Bacterial Nanocellulose Produced by Cost-Effective and Sustainable Methods and Its Applications: A Review
Abstract
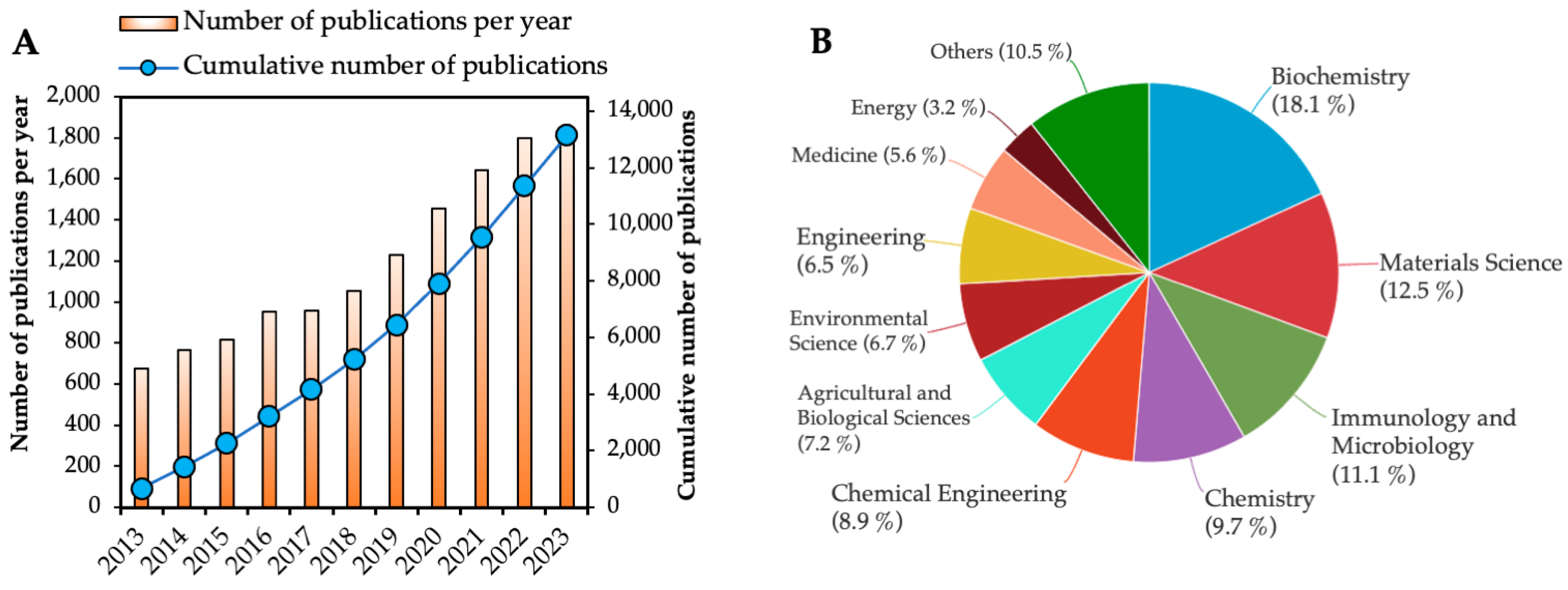
1. Introduction
2. Industrial Wastes Used in Fermentation
| Waste Source | Bacteria Name | Cultivation Condition | BC Yield (g/L) | Ref. |
|---|---|---|---|---|
| Grape pomace hydrolysate | K. melomenusus | Static culture, 3 days, 30 °C | 1.2 | [32] |
| Potato peel | G. xylinus | Static culture, pH 6.0, 4 days, 30 °C | 1.27–2.61 HS a: 1.21 | [37] |
| Potato juice | K. xylinus | Static culture, pH 6.0, 7 days, 28 °C | 2–4 HS a: 3–5 | [38] |
| Straw biomass (sugarcane bagasse, bamboo, corncob, wheat straw, and rice straw) | A. xylinus | Static culture, 6 days, 30 °C | 1.9–2.6 HS a: 1.7 | [39] |
| Orange peel waste | G. xylinus K. sucrofermentants | Static culture, 8 days, 30 °C Dynamic culture, 30 °C | 0.9–6.1 HS a: 0.9 1.9–11.6 | [40,41,42,43,44] |
| Pineapple peel waste | G. xylinus | Static culture, pH 4.5, 14 days, 30 °C | 3.8 HS a: 2.1 | [45,46,47] |
| Pineapple core | K. xylinus | Static culture, pH 4, 15 days, room temperature | 2.4–2.5 HS a: 2.5 | [48] |
| Mango waste | K. xylinus | Static culture, 16 days, 30 °C | 1–6 HS a: 2.0 | [49,50] |
| Pear peel waste | L. plantarum | Static culture, 8 days | 0.7–3.5 HS a: 1.9 | [14] |
| Watermelon waste | G. xylinus | Static culture, 16 days | 5.8 | [51] |
| Asparagus waste | K. rhaeticus | Static culture, pH 4.5, 25 days, 30 °C | 1.0–2.5 | [52] |
| Sweet lime pulp waste | A. xylinus | Dynamic culture, pH 5–6, 7 days, 25–35 °C | 5.2–7.0 | [53] |
| Jasminum sambac and Camellia sinensis | K. intermedius | Static culture, 7 days, 30 °C | 3.7–7.1 HS a: 5.6 | [54] |
| Coffee ground | K. rhaeticus | Static culture, 7 days, 30 °C | 0.5–11 | [55,56] |
| Liquid wastes from preserved tamarind and preserved mango | A. xylinus | Static culture, 10 days, 30 °C | 4.7 and 4.5 HS a: 2.5 | [57] |
| Olive oil mill wastewater | K. xylinus | Static culture, pH 4.5, 7 days, 30 °C | 1–5 HS a: 1 | [58] |
| Sago residue | G. xylinus | Static culture, pH 6.0, 14 days, 30 °C | 1.55 HS a: 1.57 | [59,60] |
| Pecan nutshell | G. entanii | Static culture, pH 3.5, 28 days, 30 °C | 2.8 | [61] |
| Residue of cashew apple juice processing | K. xylinus | Static culture, pH 4.3, 12 days, 30 °C | 1–3 HS a: 0.5–3 | [62] |
| Brewing by-products | K. rhaeticus | Static culture, pH 6, 10 days, 30 °C | 4.0 | [63] |
| Beet molasses, vinasse, and waste beer fermentation broth | K. xylinus | Dynamic culture, pH 5, 7 days, 30 °C | 8.2 HS a: 1.7 | [64] |
| Mulberry pomace waste extract | K. xylinus | Static culture, pH 8, 10 days, 30 °C | 1.5 | [65] |
| Cheese whey | G. xylinus | Static culture, pH 5.5, 14 days, 28 °C | 1–3.55 HS a: 3.26 | [66] |
| Tofu wastewater | K. xylinus | Static culture, pH 4.5, 15 days, 30 °C | 10.6 | [67] |
| Soybean residue | G. xylinus | Static culture, pH 4.5, 15 days, 30 °C | 1.5–2.8 HS a: 5.1 | [68] |
| Waste and by-product streams from biodiesel and confectionery industries | G. xylinus | Static culture, pH 5.0, 7 days, 30 °C | 1–7.32 | [69] |
| Tobacco waste extract | G. xylinus | Static–shaking (150 rpm) cultures, pH 6.5, 7–15 days, 30 °C | 0.5–5.2 HS a: 3.26 | [70] |
| Rice-washed water | K. xylinus | Static culture, pH 4.5, 15 days, 30 °C | 6.57 | [67] |
| Kitchen waste | A. xylinum K. rhaeticus | Static culture, 15 days, 30 °C Static culture, 10 days, 30 °C | 2.0 4.7 | [71,72] |
| Paper waste sludge hydrolysate | A. xylinum | Static culture, pH 4.8, 15 days, 30 °C | 12.0 | [73] |
| Beeswax recycling wastewater | K. xylinus | Static culture, pH 6, 14 days, 28 °C | 2.6 HS a: 2.7 | [74] |
| Spent black liquor from cotton pulping | G. xylinus | Static culture, pH 5.5, 2–3 weeks, 30 °C | 0.2–3.25 HS a: 4.66 | [75] |
| Textile waste | T. mepensis | Static culture, 14 days, 30 °C | 1–2.2 HS a: 2.2 | [76] |
3. Strategies to Improve BC Yield
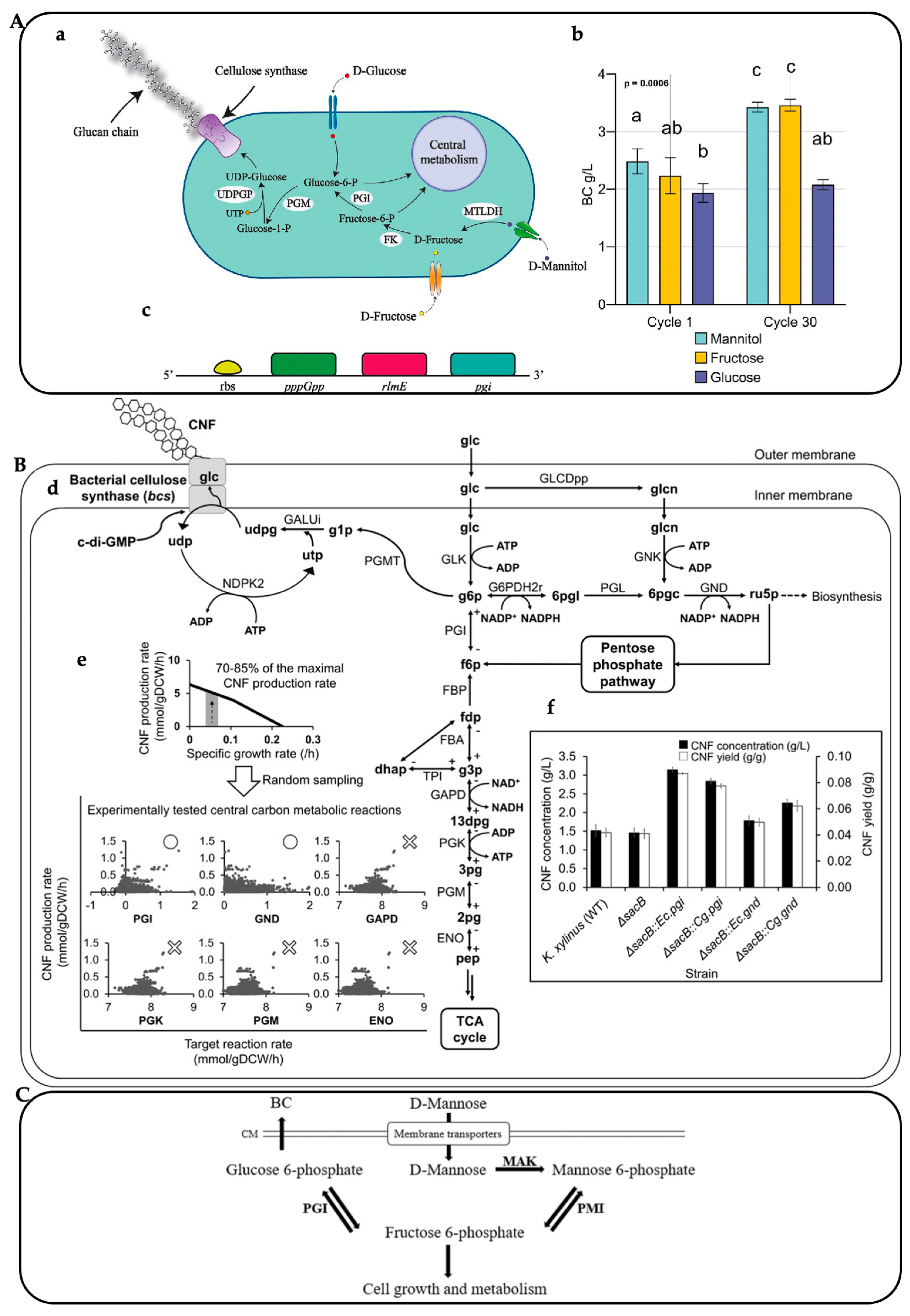
3.1. Dynamic Bioreactor
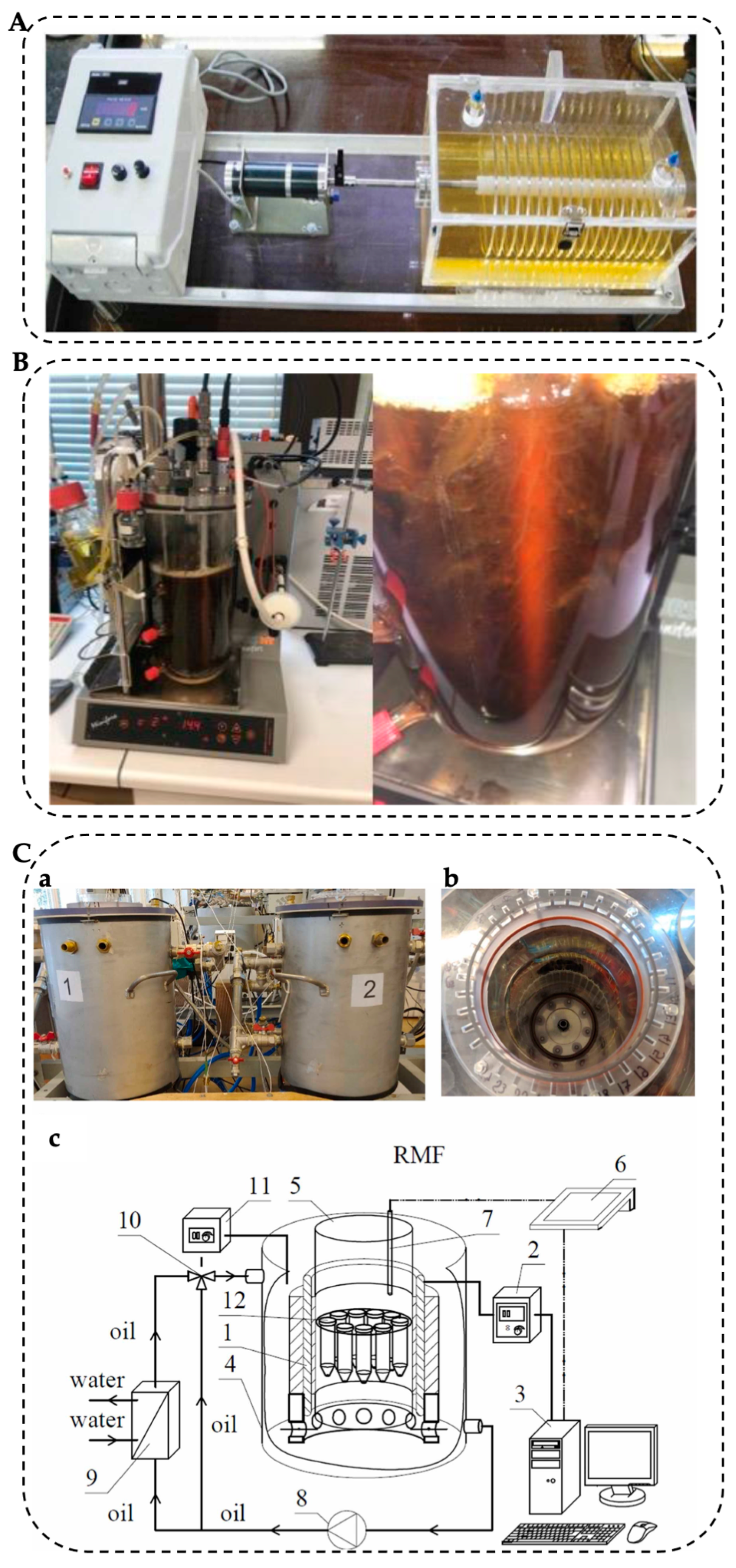
3.2. Genetic Engineering
4. Environmental Impact Assessment
5. Applications
5.1. Medical Application
| Natutal Products | Incoporation Methods | Improved Properties | Potential Applications | Ref. |
|---|---|---|---|---|
| Propolis | Impregnation of BC into propolis/ZnO solution | Antimicrobial activity against E. coli, Bacillus subtilis (B. subtilis), and Candida albicans (C. albicans) | Antimicrobial film | [150] |
| Chitosan | Impregnation of BC into chitosan solution | Antimicrobial activity against S. aureus, Pseudomonas aeruginosa (P. aeruginosa), and C. albicans | Antimicrobial dressing | [151] |
| Curcumin | Impregnation of BC into curcumin solution | Antimicrobial activity against E. coli, P. aeruginosa, Salmonella typhimurium (S. typhimurium), and S. aureus, wound healing property (accelerated wound closure up to 64% after 15 days) | Antimicrobial wound dressing | [152,153] |
| Lignin | Impregnation of BC into lignin solution | Antimicrobial activity against P. aeruginosa, S. aureus, Serratia sp., Listeria monocytogenes (L. monocytogenes), and Salmonella typhimurium (S. typhimurium) | Antimicrobial chronic wound dressing | [154] |
| Quercetin | Phase inversion of mixed BC and quercetin solution | Drug loading capacity and controlled drug release properties | Controlled-release drug delivery | [155] |
| Carrageenan | Impregnation of BC into carrageenan/gelatin solution | Antimicrobial activity against E. coli, S. aureus, and Klebsiella pneumonia, drug loading capacity and controlled drug release properties | Wound dressing and tissue regeneration | [156] |
| Green tea leaf extract, roselle flower petals, and Hibiscus rosa-sinensis L. flower extract | In situ biosynthesis with BC | Antimicrobial activity against P. aeruginosa and E. coli and antioxidant property | Wound dressing and face mask | [157] |
| Combretaceae and Solanaceae extract | Impregnation of BC into the plant extract solution | Antimicrobial activity against E. coli and S. aureus | Biomedical materials | [158] |
| Pomegranate peel extract | Impregnation of BC into the plant extract solution | Antimicrobial activity against E. coli and S. aureus | Antimicrobial wound dressing | [163] |
| Mangosteen peel extract | Impregnation of BC into the plant extract solution | Wound healing property (accelerated wound closure up to >90% after 15 days) | Wound dressing | [164] |
| Euclea schimperi extract | Impregnation of BC into the plant extract solution | Antimicrobial activity against E. coli and S. aureus | Antimicrobial wound dressing | [167] |
| Asparagaceae leave extract | Impregnation of BC into the plant extract solution | Antimicrobial activity against E. coli and S. aureus | Antimicrobial wound dressing | [168] |
5.2. Energy Storage and Electronics
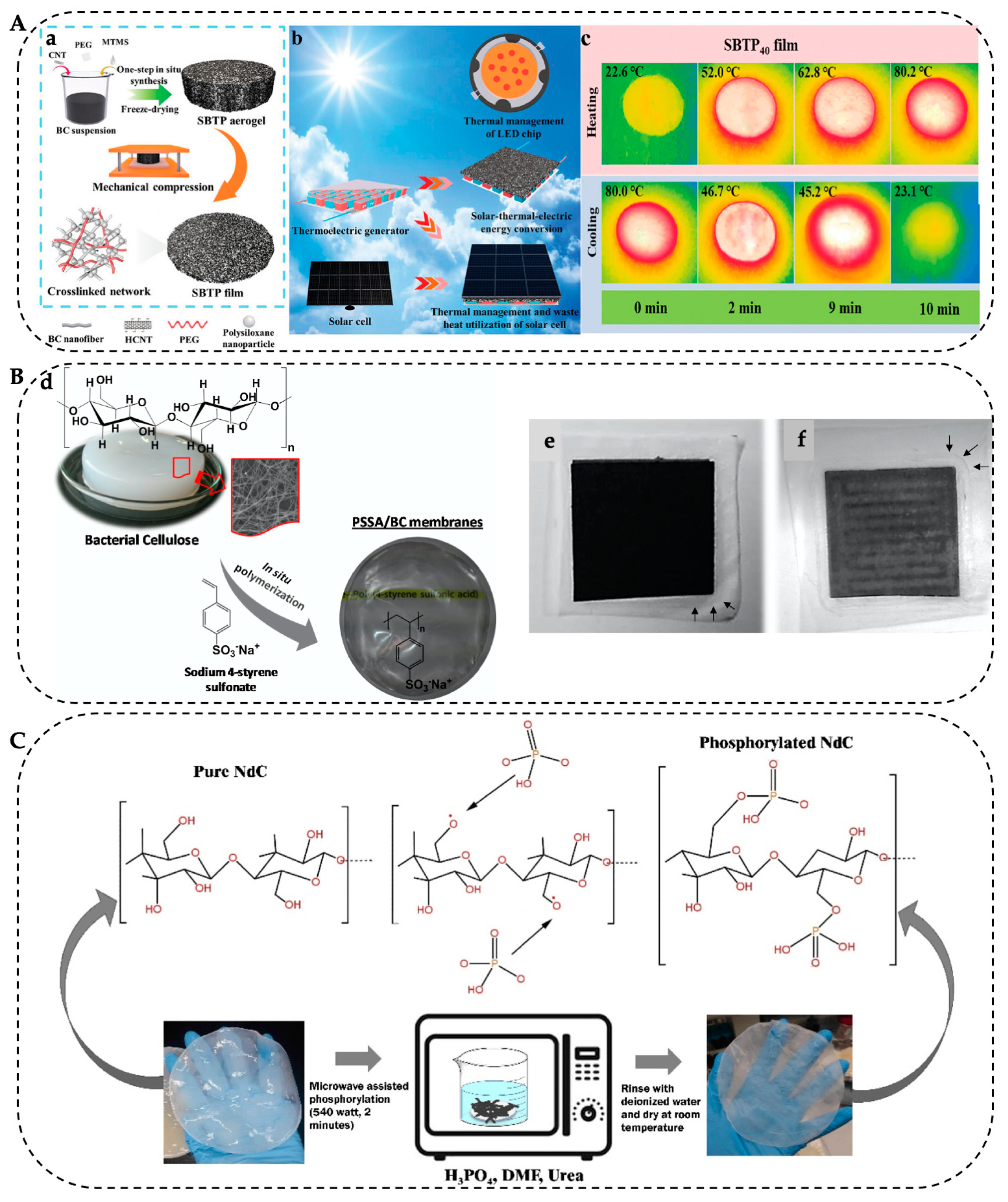
5.3. Filtration Membranes
5.4. Food Packaging Materials
6. Conclusions and Challenges
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, F.D.B. The role of plastic concerning the sustainable development goals: The literature point of view. Clean. Responsible Consum. 2021, 3, 100020. [Google Scholar] [CrossRef]
- Morrow, R.; Ribul, M.; Eastmond, H.; Lanot, A.; Baurley, S. Bio-Producing Bacterial Cellulose Filaments through Co-Designing with Biological Characteristics. Materials 2023, 16, 4893. [Google Scholar] [CrossRef] [PubMed]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Luo, H.; Xie, J.; Xiong, L.; Zhu, Y.; Yang, Z.; Wan, Y. Fabrication of flexible, ultra-strong, and highly conductive bacterial cellulose-based paper by engineering dispersion of graphene nanosheets. Compos. Part B Eng. 2019, 162, 484–490. [Google Scholar] [CrossRef]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and Properties of Nanofibrous Bacterial Cellulose-Containing Polymer Composites: A Review of Recent Advances for Biomedical Applications. Polym. Rev. 2020, 60, 144–170. [Google Scholar] [CrossRef]
