Abstract
This study aimed to investigate the effects of yeast cultures on the antioxidant capacity, rumen fermentation, and growth performance of goats in the summer. An in vitro experiment was conducted using yeast culture supplemented at 0% (control), 0.6% (test 1), 0.9% (test 2), and 1.2% (test 3) of the dry matter (DM) weight of the basal diet. With a 24 h fermentation, the pH value; the total short-chain fatty acid, acetic acid, propionic acid, and butyric acid concentrations; and the degradability of the DM, the neutral detergent fiber, and the acid detergent fiber were significantly increased (p < 0.05) in tests 2 and 3 compared with the control group. In the feeding experiment, thirty-six crossbreed goats aged 3.0 ± 0.5 months with a body weight of 11.08 ± 1.41 kg were divided, and the yeast culture was supplemented at 0% (control), 0.90% (test 1), and 1.20% (test 2) of the basal diet. Similar effects on rumen fermentation parameters were obtained in test 1 and 2 groups compared to the in vitro experiment. Moreover, the dry matter intake, average daily gain, serum total antioxidant capacity, and the activities of total superoxide dismutase and glutathione peroxidase were significantly higher and the malondialdehyde concentration was significantly lower (p < 0.05) in tests 1 and 2 compared with the control. The results indicated that yeast culture (0.90%) could improve the antioxidant capacity, rumen fermentation, and growth performance of goats in summer. The optimal supplementation concentration is 0.90% DM.
Keywords:
yeast culture; summer; oxidative stress; in vitro; in vivo; rumen fermentation; growth performance 1. Introduction
In summer, the heat generated by the animal body can not be effectively dispersed to the environment under environmental conditions with high temperatures, increasing the internal heat load of the body. As a result, heat stress will occur [1]. Heat stress is a series of non-specific physiological reactions the body makes after high-temperature stimulation [2]. As one of the most heat-resistant species, goats have a wide isothermal zone and high heat resistance. However, too high of an ambient temperature and humidity will still cause heat stress [3,4]. A previous study showed that heat stress would occur when goats were exposed to an ambient temperature of 33.3 ± 1.2 °C, a relative humidity of 72.3 ± 2.3%, and a temperature and humidity index of 84.76 for 15 days [5]. During the heat stress period, the body’s antioxidant enzyme system is damaged, and many reactive oxygen species that cannot be cleared in time cause an oxidation–antioxidant imbalance in the body, resulting in an oxidative stress reaction [4]. Studies have shown that heat stress can reduce the activity of SOD, GSH-Px, and T-AOC in the serum of Tibetan sheep and goats; impair their antioxidant function; and induce oxidative stress in the body [6]. Further studies have found that oxidative stress can cause lipid oxidation and DNA damage; and the excessive free radicals produced can induce enteric-related diseases such as enteritis and increased intestinal mucosal permeability in vivo and can reduce animal growth performance [7]. The rumen, an essential digestive organ of ruminants, maintains physiological health and growth performance [4]. Under normal physiological conditions, bacteria, fungi, protozoa, and other microorganisms inhabiting the rumen are in dynamic balance [8]. However, under heat stress, the abundance of fibrolytic bacteria in the rumen decrease, which reduces the ability of the rumen to decompose fibers and affects the production of rumen VFA [8]. A previous study reported that the concentration of total fatty acids, acetic acid, and propionic acid in the rumen of heat-stressed goats decreased significantly [9]. In intensive goat farming, growth performance is a crucial aspect and is of great significance for the operation and management of the farming industry. Dry matter intake (DMI) is the most direct indicator of the growth performance of animals [9]. Goats were kept in a hot environment for a few days, and the DMI decreased significantly [9,10,11]. Previous studies reported that the digestibility of dry matter (DM), neutral detergent fiber (NDF), and acidic detergent fiber (ADF) is significantly decreased both in dairy and meat goats in hot environments [9,12,13,14]. Previous studies reported that heat stress significantly reduced body weight in Malpura ewes; West African dwarf goats; Osmanabadi, Malabari, and Salem Black goats; and Macheng–Boer crossed goats [9,14,15,16,17]. Therefore, we can determine that heat stress has a systemic adverse effect on ruminants. As a result, seeking an efficient and practical method to alleviate these adverse effects is necessary.
In high temperatures and in high-humidity climates in the summer, yeast can effectively alleviate the adverse effects of heat stress on rumen fermentation and the growth performance of goats [5,14,18,19,20]. Yeast culture is rich in amino acids, small molecular peptides, oligosaccharides, vitamins, minerals, digestive enzymes, and growth-promoting factors [21]. Therefore, it is widely used to regulate rumen function, and the effect is more evident under heat stress conditions [22]. Yeast can enhance the DMI and digestibility of NDF and ADF. Therefore, it significantly promotes the growth and development of goats [23,24,25]. So far, few studies have been conducted on the application of yeast cultures in heat-stressed goats. This study aimed to investigate the impact of yeast cultures on the antioxidant capacity, rumen fermentation, and growth performance of goats in the summer. We hypothesized that the yeast culture has a positive promoting effect on these parameters. In that case, this study will provide scientific guidance for improving the rumen fermentation and growth promotion of goats by yeast culture in the summer.
