Advancing Thermophilic Anaerobic Digestion of Corn Whole Stillage: Lignocellulose Decomposition and Microbial Community Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Substrate and Inoculum
2.2. Advanced Wet Oxidation and Steam Explosion (AWOEx) Pretreatment
2.3. Reactors Setup and Operation
2.4. Analytical Methods
2.4.1. Characterization of Whole Stillage, Reactor Feed, and Inoculum
2.4.2. Biogas Production Measurements and Composition Analysis
2.4.3. Digestate Characterization
2.4.4. DNA Isolation
2.4.5. 16S rDNA Sequencing
2.4.6. Taxonomic Identification of Amplicon Sequence Variants
2.4.7. Statistical Analysis and Calculations
3. Results and Discussion
3.1. Feed Substrate Properties
3.2. Biogas Production and Composition
3.3. Digestate Characteristics
3.3.1. Volatile Fatty Acids
3.3.2. Organic Matter Conversions (VS, COD, and Carbohydrates)
3.3.3. Microbial Community Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The Role of Renewable Energy in the Global Energy Transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Popp, J.; Kovács, S.; Oláh, J.; Divéki, Z.; Balázs, E. Bioeconomy: Biomass and Biomass-Based Energy Supply and Demand. New Biotechnol. 2021, 60, 76–84. [Google Scholar] [CrossRef]
- Renewable Fuels Association (RFA). Annual Ethanol Production U.S. and World Ethanol Production. 2021. Available online: https://ethanolrfa.org/markets-and-statistics/annual-ethanol-production (accessed on 16 April 2024).
- Buenavista, R.M.E.; Siliveru, K.; Zheng, Y. Utilization of Distiller’s Dried Grains with Solubles: A Review. J. Agric. Food Res. 2021, 5, 100195. [Google Scholar] [CrossRef]
- Toraman, H.E. Alternative Fuels from Biomass Sources; Energy Institute, Pennsylvania State University: University Park, PA, USA, 2023. [Google Scholar]
- Reis, C.E.R.; Rajendran, A.; Hu, B. New Technologies in Value Addition to the Thin Stillage from Corn-to-Ethanol Process. Rev. Environ. Sci. Biotechnol. 2017, 16, 175–206. [Google Scholar] [CrossRef]
- U.S. Department, of Agriculture (USDA). National Agricultural Statistics Service, Grain Crushings and Co-Products Production 2021 Summary; USDA: Washington, DC, USA, 2022; ISSN 2470-9913.
- Drosg, B.; Fuchs, W.; Meixner, K.; Waltenberger, R.; Kirchmayr, R.; Braun, R.; Bochmann, G. Anaerobic Digestion of Stillage Fractions—Estimation of the Potential for Energy Recovery in Bioethanol Plants. Water Sci. Technol. 2013, 67, 494–505. [Google Scholar] [CrossRef]
- Zangaro, C.A.; Patterson, R.; Gibbons, W.R.; Woyengo, T.A. Enhancing the Nutritive Value of Corn Whole Stillage for Pigs via Pretreatment and Predigestion. J. Agric. Food Chem. 2018, 66, 9409–9417. [Google Scholar] [CrossRef]
- Jerez-Bogota, K.S.; Gibbons, W.; Woyengo, T.A. Chemical Composition and Porcine in Vitro Digestibility of Corn Whole Stillage Pretreated with Heat at Various Temperatures and Times. Anim. Feed Sci. Technol. 2021, 280, 115041. [Google Scholar] [CrossRef]
- Fuess, L.T.; Garcia, M.L. Implications of Stillage Land Disposal: A Critical Review on the Impacts of Fertigation. J. Environ. Manage 2014, 145, 210–229. [Google Scholar] [CrossRef]
- Agler, M.T.; Garcia, M.L.; Lee, E.S.; Schlicher, M.; Angenent, L.T. Thermophilic Anaerobic Digestion to Increase the Net Energy Balance of Corn Grain Ethanol. Environ. Sci. Technol. 2008, 42, 6723–6729. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Kennedy, K.J.; Marin, J.; Strehler, B. Anaerobic Digestion of Whole Stillage from Dry-Grind Corn Ethanol Plant under Mesophilic and Thermophilic Conditions. Bioresour. Technol. 2011, 102, 1079–1086. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Ghorbani, M. Effect of Inoculum/Substrate Ratio on Mesophilic Anaerobic Digestion of Bioethanol Plant Whole Stillage in Batch Mode. Process Biochem. 2011, 46, 1682–1687. [Google Scholar] [CrossRef]