- Verma, J.; Petru, M.; Goel, S. Cellulose based materials to accelerate the transition towards sustainability. Ind. Crops Prod. 2024, 210, 118078. [Google Scholar] [CrossRef]
- Camargo, M.S.A.; Cercal, A.P.; Silveira, V.F.; Mancinelli, K.C.B.; Gern, R.M.M.; Garcia, M.C.F.; Apati, G.P.; dos Santos Schneider, A.L.; Pezzin, A.P.T. Evaluation of Wet Bacterial Cellulose Degradation in Different Environmental Conditions. Macromol. Symp. 2020, 394, 2000149. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Greser, A.B.; Avcioglu, N.H. Optimization and physicochemical characterization of bacterial cellulose by Komagataeibacter nataicola and Komagataeibacter maltaceti strains isolated from grape, thorn apple and apple vinegars. Arch. Microbiol. 2022, 204, 465. [Google Scholar] [CrossRef]
- Cannazza, P.; Rissanen, A.J.; Guizelini, D.; Losoi, P.; Sarlin, E.; Romano, D.; Santala, V.; Mangayil, R. Characterization of Komagataeibacter Isolate Reveals New Prospects in Waste Stream Valorization for Bacterial Cellulose Production. Microorganisms 2021, 9, 2230. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ullah, M.W.; Ul-Islam, M.; Khan, S.; Jang, J.H.; Park, J.K. Self-assembly of bio-cellulose nanofibrils through intermediate phase in a cell-free enzyme system. Biochem. Eng. J. 2019, 142, 135–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Cao, G.; Ma, X.; Zhou, J.; Xu, W. Bacterial cellulose production from terylene ammonia hydrolysate by Taonella mepensis WT-6. Int. J. Biol. Macromol. 2021, 166, 251–258. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Salama, A.; El-Fakharany, E.M.; Saleh, A.K. Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr. Polym. 2023, 302, 120383. [Google Scholar] [CrossRef] [PubMed]
- Ammar, G.A.G.; Saleh, A.K.; Taha, T.H.; El-Zawawy, W.K.; Abdel-Fattah, Y.R. Developed applicability of a bacterial cellulose matrix as a gelling substitute for plant tissue culture media. Cellulose 2022, 29, 7883–7900. [Google Scholar] [CrossRef]
- Płoska, J.; Garbowska, M.; Klempová, S.; Stasiak-Różańska, L. Obtaining Bacterial Cellulose through Selected Strains of Acetic Acid Bacteria in Classical and Waste Media. Appl. Sci. 2023, 13, 6429. [Google Scholar] [CrossRef]
- Sari, A.K.; Majlan, E.H.; Loh, K.S.; Wong, W.Y.; Alva, S.; Khaerudini, D.S.; Yunus, R.M. Effect of acid treatments on thermal properties of bacterial cellulose produced from cassava liquid waste. Mater. Today Proc. 2022, 57, 1174–1178. [Google Scholar] [CrossRef]
- Ajkidkarn, P.; Manuspiya, H. Solution plasma synthesis of bacterial cellulose acetate derived from nata de coco waste incorporated with polyether block amide. Int. J. Biol. Macromol. 2022, 209, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, V.; Gorgieva, S.; Trček, J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers 2023, 15, 3466. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Comparative study of plant and bacterial cellulose pellicles regenerated from dissolved states. Int. J. Biol. Macromol. 2019, 137, 247–252. [Google Scholar] [CrossRef]
- Taokaew, S.; Thienchaimongkol, J.; Nakson, N.; Kobayashi, T. Valorisation of okara Waste as an Alternative Nitrogen Source in the Biosynthesis of Nanocellulose. Chem. Eng. Trans. 2022, 92, 649–654. [Google Scholar]
- Sperotto, G.; Stasiak, L.G.; Godoi, J.P.M.G.; Gabiatti, N.C.; De Souza, S.S. A review of culture media for bacterial cellulose production: Complex, chemically defined and minimal media modulations. Cellulose 2021, 28, 2649–2673. [Google Scholar] [CrossRef]
- Fernandes, I.d.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Katyal, M.; Singh, R.; Mahajan, R.; Sharma, A.; Gupta, R.; Aggarwal, N.K.; Yadav, A. Bacterial cellulose: Nature’s greener tool for industries. Biotechnol. Appl. Biochem. 2023, 70, 1629–1640. [Google Scholar] [CrossRef]
- Patel, A.; Patel, P.; Shukla, A.; Wong, J.W.C.; Varjani, S.; Gosai, H. Sustainable Bioconversion of Industrial Wastes into Bacterial Cellulose for Diverse Applications: A Way Towards Pollution Control and Abatement. Curr. Pollut. Rep. 2023, 9, 226–242. [Google Scholar] [CrossRef]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crops Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Iyyappan, J.; Jayamuthunagai, J.; Kumar, R.P.; Sirohi, R.; Gnansounou, E.; Pandey, A. Critical review on bioconversion of winery wastes into value-added products. Ind. Crops Prod. 2020, 158, 112954. [Google Scholar] [CrossRef]
- Arun, K.B.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; R, R.; Sirohi, R. Remodeling agro-industrial and food wastes into value-added bioactives and biopolymers. Ind. Crops Prod. 2020, 154, 112621. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Islam, M.U.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for cost-effective and enhanced production of bacterial cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Jančič, U.; Cepec, E.; Trček, J. Production efficiency and properties of bacterial cellulose membranes in a novel grape pomace hydrolysate by Komagataeibacter melomenusus AV436T and Komagataeibacter xylinus LMG 1518. Int. J. Biol. Macromol. 2023, 244, 125368. [Google Scholar] [CrossRef]
- Ogrizek, L.; Lamovšek, J.; Čuš, F.; Leskovšek, M.; Gorjanc, M. Properties of Bacterial Cellulose Produced Using White and Red Grape Bagasse as a Nutrient Source. Processes 2021, 9, 1088. [Google Scholar] [CrossRef]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Ionete, R.-E. An Overview on Management and Valorisation of Winery Wastes. Appl. Sci. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Cazón, P.; Puertas, G.; Vázquez, M. Production and Characterization of Active Bacterial Cellulose Films Obtained from the Fermentation of Wine Bagasse and Discarded Potatoes by Komagateibacter xylinus. Polymers 2022, 14, 5194. [Google Scholar] [CrossRef] [PubMed]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ciecholewska-Juśko, D.; Broda, M.; Żywicka, A.; Styburski, D.; Sobolewski, P.; Gorący, K.; Migdał, P.; Junka, A.; Fijałkowski, K. Potato Juice, a Starch Industry Waste, as a Cost-Effective Medium for the Biosynthesis of Bacterial Cellulose. Int. J. Mol. Sci. 2021, 22, 10807. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Chen, J.; Cao, Y.; Huang, C.; Feng, S.; Yang, H.; Tian, D. Valorization of straw biomass into lignin bio-ink, bacterial cellulose and activated nanocarbon through the trade-off alkali-catalyzed glycerol organosolv biorefinery. Chem. Eng. J. 2024, 484, 149549. [Google Scholar] [CrossRef]
- Papadaki, A.; Lappa, I.K.; Manikas, A.C.; Pastore Carbone, M.G.; Natsia, A.; Kachrimanidou, V.; Kopsahelis, N. Grafting bacterial cellulose nanowhiskers into whey protein/essential oil film composites: Effect on structure, essential oil release and antibacterial properties of films. Food Hydrocoll. 2024, 147, 109374. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Yu, Y.; Li, H.; Li, H.; Bai, J.; Shi, F.; Liu, J. Bacterial cellulose biomass aerogels for oil-water separation and thermal insulation. J. Environ. Chem. Eng. 2023, 11, 110403. [Google Scholar] [CrossRef]
