sienkiewicz, M., Lichota, A., & Łopusiewicz, Ł. (2024). Health-Promoting Effects of Lactobacillus acidophilus and Its Technological Applications in Fermented Food Products and Beverages. Fermentation, 10(8), 380. https://doi.org/10.3390/fermentation10080380