- Gyenge, L.; Raduly, B.; Crognale, S.; Stazi, S.-R.; Lanyi, S.; Abraham, B. Biogas production from corn bioethanol whole stillage: Evaluation of two different inocula. Environ. Eng. Manag. J. 2018, 17, 1021–1028. [Google Scholar] [CrossRef]
- Lee, P.; Bae, J.; Kim, J.; Chen, W. Mesophilic Anaerobic Digestion of Corn Thin Stillage: A Technical and Energetic Assessment of the Corn-to-ethanol Industry Integrated with Anaerobic Digestion. J. Chem. Technol. Biotechnol. 2011, 86, 1514–1520. [Google Scholar] [CrossRef]
- Schaefer, S.H.; Sung, S. Retooling the Ethanol Industry: Thermophilic Anaerobic Digestion of Thin Stillage for Methane Production and Pollution Prevention. Water Environ. Res. 2008, 80, 101–108. [Google Scholar] [CrossRef]
- Wilkie, A.C.; Riedesel, K.J.; Owens, J.M. Stillage Characterization and Anaerobic Treatment of Ethanol Stillage from Conventional and Cellulosic Feedstocks. Biomass Bioenergy 2000, 19, 63–102. [Google Scholar] [CrossRef]
- Wu, B.; Lin, R.; Kang, X.; Deng, C.; Xia, A.; Dobson, A.D.W.; Murphy, J.D. Graphene Addition to Digestion of Thin Stillage Can Alleviate Acidic Shock and Improve Biomethane Production. ACS Sustain. Chem. Eng. 2020, 8, 13248–13260. [Google Scholar] [CrossRef]
- Westerholm, M.; Hansson, M.; Schnürer, A. Improved Biogas Production from Whole Stillage by Co-Digestion with Cattle Manure. Bioresour. Technol. 2012, 114, 314–319. [Google Scholar] [CrossRef]
- Town, J.R.; Dumonceaux, T.J. Laboratory-Scale Bioaugmentation Relieves Acetate Accumulation and Stimulates Methane Production in Stalled Anaerobic Digesters. Appl. Microbiol. Biotechnol. 2016, 100, 1009–1017. [Google Scholar] [CrossRef]
- Gáspár, M.; Kálmán, G.; Réczey, K. Corn Fiber as a Raw Material for Hemicellulose and Ethanol Production. Process Biochem. 2007, 42, 1135–1139. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, G.; Ning, Y.; Li, X.; Hu, S.; Zhao, J.; Qu, Y. Production of Cellulosic Ethanol and Value-Added Products from Corn Fiber. Bioresour. Bioprocess. 2022, 9, 81. [Google Scholar] [CrossRef]
- Akizuki, S.; Suzuki, H.; Fujiwara, M.; Toda, T. Impacts of Steam Explosion Pretreatment on Semi-Continuous Anaerobic Digestion of Lignin-Rich Submerged Macrophyte. J. Clean. Prod. 2023, 385, 135377. [Google Scholar] [CrossRef]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet Explosion: A Universal and Efficient Pretreatment Process for Lignocellulosic Biorefineries. BioEnergy Res. 2015, 8, 1101–1116. [Google Scholar] [CrossRef]
- Rana, D.; Rana, V.; Ahring, B.K. Producing High Sugar Concentrations from Loblolly Pine Using Wet Explosion Pretreatment. Bioresour. Technol. 2012, 121, 61–67. [Google Scholar] [CrossRef]
- Ahring, B.K.; Munck, J. Method for Treating Biomass and Organic Waste with the Purpose of Generating Desired Biologically Based Products. U.S. Patent 8,506,716, 13 August 2013. [Google Scholar]
- Usman Khan, M.; Kiaer Ahring, B. Anaerobic Digestion of Biorefinery Lignin: Effect of Different Wet Explosion Pretreatment Conditions. Bioresour. Technol. 2020, 298, 122537. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass. Natl. Renew. Energy Lab. 2008, 1617, 1–16. [Google Scholar]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Dutta, N.; Garrison, R.; Usman, M.; Ahring, B.K. Enhancing Methane Production of Anaerobic Digested Sewage Sludge by Advanced Wet Oxidation & Steam Explosion Pretreatment. Environ. Technol. Innov. 2022, 28, 102923. [Google Scholar] [CrossRef]
- ThermoFisher Scientific. How to Use Phenol/Chloroform for DNA Purification. 2023. Available online: https://www.thermofisher.com/us/en/home/references/protocols/nucleic-acid-purification-and-analysis/dna-extraction-protocols/phenol-chloroform-extraction.html (accessed on 15 January 2023).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- Bisanz, J.E. qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. 2018. Available online: https://Github.Com/Jbisanz/qiime2R (accessed on 12 March 2024).