- Karanicola, P.; Patsalou, M.; Stergiou, P.-Y.; Kavallieratou, A.; Evripidou, N.; Christou, P.; Panagiotou, G.; Damianou, C.; Papamichael, E.M.; Koutinas, M. Ultrasound-assisted dilute acid hydrolysis for production of essential oils, pectin and bacterial cellulose via a citrus processing waste biorefinery. Bioresour. Technol. 2021, 342, 126010. [Google Scholar] [CrossRef] [PubMed]
- Tsouko, E.; Maina, S.; Ladakis, D.; Kookos, I.K.; Koutinas, A. Integrated biorefinery development for the extraction of value-added components and bacterial cellulose production from orange peel waste streams. Renew. Energy 2020, 160, 944–954. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Wang, H.-M.D.; Tseng, C.-Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Le, H.V.; Dao, N.T.; Bui, H.T.; Kim Le, P.T.; Le, K.A.; Tuong Tran, A.T.; Nguyen, K.D.; Mai Nguyen, H.H.; Ho, P.H. Bacterial Cellulose Aerogels Derived from Pineapple Peel Waste for the Adsorption of Dyes. ACS Omega 2023, 8, 33412–33425. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patel, P.; Parmar, M.; Gosai, H. Employing RSM and ANN-based applications for modelling enhanced bacterial cellulose production from pineapple peel waste using Komagateibacter saccharivorans APPK1. Chem. Eng. J. 2024, 480, 148057. [Google Scholar] [CrossRef]
- Anwar, B.; Bundjali, B.; Sunarya, Y.; Arcana, I.M. Properties of Bacterial Cellulose and Its Nanocrystalline Obtained from Pineapple Peel Waste Juice. Fibers Polym. 2021, 22, 1228–1236. [Google Scholar] [CrossRef]
- Mardawati, E.; Rahmah, D.M.; Rachmadona, N.; Saharina, E.; Pertiwi, T.Y.R.; Zahrad, S.A.; Ramdhani, W.; Srikandace, Y.; Ratnaningrum, D.; Endah, E.S.; et al. Pineapple core from the canning industrial waste for bacterial cellulose production by Komagataeibacter xylinus. Heliyon 2023, 9, e22010. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable bacterial cellulose production by Achromobacter using mango peel waste. Microb. Cell Factories 2023, 22, 24. [Google Scholar] [CrossRef]
- García-Sánchez, M.E.; Robledo-Ortíz, J.R.; Jiménez-Palomar, I.; González-Reynoso, O.; González-García, Y. Production of bacterial cellulose by Komagataeibacter xylinus using mango waste as alternative culture medium. Rev. Mex. Ing. Quim. 2020, 19, 851–865. [Google Scholar] [CrossRef]
- Nasharudin, M.I.H.; Mahmud, N.; Rahim, M.H.A. Watermelon waste as a growth media substitute for bacterial cellulose production. AIP Conf. Proc. 2024, 3023, 020001. [Google Scholar]
- Quiñones-Cerna, C.; Rodríguez-Soto, J.C.; Barraza-Jáuregui, G.; Huanes-Carranza, J.; Cruz-Monzón, J.A.; Ugarte-López, W.; Hurtado-Butrón, F.; Samanamud-Moreno, F.; Haro-Carranza, D.; Valdivieso-Moreno, S.; et al. Bioconversion of Agroindustrial Asparagus Waste into Bacterial Cellulose by Komagataeibacter rhaeticus. Sustainability 2024, 16, 736. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, A.; Singh, M.K. Novel low-cost green method for production bacterial cellulose. Polym. Bull. 2023, 81, 6721–6741. [Google Scholar] [CrossRef]
- Avcioglu, N.H. Eco-friendly Production of Bacterial Cellulose with Komagataeibacter intermedius Strain by Using Jasminum sambac and Camellia sinensis Plants. J. Polym. Environ. 2024, 32, 460–477. [Google Scholar] [CrossRef]
- Agüero, A.; Lascano, D.; Ivorra-Martinez, J.; Gómez-Caturla, J.; Arrieta, M.P.; Balart, R. Use of bacterial cellulose obtained from kombucha fermentation in spent coffee grounds for active composites based on PLA and maleinized linseed oil. Ind. Crops Prod. 2023, 202, 116971. [Google Scholar] [CrossRef]
- De Souza, K.C.; Trindade, N.M.; De Amorim, J.D.P.; Do Nascimento, H.A.; Costa, A.F.S.; Henrique, M.A.; Caetano, V.F.; Sarubbo, L.A.; Vinhas, G.M. Kinetic study of a bacterial cellulose production by komagataeibacter rhaeticus using coffee grounds and sugarcane molasses. Mater. Res. 2021, 24, e202000454. [Google Scholar] [CrossRef]
- Singhaboot, P.; Phanomarpornchai, A.; Phuangsiri, C.; Boonthongtho, K.; Kroeksakul, P. The Potential of Liquid Waste from the Fruit Preserves Production Process as a Low-cost Raw Material for the Production of Bacterial Cellulose. Pertanika J. Trop. Agric. Sci. 2022, 45, 1125–1136. [Google Scholar] [CrossRef]
- Sar, T.; Yesilcimen Akbas, M. Potential use of olive oil mill wastewater for bacterial cellulose production. Bioengineered 2022, 13, 7659–7669. [Google Scholar] [CrossRef]
- Voon, W.W.Y.; Muhialdin, B.J.; Yusof, N.L.; Rukayadi, Y.; Meor Hussin, A.S. Bio-cellulose Production by Beijerinckia fluminensis WAUPM53 and Gluconacetobacter xylinus 0416 in Sago By-product Medium. Appl. Biochem. Biotechnol. 2019, 187, 211–220. [Google Scholar] [CrossRef]
- Yanti, N.A.; Ahmad, S.W.; Muhiddin, N.H.; Ramadhan, L.O.A.N.; Walhidayah, T. Characterization of bacterial cellulose produced by acetobacter xylinum strain lkn6 using sago liquid waste as nutrient source. Pak. J. Biol. Sci. 2021, 24, 335–344. [Google Scholar] [CrossRef]
- Dórame-Miranda, R.F.; Gámez-Meza, N.; Medina-Juárez, L.Á.; Ezquerra-Brauer, J.M.; Ovando-Martínez, M.; Lizardi-Mendoza, J. Bacterial cellulose production by Gluconacetobacter entanii using pecan nutshell as carbon source and its chemical functionalization. Carbohydr. Polym. 2019, 207, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.T.; de Oliveira Barros, M.; de Araújo e Silva, R.; Silva, S.M.F.; de Almeida, J.S.; de Freitas Rosa, M.; Gonçalves, L.R.B.; Brígida, A.I.S. Superabsorbent bacterial cellulose film produced from industrial residue of cashew apple juice processing. Int. J. Biol. Macromol. 2023, 242, 124405. [Google Scholar] [CrossRef] [PubMed]
- Tsouko, E.; Pilafidis, S.; Dimopoulou, M.; Kourmentza, K.; Sarris, D. Bioconversion of underutilized brewing by-products into bacterial cellulose by a newly isolated Komagataeibacter rhaeticus strain: A preliminary evaluation of the bioprocess environmental impact. Bioresour. Technol. 2023, 387, 129667. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, R.L.; Lammers, D.; Gottschling, M.; Dohnt, K. Effect of food industry by-products on bacterial cellulose production and its structural properties. Cellulose 2023, 30, 4159–4179. [Google Scholar] [CrossRef]
- Unlu, F.; Boran, F.; Yesilada, O.; Koytepe, S. Laccase immobilization on bacterial cellulose produced in a mulberry pomace waste extract medium: Characterization and use for dye decolorization. J. Appl. Polym. Sci. 2023, 140, e53952. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Apriyana, A.Y.; Andriani, D.; Karina, M. Production of bacterial cellulose from tofu liquid waste and rice-washed water: Morphological property and its functional groups analysis. IOP Conf. Ser. Earth Environ. Sci. 2020, 483, 012005. [Google Scholar] [CrossRef]
- Taokaew, S.; Nakson, N.; Thienchaimongkol, J.; Kobayashi, T. Enhanced production of fibrous bacterial cellulose in Gluconacetobacter xylinus culture medium containing modified protein of okara waste. J. Biosci. Bioeng. 2023, 135, 71–78. [Google Scholar] [CrossRef]
- Ho Jin, Y.; Lee, T.; Kim, J.R.; Choi, Y.-E.; Park, C. Improved production of bacterial cellulose from waste glycerol through investigation of inhibitory effects of crude glycerol-derived compounds by Gluconacetobacter xylinus. J. Ind. Eng. Chem. 2019, 75, 158–163. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Zheng, J.; Mao, D.; Yang, X. Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium. Bioresour. Technol. 2019, 274, 518–524. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Azi, F.; Ge, Z.-W.; Liu, Y.-F.; Yin, X.-T.; Dong, M.-S. Bio-conversion of kitchen waste into bacterial cellulose using a new multiple carbon utilizing Komagataeibacter rhaeticus: Fermentation profiles and genome-wide analysis. Int. J. Biol. Macromol. 2021, 191, 211–221. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, D.; Hu, J.; Huang, M.; Shen, F.; Zeng, Y.; Yang, G.; Zhang, Y.; He, J. Harvesting Bacterial Cellulose from Kitchen Waste to Prepare Superhydrophobic Aerogel for Recovering Waste Cooking Oil toward a Closed-Loop Biorefinery. ACS Sustain. Chem. Eng. 2020, 8, 13400–13407. [Google Scholar] [CrossRef]