2. Materials and Methods
2.1. In Vitro Fermentation
Twelve goats, aged 3.0 ± 0.5 months with a body weight of 10.02 ± 1.29 kg, were divided into a non-heat stress group (NHS) and a heat stress group (HS). These goats were kept in two different thermally controlled (with an air-conditioner and an air humidifier) rooms. For the NHS group, the room temperature and relative humidity were maintained around 25.24 °C and 60.31%, respectively. For the HS group, the room temperature and relative humidity were maintained around 33.32 °C and 74.45%, respectively. The goats were fed twice daily (8:00 h; 17:00 h) with a 1.20 kg/day maintenance diet and had free access to water. According to the LPHSIs [26], the temperature–humidity index (THI) of the NHS and HS groups were 73.18 and 87.20, respectively. When the THI is greater than 82, the goats are considered to be suffering from heat stress. The rectal and skin temperatures, pulse, and respiratory rate were measured at 8:00, 12:00, and 17:00 every day during the 14-day period. The rectal temperature was measured using a thermometer (Fangda Pharmaceutical Machinery Co., Ltd., Hefei, China). The skin temperature was measured with an infrared thermometer (Omega, GA, USA). A stethoscope (YuwellCo., Ltd., Shanghai, China) was placed laterally in the thoracic area to monitor inhalation and exhalation and was placed ventrally to measure the pulse. On day 14, blood samples were taken from the jugular vein of all the goats of both the NHS and HS groups after 12 h of fasting. The measurement of the HSP 70 family of genes in the blood lymphocytes of goats was described by Peng et al. [27]. Peripheral blood lymphocytes were isolated from the whole blood using a kit provided by Solarbio Science & Technology (Beijing, China), following the manufacturer’s instructions. The total RNA of the peripheral blood lymphocytes was extracted using TRIzol® Reagent (Life Technologies, Carlsbad, CA, USA). A Revert Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) was used for the reverse transcription, following the manufacturer’s instructions. Primer 5.0 software (Primers, ON, Canada) was used for the primer design. The primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The primer sequences of the heat-stress-related genes are shown in Table 1 An SYBR RT-PCR Kit (Bio-Rad, Hercules, CA, USA) and an ABI QuanStudio TM6 flex real-time fluorescent quantitative PCR system (Life Technologies, Carlsbad, CA, USA) were used for conducting the RT-PCR processes. The levels of relative expression were quantified using the 2−ΔΔCt method [28], and each sample was analyzed in sextuplicate to ensure the accuracy of the results. The blood samples were centrifuged at 1800× g for 10 min to obtain the serum. The cortisol concentration was measured in serum samples [29] using a cortisol assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the manufacturer’s instructions.

Table 1.
The primer sequences of heat stress-related genes.
On day 14, rumen contents were collected from all the goats of the HS group. The rumen contents were collected using a negative pressure suction device, which we made, consisting of a flexible stomach tube and a vacuum pump (Jin Teng GM-0.33A, Tianjin, China). In order to avoid contamination of the rumen contents by saliva, the initial 20 mL of the collected rumen contents were discarded. Then, the rumen contents were extrusion-filtered through four layers of gauze. Next, 400 mg of dry feed substrates, 8.0 mL of rumen fluid, 32.0 mL of McDougall’s buffer [30], and yeast culture were added to a 100 mL flask, and all the flasks were prewarmed using a water bath at 39 °C. The commercial yeast culture (Hubei Lucnova Biotechnology Co., Ltd., Huanggang, China) was supplemented at concentrations of 0% (control), 0.60% (test 1), 0.90% (test 2), and 1.20% (test 3) of the DM concentration in the basal diet for the in vitro incubation. Finally, CO2 was flushed through the flask to remove oxygen, and the rubber stoppers and aluminum foil were used to close the flask. Then, the flasks were shaken and incubated at 39 °C, 271× g for 24 h. Six flasks were prepared for each group. The flasks were placed in an ice water bath for 15 min to stop the incubation before the fermentation broth was collected. The above methods of in vitro fermentation are as described by Xue et al. [5].
2.2. Feeding Experiment
This study was carried out in August and was approved by the Laboratory Animal Welfare and Ethics Committee of Jilin Agricultural University (approval code number: 20211020001). There were thirty-six crossbred goats aged 3.0 ± 0.5 months with a body weight of 11.08 ± 1.41 kg. According to the supplementation concentrations of yeast culture, these goats were divided into three groups: 0% (control), 0.90% (test 1), and 1.20% (test 2). The yeast culture was provided by Hubei Lucnova Biotechnology Co., Ltd. (Huanggang, China). This product is characterized by a content of glutathione at 1.0 mg/g. The goats were fed twice daily (8:00 h; 17:00 h) with a 1.20 kg/day maintenance diet and had free access to water. The feeding experiments lasted for 30 days, and during this period the temperature, relative humidity, and THI were 31.45 ± 2.1 °C, 71.23 ± 4.76%, and 84.23 ± 2.32, respectively. The ingredients and nutritional concentrations of the diet are given in Table 2. Five grams of chromium oxide were added as a marker to the diet of days 27 to 29 to determine the digestibility of the feed [5].