- Smith, S. Phylosmith: An R-Package for Reproducible and Efficient Microbiome Analysis with Phyloseq-Objects. J. Open Source Softw. 2019, 4, 1442. [Google Scholar] [CrossRef]
- Liu, S.; Feng, H.; Li, T.; Wang, Y.; Rong, N.; Yang, W. Highly Selective Production of Propionic Acid from Lactic Acid Catalyzed by NaI. Green Chem. 2020, 22, 7468–7475. [Google Scholar] [CrossRef]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to Energy: A Focus on the Impact of Substrate Type in Biogas Production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Pereira, M.A.; Alves, M. Enhancement of Methane Production from Long Chain Fatty Acid Based Effluents. Bioresour. Technol. 2008, 99, 4086–4095. [Google Scholar] [CrossRef]
- Ziero, H.D.D.; Ampese, L.C.; Buller, L.S.; Oliani Trevisan, V.; Gouvêa, M.T.; Rosa, M.T.M.G.; Berni, M.D.; Forster-Carneiro, T. Energy Generation from Thin Stillage Anaerobic Digestion in Stand-Alone Corn Ethanol Mills. Ind. Crops Prod. 2022, 188, 115601. [Google Scholar] [CrossRef]
- Bo, Z.; Wei-min, C.; Pin-jing, H. Influence of Lactic Acid on the Two-Phase Anaerobic Digestion of Kitchen Wastes. J. Environ. Sci. 2007, 19, 244–249. [Google Scholar] [CrossRef]
- Perman, E.; Schnürer, A.; Björn, A.; Moestedt, J. Serial Anaerobic Digestion Improves Protein Degradation and Biogas Production from Mixed Food Waste. Biomass Bioenergy 2022, 161, 106478. [Google Scholar] [CrossRef]
- Granja-Salcedo, Y.T.; Ramirez-Uscategui, R.A.; Machado, E.G.; Duarte Messana, J.; Takeshi Kishi, L.; Lino Dias, A.V.; Berchielli, T.T. Studies on Bacterial Community Composition Are Affected by the Time and Storage Method of the Rumen Content. PLoS ONE 2017, 12, e0176701. [Google Scholar] [CrossRef]