- Nguyen Ngo, T.T.; Phan, T.H.; Thong Le, T.M.; Tu Le, T.N.; Huynh, Q.; Trang Phan, T.P.; Hoang, M.; Vo, T.P.; Nguyen, D.Q. Producing bacterial cellulose from industrial recycling paper waste sludge. Heliyon 2023, 9, e17663. [Google Scholar] [CrossRef]
- Peiravi-Rivash, O.; Mashreghi, M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Producing bacterial nano-cellulose and keratin from wastes to synthesize keratin/cellulose nanobiocomposite for removal of dyes and heavy metal ions from waters and wastewaters. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130355. [Google Scholar] [CrossRef]
- Garmaroody, E.R.; Jafarzadeh, A.E.; Kermanian, H.; Ramezani, O. Spent black liquor as an alternative carbon source for the synthesis of bacterial cellulose. Cellul. Chem. Technol. 2022, 56, 749–756. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Zhang, Y.; Sun, S.; Ullah, M.W.; Xu, W. Biotransformation of nylon-6,6 hydrolysate to bacterial cellulose. Green Chem. 2021, 23, 7805–7815. [Google Scholar] [CrossRef]
- Ojo, A.O. An Overview of Lignocellulose and Its Biotechnological Importance in High-Value Product Production. Fermentation 2023, 9, 990. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production- A review. Biomass Convers. Biorefinery 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef]
- Arminda, M.; Josúe, C.; Cristina, D.; Fabiana, S.; Yolanda, M. Use of activated carbons for detoxification of a lignocellulosic hydrolysate: Statistical optimisation. J. Environ. Manag. 2021, 296, 113320. [Google Scholar] [CrossRef]
- Lin, S.-P.; Huang, S.-H.; Ting, Y.; Hsu, H.-Y.; Cheng, K.-C. Evaluation of detoxified sugarcane bagasse hydrolysate by atmospheric cold plasma for bacterial cellulose production. Int. J. Biol. Macromol. 2022, 204, 136–143. [Google Scholar] [CrossRef]
- Santoso, S.P.; Lin, S.-P.; Wang, T.-Y.; Ting, Y.; Hsieh, C.-W.; Yu, R.-C.; Angkawijaya, A.E.; Soetaredjo, F.E.; Hsu, H.-Y.; Cheng, K.-C. Atmospheric cold plasma-assisted pineapple peel waste hydrolysate detoxification for the production of bacterial cellulose. Int. J. Biol. Macromol. 2021, 175, 526–534. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Huang, C.; Guo, H.-J.; Xiong, L.; Luo, J.; Wang, B.; Lin, X.-Q.; Chen, X.-F.; Chen, X.-D. Bacterial cellulose production from the litchi extract by Gluconacetobacter xylinus. Prep. Biochem. Biotechnol. 2016, 46, 39–43. [Google Scholar] [CrossRef]
- Panesar, P.S.; Chavan, Y.; Chopra, H.K.; Kennedy, J.F. Production of microbial cellulose: Response surface methodology approach. Carbohydr. Polym. 2012, 87, 930–934. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Santoso, S.P.; Chou, C.-C.; Lin, S.-P.; Soetaredjo, F.E.; Ismadji, S.; Hsieh, C.-W.; Cheng, K.C. Enhanced production of bacterial cellulose by Komactobacter intermedius using statistical modeling. Cellulose 2020, 27, 2497–2509. [Google Scholar] [CrossRef]
- Nie, W.; Zheng, X.; Feng, W.; Liu, Y.; Li, Y.; Liang, X. Characterization of bacterial cellulose produced by Acetobacter pasteurianus MGC-N8819 utilizing lotus rhizome. LWT 2022, 165, 113763. [Google Scholar] [CrossRef]
- Amândio, M.S.T.; Rocha, J.M.S.; Xavier, A.M.R.B. Enzymatic Hydrolysis Strategies for Cellulosic Sugars Production to Obtain Bioethanol from Eucalyptus globulus Bark. Fermentation 2023, 9, 241. [Google Scholar] [CrossRef]
- Efthymiou, M.-N.; Tsouko, E.; Pateraki, C.; Papagiannopoulos, A.; Tzamalis, P.; Pispas, S.; Bethanis, K.; Mantala, I.; Koutinas, A. Property evaluation of bacterial cellulose nanostructures produced from confectionery wastes. Biochem. Eng. J. 2022, 186, 108575. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Pawar, K.D. Production of microcrystalline cellulose and bacterial nanocellulose through biological valorization of lignocellulosic biomass wastes. J. Clean. Prod. 2021, 327, 129462. [Google Scholar] [CrossRef]
- Yan, S.; Xu, Y.; Yu, X.-W. Rational engineering of xylanase hyper-producing system in Trichoderma reesei for efficient biomass degradation. Biotechnol. Biofuels 2021, 14, 90. [Google Scholar] [CrossRef]
- Saleh, A.K.; Salama, A.; Badawy, A.S.; Diab, M.A.; El-Gendi, H. Paper sludge saccharification for batch and fed-batch production of bacterial cellulose decorated with magnetite for dye decolorization by experimental design. Cellulose 2023, 30, 10841–10866. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, F.; Yang, H.; Cao, G.; Xu, W.; Sun, S.; Zhang, Y. Sequential fermentation strategy improves microbial conversion of waste jasmine flower to bacterial cellulose with antibacterial properties. Ind. Crops Prod. 2022, 185, 115147. [Google Scholar] [CrossRef]
- Duncan, S.M.; Alkasrawi, M.; Gurram, R.; Almomani, F.; Wiberley-Bradford, A.E.; Singsaas, E. Paper Mill Sludge as a Source of Sugars for Use in the Production of Bioethanol and Isoprene. Energies 2020, 13, 4662. [Google Scholar] [CrossRef]
- Saleh, A.K.; El-Gendi, H.; Ray, J.B.; Taha, T.H. A low-cost effective media from starch kitchen waste for bacterial cellulose production and its application as simultaneous absorbance for methylene blue dye removal. Biomass Convers. Biorefinery 2023, 13, 12437–12449. [Google Scholar] [CrossRef]
- El-Bestawy, E.; Eltaweil, A.S.; Khallaf, N.S. Effective production of bacterial cellulose using acidic dairy industry by-products and agro wastes. Sustain. Chem. Pharm. 2023, 33, 101064. [Google Scholar] [CrossRef]
- Ganta, A.; Bashir, Y.; Das, S. Dairy Wastewater as a Potential Feedstock for Valuable Production with Concurrent Wastewater Treatment through Microbial Electrochemical Technologies. Energies 2022, 15, 9084. [Google Scholar] [CrossRef]
- Astuti, E.N.J.; Nugroho, D.A.; Ahmadi, T.P. Application of real-time image processing for monitoring bacterial cellulose growth in various nitrogen sources using soybean-boiled wastewater medium during fermentation. IOP Conf. Ser. Earth Environ. Sci. 2023, 1183, 012064. [Google Scholar] [CrossRef]
- Jin Chung, W.; Shim, J.; Ravindran, B. Characterization of cheese processed wastewater and treatment using calcium nanoparticles synthesised by Senna auriculata L flower extract. J. King Saud Univ. Sci. 2022, 34, 101793. [Google Scholar] [CrossRef]
- Lotfy, V.F.; Basta, A.H.; Abdel-Monem, M.O.; Abdel-Hamed, G.Z. Utilization of bacteria in rotten Guava for production of bacterial cellulose from isolated and protein waste. Carbohydr. Polym. Technol. Appl. 2021, 2, 100076. [Google Scholar] [CrossRef]
- Taokaew, S.; Zhang, X.; Chuenkaek, T.; Kobayashi, T. Chitin from fermentative extraction of crab shells using okara as a nutrient source and comparative analysis of structural differences from chemically extracted chitin. Biochem. Eng. J. 2020, 159, 107588. [Google Scholar] [CrossRef]
- Skiba, E.A.; Shavyrkina, N.A.; Budaeva, V.V.; Sitnikova, A.E.; Korchagina, A.A.; Bychin, N.V.; Gladysheva, E.K.; Pavlov, I.N.; Zharikov, A.N.; Lubyansky, V.G.; et al. Biosynthesis of Bacterial Cellulose by Extended Cultivation with Multiple Removal of BC Pellicles. Polymers 2021, 13, 2118. [Google Scholar] [CrossRef] [PubMed]
