Bioactive Compounds Produced by Macromycetes for Application in the Pharmaceutical Sector: Patents and Products
Abstract
1. Introduction
2. Macromycetes as Sources of Bioactive Compounds
3. Polysaccharides from Mushrooms
3.1. Glucans
3.1.1. β-Glucans
3.1.2. α-Glucans
3.2. Glycans
3.3. Exopolysaccharides
4. Peptides from Mushrooms
5. Phenols and Polyphenols from Mushrooms
6. Terpenes from Mushrooms
| Species of Macromycetes | Class | Terpenoids | Pharmaceutical Properties | References |
|---|---|---|---|---|
| Astraeus odoratus | Triterpenes | Astraodoric acids A–D, 5-hydroxyhypaphorine | Antibacterial and cytotoxic activities | [129] |
| Anthracophyllum sp. | Sesquiterpenes | Anthracophyllic acid, anthracophyllone, aurisins A, G, K, nambinones A, C, axinysones A, and B | Antimalarial, antibacterial, citotoxicity activities | [112] |
| Agaricus bisporus | Monoterpenes | m-Xylene, β-Myrcene, DL-Limonene, Styrene, β-Terpinene, (E)-Limonene oxide, Dihydromyrcenol, Linalool, D-Carvone | Antioxidant activity | [135] |
| Cyathus africanus | Diterpenes | Neosarcodonin O, cyathatriol, 11-O-acetylcyathatriol, cyathins D–H | Cytotoxicity activity | [122] |
| Dentipellis fragilis | Diterpenes | Dentipellin, erinacines A-C | Antibacterial and antifungal activities | [136] |
| Ganoderma lucidum | Triterpenes | Ganoderate A acetonide and n-butyl ganoderate H | Inhibitor of acetylcholinesterase | [132] |
| Ganoderma orbiforme | Triterpenes | Ganorbiformins A–G | Antimycobacterial activity | [133] |
| Flammulina velutipes | Sesquiterpenes | Enokipodins A and B | Antimicrobial activity | [113] |
| Flammulina velutipes | Sesquiterpenes | Enokipodins E–J, sterpurols A and B | Antibacterial, antifungal anticancer and antioxidant activities | [115] |
| Panus strigellus formerly Lentinus strigellus | Polyketides | Lentinoids A–D | Antibacterial activity | [137] |
| Pleurotus eryngii | Diterpenes | Eryngiolide A | Cytotoxicity activity | [123] |
| Pleurotus cornucopiae | Monoterpenes and Sesquiterpenes | Monoterpenoids and Sesquiterpenoids | Inhibitory activity against nitric oxide | [138] |
| Pleurotus ostreatus | Monoterpenes | m-Xylene, β-Myrcene, DL-Limonene, Styrene, (E)-Limonene oxide, Dihydromyrcenol, Linalool, (E)-Caryophyllene, α-Humulene, γ-Muurolene, (E)-Carveol, (Z)-Carveol | Antioxidant activity | [135] |
| Montagnula donacina | Sesquiterpenes | Donacinolides A and B, donacinoic acids A and B, donacinols AeC and a meroterpenoid (Z)-4-hydroxy-3-(3-hydroxy-3-methylbut-1-en-1-yl)benzoic acid | Antibacterial activity | [114] |
| Neonothopanus nambi | Sesquiterpenes | Nambinones A-D, 1-epi-nambinone B, aurisin K | Antimalarial and antimycobacterial activities | [116] |
| Tapinella atrotomentosa | Sesquiterpenes | 5-hydroxy-hex-2-en-4-olide, spiromentins C and B | Antibacterial and antioxidant activity | [139] |
| Hydnellum scabrosum (formely Sarcodon scabrosus) | Diterpene | Neosarcodonin O, 19-O-linoleoyl, 19-O-oleoyl and 19-O-stearoyl derivatives of sarcodonin | Anti-inflammatory activity | [125] |
| Hydnellum glaucopus (formerly Sarcodon glaucopus) | Diterpene | Glaucopine C | Anti-inflammatory acitvity | [126] |
| Hydnellum scabrosum (formely Sarcodon scabrosus) | Diterpene | Scabronines H, K, L and sarcodonins G, A, M | Neurite activity | [128] |
| Strobilurus ohshimae | Sesquiterpenes | Strobilols A–D | Not showed antibacterial activity | [120] |
7. Alkaloids from Mushrooms
8. Sterols from Mushrooms
| Compounds/Origin | Biological Property | Type of Disease | Model | Dose (Ergosterol) | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Ergosterol (Monascus anka) | Antioxidant | - | H2O2-induced fibroblast | 200 mg/mL and 400 mg/mL | Inhibition of lipid peroxidation | [178] |
| Industrial-Ergosterol | Antioxidant | - | In vitro assay | 11 µM | Ergosterol is involved in yeast resistance to tert-butyl hydroperoxide and protects lipids against oxidation in liposomes | [165] |
| Ergosterol (Amaouroderma rude) | Anticancer | Carcinoma | MDA-MB-231 cancer cells | In vitro test with MCF-7 cells with IC50 of 43.51 µg/mL | Ergosterol isolate inhibits the growth of MDA-MB-231 cancer cells through apoptotic pathways by increasing FOXO3 expression | [179] |
| Industrial-Ergosterol | Anticancer | Bladder cancer | Rats were given an N-butyl-N-(4-hydroxybutyl) nitrosamine | Oral and intraperitoneal administration (15 µg/kg/day, for 21 days) | Inhibition of androgen signaling pathways | [180] |
| Industrial-Ergosterol | Anticancer | Chronic obstructive pulmonary disease | Rats were exposed 5 days a week to main smoke from five cigarettes, four times a day with a 10 min smoke session. | The mice were treated with doses of 25 mg/kg/day and 50 mg/kg/day, intragastric administration, for 4 weeks | Ergosterol inhibited pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in serum and lung | [168] |
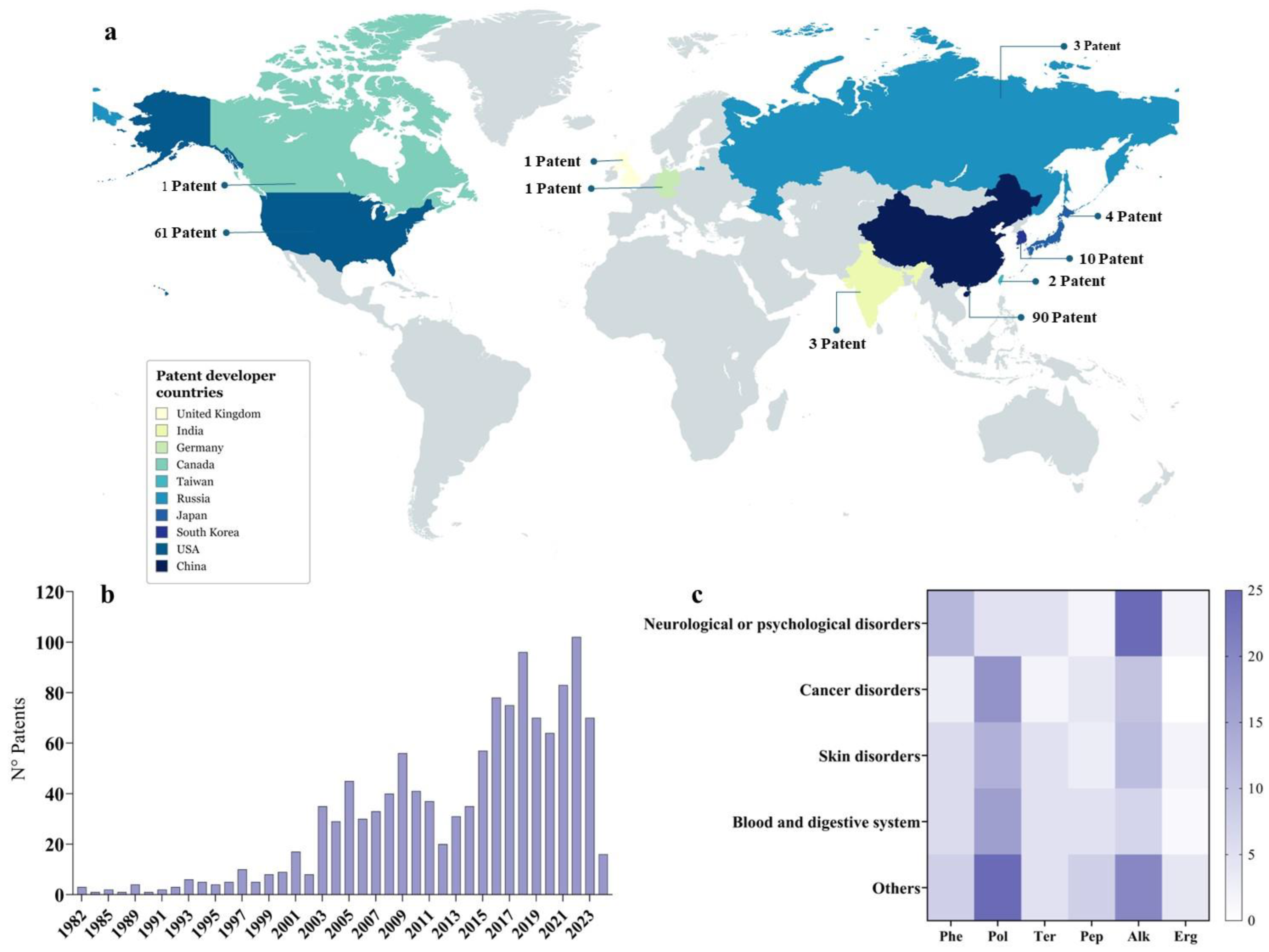
9. Patents: Technological Development and Innovation
10. Commercial Products with Pharmaceutical Properties Obtained from Macromycetes
| Compounds/Origin | Biological Property | Type of Disease | Model | Company | Reference |
|---|---|---|---|---|---|
| Mico Rei | Food supplement with the highest concentration of active ingredients from Reishi (Ganoderma lucidum), “the mushroom of eternal youth”. | - | The product contains alpha, beta and D-glucans, triterpenoid compounds, ergosterol, ganoderic acids (A, F, C2 and D) and alpha-linolenic acid C18:3n6. | Hifas da Terra (E.U) | [218] |
| Mico-Five | Food supplement with the highest concentration of active ingredients from Chaga, Reishi, Maitake, Shiitake and Sun Mushroom. | Greater activation of host cell receptors (high concentration of immunomodulatory compounds). | The product contains organic extract, Ganoderma lucidum, Lentinula edodes, Grifola frondosa, Agaricus blazei, Inonotus obliquus. | Hifas da Terra (E.U) | [219] |
| Mico-Mix | Powerful formula of organic Shiitake, Reishi and Maitake extracts. This product supplement has alpha, beta and D-glucans, ergosterol, ganoderic acid, grifolan and lentinan. | Mico-Mix supported a phase of insulin resistance, bringing it under control well. | The product has alpha, beta and D-glucans, ergosterol, ganoderic acid, grifolan and lentinan. | Hifas da Terra (E.U) | [220] |
| The Real Thing—Medical Mushrooms 60s | - | The product helps boosting immunity, assists in providing cyto protective activity and helps maintain healthy cellular function. | The product has G. lucidum, A. blazei, C. versicolor, L. edodes, G. frondosa, I. obliquus, C. militaris, H. erinaceus, P. linteus, A. auricula, P. eryngii, P. ostreatus | Wellness-Warehouse | [221] |
| Agarikon.1 | Myko San has been the leader in producing high-quality medicinal mushroom supplements for promoting health. Our expert-developed, scientifically-tested medicinal mushroom extract blends feature the most important medicinal mushrooms, including shiitake (Lentinula edodes), reishi (Ganoderma lucidum), maitake (Grifola frondosa), turkey tail (Trametes versicolor) and many others. | Agarikon.1 is a potent medicinal mushroom extract blend scientifically formulated for patients with cancer and other critical conditions. | The product contains extracts from edible and medicinal mushrooms Ganoderma lucidum (reishi), Lentinula edodes (shiitake), Grifola frondosa (maitake), Agaricus blazei Murill (=A. brasiliensis = A. subrufescens), Trametes versicolor (turkey tail) and Pleurotus ostreatus (oyster mushroom). The addition of immunity-boosting vitamin C enhances the absorption of the mushroom extracts in Agarikon.1. | Mykosan | [222] |