Table 2.
The ingredients and nutritional concentrations of the diet.
2.3. Measurements
Fecal samples were collected from the rectum of the goats during the afternoon and morning feedings of days 28 to 30 and were put together from the same group. On the morning of day 30, blood samples were collected. On the same day, the rumen contents were collected by a negative pressure suction device after a morning feed of 4 h. Then, the blood samples were centrifuged at 1800× g for 10 min, and four layers of gauze were used to strain the rumen contents to obtain serum and rumen fluid. These samples were immediately transferred to liquid nitrogen and then stored at −20 °C for further analysis.
The serum total antioxidant capacity (T-AOC) and glutathione peroxidase (GSH-Px) were determined by colorimetric method. The activity of total superoxide dismutase (T-SOD) was determined by the hydroxylamine method. The concentration of malondialdehyde (MDA) was determined by the thiobarbituric acid method. All the above indices were determined by test kits purchased from the Nanjing Jiengcheng Institute of Biological Engineering (Nanjing, China). The measurement procedure was carried out according to the instructions. The pH of the fermentation broth of the rumen fluids was measured with a pH meter immediately after the rumen contents were collected (Lichen, Changsha, China). The concentrations of acetic acid, propionic acid, and butyric acid were determined as described by Yang et al. [31] using gas chromatography. In brief, the fermentation broth of the rumen fluids was centrifuged at 12,000× g at 4 °C for 15 min, and the supernatants were collected. In total, 0.20 mL of this supernatant was added to 1.00 mL of 25% (w/v) metaphosphoric acid and centrifuged at 10,000 r/min for 10 min. Then, the mixed liquor was injected into a Chrompack CP-Wax 52 fused silica column (30 m × 0.53 mm × 1.00 µm) of gas chromatography equipped with a flame ionization detector (Model 2010, Shimazu, Japan). As described by Wang and Wang [32], the activities of avicelase, CMCase, cellobiase, and xylanase in the fermentation broth of the rumen fluid were measured. The dry matter intake (DMI) was obtained by subtracting the weight of the remaining feed from the feed weight provided each day. The body weights of the goats were measured weekly in the morning before offering feed and water. As described by Zhang et al. [33], the DM, NDF, and ADF of the feed, fermentation broth, and fecal samples were analyzed. The digestibility of the DM, NDF, and ADF was calculated as follows [18]: (DM content in feedstuff-DM content in feces or fermentation broth)/DM content in feedstuff × 100 = DM digestibility (%); (NDF or ADF content in feedstuff-NDF or ADF content in feces or fermentation broth)/NDF or ADF content in feedstuff × 100 = NDF or ADF digestibility (%).
2.4. Statistical Analysis
The “stats” package in R software (v.4.0.2) was used for the statistical analysis. The physiological index, heat stress-related gene expression, and serum cortisol concentration were compared between the NHS and HS groups using two-tailed Student’s t-tests. To reveal significant differences in the parameters of antioxidant capacity, rumen fermentation, and growth performance among different yeast culture-supplemented groups, two-way analysis of variance (ANOVA) tests followed by a post hoc Dunn test for multiple pairwise comparisons were performed. The p values of less than 0.05 were considered statistically significant.
3. Results
3.1. Goats Meet the Criteria to Be Rumen Fluid Donors
The skin temperature, pulse, and respiratory rate were significantly increased (p < 0.05; Figure 1A,C,D), while there was no significant difference in the rectal temperature (p > 0.05; Figure 1B) of the HS group compared to the NHS group. The expression levels of HSPA 1 in the blood and HSPA 70 in the rumen fluid were significantly increased (p < 0.05; Figure 1E), while there were no differences (p > 0.05; Figure 1E) in the expression of HSPA 6 and HSPA 8 in the blood of the HS goats compared to in the NHS goats. Moreover, the cortisol concentrations in the serum were significantly increased (p < 0.01; Figure 1F) in the serum of the HS goats compared to in the NHS goats.
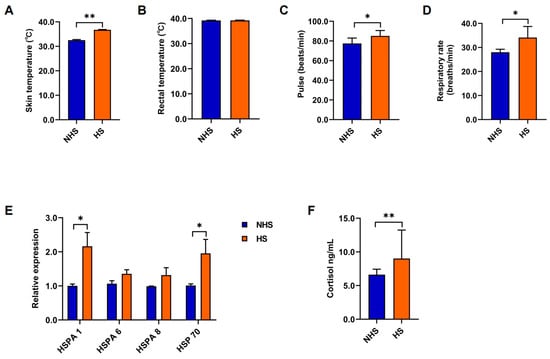
Figure 1.