- Shavyrkina, N.A.; Skiba, E.A.; Kazantseva, A.E.; Gladysheva, E.K.; Budaeva, V.V.; Bychin, N.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Mironova, G.F.; Korchagina, A.A.; et al. Static Culture Combined with Aeration in Biosynthesis of Bacterial Cellulose. Polymers 2021, 13, 4241. [Google Scholar] [CrossRef] [PubMed]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized culture conditions for bacterial cellulose production by Acetobacter senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Babaeipour, V.; Soleimani, A. Optimization of Bacterial Nano-Cellulose Production in Bench-Scale Rotating Biological Contact Bioreactor by Response Surface Methodology. Iran. J. Chem. Chem. Eng. 2021, 40, 407–416. [Google Scholar]
- Kumar, V.; Sharma, D.K.; Bansal, V.; Mehta, D.; Sangwan, R.S.; Yadav, S.K. Efficient and economic process for the production of bacterial cellulose from isolated strain of Acetobacter pasteurianus of RSV-4 bacterium. Bioresour. Technol. 2019, 275, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Han, K.-A. Optimization of bacterial cellulose production from alcohol lees by intermittent feeding strategy. Braz. J. Chem. Eng. 2023, 40, 685–694. [Google Scholar] [CrossRef]
- Adnan, A.; Nair, G.; Lay, M.; Swan, J. Bacterial Cellulose Synthesis by Gluconacetobacter xylinus: Enhancement via Fed-batch Fermentation Strategies in Glycerol Media. Trends Sci. 2021, 18, 453. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Chegeni, A.; Babaeipour, V. Mathematical modeling and simulation of oxygen mass transfer in rotating biological contactor (RBC) for bacterial cellulose production. Biochem. Eng. J. 2023, 200, 109076. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Aqsha, A.; Yub Harun, N.; Ayoub, M.; Wirzal, M.D.H.; Jaafar, J.; Mulyati, S.; Elma, M. Effect of membrane properties in a membrane rotating biological contactor for wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104869. [Google Scholar] [CrossRef]
- Han, Y.; Ma, J.; Xiao, B.; Huo, X.; Guo, X. New Integrated Self-Refluxing Rotating Biological Contactor for rural sewage treatment. J. Clean. Prod. 2019, 217, 324–334. [Google Scholar] [CrossRef]
- Soleimani, A.; Hamedi, S.; Babaeipour, V.; Rouhi, M. Design, construction and optimization a flexible bench-scale rotating biological contactor (RBC) for enhanced production of bacterial cellulose by Acetobacter Xylinium. Bioprocess Biosyst. Eng. 2021, 44, 1071–1080. [Google Scholar] [CrossRef]
- Vázquez, M.; Puertas, G.; Cazón, P. Processing of Grape Bagasse and Potato Wastes for the Co-Production of Bacterial Cellulose and Gluconic Acid in an Airlift Bioreactor. Polymers 2023, 15, 3944. [Google Scholar] [CrossRef]
- Kthiri, A.; Hidouri, S.; Wiem, T.; Jeridi, R.; Sheehan, D.; Landouls, A. Biochemical and biomolecular effects induced by a static magnetic field in Saccharomyces cerevisiae: Evidence for oxidative stress. PLoS ONE 2019, 14, e0209843. [Google Scholar] [CrossRef]
- Żywicka, A.; Ciecholewska-Juśko, D.; Drozd, R.; Rakoczy, R.; Konopacki, M.; Kordas, M.; Junka, A.; Migdał, P.; Fijałkowski, K. Preparation of Komagataeibacter xylinus Inoculum for Bacterial Cellulose Biosynthesis Using Magnetically Assisted External-Loop Airlift Bioreactor. Polymers 2021, 13, 3950. [Google Scholar] [CrossRef]
- Konopacki, M.; Grygorcewicz, B.; Kordas, M.; Ossowicz-Rupniewska, P.; Nowak, A.; Perużyńska, M.; Rakoczy, R. Intensification of bacterial cellulose production process with sequential electromagnetic field exposure aided by dynamic modelling. Biochem. Eng. J. 2022, 182, 108432. [Google Scholar] [CrossRef]
- Drozd, R.; Rakoczy, R.; Wasak, A.; Junka, A.; Fijałkowski, K. The application of magnetically modified bacterial cellulose for immobilization of laccase. Int. J. Biol. Macromol. 2018, 108, 462–470. [Google Scholar] [CrossRef]
- Drozd, R.; Szymańska, M.; Żywicka, A.; Kowalska, U.; Rakoczy, R.; Kordas, M.; Konopacki, M.; Junka, A.F.; Fijałkowski, K. Exposure to non-continuous rotating magnetic field induces metabolic strain-specific response of Komagataeibacter xylinus. Biochem. Eng. J. 2021, 166, 107855. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Rakoczy, R.; Żywicka, A.; Drozd, R.; Zielińska, B.; Wenelska, K.; Cendrowski, K.; Peitler, D.; Kordas, M.; Konopacki, M.; et al. Time Dependent Influence of Rotating Magnetic Field on Bacterial Cellulose. Int. J. Polym. Sci. 2016, 2016, 7536397. [Google Scholar] [CrossRef]
- Lechowska, J.; Kordas, M.; Konopacki, M.; Fijałkowski, K.; Drozd, R.; Rakoczy, R. Hydrodynamic studies in magnetically assisted external-loop airlift reactor. Chem. Eng. J. 2019, 362, 298–309. [Google Scholar] [CrossRef]
- Gullo, M.; La China, S.; Petroni, G.; Di Gregorio, S.; Giudici, P. Exploring K2G30 Genome: A High Bacterial Cellulose Producing Strain in Glucose and Mannitol Based Media. Front. Microbiol. 2019, 10, 58. [Google Scholar] [CrossRef]
- Ryngajłło, M.; Jędrzejczak-Krzepkowska, M.; Kubiak, K.; Ludwicka, K.; Bielecki, S. Towards control of cellulose biosynthesis by Komagataeibacter using systems-level and strain engineering strategies: Current progress and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 6565–6585. [Google Scholar] [CrossRef]
- Singh, A.; Walker, K.T.; Ledesma-Amaro, R.; Ellis, T. Engineering Bacterial Cellulose by Synthetic Biology. Int. J. Mol. Sci. 2020, 21, 9185. [Google Scholar] [CrossRef]
- Anguluri, K.; La China, S.; Brugnoli, M.; Cassanelli, S.; Gullo, M. Better under stress: Improving bacterial cellulose production by Komagataeibacter xylinus K2G30 (UMCC 2756) using adaptive laboratory evolution. Front. Microbiol. 2022, 13, 994097. [Google Scholar] [CrossRef]
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular aspects of bacterial nanocellulose biosynthesis. Microb. Biotechnol. 2019, 12, 633–649. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Xie, Y.; Jia, S.; Hou, Y.; Zou, Y.; Zhong, C. Enhanced bacterial cellulose production by Gluconacetobacter xylinus via expression of Vitreoscilla hemoglobin and oxygen tension regulation. Appl. Microbiol. Biotechnol. 2018, 102, 1155–1165. [Google Scholar] [CrossRef]
- Jang, W.D.; Kim, T.Y.; Kim, H.U.; Shim, W.Y.; Ryu, J.Y.; Park, J.H.; Lee, S.Y. Genomic and metabolic analysis of Komagataeibacter xylinus DSM 2325 producing bacterial cellulose nanofiber. Biotechnol. Bioeng. 2019, 116, 3372–3381. [Google Scholar] [CrossRef]
- Yang, F.; Cao, Z.; Li, C.; Chen, L.; Wu, G.; Zhou, X.; Hong, F.F. A recombinant strain of Komagataeibacter xylinus ATCC 23770 for production of bacterial cellulose from mannose-rich resources. New Biotechnol. 2023, 76, 72–81. [Google Scholar] [CrossRef]
- Lu, T.; Gao, H.; Liao, B.; Wu, J.; Zhang, W.; Huang, J.; Liu, M.; Huang, J.; Chang, Z.; Jin, M.; et al. Characterization and optimization of production of bacterial cellulose from strain CGMCC 17276 based on whole-genome analysis. Carbohydr. Polym. 2020, 232, 115788. [Google Scholar] [CrossRef]
- Summary for Policymakers. In Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2023; pp. 3–34. [Google Scholar]
- Forte, A.; Dourado, F.; Mota, A.; Neto, B.; Gama, M.; Ferreira, E.C. Life cycle assessment of bacterial cellulose production. Int. J. Life Cycle Assess. 2021, 26, 864–878. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. Microcrystalline cellulose production from sugarcane bagasse: Sustainable process development and life cycle assessment. J. Clean. Prod. 2020, 249, 119342. [Google Scholar] [CrossRef]