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Si, J.; Meng, G.; Wu, Y.; Ma, H.F.; Cui, B.K.; Dai, Y.C. Medium Composition Optimization, Structural Characterization, and Antioxidant Activity of Exopolysaccharides from the Medicinal Mushroom Ganoderma Lingzhi. Int. J. Biol. Macromol. 2019, 124, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Zavadinack, M.; de Lima Bellan, D.; da Rocha Bertage, J.L.; da Silva Milhorini, S.; da Silva Trindade, E.; Simas, F.F.; Sassaki, G.L.; Cordeiro, L.M.C.; Iacomini, M. An α-D-Galactan and a β-D-Glucan from the Mushroom Amanita Muscaria: Structural Characterization and Antitumor Activity against Melanoma. Carbohydr. Polym. 2021, 274, 118647. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, M.; Wu, S.; Liao, X.; Wang, J.; Wu, Q.; Zhuang, M.; Ding, Y. A Review on Mushroom-Derived Bioactive Peptides: Preparation and Biological Activities. Food Res. Int. 2020, 134, 109230. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Kimatu, B.M.; Fang, D.; Zhao, L.; Hu, Q. Agaricus bisporus Peptide Fractions Confer Cytoprotective Ability against Hydrogen Peroxide-Induced Oxidative Stress in HepG2 and Caco-2 Cells. J. Food Meas. Charact. 2020, 14, 2503–2519. [Google Scholar] [CrossRef]
- Pilafidis, S.; Diamantopoulou, P.; Gkatzionis, K.; Sarris, D. Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy. Appl. Sci. 2022, 12, 11426. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Fungal Kingd. 2017, 79–95. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. Application of Vibrational Spectroscopy for Classification, Authentication and Quality Analysis of Mushroom: A Concise Review. Food Chem. 2019, 289, 545–557. [Google Scholar] [CrossRef]
- Gargano, M.L.; van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal Mushrooms: Valuable Biological Resources of High Exploitation Potential. Plant Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom Nutraceuticals for Improved Nutrition and Better Human Health: A Review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J. Bacterial Exopolysaccharides: Chemical Structures, Gene Clusters and Genetic Engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.A. Immunomodulating and Anticancer Agents in the Realm of Macromycetes Fungi (Macrofungi). Int. Immunopharmacol. 2007, 7, 701–724. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhou, Z.; Zhang, L. An Overview of Fungal Glycan-Based Therapeutics. Prog. Mol. Biol. Transl. Sci. 2019, 163, 135–163. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S. Characterization and Applications of Mushroom Exopolysaccharides. In New and Future Developments in Microbial Biotechnology and Bioengineering: Recent Advances in Application of Fungi and Fungal Metabolites: Environmental and Industrial Aspects; Elsevier: Amsterdam, The Netherlands, 2020; pp. 171–181. [Google Scholar] [CrossRef]
- Vetter, J. The Mushroom Glucans: Molecules of High Biological and Medicinal Importance. Foods 2023, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.M.; Free, S.J. The Structure and Synthesis of the Fungal Cell Wall. BioEssays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell Wall Glucans of Fungi. A Review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Hou, Y.K.; Wang, X.; Zhou, X.T.; Yin, J.Y.; Nie, S.P. Recent Advances in the Biosynthesis of Fungal Glucan Structural Diversity. Carbohydr. Polym. 2024, 329, 121782. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Rodrigues, H.; Sousa, A.S.; Relvas, J.B.; Tavaria, F.K.; Pintado, M. An Overview on Mushroom Polysaccharides: Health-Promoting Properties, Prebiotic and Gut Microbiota Modulation Effects and Structure-Function Correlation. Carbohydr. Polym. 2024, 333, 121978. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Kim, E.H.; Cheong, C.; Williams, D.L.; Kim, C.W.; Lim, S.T. Structural Characterization of β-d-(1→3, 1→6)-Linked Glucans Using NMR Spectroscopy. Carbohydr. Res. 2000, 328, 331–341. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans from the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J. Biosynthesis of β-Glucans in Fungi. Antonie Leeuwenhoek 1991, 60, 73–81. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; Van Griensven, L.J.L.D. Antioxidative Activities and Chemical Characterization of Polysaccharides Extracted from the Basidiomycete Schizophyllum Commune. LWT-Food Sci. Technol. 2011, 44, 2005–2011. [Google Scholar] [CrossRef]
- Rasmy, G.E.; Botros, W.; Kabeil, S.S.; Daba, A. Preparation of Glucan from Lentinula Edodes Edible Mushroom and Elucidation of Its Medicinal Value. Aust. J. Basic Appl. Sci. 2010, 4, 5717–5726. [Google Scholar]
- Cha, Y.J.; Alam, N.; Lee, J.S.; Lee, K.R.; Shim, M.J.; Lee, M.W.; Kim, H.Y.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; et al. Anticancer and Immunopotentiating Activities of Crude Polysaccharides from Pleurotus Nebrodensis on Mouse Sarcoma 180. Mycobiology 2012, 40, 236–243. [Google Scholar] [CrossRef]
- Masuda, Y.; Togo, T.; Mizuno, S.; Konishi, M.; Nanba, H. Soluble β-Glucan from Grifola Frondosa Induces Proliferation and Dectin-1/Syk Signaling in Resident Macrophages via the GM-CSF Autocrine Pathway. J. Leukoc. Biol. 2012, 91, 547–556. [Google Scholar] [CrossRef]
- Komatsu, N.; Okubo, S.; Kikumoto, S.; Kimura, K.; Saito, G.; Sakai, S. Host-Mediated Antitumor Action of Schizophyllan, a Glucan Produced by Schizophyllum Commune. GANN Jpn. J. Cancer Res. 1969, 60, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guang Li, C.; Liang, H.; Reddy, N. Bioactive Mushroom Polysaccharides: Immunoceuticals to Anticancer Agents. J. Nutraceuticals Food Sci. 2017, 2, 1–5. [Google Scholar]
- Sasaki, S.; Izumi, H.; Morimoto, Y.; Sakurai, K.; Mochizuki, S. Induction of Potent Cell Growth Inhibition by Schizophyllan/K-Ras Antisense Complex in Combination with Gemcitabine. Bioorg. Med. Chem. 2020, 28, 115668. [Google Scholar] [CrossRef]
- Negahban, Z.; Shojaosadati, S.A.; Hamedi, S. A Novel Self-Assembled Micelles Based on Stearic Acid Modified Schizophyllan for Efficient Delivery of Paclitaxel. Colloids Surf. B Biointerfaces 2021, 199, 111524. [Google Scholar] [CrossRef]
- Pirzadeh-Naeeni, S.; Mozdianfard, M.R.; Shojaosadati, S.A.; Khorasani, A.C.; Saleh, T. A Comparative Study on Schizophyllan and Chitin Nanoparticles for Ellagic Acid Delivery in Treating Breast Cancer. Int. J. Biol. Macromol. 2020, 144, 380–388. [Google Scholar] [CrossRef]
- Wang, K.P.; Wang, J.; Li, Q.; Zhang, Q.L.; You, R.X.; Cheng, Y.; Luo, L.; Zhang, Y. Structural Differences and Conformational Characterization of Five Bioactive Polysaccharides from Lentinus Edodes. Food Res. Int. 2014, 62, 223–232. [Google Scholar] [CrossRef]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Shiio, T.; Suga, T.; Takasuka, N.; Sasaki, T. Antitumor and Metastasis-Inhibitory Activities of Lentinan as an Immunomodulator: An Overview. Cancer Detect. Prev. Suppl. 1987, 1, 423–443. [Google Scholar] [PubMed]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.H.; Magee, A.S.; Danielson, M.E.; et al. Activation of the Innate Immune Receptor Dectin-1 upon Formation of a ‘Phagocytic Synapse’. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef] [PubMed]
- LeibundGut-Landmann, S.; Osorio, F.; Brown, G.D.; Sousa, C.R.E. Stimulation of Dendritic Cells via the Dectin-1/Syk Pathway Allows Priming of Cytotoxic T-Cell Responses. Blood 2008, 112, 4971–4980. [Google Scholar] [CrossRef] [PubMed]
- Park, G.S.; Shin, J.; Hong, S.; Gopal, J.; Oh, J.W. Anticarcinogenic Potential of the Mushroom Polysaccharide Lentinan on Gastric and Colon Cancer Cells: Antiproliferative, Antitumorigenic, Mu-2-Related Death-Inducing Gene, MUDENG Ramifications. J. Ind. Eng. Chem. 2024, 135, 122–130. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, L.; Gu, D.; Li, X.; Wen, T.; Li, W. Lentinan-Loaded GelMA Hydrogel Accelerates Diabetic Wound Healing through Enhanced Angiogenesis and Immune Microenvironment Modulation. Int. J. Biol. Macromol. 2024, 264, 130716. [Google Scholar] [CrossRef] [PubMed]