Physiological parameters and gene expression levels of goats as rumen fluid donors. The (A) skin temperature, (B) rectal temperature, (C) pulse, and (D) respiratory rate of NHS and HS goats. (E) The expression levels of HSPA 1, HSPA 6, and HSPA 8 in the blood and HSP 70 in the rumen content of NHS and HS goats. (F) Cortisol concentrations in the serum of NHS and HS goats. The data are expressed as the mean ± SEM; * p < 0.05, ** p < 0.01.
3.2. Heat Stress Adversely Affects the Antioxidant Capacity and Rumen Fermentation of Goats
In terms of the antioxidant parameters, the goats exhibited significantly lower (p < 0.05) T-AOC, T-SOD, and GSH-Px activities but significantly higher (p < 0.05) MDA concentrations in the HS group than they exhibited in the NHS group. In terms of the rumen fermentation parameters, the pH value; the concentrations of total SCFA, acetic acid, propionic acid, and butyric acid; and the activities of acylase, CMCaes, cellobiase, and xylanase were significantly lower (p < 0.05) in the HS group than in the NHS group. Regarding digestibility, the digestibility of DM, NDF, and ADF significantly decreased (p < 0.05) in the HS group compared to that in the NHS group. The antioxidant capacity, rumen fermentation, and digestibility parameters of goats in the NHS and HS groups are shown in Table 3

Table 3.
Antioxidant capacity, rumen fermentation, and digestibility parameters of goats in NHS and HS groups.
3.3. Rumen Fermentation with Yeast Culture Supplementation In Vitro
After 24 h of incubation, the pH and the concentrations of total SCFA, acetic acid, propionic acid, and butyric acid in the fermentation broth were significantly increased (p < 0.05) in tests 2 and 3 compared with the control and test 1. Moreover, the degradability of DM, NDF, and ADF were significantly increased (p < 0.05) in tests 2 and 3 compared with the control and test 1. The rumen avicelase, CMCase, cellobiase, and xylanase activities in tests 2 and 3 were significantly higher (p < 0.05) in the fermentation broth of tests 2 and 3 compared with in the control and test 1. The fermentation parameters in the fermentation broth with yeast culture incubation in vitro are shown in Table 4.

Table 4.
Fermentation parameters in fermentation broth with yeast culture in vitro.
3.4. Effects of Diets Supplemented with Yeast Culture on the Serum Antioxidant Indices of Goats in the Summer
The activities of serum T-AOC, T-SOD, and GSH-Px were significantly higher and the concentration of MDA was significantly lower in tests 1 and 2 than in the control (p < 0.05) in all the test groups. However, there was no significant difference in these indices between the two test groups (p > 0.05). The serum antioxidant indices of goats with yeast culture supplements in the summer are shown in Table 5.

Table 5.
Serum antioxidant indices of goats with yeast culture supplementation in the summer.
3.5. Diets Supplemented with Yeast Culture Improved the Rumen Fermentation of Goats in the Summer
The pH value in the rumen was significantly higher (p < 0.05) in the two test groups than in the control. The total SCFA, acetic acid, propionic acid, and butyric acid concentrations significantly increased (p < 0.05) in tests 1 and 2 compared with in the control. The rumen avicelase, CMCase, cellobiase, and xylanase activities were significantly higher (p < 0.05) in tests 1 and 2 than in the rumen of the control group. The rumen fermentation parameters mentioned above were not significantly different among the test groups (p > 0.05). The rumen fermentation parameters of goats with yeast culture supplementation are shown in Table 6.

Table 6.
Rumen fermentation parameters of goats with yeast culture supplementation in the summer.
3.6. Effects of Diets Supplemented with Antioxidant Yeast Culture on the Growth Performance of Goats in the Summer
The DMI, ADG, and digestibility of DM, NDF, and ADF were significantly increased (p < 0.05) in tests 1 and 2 compared with in the control. However, there was no significant difference in these indices between the two test groups (p > 0.05). The growth performance parameters of goats with yeast culture supplements are shown in Table 7.

Table 7.
The DMI, ADG, and the apparent digestibility of DM, NDF, and ADF of goats with yeast culture supplementation in the summer.
4. Discussion
In recent years, intensive goat farming has bloomed in China. However, in most areas that developed intensive goat farming, goats are primarily kept in naturally ventilated goat houses, so heat stress inevitably occurs during hot summers [8,9,34]. The metabolic rate of heat-stressed animals increases, increasing free radical production [35]. Studies have shown that when animals are subjected to heat stress, the body’s antioxidant system is adversely affected, resulting in oxidative stress [6,36,37,38] and ultimately causing damage to organism tissue cells and biological macromolecules such as protein and nucleic acid [35]. This study found that when goats suffered heat stress, the antioxidant capacity significantly decreased; for example, the activities of T-AOC, T-SOD, and GSH-Px decreased, whereas the MDA significantly increased. This result is consistent with previous reports. The rumen is a vital digestive organ of ruminants. When goats are exposed to heat, their internal environment changes significantly, and the fermentation function is adversely affected. In this study, the results showed that the pH value; the concentrations of total SCFA, acetic acid, propionic acid, and butyric acid; and the activities of acylase, CMCaes, cellobiase, and xylanase were significantly decreased in heat-stressed goats compared to that of non-heat-stressed goats. This result is consistent with previous studies. The decline in goat growth performance will eventually reflect the adverse effects of heat stress and will lead to the deterioration of the economic benefits of intensive goat farming [9].