- Hahnke, S.; Langer, T.; Koeck, D.E.; Klocke, M. Description of Proteiniphilum Saccharofermentans Sp. Nov., Petrimonas Mucosa Sp. Nov. and Fermentimonas Caenicola Gen. Nov., Sp. Nov., Isolated from Mesophilic Laboratory-Scale Biogas Reactors, and Emended Description of the Genus Proteiniphilum. Int. J. Syst. Evol. Microbiol. 2016, 66, 1466–1475. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, H.; Wang, H.; Zhou, W.; Zhao, D.; Qiao, Z.; Zheng, J.; Ren, C.; Xu, Y. Proteiniphilum Propionicum Sp. Nov., a Novel Member of the Phylum Bacteroidota Isolated from Pit Clay Used to Produce Chinese Liquor. Int. J. Syst. Evol. Microbiol. 2022, 72, 005612. [Google Scholar] [CrossRef]
- Hania, W.B.; Bouanane-Darenfed, A.; Cayol, J.-L.; Ollivier, B.; Fardeau, M.-L. Reclassification of Anaerobaculum Mobile, Anaerobaculum Thermoterrenum, Anaerobaculum Hydrogeniformans as Acetomicrobium Mobile Comb. Nov., Acetomicrobium Thermoterrenum Comb. Nov. and Acetomicrobium Hydrogeniformans Comb. Nov., Respectively, and Emendation of the Genus Acetomicrobium. Int. J. Syst. Evol. Microbiol. 2016, 66, 1506–1509. [Google Scholar] [CrossRef]
- Dyksma, S.; Jansen, L.; Gallert, C. Syntrophic Acetate Oxidation Replaces Acetoclastic Methanogenesis during Thermophilic Digestion of Biowaste. Microbiome 2020, 8, 105. [Google Scholar] [CrossRef]
- Lu, J.; Jia, Z.; Wang, P.; Yang, X.; Lin, P.; Ren, L.; Farghali, M. Restoration of Acidified Dry Anaerobic Digestion of Food Waste: Bioaugmentation of Butyric Acid-Resistant Microbes. J. Environ. Chem. Eng. 2022, 10, 106935. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Guo, X.; Liu, T.; Wu, J.; Ren, N. Efficient Biogas Production from Cattle Manure in a Plug Flow Reactor: A Large Scale Long Term Study. Bioresour. Technol. 2019, 278, 450–455. [Google Scholar] [CrossRef]