- Grün, C.H. Structure and Biosynthesis of Fungal α-Glucans. Ph.D. Thesis, Universiteit Utrecht, Utrecht, The Netherlands, 2003. [Google Scholar]
- Reddy Shetty, P.; Batchu, U.R.; Buddana, S.K.; Sambasiva Rao, K.R.S.; Penna, S. A Comprehensive Review on α-D-Glucans: Structural and Functional Diversity, Derivatization and Bioapplications. Carbohydr. Res. 2021, 503, 108297. [Google Scholar] [CrossRef]
- Patra, S.; Maity, P.; Chakraborty, I.; Sen, I.K.; Ghosh, D.; Rout, D.; Bhanja, S.K. Structural Studies of Immunomodulatory (1→3)-, (1→4)-α Glucan from an Edible Mushroom Polyporus Grammocephalus. Int. J. Biol. Macromol. 2021, 168, 649–655. [Google Scholar] [CrossRef]
- Samuelsen, A.B.C.; Rise, F.; Wilkins, A.L.; Teveleva, L.; Nyman, A.A.T.; Aachmann, F.L. The Edible Mushroom Albatrellus Ovinus Contains a α-l-Fuco-α-d-Galactan, α-d-Glucan, a Branched (1→6)-β-d-Glucan and a Branched (1→3)-β-d-Glucan. Carbohydr. Res. 2019, 471, 28–38. [Google Scholar] [CrossRef]
- Palacios, I.; García-Lafuente, A.; Guillamón, E.; Villares, A. Novel Isolation of Water-Soluble Polysaccharides from the Fruiting Bodies of Pleurotus ostreatus Mushrooms. Carbohydr. Res. 2012, 358, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Smiderle, F.R.; Sassaki, G.L.; Van Arkel, J.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. High Molecular Weight Glucan of the Culinary Medicinal Mushroom Agaricus bisporus Is an α-Glucan That Forms Complexes with Low Molecular Weight Galactan. Molecules 2010, 15, 5818–5830. [Google Scholar] [CrossRef] [PubMed]
- Milhorini, S.d.S.; Smiderle, F.R.; Biscaia, S.M.P.; Rosado, F.R.; Trindade, E.S.; Iacomini, M. Fucogalactan from the Giant Mushroom Macrocybe Titans Inhibits Melanoma Cells Migration. Carbohydr. Polym. 2018, 190, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Luo, L.; Zhang, L. A New Galactoglucomannan from the Mycelium of the Medicinal Parasitic Fungus Cordyceps Cicadae and Its Immunomodulatory Activity In Vitro and In Vivo. Molecules 2023, 28, 3867. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps Sinensis: A Review. J. Funct. Foods 2013, 5, 550–569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic Activity and Gut Microbiota Regulation of a Novel Polysaccharide from Grifola Frondosa in Type 2 Diabetic Mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.V.; Islam, J.; Narzary, P.; Sharma, D.; Sultana, F. Bioactive Compounds, Nutraceutical Values and Its Application in Food Product Development of Oyster Mushroom. J. Future Foods 2024, 4, 335–342. [Google Scholar] [CrossRef]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An Insight into the Nutritional and Medicinal Value of Edible Mushrooms: A Natural Treasury for Human Health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef]
- Sonawane, H.; Arya, S.; Ghole, V.; Apte, K.; Shelke, D.; Chaskar, M. Hypoglycemic and Anticataract Activity of Crude Exopolysaccharides of Medicinal Mushroom Phellinus Badius on Streptozotocin-Induced Diabetic Rats and Goat Eye Lenses Respectively. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100241. [Google Scholar] [CrossRef]
- Abdel-Monem, N.M.; El-Saadani, M.A.; Daba, A.S.; Saleh, S.R.; Aleem, E. Exopolysaccharide-Peptide Complex from Oyster Mushroom (Pleurotus ostreatus) Protects against Hepatotoxicity in Rats. Biochem. Biophys. Rep. 2020, 24, 100852. [Google Scholar] [CrossRef]
- Lidbecq, C. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Nomenclature and Symbolism for Amino Acids and Peptides—Recommendations 1983. Eur. J. Biochem. 1984, 138, 9–37. [Google Scholar]
- Landi, N.; Clemente, A.; Pedone, P.V.; Ragucci, S.; Di Maro, A. An Updated Review of Bioactive Peptides from Mushrooms in a Well-Defined Molecular Weight Range. Toxins 2022, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, Y.; Lu, H.; Chen, Q. A Critical Review of Fungal Proteins: Emerging Preparation Technology, Active Efficacy and Food Application. Trends Food Sci. Technol. 2023, 141, 104178. [Google Scholar] [CrossRef]
- Li, H.; Gao, J.; Zhao, F.; Liu, X.; Ma, B. Bioactive Peptides from Edible Mushrooms—The Preparation, Mechanisms, Structure—Activity Relationships and Prospects. Foods 2023, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Qi, G.; Yang, Z.; Wang, H.; Wang, S.; Chen, G. Preparation, Separation and Antioxidant Properties of Hydrolysates Derived from Grifola Frondosa Protein. Czech J. Food Sci. 2015, 33, 500–506. [Google Scholar] [CrossRef]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant Potential of Edible Mushroom (Agaricus bisporus) Protein Hydrolysates and Their Ultrafiltration Fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, P.; Khanahamadi, M.; Ehsani, M.R.; Sharifan, A. Bioactive Properties of Agaricus bisporus and Terfezia claveryi Proteins Hydrolyzed by Gastrointestinal Proteases. LWT 2018, 91, 322–329. [Google Scholar] [CrossRef]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess Challenges to the Isolation and Purification of Bioactive Peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel Technologies for the Production of Bioactive Peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Peng, K.; Wang, X.L.; Ding, Z.; Liu, L.; Xu, P.; Liu, G.Q. Isolation and Characterization of Three Antihypertension Peptides from the Mycelia of Ganoderma Lucidum (Agaricomycetes). J. Agric. Food Chem. 2019, 67, 8149–8159. [Google Scholar] [CrossRef]
- Choi, H.S.; Cho, H.Y.; Yang, H.C.; Ra, K.S.; Suh, H.J. Angiotensin I-Converting Enzyme Inhibitor from Grifola Frondosa. Food Res. Int. 2001, 34, 177–182. [Google Scholar] [CrossRef]
- Lau, C.C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Novel Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Edible Mushroom Agaricus bisporus (J.E. Lange) Imbach Identified by LC-MS/MS. Food Chem. 2014, 148, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Jeong, S.C.; Kim, J.H.; Lee, Y.H.; Ju, Y.C.; Lee, J.S. Characterisation of a New Antihypertensive Angiotensin I-Converting Enzyme Inhibitory Peptide from Pleurotus Cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.C.; Abdullah, N.; Shuib, A.S. Novel Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from an Edible Mushroom, Pleurotus Cystidiosus O.K. Miller Identified by LC-MS/MS. BMC Complement. Altern. Med. 2013, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lu, X.; Yuan, G.; Zhang, Q.; An, L. Effects of Agaricus Blazei Polypeptide on Cell Senescence by Regulation of Keap1/Nrf2/ARE and TLR4/NF-ΚBp65 Signaling Pathways and Its Mechanism in D-Gal-Induced NIH/3T3 Cells. J. Funct. Foods 2020, 72, 104037. [Google Scholar] [CrossRef]
- Sun, J.; He, H.; Bi, J.X. Novel Antioxidant Peptides from Fermented Mushroom Ganoderma Lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective Effects of Ganoderma Lucidum Peptides against D-Galactosamine-Induced Liver Injury in Mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef] [PubMed]
- He, H.; He, J.-P.; Sui, Y.-J.; Zhou, S.-Q.; Wang, J. The Hepatoprotective Effects of Ganoderma Lucidum Peptides against Carbon Tetrachloride-Induced Injury in Mice. J. Food Biochem. 2008, 32, 628–641. [Google Scholar] [CrossRef]
- Mishra, J.; Rajput, R.; Singh, K.; Puri, S.; Goyal, M.; Bansal, A.; Misra, K. Antibacterial Natural Peptide Fractions from Indian Ganoderma Lucidum. Int. J. Pept. Res. Ther. 2018, 24, 543–554. [Google Scholar] [CrossRef]