Studies have shown that yeast culture can significantly increase plasma SOD and GSH-Px enzyme activities and significantly decrease plasma MDA in cows with mastitis [39]. A few studies also showed that yeast culture significantly increased the activity of serum SOD and T-AOC in each group and decreased the MDA concentration of cashmere goats in the initial stage of the experiment in the high-concentrate group [40]. It was found that the GSH-Px and SOD activities and T-AOC in the plasma of dairy goats were significantly decreased under heat stress [41]. In this study, it was found that the contents of T-AOC, SOD, and GSH-Px were significantly increased, while the contents of MDA were significantly decreased in the serum of goats supplemented with yeast culture in a hot summer. These results were consistent with previous studies. The antioxidant effect of yeast culture is related to its rich nutrients, such as vitamins (vitamins C, E, and B2), glutathione, trace elements (Zn, Se, Fe, Cu, Mn, etc.), enzymes, and coenzymes. Among them, vitamin C, vitamin E, vitamin B2, and trace elements Se and Zn can be used as low molecular reduction substances to have antioxidant effects. Glutathione can be used as a reducing agent to promote a reduction in hydrogen peroxide, organic hydroperoxide, and lipid hydroperoxide and reduce the oxidative damage of cells [42]. The yeast culture used in this study is rich in glutathione with a concentration of 1.0mg/g, which shows that this product may effectively improve the antioxidant capacity of summer goats. Our follow-up studies will confirm this point.
Dietary supplementation with yeast is one effective way to improve rumen fermentation [43]. The rumen pH significantly decreases when goats are exposed to high temperatures in the summer due to changes in physiological activities [9]. Studies have shown that supplementing Saccharomyces cerevisiae or a mixture of Saccharomyces cerevisiae and Clostridium butyricum to the diet of heat-stressed goats can significantly increase the ruminal pH [5,18,19,20]. In this study, yeast culture supplementation can also increase the goats’ rumen pH in a hot summer. It is consistent with the previously reported results of supplementation with live saccharomyces cerevisiae in the diet of heat-stressed goats. It is because yeast culture mainly promotes the growth of lactic acid utilization bacteria by changing the structure of the microbial flora in the rumen, so that lactic acid produced in the rumen can be utilized and the rumen pH can be increased to promote rumen fermentation [21,42,44]. The supplementation with yeast or yeast culture can increase the concentration of acetic acid, propionic acid, butyric acid, and total SCFA and reduce the ratio of acetic acid to propionic acid [5,18,19,20,44]. This present study showed that the rumen pH and SCFA production increased significantly after yeast culture supplemented the goats’ diet in the hot summer. These results were consistent with previous studies. This is due to the fact that yeast cultures can effectively increase the abundance of cellulolytic bacteria in the rumen, such as Ruminococcus flavefaciens, Ruminococcus albus, and Fibrobacter succinogenes, which contribute to the decomposition of crude fibers and enhance the abundance of lactic acid utilization bacteria [21,44]. This study did not study the changes in rumen microbial composition and function under heat stress conditions, nor did it compare the changes in rumen microbial function and composition before and after yeast culture supplementation. Therefore, the effects of rumen microorganisms on rumen fermentation are all referred to in previous studies, and the changes of microorganisms will be studied in our subsequent studies.
Studies have shown that supplementation with 1.5% and 3% yeast cultures in sheep diets can promote the DMI [45]. The supplementation of yeast culture in a 3-month-old calf’s diet significantly increased the calf’s DM, ADG, and feed efficiency and played a positive role in the feeding and growth of calves [46]. The ADG of meat sheep can be increased by adding yeast culture to the diet [47]. In this study, supplementation with yeast culture significantly increased the DMI and ADG of goats. This result is similar to that of previous studies above. This is because the yeast culture has a good flavor; adding it to the diet improves the diet’s palatability and promotes the feeding of summer goats [48]. The yeast culture contains a cellulase system, hemicellulase (xylanase), and neutral protease. Hemicellulase and cellulase have higher activity, so they can help improve the digestion of the cellulose components in the diet [48,49]. In this study, the activities of cellulolytic enzymes were enhanced in the rumen by the supplementation of yeast culture, which is speculated to be the primary cause of the increased digestibility of DM, NDF, and ADF. It was confirmed that supplementing with yeast culture could improve the degradation function of rumen fiber. In addition, the ability of rumen microorganisms to synthesize B vitamins decreased in goats under high-temperature environments. However, vitamins B1 and B2 and niacin concentrations in the rumen were significantly increased after yeast culture was added [14]. Such an effect may be because yeast cells contain glucose, furan mannose, and chitin, which can be used as fermentation substrates for rumen microbiota to promote the production of B vitamins [46,50,51]. Moreover, the yeast is rich in B vitamins, which also directly provide B vitamins for rumen microbiota and ruminants [52]. Since vitamin B involves the processes of the nutritional metabolism, including the synthesis of fatty acids, the catabolism of branched-chain amino acids, and gluconeogenesis [53,54], adding yeast culture can promote the growth of goats by promoting the synthesis of rumen B vitamins. In summary, yeast culture significantly affects the antioxidant performance, rumen fermentation, and production performance of goats. Nevertheless, different yeast strains, dietary concentrate-to-concentrate ratios, and dietary types have significant differences in the feeding effects of yeast culture. Therefore, given the problems existing in the feeding and specific growth stages of goats, the selection of excellent yeast strains and the optimal addition amount of their cultures based on the targeted test results have a positive guiding significance for intensive goat culture.