- Turk, J.; Oven, P.; Poljanšek, I.; Lešek, A.; Knez, F.; Malovrh Rebec, K. Evaluation of an environmental profile comparison for nanocellulose production and supply chain by applying different life cycle assessment methods. J. Clean. Prod. 2020, 247, 119107. [Google Scholar] [CrossRef]
- de Araújo e Silva, R.; Santa Brígida, A.I.; de Freitas Rosa, M.; da Silva Neto, R.M.; Spinosa, W.A.; Benício de Sá Filho, E.; Brito de Figueirêdo, M.C. An approach for implementing ecodesign at early research stage: A case study of bacterial cellulose production. J. Clean. Prod. 2020, 269, 122245. [Google Scholar] [CrossRef]
- Martínez, E.; Posada, L.; Botero, J.C.; Rios-Arango, J.A.; Zapata-Benabithe, Z.; López, S.; Molina-Ramírez, C.; Osorio, M.A.; Castro, C.I. Nata de fique: A cost-effective alternative for the large-scale production of bacterial nanocellulose. Ind. Crops Prod. 2023, 192, 116015. [Google Scholar] [CrossRef]
- Aragão, J.V.S.; Costa, A.F.S.; Silva, G.L.; Silva, S.M.; Macêdo, J.S.; Galdino, C.J.S.; Milanez, V.F.A.; Sarubbo, L.A. Analysis of the environmental life cycle of bacterial cellulose production. Chem. Eng. Trans. 2020, 79, 445–450. [Google Scholar]
- Wada, N.; Fujie, T.; Sasaki, R.; Matsushima, T.; Takahashi, K. Direct synthesis of a robust cellulosic composite from cellulose acetate and a nanofibrillated bacterial cellulose sol. Polym. J. 2022, 54, 735–740. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, W.; Cui, Y.; Zhou, Y.; Zhang, Y.; Xu, S.; Lin, L.; Zhou, M.; Li, Z. Reversible wettability control of self-assembled TiO2 scaffolds on bacterial cellulose from superhydrophobicity to superhydrophilicity. Cellulose 2024, 31, 2907–2920. [Google Scholar] [CrossRef]
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B.; Alemtoshi. PHA-Based Bioplastic: A Potential Alternative to Address Microplastic Pollution. Water Air Soil Pollut. 2022, 234, 21. [Google Scholar] [CrossRef]
- Patrício Silva, A.L.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar] [CrossRef]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Advances in Biodegradable Polymers and Biomaterials for Medical Applications—A Review. Molecules 2023, 28, 6213. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Zhang, M.; Ullah, M.W.; Liu, L.; Zhao, W.; Li, Y.; Ahmed, A.A.Q.; Cheng, H.; Shi, Z.; et al. In Situ Synthesized Selenium Nanoparticles-Decorated Bacterial Cellulose/Gelatin Hydrogel with Enhanced Antibacterial, Antioxidant, and Anti-Inflammatory Capabilities for Facilitating Skin Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100402. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.; Burdiles, P.A.; Quero, F.; Palma, P.; Olate-Moya, F.; Palza, H. 3D Printing of Antimicrobial Alginate/Bacterial-Cellulose Composite Hydrogels by Incorporating Copper Nanostructures. ACS Biomater. Sci. Eng. 2019, 5, 6290–6299. [Google Scholar] [CrossRef] [PubMed]
- Supanakorn, G.; Taokaew, S.; Phisalaphong, M. Multifunctional Cellulosic Natural Rubber and Silver Nanoparticle Films with Superior Chemical Resistance and Antibacterial Properties. Nanomaterials 2023, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Dincă, V.; Mocanu, A.; Isopencu, G.; Busuioc, C.; Brajnicov, S.; Vlad, A.; Icriverzi, M.; Roseanu, A.; Dinescu, M.; Stroescu, M.; et al. Biocompatible pure ZnO nanoparticles-3D bacterial cellulose biointerfaces with antibacterial properties. Arab. J. Chem. 2020, 13, 3521–3533. [Google Scholar] [CrossRef]
- Windarsih, A.; Indrianingsih, A.W.; Maryana, R.; Apriyana, W.; Rosyida, V.T.; Nurhayati, S.; Jatmiko, T.H.; Ratih, D.; Suwanto, A. Gold modified bacterial cellulose from coconut water waste and its antibacterial activity. Waste Biomass Valorization 2022, 13, 4157–4164. [Google Scholar] [CrossRef]
- Skeeters, S.S.; Rosu, A.C.; Divyanshi; Yang, J.; Zhang, K. Comparative Determination of Cytotoxicity of Sub-10 nm Copper Nanoparticles to Prokaryotic and Eukaryotic Systems. ACS Appl. Mater. Interfaces 2020, 12, 50203–50211. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jin, W.; Qasim, A.M.; Gao, A.; Peng, X.; Li, W.; Feng, H.; Chu, P.K. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials 2017, 124, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants 2017, 6, 16. [Google Scholar] [CrossRef]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.-M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef]
- Cabañas-Romero, L.V.; Valls, C.; Valenzuela, S.V.; Roncero, M.B.; Pastor, F.I.J.; Diaz, P.; Martínez, J. Bacterial Cellulose–Chitosan Paper with Antimicrobial and Antioxidant Activities. Biomacromolecules 2020, 21, 1568–1577. [Google Scholar] [CrossRef]
- Sajjad, W.; He, F.; Ullah, M.W.; Ikram, M.; Shah, S.M.; Khan, R.; Khan, T.; Khalid, A.; Yang, G.; Wahid, F. Fabrication of Bacterial Cellulose-Curcumin Nanocomposite as a Novel Dressing for Partial Thickness Skin Burn. Front. Bioeng. Biotechnol. 2020, 8, 553037. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.; Spasojević, D.; Orlovska, I.; Kozyrovska, N.; Soković, M.; Glamočlija, J.; Dmitrović, S.; Matović, B.; Tasić, N.; Maksimović, V.; et al. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Jantarat, C.; Attakitmongkol, K.; Nichsapa, S.; Sirathanarun, P.; Srivaro, S. Molecularly imprinted bacterial cellulose for sustained-release delivery of quercetin. J. Biomater. Sci. Polym. Ed. 2020, 31, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Ngwabebhoh, F.A.; Patwa, R.; Zandraa, O.; Saha, N.; Saha, P. Preparation and characterization of injectable self-antibacterial gelatin/carrageenan/bacterial cellulose hydrogel scaffolds for wound healing application. J. Drug Deliv. Sci. Technol. 2021, 63, 102415. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Rosyida, V.T.; Apriyana, W.; Hayati, S.N.; Darsih, C.; Nisa, K.; Ratih, D. Antioxidant and antibacterial properties of bacterial cellulose—Indonesian plant extract composites for mask sheet. J. Appl. Pharm. Sci. 2020, 10, 037–042. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Ul-Islam, M.; Kamal, T.; Ahmad, M.W.; Abbas, Y.; Manan, S.; Ullah, M.W.; Yang, G. Ex situ development and characterization of green antibacterial bacterial cellulose-based composites for potential biomedical applications. Adv. Compos. Hybrid Mater. 2022, 5, 307–321. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ahmad, F.; Fatima, A.; Shah, N.; Yasir, S.; Ahmad, M.W.; Manan, S.; Ullah, M.W. Ex situ Synthesis and Characterization of High Strength Multipurpose Bacterial Cellulose-Aloe vera Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 601988. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Daniloski, D.; Pratibha; Neeraj; D’Cunha, N.M.; Naumovski, N.; Petkoska, A.T. Pomegranate peel extract—A natural bioactive addition to novel active edible packaging. Food Res. Int. 2022, 156, 111378. [Google Scholar] [CrossRef]
- Glazer, I.; Masaphy, S.; Marciano, P.; Bar-Ilan, I.; Holland, D.; Kerem, Z.; Amir, R. Partial Identification of Antifungal Compounds from Punica granatum Peel Extracts. J. Agric. Food Chem. 2012, 60, 4841–4848. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Gendi, H.; El-Fakharany, E.M.; El-Ziney, M.G.; El-Yazbi, A.F.; Al Meqbaali, F.T.; Ibrahim, W.H. Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate. Nutrients 2023, 15, 2709. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Alhajaim, W.; Fatima, A.; Yasir, S.; Kamal, T.; Abbas, Y.; Khan, S.; Khan, A.H.; Manan, S.; Ullah, M.W.; et al. Development of low-cost bacterial cellulose-pomegranate peel extract-based antibacterial composite for potential biomedical applications. Int. J. Biol. Macromol. 2023, 231, 123269. [Google Scholar] [CrossRef] [PubMed]