- Bondaryk, M.; Staniszewska, M.; Zielińska, P.; Urbańczyk-Lipkowska, Z. Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds. J. Fungi 2017, 3, 46. [Google Scholar] [CrossRef]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin Is a Peptide Antibiotic with Therapeutic Potential from a Saprophytic Fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.W.; Ng, T.B.; Wang, H.X. Antiproliferative and Antimitogenic Activities in a Peptide from Puffball Mushroom Calvatia Caelata. Biochem. Biophys. Res. Commun. 2001, 289, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.H.K.; Zhao, Z.; Ng, T.B. Agrocybin, an Antifungal Peptide from the Edible Mushroom Agrocybe Cylindracea. Peptides 2005, 26, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hu, Q.; Liu, J.; Su, A.; Xu, H.; Li, X.; Huang, Q.; Zhou, J.; Mariga, A.M.; Yang, W. Isolation, Purification and Identification of Immunologically Active Peptides from Hericium Erinaceus. Food Chem. Toxicol. 2021, 151, 112111. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Ng, T.B. A Novel Peptide with Ribonuclease and Translation-Inhibitory Activities from Fruiting Bodies of the Oyster Mushroom Pleurotus ostreatus. J. Pept. Sci. 2002, 8, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.T.; Xia, L.; Ng, T.B. Pleurostrin, an Antifungal Peptide from the Oyster Mushroom. Peptides 2005, 26, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.M.; Cao, W.; Yao, K.W.; Liu, Z.Q.; Guo, J.Y. Anti-Inflammation and Antioxidant Effect of Cordymin, a Peptide Purified from the Medicinal Mushroom Cordyceps Sinensis, in Middle Cerebral Artery Occlusion-Induced Focal Cerebral Ischemia in Rats. Metab. Brain Dis. 2012, 27, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, C.; Wang, T.; Sun, Y.; Li, T.; Fan, G. Improvement of Biological Activity of Morchella Esculenta Protein Hydrolysate by Microwave-Assisted Selenization. J. Food Sci. 2019, 84, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wu, Y.; Zhang, Z.; Song, S.; Zhuang, H.; Xu, Z.; Yao, L.; Sun, M. Purification, Identification, and Sensory Evaluation of Kokumi Peptides from Agaricus bisporus Mushroom. Foods 2019, 8, 43. [Google Scholar] [CrossRef]
- Kang, M.G.; Kim, Y.H.; Bolormaa, Z.; Kim, M.K.; Seo, G.S.; Lee, J.S. Characterization of an Antihypertensive Angiotensin I-Converting Enzyme Inhibitory Peptide from the Edible Mushroom Hypsizygus Marmoreus. Biomed. Res. Int. 2013, 2013, 283964. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.H.; Park, J.S.; Choi, Y.J.; Lee, J.S. Isolation and Characterization of a Novel Angiotensin I-Converting Enzyme Inhibitory Peptide Derived from the Edible Mushroom Tricholoma Giganteum. Peptides 2004, 25, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.-C.; Lee, D.-H.; Kim, J.-H.; Yu, H.-E.; Park, J.-S.; Lee, J.-S. Production and Characterization of Antihypertensive Angiotensin I-Converting Enzyme Inhibitor from Pholiota Adiposa. J. Microbiol. Biotechnol. 2006, 16, 757–763. [Google Scholar]
- Geng, X.; Tian, G.; Zhang, W.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. A Tricholoma Matsutake Peptide with Angiotensin Converting Enzyme Inhibitory and Antioxidative Activities and Antihypertensive Effects in Spontaneously Hypertensive Rats. Sci. Rep. 2016, 6, 24130. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Li, W. Antioxidant, Antitumor and Immunostimulatory Activities of the Polypeptide from Pleurotus Eryngii Mycelium. Int. J. Biol. Macromol. 2017, 97, 323–330. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Eryngin, a Novel Antifungal Peptide from Fruiting Bodies of the Edible Mushroom Pleurotus Eryngii. Peptides 2004, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B.; Liu, Q. Alveolarin, a Novel Antifungal Polypeptide from the Wild Mushroom Polyporus Alveolaris. Peptides 2004, 25, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B.; Wang, H.; Sze, S.C.W.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an Antifungal Peptide from the Medicinal Fungus Cordyceps Militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.X.; Ng, T.B. A Peptide with HIV-1 Reverse Transcriptase Inhibitory Activity from the Medicinal Mushroom Russula Paludosa. Peptides 2007, 28, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.H.K.; Wang, H.X.; Ng, T.B. Purification and Characterization of a Ubiquitin-like Peptide with Macrophage Stimulating, Antiproliferative and Ribonuclease Activities from the Mushroom Agrocybe Cylindracea. Peptides 2003, 24, 639–645. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A Comprehensive Review on Phenolic Compounds from Edible Mushrooms: Occurrence, Biological Activity, Application and Future Prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Zhou, Y.; Chu, M.; Ahmadi, F.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A Comprehensive Review on Phytochemical Profiling in Mushrooms: Occurrence, Biological Activities, Applications and Future Prospective. Food Rev. Int. 2024, 40, 924–951. [Google Scholar] [CrossRef]
- Michalak, A. Heavy Metals Toxicity Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and Quantification of Phenolic Acid Compounds of Twenty-Six Mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Sommano, S.R.; Suksathan, R.; Sombat, T.; Seehanam, P.; Sirilun, S.; Ruksiriwanich, W.; Wangtueai, S.; Leksawasdi, N. Novel Perspective of Medicinal Mushroom Cultivations: A Review Case for ‘Magic’ Mushrooms. Agronomy 2022, 12, 3185. [Google Scholar] [CrossRef]
- Pukalski, J.; Latowski, D. Secrets of Flavonoid Synthesis in Mushroom Cells. Cells 2022, 11, 3052. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marín, F.R.; Soler-Rivas, C. Mushrooms Do Not Contain Flavonoids. J. Funct. Foods 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Sifat, N.; Lovely, F.; Zihad, S.M.N.K.; Hossain, M.d.G.; Shilpi, J.A.; Grice, I.D.; Mubarak, M.S.; Uddin, S.J. Investigation of the Nutritional Value and Antioxidant Activities of Common Bangladeshi Edible Mushrooms. Clin. Phytosci. 2020, 6, 88. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Oguoma, L.M.O.; Adigwe, P.C.; Anthony, B.B. Phytochemical Properties and In-Vitro Antimicrobial Potency of Wild Edible Mushrooms (Pleurotus ostreatus) Obtained from Yenagoa, Nigeria. J. Phytopharm. 2021, 10, 180–184. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, I.K.; Yun, B.S. Phenolic Compounds from the Fungus Inonotus Obliquus and Their Antioxidant Properties. J. Antibiot. 2016, 69, 108–110. [Google Scholar] [CrossRef]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H. Bin Antioxidant Capacities, Phenolic Compounds and Polysaccharide Contents of 49 Edible Macro-Fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef]
- Ukaegbu, C.I.; Shah, S.R.; Hamid, H.A.; Alara, O.R.; Sarker, M.Z.I. Phenolic Compounds of Aqueous and Methanol Extracts of Hypsizygus tessellatus (Brown and White Var.) and Flammulina velutipes Caps: Antioxidant and Antiproliferative Activities. Pharm. Chem. J. 2020, 54, 170–183. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Wójciak, M.; Mroziak-Lal, K.; Zagórska-Dziok, M.; Bujak, T.; Nizioł-Łukaszewska, Z.; Szczepanek, D.; Sowa, I. Assessment of Cosmetic Properties and Safety of Use of Model Washing Gels with Reishi, Maitake and Lion’s Mane Extracts. Molecules 2022, 27, 5090. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, E.; Oz, E.; Abd El-Aty, A.M.; Proestos, C.; Brennan, C.; Zeng, M.; Tomasevic, I.; Elobeid, T.; Çadırcı, K.; Bayrak, M.; et al. Exploring the Potential Medicinal Benefits of Ganoderma Lucidum: From Metabolic Disorders to Coronavirus Infections. Foods 2023, 12, 1512. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, L.; Liang, H.; Chen, S. dong In Silico Screening of Potential Anti–COVID-19 Bioactive Natural Constituents from Food Sources by Molecular Docking. Nutrition 2021, 82, 111049. [Google Scholar] [CrossRef]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Mushroom Ethanolic Extracts as Cosmeceuticals Ingredients: Safety and Ex Vivo Skin Permeation Studies. Food Chem. Toxicol. 2019, 127, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Machado-Carvalho, L.; Martins, T.; Aires, A.; Marques, G. Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus Hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites 2023, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.Y.; Xie, T.; Bai, R. Natural Terpenoids with Anti-Inflammatory Activities: Potential Leads for Anti-Inflammatory Drug Discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyn, G.T.; Bloch, S.E.; Schmidt-Dannert, C. Discovery and Characterization of Terpenoid Biosynthetic Pathways of Fungi. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2012; Volume 515, pp. 83–105. [Google Scholar]