5. Conclusions
Supplementation with yeast culture could enhance the antioxidant capacity by prompting serum antioxidant enzyme activities; improving rumen fermentation by increasing the pH value, SCFAs concentrations, and cellulolytic enzymes; and promoting growth performance by elevating the digestibility of DM, DNF, and ADF. Thereafter, the growth performances were enhanced by increasing the DMI and ADG of goats in the summer. Supplementation with yeast culture can effectively alleviate the adverse effects of hot and humid environments on the rumen fermentation of goats in the summer. The optimal supplementation amount is 0.9%DM.
Author Contributions
Conceptualization, S.Z. and G.H.; methodology, Y.L.; software, Y.L.; validation, S.Z. and Y.G.; formal analysis, D.W.; investigation, D.W.; resources, S.Z.; data curation, Y.G.; writing—original draft preparation, S.Z.; writing—review and editing, S.Z. and G.H.; visualization, Y.G.; supervision, G.H.; project administration, S.Z.; and funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Scientific and Technological Development Plan from the Jilin Provincial Science and Technology Department of Jilin, China (grant number: 20230101257JC).
Institutional Review Board Statement
This feeding experiment was carried out from June to August and was approved by the Laboratory Animal Welfare and Ethics Committee of Jilin Agricultural University (approval code number: 20211020001).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to we will to do further research on the rumen microbiome based on these data.
Acknowledgments
The authors would like to thank Liyuan Cai, technical director of Hubei Lucnova Biotechnology Co., Ltd. (Huanggang, China), for helping design the animal feeding experiments. The authors would also like to thank the R&D team in the Technical Department of Hubei Lucnova Biotechnology Co., Ltd. for providing us with the latest research products.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Caulfield, M.P.; Cambridge, H.; Foster, S.F.; Susan, F.F.; Paul, D.M. Heat stress: A major contributor to poor animal welfare associated with long-haul live export voyages. Vet. J. 2014, 199, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kovats, R.S.; Hajat, S. Heat stress and public health: A critical review. Annu. Rev. Publ. Health 2008, 29, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.I.; Nonaka, K.; Higuchi, N.; Takusari, M.; Kurihara, A.; Takenak, M.; Mitsumori, H.; Kajikawa, R.I.; Aminov, R.I. Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe 2007, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Xu, Y.Y.; Wang, Z.Q.; Shi, B.L. Effects of oxidative stress induced by heat stress and its mechanism in sheep and goats. Chin. J. Anim. Nutr. 2019, 31, 3016–3022. [Google Scholar]
- Xue, L.G.; Zhou, S.Y.; Wang, D.; Zhang, F.Y.; Li, J.F.; Cai, L.Y. The low dose of Saccharomyces cerevisiae is beneficial for rumen fermentation (both in vivo and in vitro) and the growth performance of heat-stressed goats. Microorganisms 2022, 10, 1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.S.; Peng, Q.H.; Zou, H.W.; Jing, X.P.; Pu, Q.J. Effects of moist-heat stress on growth performance, oxidation resistance and immunity of Tibetan sheep and goats. Chin. J. Anim. Nutr. 2017, 29, 2179–2187. [Google Scholar]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2014, 16, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Y. Environmental Control of Intensive Goat Buildings and the Effects of Heat Stress on Rumen Fermentation of Goats. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Cai, L.Y.; Yu, J.K.; Hartanto, R.; Zhang, J.; Yang, A.; Qi, D.S. Effects of heat challenge on growth performance, ruminal, blood and physiological parameters of Chinese crossbred goats. Small Rumin. Res. 2019, 174, 125–130. [Google Scholar] [CrossRef]
- Yadav, B. Impact of heat stress on rumen functions. Vet. World 2013, 6, 992–996. [Google Scholar] [CrossRef]
- Hooda, O.K.; Upadhyay, R.C. Physiological responses, growth rate and blood metabolites under feed restriction and thermal exposure in kids. J. Stress Physiol. Biochem. 2014, 10, 214–227. [Google Scholar]