- Crocker, A.W.; Harty, C.E.; Hammond, J.H.; Willger, S.D.; Salazar, P.; Botelho, N.J.; Jacobs, N.J.; Hogan, D.A. Pseudomonas Aeruginosa Ethanol Oxidation by AdhA in Low-Oxygen Environments. J. Bacteriol. 2019, 201, 10–1128. [Google Scholar] [CrossRef]
- Moset, V.; Poulsen, M.; Wahid, R.; Højberg, O.; Møller, H.B. Mesophilic versus Thermophilic Anaerobic Digestion of Cattle Manure: Methane Productivity and Microbial Ecology. Microb. Biotechnol. 2015, 8, 787–800. [Google Scholar] [CrossRef]
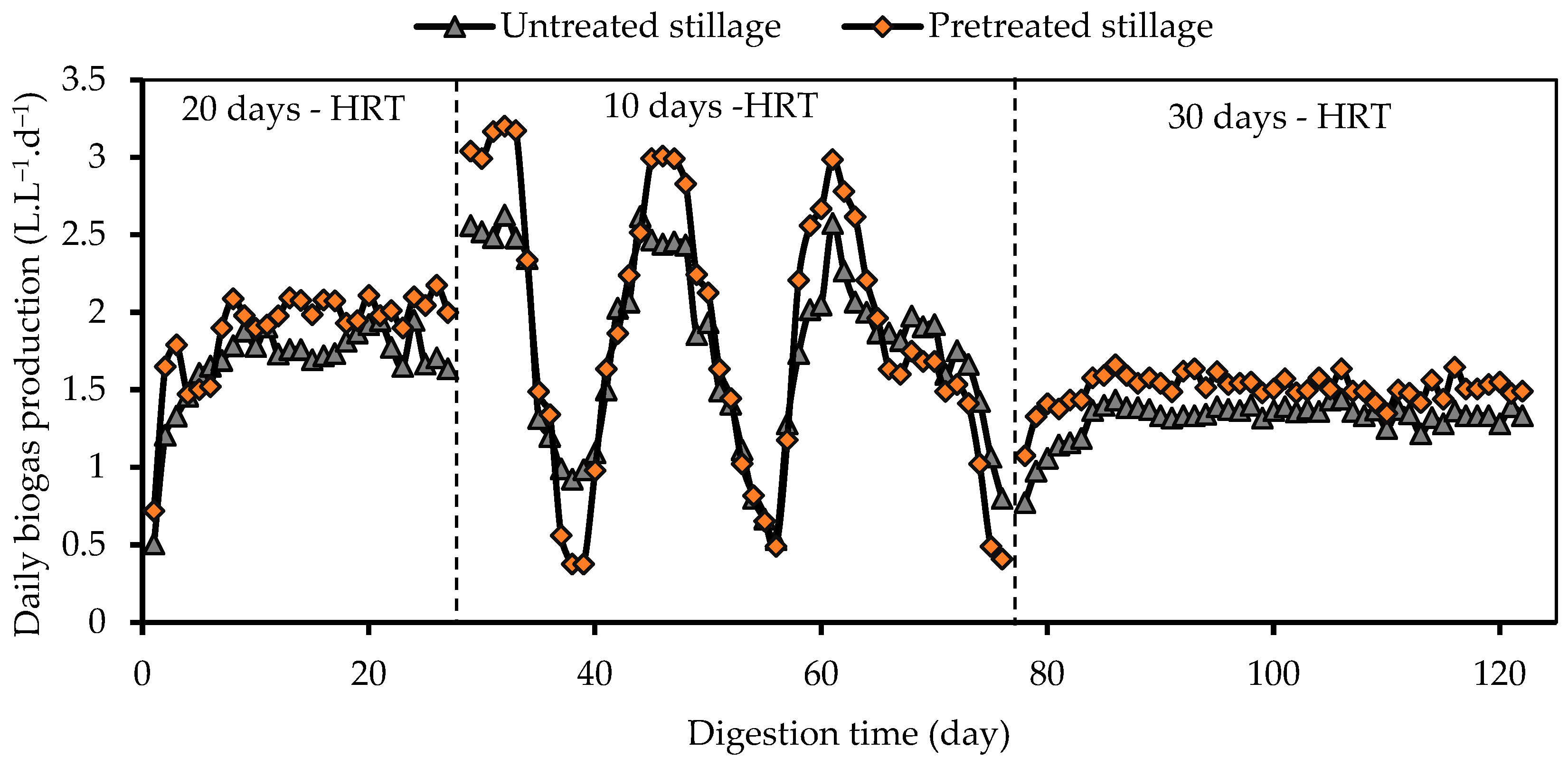
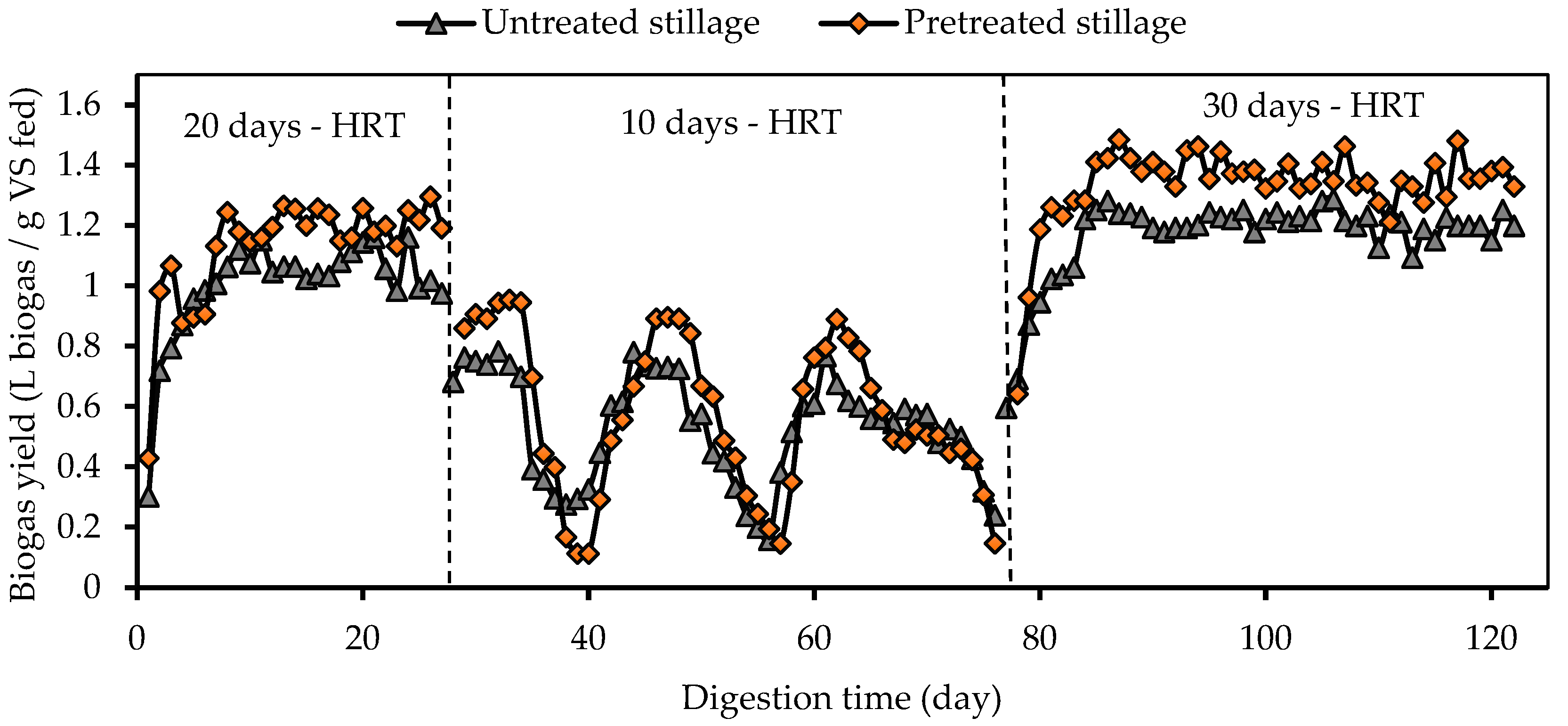

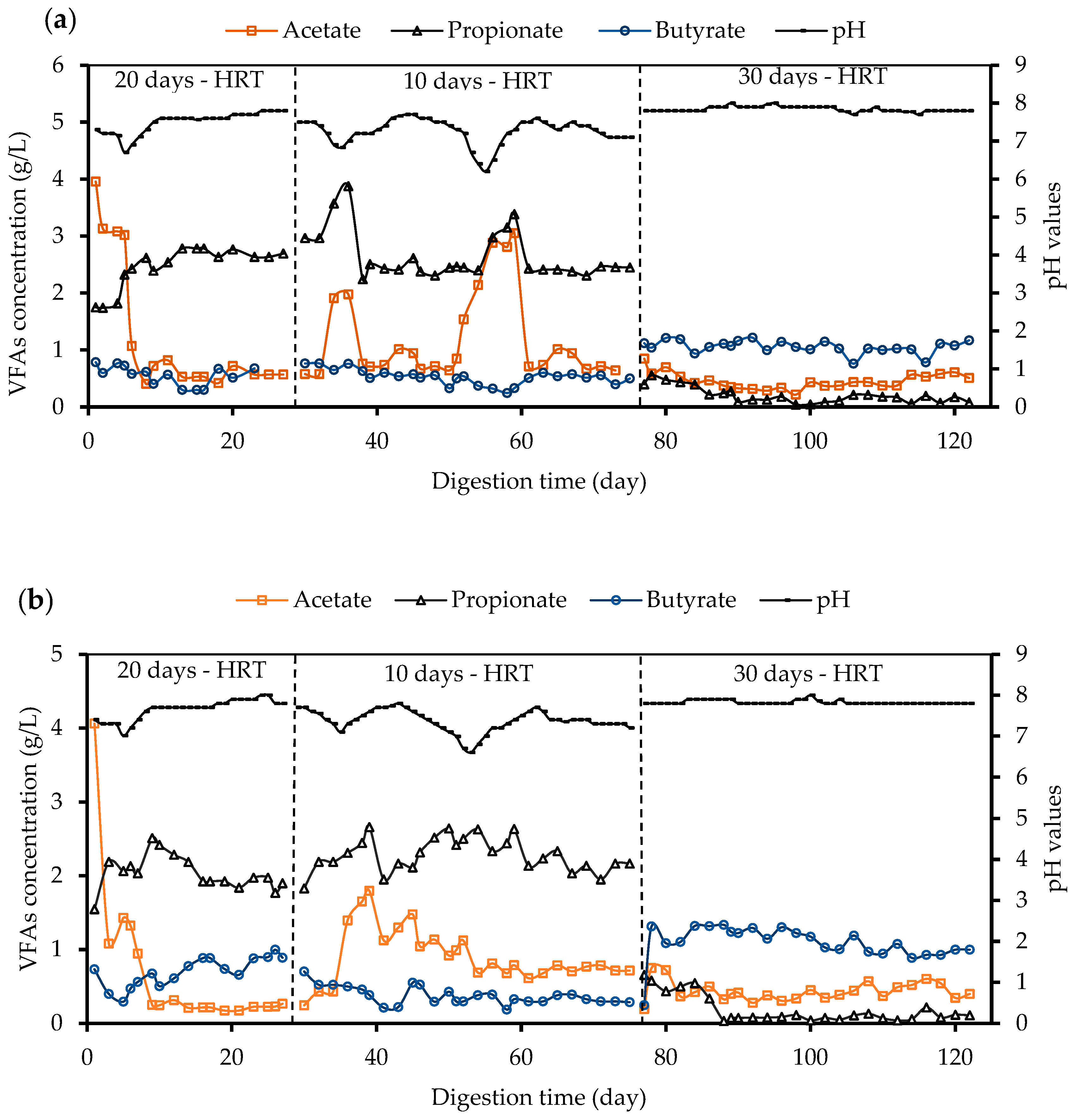


| Parameter | Pretreated Whole Stillage | Crude Whole Stillage | Inoculum |
|---|---|---|---|
| TS (%, w/w) | 9.05 ± 0.02 | 11.01 ± 0.26 | 3.50 ± 0.06 |
| VS (%, w/w) | 8.40 ± 0.0.04 | 10.3 ± 0.20 | 2.18 ± 0.03 |
| VS/TS | 93 ± 0.22 | 94 ± 0.47 | 62.38 ± 0.33 |
| TCOD (g/L) | 112.38 ± 3.86 | 116.22 ± 0.87 | 75.64 ± 0.24 |
| SCOD (g/L) | 76.68 ± 6.76 | 69.48 ± 0.77 | 18.39 ± 0.54 |
| Acetic acid (g/L) | 1.48 ± 0.07 | 1.22 ± 0.03 | 0.38 ± 0.03 |
| Propionic acid (g/L) | 0.86 ± 0.07 | 0.37 ± 0.14 | 0.81 ± 0.12 |
| Butyric acid (g/L) | 1.60 ± 0.10 | 1.98 ± 0.12 | 1.07 ± 0.14 |
| Isocaproic acid (g/L) | – | 0.05 ± 0.01 | – |
| Caproic acid (g/L) | – | 0.17 ± 0.02 | 0.14 ± 0.00 |
| TVFA (g/L) | 3.94 | 3.79 | 2.40 |
| Lactic acid (g/L) | 3.56 ± 0.12 | 4.27 ± 0.25 | – |
| Cellulose (%) | 11.66 ± 0.70 | 10.04 ± 1.33 | 8.82 ± 1.58 |
| Hemicellulose (%) | 8.64 ± 1.34 | 16.36 ± 2.27 | 7.96 ± 1.31 |