- Trepa, M.; Sułkowska-Ziaja, K.; Kała, K.; Muszyńska, B. Therapeutic Potential of Fungal Terpenes and Terpenoids: Application in Skin Diseases. Molecules 2024, 29, 1183. [Google Scholar] [CrossRef]
- Intaraudom, C.; Boonyuen, N.; Supothina, S.; Tobwor, P.; Prabpai, S.; Kongsaeree, P.; Pittayakhajonwut, P. Novel Spiro-Sesquiterpene from the Mushroom Anthracophyllum Sp. BCC18695. Phytochem. Lett. 2013, 6, 345–349. [Google Scholar] [CrossRef]
- Ishikawa, N.K.; Fukushi, Y.; Yamaji, K.; Tahara, S.; Takahashi, K. Antimicrobial Cuparene-Type Sesquiterpenes, Enokipodins C and D, from a Mycelial Culture of Flammulina velutipes. J. Nat. Prod. 2001, 64, 932–934. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Zhao, K.; Chen, H.P.; Bai, X.; Zhang, L.; Liu, J.K. Terpenoids from the Mushroom-Associated Fungus Montagnula Donacina. Phytochemistry 2018, 147, 21–29. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, L.; Yang, X.; Li, L.; Li, S.; Gao, H.; Yao, X.S.; Wen, H.; Liu, H.W. Bioactive Sesquiterpenoids from the Solid Culture of the Edible Mushroom Flammulina velutipes Growing on Cooked Rice. Food Chem. 2012, 132, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Kanokmedhakul, S.; Lekphrom, R.; Kanokmedhakul, K.; Hahnvajanawong, C.; Bua-Art, S.; Saksirirat, W.; Prabpai, S.; Kongsaeree, P. Cytotoxic Sesquiterpenes from Luminescent Mushroom Neonothopanus Nambi. Tetrahedron 2012, 68, 8261–8266. [Google Scholar] [CrossRef]
- Wang, S.J.; Bao, L.; Han, J.J.; Wang, Q.X.; Yang, X.L.; Wen, H.A.; Guo, L.D.; Li, S.J.; Zhao, F.; Liu, H.W. Pleurospiroketals A-E, Perhydrobenzannulated 5,5-Spiroketal Sesquiterpenes from the Edible Mushroom Pleurotus Cornucopiae. J. Nat. Prod. 2013, 76, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Cassino, C.; Corana, F.; Vidari, G. Terpenoids from Russula Lepida and R. amarissima (Basidiomycota, Russulaceae). Phytochemistry 2012, 84, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Li, Z.H.; Dong, Z.J.; Li, X.Y.; Su, J.; Li, Y.; Liu, J.K. Two Novel Fomannosane-Type Sesquiterpenoids from the Culture of the Basidiomycete Agrocybe Salicacola. Nat. Prod. Bioprospect 2012, 2, 130–132. [Google Scholar] [CrossRef]
- Hiramatsu, F.; Murayama, T.; Koseki, T.; Shiono, Y. Strobilols A-D: Four Cadinane-Type Sesquiterpenes from the Edible Mushroom Strobilurus Ohshimae. Phytochemistry 2007, 68, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.S.; Lee, I.K.; Cho, Y.; Cho, S.M.; Yoo, I.D. New Tricyclic Sesquiterpenes from the Fermentation Broth of Stereum Hirsutum. J. Nat. Prod. 2002, 65, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, Y.; Bao, L.; Yang, X.; Liu, D.; Li, S.; Zhao, F.; Liu, H. Anti-Inflammatory and Cytotoxic Cyathane Diterpenoids from the Medicinal Fungus Cyathus Africanus. Fitoterapia 2013, 84, 22–31. [Google Scholar] [CrossRef]
- Wang, S.J.; Li, Y.X.; Bao, L.; Han, J.J.; Yang, X.L.; Li, H.R.; Wang, Y.Q.; Li, S.J.; Liu, H.W. Eryngiolide A, a Cytotoxic Macrocyclic Diterpenoid with an Unusual Cyclododecane Core Skeleton Produced by the Edible Mushroom Pleurotus Eryngii. Org. Lett. 2012, 14, 3672–3675. [Google Scholar] [CrossRef]
- Shibata, H.; Irie, A.; Morita, Y. New Antibacterial Diterpenoids from the Sarcodon Scabrosus Fungus. Biosci. Biotechnol. Biochem. 1998, 62, 2450–2452. [Google Scholar] [CrossRef] [PubMed]
- Kamo, T.; Imura, Y.; Hagio, T.; Makabe, H.; Shibata, H.; Hirota, M. Anti-Inflammatory Cyathane Diterpenoids from Sarcodon Scabrosus. Biosci. Biotechnol. Biochem. 2004, 68, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Marcotullio, M.C.; Pagiotti, R.; Campagna, V.; Maltese, F.; Fardella, G.; Altinier, G.; Tubaro, A. Glaucopine C, a New Diterpene from the Fruiting Bodies of Sarcodon Glaucopus. Nat. Prod. Res. 2006, 20, 917–921. [Google Scholar] [CrossRef]
- Marcotullio, M.C.; Pagiotti, R.; Maltese, F.; Oball-Mond Mwankie, G.N.; Hoshino, T.; Obara, Y.; Nakahata, N. Cyathane Diterpenes from Sarcodon Cyrneus and Evaluation of Their Activities of Neuritegenesis and Nerve Growth Factor Production. Bioorg. Med. Chem. 2007, 15, 2878–2882. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Liu, L.; Gao, J.M.; Zhang, A.L. Cyathane Diterpenes from Chinese Mushroom Sarcodon Scabrosus and Their Neurite Outgrowth-Promoting Activity. Eur. J. Med. Chem. 2011, 46, 3112–3117. [Google Scholar] [CrossRef] [PubMed]
- Arpha, K.; Phosri, C.; Suwannasai, N.; Mongkolthanaruk, W.; Sodngam, S. Astraodoric Acids A-D: New Lanostane Triterpenes from Edible Mushroom Astraeus Odoratus and Their Anti-Mycobacterium Tuberculosis H37Ra and Cytotoxic Activity. J. Agric. Food Chem. 2012, 60, 9834–9841. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Ali, N.A.A.; Jansen, R.; Wegner, U.; Mentel, R.; Lindequist, U.; Kreisel, H. Antiviral Lanostanoid Triterpenes from the Fungus Ganoderma Pfeifferi. Fitoterapia 2003, 74, 177–180. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.J.; Lindequist, U.; Mentel, R.; Gördes, D.; Schmidt, E.; Thurow, K.; Lalk, M. Antiviral Terpenoid Constituents of Ganoderma Pfeifferi. J. Nat. Prod. 2005, 68, 1728–1731. [Google Scholar] [CrossRef]
- Lee, I.S.; Ahn, B.R.; Choi, J.S.; Hattori, M.; Min, B.; Bae, K.H. Selective Cholinesterase Inhibition by Lanostane Triterpenes from Fruiting Bodies of Ganoderma Lucidum. Bioorg. Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Kongthong, S.; Srichomthong, K.; Choeyklin, R. Lanostane Triterpenes from Cultures of the Basidiomycete Ganoderma Orbiforme BCC 22324. Phytochemistry 2013, 87, 133–139. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Lanostane Triterpenoids from the Mushroom Naematoloma Fasciculare. J. Nat. Prod. 2013, 76, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the Key Aroma and Phenolic Compounds of Champignon (Agaricus bisporus) and Oyster (Pleurotus ostreatus) Mushrooms after Two Cooking Treatments as Elucidated by GC–MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021, 354, 129576. [Google Scholar] [CrossRef] [PubMed]
- Ha, L.S.; Ki, D.W.; Kim, J.Y.; Choi, D.C.; Lee, I.K.; Yun, B.S. Dentipellin, a New Antibiotic from Culture Broth of Dentipellis Fragilis. J. Antibiot. 2021, 74, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, R.; Rios, N.; Solano, G.; Cubilla-Rios, L. Lentinoids A–D, New Natural Products Isolated from Lentinus Strigellus. Molecules 2018, 23, 773. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bao, L.; Zhao, F.; Wang, Q.; Li, S.; Ren, J.; Li, L.; Wen, H.; Guo, L.; Liu, H. Isolation, Identification, and Bioactivity of Monoterpenoids and Sesquiterpenoids from the Mycelia of Edible Mushroom Pleurotus Cornucopiae. J. Agric. Food Chem. 2013, 61, 5122–5129. [Google Scholar] [CrossRef] [PubMed]