- Maloiy, G.M.O.; Kanui, T.I.; Towett, P.K.; Wambugua, S.N.; Miarona, J.O.; Wanyoike, M.M. Effects of dehydration and heat stress on food intake and dry matter digestibility in East African ruminants. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 2, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Du, R.P.; Gao, P. Effect of heat stress on dairy goat performance and rumen epithelial cell morphology. Chin. Agr. Sci. 2013, 21, 4486–4495. [Google Scholar]
- Cai, L.Y.; Hartanto, R.; Xu, Q.B.; Zhang, J.; Qi, D.S. Saccharomyces cerevisiae and Clostridium butyricum could improve B-vitamin production in the rumen and growth performance of heat-stressed goats. Metabolites 2022, 12, 766. [Google Scholar] [CrossRef] [PubMed]
- Indu, S.; Sejian, V.; Naqvi, S.M.K. Impact of simulated heat stress on growth, physiological adaptability, blood metabolites and endocrine responses in Malpura ewes under semiarid tropical environment. Anim. Prod. Sci. 2015, 55, 766. [Google Scholar] [CrossRef]
- Popoola, M.A.; Bolarinwa, M.O.; Yahaya, M.O.; Adebisi, G.L.; Saka, A.A. Thermal comfort effects on physiological adaptations and growth performance of West African dwarf goats raised in Nigeria. Eur. Sci. J. 2014, 10, 275–281. [Google Scholar]
- Pragna, P.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Soren, N.M.; Beena, V.; Bhatta, R. Comparative assessment of growth performance of three different indigenous goat breeds exposed to summer heat. Stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.G.; Wang, D.; Zhang, F.Y.; Cai, L.Y. Prophylactic feeding of Clostridium butyricum and Saccharomyces cerevisiae were advantageous in resisting the adverse effects of heat stress on rumen fermentation and growth performance of goats. Animals 2022, 12, 2455. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Y.; Yu, J.K.; Hartanto, R.; Qi, D.S. Dietary supplementation with Saccharomy ceserevisiae, Clostridium butyricum and their combination ameliorate rumen fermentation and growth performance of heat-stressed goats. Animals 2021, 11, 2116. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Y.; Li, M.; Zhou, S.Y.; Xu, Q.B. The mixture of Saccharomyces cerevisiae and Clostridium butyricum could promote rumen fermentation and improve the growth performance of goats in hot summer. Metabolites 2023, 13, 104. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhou, X.F.; Zhen, Y.G.; Wang, L.H. The application of yeast culture in ruminant production. Chin. J. Vet. Sci. 2016, 36, 1986–1989. [Google Scholar]
- Schingoethe, D.J.; Linke, K.N.; Kalscheur, K.F.; Hippen, A.R.; Rennich, D.R.; Yoon, I. Feed efficiency of mid-lactation dairy cows fed yeast culture during summer. J. Dairy Sci. 2004, 87, 4178–4181. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.C.; Pang, X.D.; Zhuang, S.; Wang, T. Effects of yeast culture on rumen cellulase activity and volatile fatty acids of goats. Chin. J. Anim. Sci. 2006, 42, 34–38. [Google Scholar]
- Kou, H.J.; Chen, Y.L.; Liu, J.M.; Cao, B.H.; Zhou, G.X.; Zhang, S.H.; Zhang, E.P. Effects of yeast culture on performance, nutrient performance digestibility and rumen development of lambs. J. Northwest A F Univ. (Nat. Sci. Ed.) 2011, 8, 45–50. [Google Scholar]
- Xiang, H.; Lin, Y.H.; Zhang, X.D.; Ding, J.P.; Zhang, X.R. effects of silage on the nutrient preservation of corn harvested in different period. Chin. Herb. Sci. 2012, 2, 33–36. [Google Scholar]
- LPHSI. Livestock and Poultry Heat Stress Indices Agriculture Engineering Technology Guide; Clemson University: Clemson, SC, USA, 1990. [Google Scholar]
- Peng, X.K.; Zhao, T.; Huang, X.Y.; Zhang, Y.; Xing, X.; Zhang, E. Effects of acute heat stress on blood biochemistry indices and expression of HSP70 family genes in blood lymphocytes in goats. Acta Vet. Zootech. Sin. 2019, 50, 1219–1229. [Google Scholar]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Wang, Z.S.; Wang, L.Z.; Liu, J.H.; Xu, L.X. Effects of temperature and humidity index in different seasons on production performance and physiological and biochemical indexes of dairy cows. Chin. J. Anim. Sci. 2009, 45, 60–63. [Google Scholar]
- McDougall, E.I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Beauchemin, K.A.; Rode, L.M. Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion by dairy cows. J. Dairy Sci. 2001, 2, 203–216. [Google Scholar] [CrossRef]