| Lignin (%) | 17.89 ± 1.12 | 13.58 ± 1.25 | 27.64 ± 0.91 |
| Glucose (g/L) * | 0.09 ± 0.01 | – | – |
| Cellobiose (g/L) * | 0.42 ± 0.05 | 0.67 ± 0.08 | – |
| Xylose (g/L) * | 1.82 ± 0.12 | 0.16 ± 0.06 | – |
| Arabinose (g/L) * | 0.48 ± 0.09 | 0.19 ± 0.02 | – |
| Crude fat (%) | 8.2 | 7.1 | – |
| Crude protein (%) | 32.79 | 29.96 | |
| Ash (%) | 3.56 ± 0.14 | 4.15 ± 0.36 | 5.27 ± 0.32 |
| Potassium (%) | 2.64 | 2.48 | – |
| Phosphorus (%) | 1.03 | 1.12 | – |
| Calcium (%) | 0.11 | 0.11 | – |
| Magnesium (%) | 0.04 | 0.04 | – |
| pH | 4.15 ± 0.01 | 3.99 ± 0.01 | 7.3 ± 0.07 |
| Parameters | Pretreated Stillage Reactor | Untreated Stillage Reactor | ||||
|---|---|---|---|---|---|---|
| 20-Day HRT | 10-Day HRT | 30-Day HRT | 20-Day HRT | 10-Day HRT | 30-Day HRT | |
| Digestion conditions | ||||||
| Digestion duration (d) | 27 | 49 | 46 | 27 | 49 | 46 |
| Temperature (°C) | 55 | 55 | 55 | 55 | 55 | 55 |
| OLR (kg VS/m3 d) | 1.67 ± 0.01 | 3.35 ± 0.01 | 1.12 ± 0.03 | 1.67 ± 0.01 | 3.35 ± 0.01 | 1.12 ± 0.03 |
| OLR (kg TCOD/m3 d) | 2.91 ± 0.00 | 5.81 ± 0.02 | 1.94 ± 0.01 | 2.91 ± 0.00 | 5.81 ± 0.02 | 1.94 ± 0.01 |
| Solids concentration and conversion efficiency | ||||||
| TS (%) | 1.52 ± 0.03 | 1.74 ± 0.04 | 1.26 ± 0.04 | 1.53 ± 0.02 | 1.69 ± 0.02 | 1.35 ± 0.05 |
| VS (%) | 0.87 ± 0.03 | 1.15 ± 0.04 | 0.63 ± 0.06 | 1.04 ± 0.03 | 1.22 ± 0.02 | 0.71 ± 0.03 |
| VS conversion (%) | 81.20 ± 0.56 | 75.03 ± 0.64 | 86.31 ± 1.14 | 77.19 ± 0.59 | 73.56 ± 0.36 | 84.49 ± 0.57 |
| TS conversion (%) | 69.59 ± 0.52 | 65.13 ± 0.72 | 74.65 ± 0.60 | 69.25 ± 0.36 | 66.05 ± 0.39 | 72.73 ± 1.09 |
| TVFA (g/L) | 4.83 | 5.5 | 1.90 | 4.48 | 5.01 | 1.92 |