- Béni, Z.; Dékány, M.; Kovács, B.; Csupor-Löffler, B.; Zomborszki, Z.P.; Kerekes, E.; Szekeres, A.; Urbán, E.; Hohmann, J.; Ványolós, A. Bioactivity-Guided Isolation of Antimicrobial and Antioxidant Metabolites from the Mushroom Tapinella Atrotomentosa. Molecules 2018, 23, 1082. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Rapior, S. Perspectives of Biomedical Application of Macrofungi. Curr. Trends Biomed. Eng. Biosci. 2020, 19, 556024. [Google Scholar]
- Xu, W.; Gavia, D.J.; Tang, Y. Biosynthesis of Fungal Indole Alkaloids. Physiol. Behav. 2015, 176, 100–106. [Google Scholar] [CrossRef]
- Byrne, K.M.; Smith, S.K.; Ondeyka, J.G. Biosynthesis of Nodulisporic Acid A: Precursor Studies. J. Am. Chem. Soc. 2002, 124, 7055–7060. [Google Scholar] [CrossRef]
- Dunn, M.F.; Niks, D.; Ngo, H.; Barends, T.R.M.; Schlichting, I. Tryptophan Synthase: The Workings of a Channeling Nanomachine. Trends Biochem. Sci. 2008, 33, 254–264. [Google Scholar] [CrossRef]
- Cristino, A.S.; Williams, S.M.; Hawi, Z.; An, J.Y.; Bellgrove, M.A.; Schwartz, C.E.; Costa, L.D.F.; Claudianos, C. Neurodevelopmental and Neuropsychiatric Disorders Represent an Interconnected Molecular System. Mol. Psychiatry 2014, 19, 294–301. [Google Scholar] [CrossRef]
- Plazas, E.; Faraone, N. Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents. Biomedicines 2023, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO Prevalence Estimates of Mental Disorders in Conflict Settings: A Systematic Review and Meta-Analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, F.; Steinert, C.; Ioannidis, J.P.A. Toward a Paradigm Shift in Treatment and Research of Mental Disorders. Psychol. Med. 2019, 49, 2111–2117. [Google Scholar] [CrossRef]
- Guerra-Doce, E. Psychoactive Substances in Prehistoric Times: Examining the Archaeological Evidence. Time Mind 2015, 8, 91–112. [Google Scholar] [CrossRef]
- Miller, M.J.; Albarracin-Jordan, J.; Moore, C.; Capriles, J.M. Chemical Evidence for the Use of Multiple Psychotropic Plants in a 1000-Year-Old Ritual Bundle from South America. Proc. Natl. Acad. Sci. USA 2019, 166, 11207–11212. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic Effects of Psilocybin Correlate with Serotonin 2A Receptor Occupancy and Plasma Psilocin Levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kuzuyama, T. Recent Advances in the Biosynthesis of Carbazoles Produced by Actinomycetes. Biomolecules 2020, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.M.; Carvalho, F.; Bastos, M.d.L.; Guedes de Pinho, P.; Carvalho, M. The Hallucinogenic World of Tryptamines: An Updated Review. Arch. Toxicol. 2015, 89, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Dan, W.; Schneider, U.; Wang, J. β-Carboline Alkaloid Monomers and Dimers: Occurrence, Structural Diversity, and Biological Activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef]
- Cao, R.; Peng, W.; Wang, Z.; Xu, A. β-Carboline Alkaloids: Biochemical and Pharmacological Functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Carbazole Dyes. In Metal-Free Synthetic Organic Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 109–116. ISBN 9780128156476. [Google Scholar]
- Birari, R.; Roy, S.K.; Singh, A.; Bhutani, K.K. NPC Natural Product Communications. Nat. Prod. Commun. 2010, 1, 9–12. [Google Scholar]
- Yaoita, Y.; Kikuchi, M.; Machida, K. Terpenoids and Sterols from Mushrooms. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 44, pp. 1–32. ISBN 9780444634603. [Google Scholar]
- Hu, Z.; He, B.; Ma, L.; Sun, Y.; Niu, Y.; Zeng, B. Recent Advances in Ergosterol Biosynthesis and Regulation Mechanisms in Saccharomyces Cerevisiae. Indian J. Microbiol. 2017, 57, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef]
- Baker, M.A.B.; Brown, A.J. A Detour to Sterol Synthesis. Nat. Microbiol. 2019, 4, 214–215. [Google Scholar] [CrossRef]
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.P.; Lyng, J.G.; Jacquier, J.C.; Bhuyan, D.J. Recovery of Ergosterol and Vitamin D2 from Mushroom Waste—Potential Valorization by Food and Pharmaceutical Industries. Trends Food Sci. Technol. 2020, 99, 351–366. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a Novel Methodology for the Analysis of Ergosterol in Mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Shao, S.; Hernandez, M.; Kramer, J.K.G.; Rinker, D.L.; Tsao, R. Ergosterol Profiles, Fatty Acid Composition, and Antioxidant Activities of Button Mushrooms as Affected by Tissue Part and Developmental Stage. J. Agric. Food Chem. 2010, 58, 11616–11625. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-Derived Bioactive Compounds Potentially Serve as the Inhibitors of SARS-CoV-2 Main Protease: An in Silico Approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef]
- Dupont, S.; Fleurat-Lessard, P.; Cruz, R.G.; Lafarge, C.; Grangeteau, C.; Yahou, F.; Gerbeau-Pissot, P.; Abrahão Júnior, O.; Gervais, P.; Simon-Plas, F.; et al. Antioxidant Properties of Ergosterol and Its Role in Yeast Resistance to Oxidation. Antioxidants 2021, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Rangsinth, P.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S.; Tencomnao, T. Neuroprotective Effects against Glutamate-Induced Ht-22 Hippocampal Cell Damage and Caenorhabditis Elegans Lifespan/Healthspan Enhancing Activity of Auricularia Polytricha Mushroom Extracts. Pharmaceuticals 2021, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol Isolated from Undaria Pinnatifida Inhibits Lipopolysaccharide- Induced Production of Nitric Oxide and pro-Inflammatory Cytokines via the Inactivation of Nuclear Factor-ΚB and P38 Mitogen-Activated Protein Kinase in RAW264.7 Macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef]
- Huan, W.; Tianzhu, Z.; Yu, L.; Shumin, W. Effects of Ergosterol on COPD in Mice via JAK3/STAT3/NF-ΚB Pathway. Inflammation 2017, 40, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Nallathamby, N.; Guan-Serm, L.; Vidyadaran, S.; Malek, S.N.A.; Raman, J.; Sabaratnam, V. Ergosterol of Cordyceps Militaris Attenuates LPS Induced Inflammation in BV2 Microglia Cells. Nat. Prod. Commun.-NPC 2015, 10, 9–10. [Google Scholar] [CrossRef]
- Hassan, S.; Dritsas, S.; O’Dwyer, S.T.; Aziz, O.; Sutton, P.; Wang, X.; Fish, R.; Renehan, A.G.; Wilson, M.; Selvasekar, C.; et al. Open versus Closed Technique for Administration of Heated Intraperitoneal Chemotherapy (HIPEC): Morbidity and Mortality Outcomes from a High-Volume Centre. Eur. J. Surg. Oncol. 2023, 49, 106924. [Google Scholar] [CrossRef]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of Biologically Active Ganoderma Lucidum Compounds and Synthesis of Improved Derivatives That Confer Anti-Cancer Activities in Vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Q.; Xie, Y.; Ding, Y.; Du, W.W.; Sdiri, M.; Yang, B.B. Ergosterol Purified from Medicinal Mushroom Amauroderma Rude Inhibits Cancer Growth in Vitro and in Vivo by Up-Regulating Multiple Tumor Suppressors. Oncotarget 2015, 6, 17832–17846. [Google Scholar] [CrossRef]
- Yazawa, Y.; Ikarashi, N.; Hoshino, M.; Kikkawa, H.; Sakuma, F.; Sugiyama, K. Inhibitory Effect of Ergosterol on Bladder Carcinogenesis Is Due to Androgen Signaling Inhibition by Brassicasterol, a Metabolite of Ergosterol. J. Nat. Med. 2020, 74, 680–688. [Google Scholar] [CrossRef]
- Xiong, M.; Huang, Y.; Liu, Y.; Huang, M.; Song, G.; Ming, Q.; Ma, X.; Yang, J.; Deng, S.; Wen, Y.; et al. Antidiabetic Activity of Ergosterol from Pleurotus ostreatus in KK-Ay Mice with Spontaneous Type 2 Diabetes Mellitus. Mol. Nutr. Food Res. 2018, 62, 1700444. [Google Scholar] [CrossRef]