- Marisca Evalina, G.; Yulanda, A.; Yuana Elly, A. Effectivity of Patch Herbal Mixture Composed of Mangosteen Peel Extract and Bacterial Cellulose for Wound Healing. Pharmacogn. J. 2023, 15, 461–466. [Google Scholar]
- Taokaew, S.; Chiaoprakobkij, N.; Siripong, P.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Multifunctional cellulosic nanofiber film with enhanced antimicrobial and anticancer properties by incorporation of ethanolic extract of Garcinia mangostana peel. Mater. Sci. Eng. C 2021, 120, 111783. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Piyaviriyakul, S.; Siripong, P.; Phisalaphong, M. Aqueous and ethanolic extracts of mangosteen peels as natural antimicrobial/anticancer materials against pathogenic microbes and B16F10 murine melanoma. Chiang Mai J. Sci. 2018, 45, 1345–1358. [Google Scholar]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Bakhsh, E.M.; Chani, M.T.S. Development of plant extract impregnated bacterial cellulose as a green antimicrobial composite for potential biomedical applications. Ind. Crops Prod. 2022, 187, 115337. [Google Scholar] [CrossRef]
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ding, S.; Hu, H.; Wang, S. Recent advances in structural regulation and optimization of high-performance solar-driven interfacial evaporation systems. J. Mater. Chem. A 2022, 10, 18509–18541. [Google Scholar] [CrossRef]
- Wang, G.; Tang, Z.; Gao, Y.; Liu, P.; Li, Y.; Li, A.; Chen, X. Phase Change Thermal Storage Materials for Interdisciplinary Applications. Chem. Rev. 2023, 123, 6953–7024. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Liang, Q.; Song, J.; Guan, M.; Zhang, T.; Chen, S.; Wang, H. One-Step Synthesis of Multifunctional Bacterial Cellulose Film-Based Phase Change Materials with Cross-Linked Network Structure for Solar–Thermal Energy Conversion, Storage, and Utilization. Small 2023, 20, 2307259. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.H.; Chowdhury, M.A.; Hossain, N.; Islam, M.A.; Mobarak, M.H. Advances of lithium-ion batteries anode materials—A review. Chem. Eng. J. Adv. 2023, 16, 100569. [Google Scholar] [CrossRef]
- Lingappan, N.; Lee, W.; Passerini, S.; Pecht, M. A comprehensive review of separator membranes in lithium-ion batteries. Renew. Sustain. Energy Rev. 2023, 187, 113726. [Google Scholar] [CrossRef]
- Ajkidkarn, P.; Manuspiya, H. Novel bacterial cellulose nanocrystals/polyether block amide microporous membranes as separators for lithium-ion batteries. Int. J. Biol. Macromol. 2020, 164, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Kartika Sari, A.; Mohamad Yunus, R.; Majlan, E.H.; Loh, K.S.; Wong, W.Y.; Saidin, N.U.; Alva, S.; Khaerudini, D.S. Nata de Cassava Type of Bacterial Cellulose Doped with Phosphoric Acid as a Proton Exchange Membrane. Membranes 2023, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Teymouri, M.; Saboor, S.; Khalili, A.; Goodarzi, V.; Poudineh Hajipoor, F.; Khonakdar, H.A.; Shojaei, S.; Asefnejad, A.; Bagheri, H. Challenge between sequence presences of conductive additives on flexibility, dielectric and supercapacitance behaviors of nanofibrillated template of bacterial cellulose aerogels. Eur. Polym. J. 2019, 115, 335–345. [Google Scholar] [CrossRef]
- Ye, J.; Guo, L.; Zheng, S.; Feng, Y.; Zhang, T.; Yang, Z.; Yuan, Q.; Shen, G.; Zhang, Z. Synthesis of bacterial cellulose based SnO2-PPy nanocomposites as potential flexible, highly conductive material. Mater. Lett. 2019, 253, 372–376. [Google Scholar] [CrossRef]
- Sheng, N.; Chen, S.; Yao, J.; Guan, F.; Zhang, M.; Wang, B.; Wu, Z.; Ji, P.; Wang, H. Polypyrrole@TEMPO-oxidized bacterial cellulose/reduced graphene oxide macrofibers for flexible all-solid-state supercapacitors. Chem. Eng. J. 2019, 368, 1022–1032. [Google Scholar] [CrossRef]
- Vilela, C.; Silva, A.C.Q.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Loureiro, F.J.A.; Vilela, C.; Rosero-Navarro, N.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Protonic conductivity and fuel cell tests of nanocomposite membranes based on bacterial cellulose. Electrochim. Acta 2017, 233, 52–61. [Google Scholar] [CrossRef]
- Naumi, F.; Natanael, C.L.; Rahayu, I.; Indrarti, L.; Hendrana, S. Polymer Electrolyte Membrane Fuel Cell based on Sulfonated Polystyrene and Phosphoric Acid with Biocellulose as a Matrix. Res. J. Chem. Env. 2018, 22, 289–293. [Google Scholar]
- Trindade, E.C.A.; Antônio, R.V.; Brandes, R.; de Souza, L.; Neto, G.; Vargas, V.M.M.; Carminatti, C.A.; de Oliveira Souza Recouvreux, D. Carbon fiber-embedded bacterial cellulose/polyaniline nanocomposite with tailored for microbial fuel cells electrode. J. Appl. Polym. Sci. 2020, 137, 49036. [Google Scholar] [CrossRef]
- Luo, H.; Dong, J.; Xu, X.; Wang, J.; Yang, Z.; Wan, Y. Exploring excellent dispersion of graphene nanosheets in three-dimensional bacterial cellulose for ultra-strong nanocomposite hydrogels. Compos. Part A Appl. Sci. Manuf. 2018, 109, 290–297. [Google Scholar] [CrossRef]
- Luo, H.; Dong, J.; Yao, F.; Yang, Z.; Li, W.; Wang, J.; Xu, X.; Hu, J.; Wan, Y. Layer-by-Layer Assembled Bacterial Cellulose/Graphene Oxide Hydrogels with Extremely Enhanced Mechanical Properties. Nano-Micro Lett. 2018, 10, 42. [Google Scholar] [CrossRef]
- Luo, H.; Dong, J.; Zhang, Y.; Li, G.; Guo, R.; Zuo, G.; Ye, M.; Wang, Z.; Yang, Z.; Wan, Y. Constructing 3D bacterial cellulose/graphene/polyaniline nanocomposites by novel layer-by-layer in situ culture toward mechanically robust and highly flexible freestanding electrodes for supercapacitors. Chem. Eng. J. 2018, 334, 1148–1158. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Yang, Z.; Ao, H.; Xiong, L.; Luo, H. Simultaneously depositing polyaniline onto bacterial cellulose nanofibers and graphene nanosheets toward electrically conductive nanocomposites. Curr. Appl. Phys. 2018, 18, 933–940. [Google Scholar] [CrossRef]
- Tan, H.; Tang, J.; Henzie, J.; Li, Y.; Xu, X.; Chen, T.; Wang, Z.; Wang, J.; Ide, Y.; Bando, Y.; et al. Assembly of Hollow Carbon Nanospheres on Graphene Nanosheets and Creation of Iron–Nitrogen-Doped Porous Carbon for Oxygen Reduction. ACS Nano 2018, 12, 5674–5683. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, P.; Xie, J.; Yang, Z.; Huang, Y.; Hu, J.; Wan, Y.; Xu, Y. Uniformly Dispersed Freestanding Carbon Nanofiber/Graphene Electrodes Made by a Scalable Biological Method for High-Performance Flexible Supercapacitors. Adv. Funct. Mater. 2018, 28, 1803075. [Google Scholar] [CrossRef]
- Dhar, P.; Pratto, B.; Gonçalves Cruz, A.J.; Bankar, S. Valorization of sugarcane straw to produce highly conductive bacterial cellulose / graphene nanocomposite films through in situ fermentation: Kinetic analysis and property evaluation. J. Clean. Prod. 2019, 238, 117859. [Google Scholar] [CrossRef]
- Wei, Q.; Fei, N.; Islam, A.; Lei, T.; Hong, L.; Peng, R.; Fan, X.; Chen, L.; Gao, P.; Ge, Z. Small-Molecule Emitters with High Quantum Efficiency: Mechanisms, Structures, and Applications in OLED Devices. Adv. Opt. Mater. 2018, 6, 1800512. [Google Scholar] [CrossRef]
- Lim, Y.-W.; Jin, J.; Bae, B.-S. Optically Transparent Multiscale Composite Films for Flexible and Wearable Electronics. Adv. Mater. 2020, 32, 1907143. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef]
- Legnani, C.; Barud, H.S.; Caiut, J.M.A.; Calil, V.L.; Maciel, I.O.; Quirino, W.G.; Ribeiro, S.J.L.; Cremona, M. Transparent bacterial cellulose nanocomposites used as substrate for organic light-emitting diodes. J. Mater. Sci. Mater. Electron. 2019, 30, 16718–16723. [Google Scholar] [CrossRef]