| Acetic acid (g/L) | 0.58 ± 0.13 | 1.17 ± 0.19 | 0.45 ± 0.24 | 0.47 ± 0.20 | 0.99 ± 0.34 | 0.44 ± 0.12 |
| Propionic acid (g/L) | 2.47 ± 0.36 | 2.66 ± 0.41 | 0.21 ± 0.14 | 2.00 ± 0.27 | 2.29 ± 0.23 | 0.19 ± 0.09 |
| Butyric acid (g/L) | 0.56 ± 0.17 | 0.54 ± 0.14 | 1.06 ± 0.11 | 0.69 ± 0.20 | 0.38 ± 0.12 | 1.11 ± 0.22 |
| Isobutyric acid (g/L) | 0.11 ± 0.02 | 0.10 ± 0.02 | – | 0.21 ± 0.03 | 0.14 ± 0.03 | – |
| Isovaleric acid (g/L) | 0.44 ± 0.06 | 0.53 ± 0.02 | – | 0.49 ± 0.03 | 0.53 ± 0.08 | – |
| Valeric acid (g/L) | 0.12 ± 0.02 | 0.06 ± 0.00 | – | 0.06 ± 0.02 | 0.12 ± 0.03 | – |
| Isocaproic acid (g/L) | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| Caproic acid (g/L) | 0.27 ± 0.02 | 0.18 ± 0.01 | 0.13 ± 0.00 | 0.29 ± 0.17 | 0.29 ± 0.03 | 0.13 ± 0.01 |
| Heptanoic acid (g/L) | 0.23 ± 0.00 | 0.22 ± 0.01 | – | 0.22 ± 0.01 | 0.22 ± 0.01 | – |
| Effluent COD and pH | ||||||
| SCOD (g/L) | 13.89 ± 1.28 | 18.77 ± 0.42 | 8.122 ± 0.18 | 16.13 ± 0.25 | 21.85 ± 0.98 | 11.97 ± 0.83 |
| TCOD conversion (%) | 83.33 ± 1.51 | 74.55 ± 0.12 | 87.60 ± 0.45 | 82.16 ± 1.73 | 73.04 ± 0.36 | 85.98 ± 1.04 |
| pH value | 7.7 + 0.08 | 7.2 ± 0.19 | 7.8 ± 0.03 | 7.8 ± 0.13 | 7.3 ± 0.3 | 7.8 ± 0.07 |
| Lignocellulosic concentration and conversion efficiency | ||||||
| Cellulose conc. (%) | 2.73 ± 0.29 | 3.35 ± 0.17 | 1.71 ± 0.15 | 2.47 ± 0.10 | 5.00 ± 0.45 | 1.99 ± 0.31 |
| Hemicellulose conc. (%) | 2.21 ± 0.48 | 2.68 ± 0.62 | 1.16 ± 0.09 | 2.42 ± 0.09 | 4.54 ± 0.31 | 1.52 ± 0.24 |
| Lignin conc. (%) | 29.66 ± 0.11 | 35.34 ± 2.85 | 26.38 ± 0.13 | 25.10 ± 1.08 | 27.38 ± 1.62 | 24.53 ± 1.33 |
| Cellulose conversion (%) | 92.87 ± 0.75 | 89.90 ± 0.58 | 95.20 ± 1.32 | 91.70 ± 0.36 | 83.13 ± 1.22 | 94.13 ± 0.72 |