- Mbambo, B.; Odhav, B.; Mohanlall, V. Antifungal Activity of Stigmasterol, Sitosterol and Ergosterol from Bulbine Natalensis Baker (Asphodelaceae). J. Med. Plants Res. 2012, 6, 5135–5141. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.C.; Liu, J.Y.; Ma, Y.M.; Tan, R.X. Anti-Helicobacter Pylori Substances from Endophytic Fungal Cultures. World J. Microbiol. Biotechnol. 2005, 21, 553–558. [Google Scholar] [CrossRef]
- Bu, F.; Song, X.; Zhao, X.; Wang, R.; Xie, Y.; Yu, L.; Yan, X. Advances in Micro/Nanodrug Delivery Systems for the Treatment of Helicobacter Pylori Infection: From Diagnosis to Eradication. Appl. Mater. Today 2024, 37, 102138. [Google Scholar] [CrossRef]
- Yongxia, Z.; Jian, X.; Suyuan, H.; Aixin, N.; Lihong, Z. Isolation and Characterization of Ergosterol from Monascus Anka for Anti-Lipid Peroxidation Properties. J. Mycol. Medicale 2020, 30, 101038. [Google Scholar] [CrossRef] [PubMed]
- Misgiati, M.; Widyawaruyanti, A.; Raharjo, S.J.; Sukardiman, S. Ergosterol Isolated from Agaricus Blazei Murill N-Hexane Extracts as Potential Anticancer MCF-7 Activity. Pharmacogn. J. 2021, 13, 418–426. [Google Scholar] [CrossRef]
- Ikarashi, N.; Hoshino, M.; Ono, T.; Toda, T.; Yazawa, Y.; Sugiyama, K. A Mechanism by Which Ergosterol Inhibits the Promotion of Bladder Carcinogenesis in Rats. Biomedicines 2020, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, H.; Takeuchi, T.; Tanabe, O.; Iinuma, H. Carcinostatic Cpd. Basidalin. JP Patent 57047482A, 18 March 1982. [Google Scholar]
- Kagome-Company. Appreciation, Nature and Corporate Openness. Available online: https://www.kagome.co.jp/english/vision.html (accessed on 20 April 2024).
- Arimoto, Y.; Mori, Y.; Sakamoto, H.; Ishiguro, Y. Ischemic Brain Disease Improving Agent Containing Chroman Derivative as Active Component. JP Patent H09235226A, 29 February 1996. [Google Scholar]
- Sugano, N.; Iizuka, C.; Maeda, H. Anticancer Agent Comprising Xylose Based Glycoprotein Obtained from Basidiomycetes Mycelium Incubated in Solid Culture Medium. JP Patent S58118519A, 14 July 1983. [Google Scholar]
- Mizuno, T.; Kawagishi, H.; Hagiwara, T.; Nakamura, T. Ergosterol Derivative and Production Thereof. JP Patent 1246299A, 2 October 1989. [Google Scholar]
- Ikemizu, S.; Konishi, H.; Hataya, C.; Kishimoto, M. Method for Producing Angiotensin Conversion-Inhibiting Enzyme-Containing Substance Originated from Basidiomycetes. JP Patent 1995238032A, 12 September 1995. [Google Scholar]
- Niwa, K. Method of Strengthening Antitumor Activity of Crude Drug, Composition Containing Crude Drug for Strengthening Antitumor Activity, Method of Evaluating Antitumor Effectivity Treated by Crude Drug and Method of Evaluating Antitumor Effectivity of Crud Drug. JP Patent 2000159682A, 13 November 1998. [Google Scholar]
- Nagatomo, S. Method for Extracting Antitumor Active Substance of Crude Drug. JP Patent 2008105972A, 24 October 2006. [Google Scholar]
- Mulqueen, M.; Moran, M. Methods for Treating Dependence. International Application Published under the Patent Cooperation Treaty (PCT). WO2010124089A2, 28 October 2010. [Google Scholar]
- Kirkland, J. Pharmaceutical Composition Useful for Treating Patient Suffering from Anxiety, Depression, and/or Other Mental Health Conditions, Comprises Deep Eutectic Solvent and One or More Active Pharmaceutical Ingredients Derived from Fungal Biomass. CA Patent 3186405A1, 14 July 2023. [Google Scholar]
- Yoshida, H.; Sakai, S.; Inoue, S.; Kawagishi, H. Pharmaceutical Composition, Hyaluronic Acid Decomposition Inhibitor, Cosmetic, Chapped Skin or Dried Skin-Preventing Agent Each Comprising Extract of Tricholoma Orirubens, and New Compound Contained in Tricholoma Orirubens and Its Application. JP Patent 2005089391A, 19 September 2003. [Google Scholar]
- Sato, T.; Ichinose, S.; Nishizawa, Y.; Kusuoku, H.; Shibuya, Y. Apoptosis Inhibitor. JP Patent 2005068061A, 22 August 2003. [Google Scholar]
- Sun, A. Treating Malignancies and Viral Infections and Improving Immune Function Using Dietary Supplement of Soybean, Mushroom and Mung Bean. US Patent 20020127243A1, 10 January 2002. [Google Scholar]
- Bahl, A.K.; Vercellotti, S.V.; Vercellotti, J.R.; Klein, E. Purified Beta Glucan Composition Useful for Treating Conditions Associated with Bone Loss or Low Bone Density, Particularly Osteoporosis, Paget’s Disease Comprises Water Soluble Beta Glucan. US Patent 20080200429A1, 14 April 2008. [Google Scholar]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Soccol, V.T.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-State Fermentation Technology and Innovation for the Production of Agricultural and Animal Feed Bioproducts. Syst. Microbiol. Biomanuf. 2020, 1, 142–165. [Google Scholar] [CrossRef]
- Rutjes, S.A.; Vennis, I.M.; Wagner, E.; Maisaia, V. Lukas Peintner Biosafety and Biosecurity Challenges during the COVID-19 Pandemic and Beyond. Front. Bioeng. Biotechnol. 2023, 11, 1117316. [Google Scholar] [CrossRef] [PubMed]
- Justia Trademarks. Featured Companies. Available online: https://trademarks.justia.com/ (accessed on 20 April 2024).
- Xinqiao, H. Steam Equipment Multivitamin for Auxiliary Treatment of Cardiovascular Diseases and Preparation Method and Application Thereof. CN Patent 114887052A, 11 May 2022. [Google Scholar]
- Zhong, S.; Zhang, Y.; Zhang, Z.; Deng, Z.; Shen, J. Application of Oyster Mushroom Polysaccharide Selenoside-III Anticancer Active Ingredient in Preparation of Medicine for Resisting Colon Cancer. CN Patent 112315973A, 11 November 2020. [Google Scholar]
- Zhu, C.; Li, J. Composition Useful for Preventing Inflammation, Preventing Aging and Preparing Skin Care Composition Comprises Lentinus Edodes Mycelia Polysaccharide and Salvianic Acid A. CN Patent 110638687A, 23 August 2019. [Google Scholar]
- Barnhill, S.D. Composition Used for Treating Human Suffering from Inflammatory Disease e.g., Achalasia, Addisons Disease, Adult Stills Disease, Agammaglobulinemia, Alopecia Areata, Comprises One/More Edible/Medicinal Mushrooms, Cannabinoids and Flavonoids. U.S. Patent 2023293610A1, 21 September 2023. [Google Scholar]
- Greenbaum, E.; Bueno, J. Oral Formulations of Psychotropic Macrofungus Botanical Extracts with Mouthfeel Experience Enhancers. U.S. Patent 20220331344A1, 20 October 2022. [Google Scholar]
- Arnold, C.M.; Bhatt, P.; Hartman, M.S. Metered Dosing Composition for Treating or Mitigating a Neurological, Physiological, or Mental Health Condition Such as Anxiety, Post-Traumatic Stress Disorder, Attention Deficit Disorders, Depression, Comprises Psychedelic Compounds. U.S. Patent 2022304980A1, 29 September 2021. [Google Scholar]
- Thompson, S. Preventing or Treating Psychological Disorder, Comprises Administering Serotonin Agonist in Combination with Serotonin Receptor 2A Antagonist. U.S. Patent 2022273680A1, 1 September 2021. [Google Scholar]
- White, P. Treating Compulsive Eating Disorder, Comprises Administering a Compound Comprising Psilocin, Psilocybin, or Their Analogs. U.S. Patent 2023021957A1, 26 January 2021. [Google Scholar]
- Glamoclija, J.; Sokovic, M. Fungi a Source with Huge Potential for “Mushroom Pharmaceuticals”. Lek. Sirovine 2017, 37, 50–56. [Google Scholar] [CrossRef]
- Wasser, S. A Book Review: The Fungal Pharmacy: Medicinal Mushrooms of Western Canada. Int. J. Med. Mushrooms 2008, 10, 97–100. [Google Scholar] [CrossRef]
- Pandey, A.T.; Pandey, I.; Hachenberger, Y.; Krause, B.C.; Haidar, R.; Laux, P.; Luch, A.; Singh, M.P.; Singh, A.V. Emerging Paradigm against Global Antimicrobial Resistance via Bioprospecting of Mushroom into Novel Nanotherapeutics Development. Trends Food Sci. Technol. 2020, 106, 333–344. [Google Scholar] [CrossRef]
- Chem-Pharm Product Categories. 2020, p. 14970. Available online: https://Www.Chemicalbook.Com/ShowSupplierProductsList14970/0_EN.Htm (accessed on 20 April 2024).