- Cebrian, A.V.S.; Carvalho, R.S.; Barreto, A.R.J.; Maturi, F.E.; Barud, H.S.; Silva, R.R.; Legnani, C.; Cremona, M.; Ribeiro, S.J.L. Development of Conformable Substrates for OLEDs Using Highly Transparent Bacterial Cellulose Modified with Recycled Polystyrene. Adv. Sustain. Syst. 2022, 6, 2000258. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.A.; Priya, A.K.; Hawash, H.B.; Yap, P.-S. Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Al-Juboori, R.A.; Khatri, M.; Ahmed, F.E.; Ibrahim, Y.; Hilal, N. Sustainability in Membrane Technology: Membrane Recycling and Fabrication Using Recycled Waste. Membranes 2024, 14, 52. [Google Scholar] [CrossRef]
- Hou, Y.; Duan, C.; Zhu, G.; Luo, H.; Liang, S.; Jin, Y.; Zhao, N.; Xu, J. Functional bacterial cellulose membranes with 3D porous architectures: Conventional drying, tunable wettability and water/oil separation. J. Membr. Sci. 2019, 591, 117312. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.-J.; Duan, Y.-X.; Zhao, X.-Q.; Jia, S.-R.; Zhong, C. Designing of bacterial cellulose-based superhydrophilic/underwater superoleophobic membrane for oil/water separation. Carbohydr. Polym. 2021, 257, 117611. [Google Scholar] [CrossRef]
- Than-ardna, B.; Weder, C.; Manuspiya, H. Superhydrophilic bacterial cellulose membranes efficiently separate oil-in-water emulsions. J. Mater. Sci. 2023, 58, 5086–5103. [Google Scholar] [CrossRef]
- Sijabat, E.K.; Nuruddin, A.; Aditiawati, P.; Sunendar Purwasasmita, B. Flat sheet membrane composite for desalinisation applications based on Bacterial Nanocellulose (BNC) from banana peel waste, cellulose, and silica. Mater. Res. Express 2020, 7, 105004. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.-W.; Yang, Y.-L.; Bai, Z.-C.; Xia, L.-R.; Wang, M.; Lv, X.-L.; Li, L. Removal of heavy metal ions and anionic dyes from aqueous solutions using amide-functionalized cellulose-based adsorbents. Carbohydr. Polym. 2020, 230, 115619. [Google Scholar] [CrossRef] [PubMed]
- Tissera, N.D.; Wijesena, R.N.; Yasasri, H.; de Silva, K.M.N.; de Silva, R.M. Fibrous keratin protein bio micro structure for efficient removal of hazardous dye waste from water: Surface charge mediated interfaces for multiple adsorption desorption cycles. Mater. Chem. Phys. 2020, 246, 122790. [Google Scholar] [CrossRef]
- Durval, I.J.B.; Silvestre, G.U.; Medeiros, A.D.; da Silva, C.G.; Amorim, J.D.; Rufino, R.D.; Costa, A.F.S.; Sarubbo, L.A. Biosurfactant and Bacterial Cellulose Applied to Textile Effluent Treatment. Chem. Eng. Trans. 2023, 100, 415–420. [Google Scholar]
- Kim, H.; Kim, H.R. Production of coffee-dyed bacterial cellulose as a bio-leather and using it as a dye adsorbent. PLoS ONE 2022, 17, e0265743. [Google Scholar] [CrossRef] [PubMed]
- Syukri, D.; Suryanto, H.; Kurniawan, F.; Hari, P.D.; Fiana, R.M.; Rini. Bacterial reduction in river water using nanocellulose membrane from pineapple biomass with ferrous-ferric oxide reinforcement. Glob. J. Environ. Sci. Manag. 2024, 10, 643–656. [Google Scholar]
- Suryanto, H.; Kurniawan, F.; Syukri, D.; Binoj, J.S.; Hari, P.D.; Yanuhar, U. Properties of bacterial cellulose acetate nanocomposite with TiO2 nanoparticle and graphene reinforcement. Int. J. Biol. Macromol. 2023, 235, 123705. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, L.; Wang, S.; Zhang, L.; Sun, D. Citric acid cross-linked regenerated bacterial cellulose as biodegradable film for food packaging. Cellulose 2023, 30, 10273–10284. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheung, K.M.; Ngai, T. Development of strong and high-barrier food packaging films from cyclic-anhydride modified bacterial cellulose. RSC Sustain. 2024, 2, 139–152. [Google Scholar] [CrossRef]
- Yanti, N.A.; Ahmad, S.W.; Ramadhan, L.O.; Jamili; Muzuni; Walhidayah, T.; Mamangkey, J. Properties and Application of Edible Modified Bacterial Cellulose Film Based Sago Liquid Waste as Food Packaging. Polymers 2021, 13, 3570. [Google Scholar] [CrossRef]
- Rouhi, M.; Garavand, F.; Heydari, M.; Mohammadi, R.; Sarlak, Z.; Cacciotti, I.; Razavi, S.H.; Mousavi, M.; Parandi, E. Fabrication of novel antimicrobial nanocomposite films based on polyvinyl alcohol, bacterial cellulose nanocrystals, and boric acid for food packaging. J. Food Meas. Charact. 2024, 18, 2146–2161. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ul-Islam, M.U.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Silver Decorated Bacterial Cellulose Nanocomposites as Antimicrobial Food Packaging Materials. ES Food Agrofor. 2021, 6, 12–26. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef]
- Papadaki, A.; Manikas, A.C.; Papazoglou, E.; Kachrimanidou, V.; Lappa, I.; Galiotis, C.; Mandala, I.; Kopsahelis, N. Whey protein films reinforced with bacterial cellulose nanowhiskers: Improving edible film properties via a circular economy approach. Food Chem. 2022, 385, 132604. [Google Scholar] [CrossRef]
- Muhammed, A.P.; Thangarasu, S.; Manoharan, R.K.; Oh, T.H. Ex-situ fabrication of engineered green network of multifaceted bacterial cellulose film with enhanced antimicrobial properties for post-harvest preservation of table grapes. Food Packag. Shelf Life 2024, 43, 101284. [Google Scholar] [CrossRef]
- Kusuma, A.C.; Chou, Y.-C.; Hsieh, C.-C.; Santoso, S.P.; Go, A.W.; Lin, H.-T.V.; Hsiao, I.L.; Lin, S.-P. Agar-altered foaming bacterial cellulose with carvacrol for active food packaging applications. Food Packag. Shelf Life 2024, 42, 101269. [Google Scholar] [CrossRef]
- Paximada, P.; Echegoyen, Y.; Koutinas, A.A.; Mandala, I.G.; Lagaron, J.M. Encapsulation of hydrophilic and lipophilized catechin into nanoparticles through emulsion electrospraying. Food Hydrocoll. 2017, 64, 123–132. [Google Scholar] [CrossRef]
- Razavi, M.S.; Golmohammadi, A.; Nematollahzadeh, A.; Fiori, F.; Rovera, C.; Farris, S. Preparation of cinnamon essential oil emulsion by bacterial cellulose nanocrystals and fish gelatin. Food Hydrocoll. 2020, 109, 106111. [Google Scholar] [CrossRef]
- Liao, W.; Liu, X.; Zhao, Q.; Lu, Z.; Feng, A.; Sun, X. Physicochemical, antibacterial and food preservation properties of active packaging films based on chitosan/ε-polylysine-grafted bacterial cellulose. Int. J. Biol. Macromol. 2023, 253, 127231. [Google Scholar] [CrossRef]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Cazón, P.; Puertas, G.; Vázquez, M. Characterization of multilayer bacterial cellulose-chitosan films loaded with grape bagasse antioxidant extract: Insights into spectral and water properties, microstructure, and antioxidant activity. Int. J. Biol. Macromol. 2024, 268, 131774. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taokaew, S. Bacterial Nanocellulose Produced by Cost-Effective and Sustainable Methods and Its Applications: A Review. Fermentation 2024, 10, 316. https://doi.org/10.3390/fermentation10060316
Taokaew S. Bacterial Nanocellulose Produced by Cost-Effective and Sustainable Methods and Its Applications: A Review. Fermentation. 2024; 10(6):316. https://doi.org/10.3390/fermentation10060316
Chicago/Turabian StyleTaokaew, Siriporn. 2024. "Bacterial Nanocellulose Produced by Cost-Effective and Sustainable Methods and Its Applications: A Review" Fermentation 10, no. 6: 316. https://doi.org/10.3390/fermentation10060316
APA StyleTaokaew, S. (2024). Bacterial Nanocellulose Produced by Cost-Effective and Sustainable Methods and Its Applications: A Review. Fermentation, 10(6), 316. https://doi.org/10.3390/fermentation10060316