| Hemicellulose conversion (%) | 94.87 ± 1.00 | 92.78 ± 2.90 | 96.60 ± 0.27 | 93.00 ± 0.18 | 90.60 ± 0.52 | 95.18 ± 0.35 |
| Lignin conversion (%) | 49.46 ± 0.20 | 32.11 ± 2.95 | 58.92 ± 0.19 | 37.57 ± 2.69 | 31.72 ± 1.89 | 50.85 ± 2.44 |
| HRT (Day) | Richness | ACE | Shannon | Simpson (Diversity) | Fisher |
|---|---|---|---|---|---|
| 20 | 345 | 345 | 4.75 | 0.981 | 54.7 |
| 10 | 247 | 247 | 4.11 | 0.963 | 37.2 |
| 30 | 217 | 217 | 4.07 | 0.966 | 32.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokhary, A.; Ale Enriquez, F.; Garrison, R.; Ahring, B.K. Advancing Thermophilic Anaerobic Digestion of Corn Whole Stillage: Lignocellulose Decomposition and Microbial Community Characterization. Fermentation 2024, 10, 306. https://doi.org/10.3390/fermentation10060306
Bokhary A, Ale Enriquez F, Garrison R, Ahring BK. Advancing Thermophilic Anaerobic Digestion of Corn Whole Stillage: Lignocellulose Decomposition and Microbial Community Characterization. Fermentation. 2024; 10(6):306. https://doi.org/10.3390/fermentation10060306
Chicago/Turabian StyleBokhary, Alnour, Fuad Ale Enriquez, Richard Garrison, and Birgitte Kiaer Ahring. 2024. "Advancing Thermophilic Anaerobic Digestion of Corn Whole Stillage: Lignocellulose Decomposition and Microbial Community Characterization" Fermentation 10, no. 6: 306. https://doi.org/10.3390/fermentation10060306
APA StyleBokhary, A., Ale Enriquez, F., Garrison, R., & Ahring, B. K. (2024). Advancing Thermophilic Anaerobic Digestion of Corn Whole Stillage: Lignocellulose Decomposition and Microbial Community Characterization. Fermentation, 10(6), 306. https://doi.org/10.3390/fermentation10060306