- Wang, S.P.; Wang, W.J. Determination of enzyme activity related to fiber degradation in rumen. Chin. Feed 2006, 11, 31–32. [Google Scholar]
- Zhang, L.Y. Feed Analysis and Feed Quality Testing Technology; China Agricultural University Press: Beijing, China, 2007; pp. 270–274. [Google Scholar]
- Zhang, Y.F.; Qi, Z.L. Mechanism of oxidative stress in body under heat stress. Chin. J. Anim. Nutr. 2017, 2, 3051–3058. [Google Scholar]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tan, G.Y.; Fu, Y.Q.; Feng, J.H.; Zhang, M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.H.; Hao, Y.; Wang, X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: Intestinal oxidative stress. Poult. Sci. 2012, 91, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Li, J.J.; Wang, D.Q.; Li, G.Q.; Wang, G.L.; Lu, L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: Evidence for differential thermal sensitivities. Cell Stress Chaperones 2014, 19, 895–901. [Google Scholar] [CrossRef]
- Liu, D.C.; Cheng, Y.; Lu, D.X. Effects of yeast culture on immune function and antioxidant function of cows with latent mastitis. Anim. Husb. Feed Sci. 2011, 1, 165–166. [Google Scholar]
- Zhang, A.Z.; Lu, D.X.; Jiang, N.; Gao, M.; Hu, H.L. Effects of yeast culture on antioxidant capacity of cashmere goats. Chin. J. Anim. Nutr. 2010, 22, 781–786. [Google Scholar]
- Xu, X.; Li, Y.; Wang, C.; Qi, Z.L. Effects of Heat Stress on Antioxidant Capacity and HSP70 mRNA Expression and Mechanism of Organic Chromium in Dairy Goats; National Symposium on Animal Nutrition, Animal Nutrition Branch, Chinese Society of Animal Husbandry and Veterinary Medicine: Changsha, China, 2012. [Google Scholar]
- Zhang, A.Z.; Lu, D.X.; Wang, L.Z.; Ren, X.P.; Shan, D. Effect of yeast culture on rumen fermentation of cashmere goats in vitro. Chin. J. Anim. Sci. 2008, 3, 31–34. [Google Scholar]
- Desnoyers, M.; Giger-Reverdin, S.; Bertin, G.; Duvaux-Pouter, C.; Sauvant, D. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and mile productuin of ruminant. J. Dairy Sci. 2009, 92, 1620–1632. [Google Scholar] [CrossRef]
- Huang, Q.S.; Wang, J.Q. Effect of yeast cultures on fibrolytic bacterial population and activities of fiber hydrolytic enzymes in therumen. Acta Vet. Et Zootech. Sin. 2005, 36, 144–148. [Google Scholar]
- Zhang, C.J.; Liu, Z.; Hao, Z.L.; Li, F.D. Effect of supplement with yeast culture on the digestibility in sheep. Pratacultural Sci. 2007, 24, 5. [Google Scholar]
- Guo, Y.Q.; Zhao, Y.F.; Zhang, X.Y. Effect of yeast culture on growth performance and rumen fermentation of weaned calves. Feed Res. 2019, 42, 10–13. [Google Scholar]
- Zhao, G.H.; Wang, S.G.; Wang, F.; Wang, H.; Diao, Q.Y.; Wang, S.Q.; Zhang, N.F. Effects of different levels of yeast culture supplementation on growth performance, slaughter performance, visceral organ development and meat quality of fattening Hu sheep. Chin. J. Anim. Nutr. 2020, 32, 9. [Google Scholar]
- Long, L. Yeast products and their uses. Chin. Feed 2001, 5, 3. [Google Scholar]
- Wang, X.; Li, F.; Zhang, N.; Ungerfeld, E.; Guo, L.; Zhang, X.; Wang, M.; Ma, Z. Effects of supplementing a yeast culture in a pelleted total mixed ration on fiber degradation, fermentation parameters, and the bacterial community in the rumen of sheep. Anim. Feed Sci. Tech. 2023, 296, 115565. [Google Scholar] [CrossRef]
- Klis, F.M. Review: Cell wall assembly in yeast. Yeast 1994, 7, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Moukadiri, I.; Armero, J.; Abad, A.; Sentandreu, R.; Zueco, R. Dentification of a mannoprotein present in the inner layer of the cell wall of Saccharomyces cerevisiae. J. Bacteriol. 1997, 7, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.S.; Wang, Q.S.; Liao, B.L.; Yan, X.H. Application of yeast feed in animal husbandry. Feed. Ind. 2008, 4, 4–6. [Google Scholar]
- Chen, B.; Wang, C.; Wang, Y.M.; Liu, J.X. Effect of biotin on milk performance of dairy cattle: A meta-analysis. J. Dairy Sci. 2011, 94, 3537–3546. [Google Scholar] [CrossRef]
- Li, N.; Li, M.Y.; Peng, Q.H. Research Progress of Vitamin B in Ruminant Nutrition. Chin. J. Anim. Nutr. 2021, 9, 4909–4919. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).