- Feng, T.; Cai, J.L.; Li, X.M.; Zhou, Z.Y.; Li, Z.H.; Liu, J.K. Chemical Constituents and Their Bioactivities of Mushroom Phellinus Rhabarbarinus. J. Agric. Food Chem. 2016, 64, 1945–1949. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.K.; Kumar, V.; Bera, T.; Saxena, P.S.; Nath, G.; Srivastava, S.K.; Giri, R.; Srivastava, A. Study of Mechanism of Enhanced Antibacterial Activity by Green Synthesis of Silver Nanoparticles. Nanotechnology 2011, 22, 415104. [Google Scholar] [CrossRef] [PubMed]
- Halford, B. Drug Companies Are Investing Big in Psychedelics, but Can They Engineer Out the Trip? 2022. Available online: https://cen.acs.org/pharmaceuticals/drug-development/Drug-companies-investing-big-psychedelics/100/i9 (accessed on 20 April 2024).
- Chrysanthos, N.; Dow, A. Australia Becomes First Country to Recognise Psychedelics as Medicines. 2023. Available online: https://www.smh.com.au/politics/federal/australia-becomes-first-country-to-recognise-psychedelics-as-medicines-20230203-p5chs6.html (accessed on 20 April 2024).
- Chang, S.; Buswell, J. Medicinal Mushrooms: Past, Present and Future. In Biochemical Engineering and Biotechnology of Medicinal Mushrooms; Springer: Cham, Switzerland, 2022; Volume 1, pp. 1–27. [Google Scholar]
- Yadav, D.; Negi, P.S. Bioactive Components of Mushrooms: Processing Effects and Health Benefits. Food Res. Int. 2021, 148, 110559. [Google Scholar] [CrossRef] [PubMed]
- Sheikha, A.F. Nutritional Profile and Health Benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as Functional Foods: Current Scenario and Future Perspectives. Foods 2019, 11, 1030. [Google Scholar] [CrossRef]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma Lucidum (Reishi) an Edible Mushroom; a Comprehensive and Critical Review of Its Nutritional, Cosmeceutical, Mycochemical, Pharmacological, Clinical, and Toxicological Properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef]
- Hifas-da-Terra Mico Rei (Reishi Extract) Capsules. 2021, pp. 2021–2025. Available online: https://hifasdaterra.com/en/product/mico-rei-extract-of-reishi/ (accessed on 20 April 2024).
- Hifas-da-Terra Mico Five (Chaga, Reishi, Maitake, Shiitake, Sun Mushroom Capsules. 2019. Available online: https://hifasdaterra.com/en/product/mico-five-vit-c/ (accessed on 20 April 2024).
- Hifas-da-Terra Mico Mix (Shiitake, Reishi, Maitake) Capsules. Natassia. 2023. Available online: https://hifasdaterra.com/en/product/mico-mix-extract-of-3-mushrooms/ (accessed on 20 April 2024).
- Wellness-Warehouse The Real Thing—Medical Mushrooms 60s. Hifas-Da-Terra. 2024, Volume 184. Available online: https://www.wellnesswarehouse.com/the-real-thing-medical-mushrooms-60s-60s-00011120438103 (accessed on 20 April 2024).
- Myko-San Myko-San. Superior Medicinal Mushrooms Extracts. 2020. Available online: https://mykosan.com/ (accessed on 20 April 2024).



| Species | Peptide | N’-Terminal Sequence | Molecular Mass (Da) | Pharmaceutical Potential | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| Hypsizygus marmoreus | No name | LSMGSASLSP | 567.3 | antihypertensive | Non-competitive inhibition of ACE activity | [82] |
| Macrocybe gigantea (formerly Tricholoma giganteum) | No name | GQP | 301 | antihypertensive | Competitive inhibition of ACE | [83] |
| Pholiota adiposa | No name | GQGGP | 414 | antihypertensive | Competitive inhibition of ACE | [84] |
| Tricholoma matsutake | TMP | WALKGYK | 679.53 | antihypertensive | Non-competitive inhibition of ACE activity | [85] |
| Ganoderma lucidum | QLVP | QLVP | 456 | antihypertensive | Inhibition of ACE through interaction with Gln242 and Lys472 of ACE via a hydrogen bond and a salt bridge | [62] |
| Ganoderma lucidum | GLP Fraction | Not found | <10,000 | antioxidant | Inhibition of lipid peroxidation; increase of hepatic SOD and GSH | [69] |
| Pleurotus eryngii | PEMP | Not found | Not found | antioxidant; immunomodulatory; antitumor | Scavenging of reactive species; inhibition of cell proliferation; stimulation of macrophage production | [86] |
| Pleurotus eryngii | Eryngin | ATRVVYCNRR SGSVVGGDDT VYYEG | 10,000 | antifungal | Not specified | [87] |
| Neofavolus alveolaris (formerly Polyporus alveolaris) | Alveolarin | GVCDMADLA | 14,000 | antifungal | Inhibition of mycelial growth | [88] |
| Pleurotus ostreatus | Pleurostrin | VRPYLVAF | 7000 | antifungal | Inhibition of mycelial growth | [78] |
| Cordyceps militaris | Cordymin | AMAPPYGYRTPDAAQ | 10,906 | antifungal; antiviral; antitumor | Inhibition of mycelial growth; inhibition of HIV virus reverse transcriptase; decrease in cell proliferation | [89] |
| Pseudoplectania nigrella | Plectasin | GFGCNGPWDE DDMQCHNHCK SIKGYKGGYC AKGGFVCKCY | 4398.80 | anti-bacterial | Inhibition of bacterial growth; mechanisms not very well elucidated | [73] |
| Ganoderma lucidum | GLF and GLM Fractions | Not found | Not found | anti-bacterial | Generation of reactive oxygen species and induction of intracellular protein leakage | [71] |
| Russula paludosa | SU2 | KREHGQHCEF | 4500 | antiviral | Inhibition of HIV virus reverse transcriptase | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Burgos, W.J.; Montes Montes, E.; Pozzan, R.; Serra, J.L.; Torres, D.O.; Manzoki, M.C.; Vieira, R.L.; dos Reis, G.A.; Rodrigues, C.; Karp, S.G.; et al. Bioactive Compounds Produced by Macromycetes for Application in the Pharmaceutical Sector: Patents and Products. Fermentation 2024, 10, 275. https://doi.org/10.3390/fermentation10060275
Martinez-Burgos WJ, Montes Montes E, Pozzan R, Serra JL, Torres DO, Manzoki MC, Vieira RL, dos Reis GA, Rodrigues C, Karp SG, et al. Bioactive Compounds Produced by Macromycetes for Application in the Pharmaceutical Sector: Patents and Products. Fermentation. 2024; 10(6):275. https://doi.org/10.3390/fermentation10060275
Chicago/Turabian StyleMartinez-Burgos, Walter José, Everaldo Montes Montes, Roberta Pozzan, Josilene Lima Serra, Diego Ocán Torres, Maria Clara Manzoki, Ricardo Luiz Vieira, Guilherme Anacleto dos Reis, Cristine Rodrigues, Susan Grace Karp, and et al. 2024. "Bioactive Compounds Produced by Macromycetes for Application in the Pharmaceutical Sector: Patents and Products" Fermentation 10, no. 6: 275. https://doi.org/10.3390/fermentation10060275
APA StyleMartinez-Burgos, W. J., Montes Montes, E., Pozzan, R., Serra, J. L., Torres, D. O., Manzoki, M. C., Vieira, R. L., dos Reis, G. A., Rodrigues, C., Karp, S. G., & Soccol, C. R. (2024). Bioactive Compounds Produced by Macromycetes for Application in the Pharmaceutical Sector: Patents and Products. Fermentation, 10(6), 275. https://doi.org/10.3390/fermentation10060275








