Microbial Preservation and Contamination Control in the Baking Industry
Abstract
1. Introduction
2. Bread Fermentation Process
3. Bread Native Microbiology, Ingredients, and Additives
| Microorganism | Name | Reference |
|---|---|---|
| Bacteria | Bacillus cereus | [37] |
| Escherichia coli | ||
| Salmonella spp. | ||
| Actinobacteria phylum | [25,41,44] | |
| Chryseobacterium | ||
| Delftia | ||
| Enterobacteriaceae and Oxalobacteriaceae families | ||
| Enterococcus durans | ||
| Enterococcus faecium | ||
| Erwinia | ||
| Lacticaseibacillus paracasei | ||
| Lactiplantibacillus pentosus | ||
| Lactobacillus brevis | ||
| Lysinobacillus | ||
| Paenibacillus | ||
| Pediococcus | ||
| Pseudomonas | ||
| Serratia | ||
| Sphingomonas | ||
| Stenotrophomonas | ||
| Enterococcus | ||
| Lactobacillus | ||
| Lactococcus | ||
| Streptococcus | ||
| Yeasts | Aureobasidium pullulans | [24,44] |
| Candida phangngaensis | ||
| Filobasidum magnum Kazachstania | ||
| Naganishia albida | ||
| Papiliotrema rajasthanensis Pichia | ||
| Rhodotorula graminis | ||
| Rhodotorula mucilaginosa Saccharomyces | ||
| Sporidiobolus metaroseus | ||
| Vishniacozyma victoriae | ||
| Filamentous fungi | Alternaria sp. | [37] |
| Aspergillus | ||
| Penincillus | ||
| Cladosporium sp. | [45] | |
| Talaromyces rugulosum | ||
| Wallemia sebi |
4. Microorganisms’ Entry in Bread-Making
5. Preserving and Storing Microbial Cultures for Baking
5.1. Microencapsulation
5.2. Freeze-Drying
| Starter | Mo | Before Freeze-Drying (log CFU/g) | After Freeze-Drying (log CFU/g) | Survival Rate (%) | Reference |
|---|---|---|---|---|---|
| Sourdough type I | LAB | 9.50 | 8.93 | 94.00 | [73] |
| Sourdough type I | LAB | 9.17 ± 0.17 | 6.07 | 66.19 | [56] |
| Yeast | 7.53 ± 0.12 | 5.03 | 66.80 | ||
| Yeast starter | S. cerevisiae 88-4 | 7.00 | 6.65 | 95 | [74] |
| Sourdough type I | LAB | 8.7 ± 0.0 | 8.0 ± 0.6 | 91.95 | [61] |
| Yeast | 8.6 ± 0.0 | 8.0 ± 0.0 | 93.02 |
5.3. Spray-Drying
5.4. Fluidized Bed Drying
5.5. Vacuum Drying
| Strain | Protectant | Temperature | Pressure | Time | Survival Rate | Reference |
|---|---|---|---|---|---|---|
| Lc. paracasei F19 | Trehalose 25% (w/w) | 15 °C | 15 mbar | 22 h | 70% | [91] |
| Lactobacillus helveticus | Sorbitol (1% w/w) | 43 °C | 100 mbar | 12 h | 18% | [92] |
| L. acidophilus | Trehalose (20% w/w) | Room temperature | 0.11 mbar | 96 h | 37.9% | [93] |
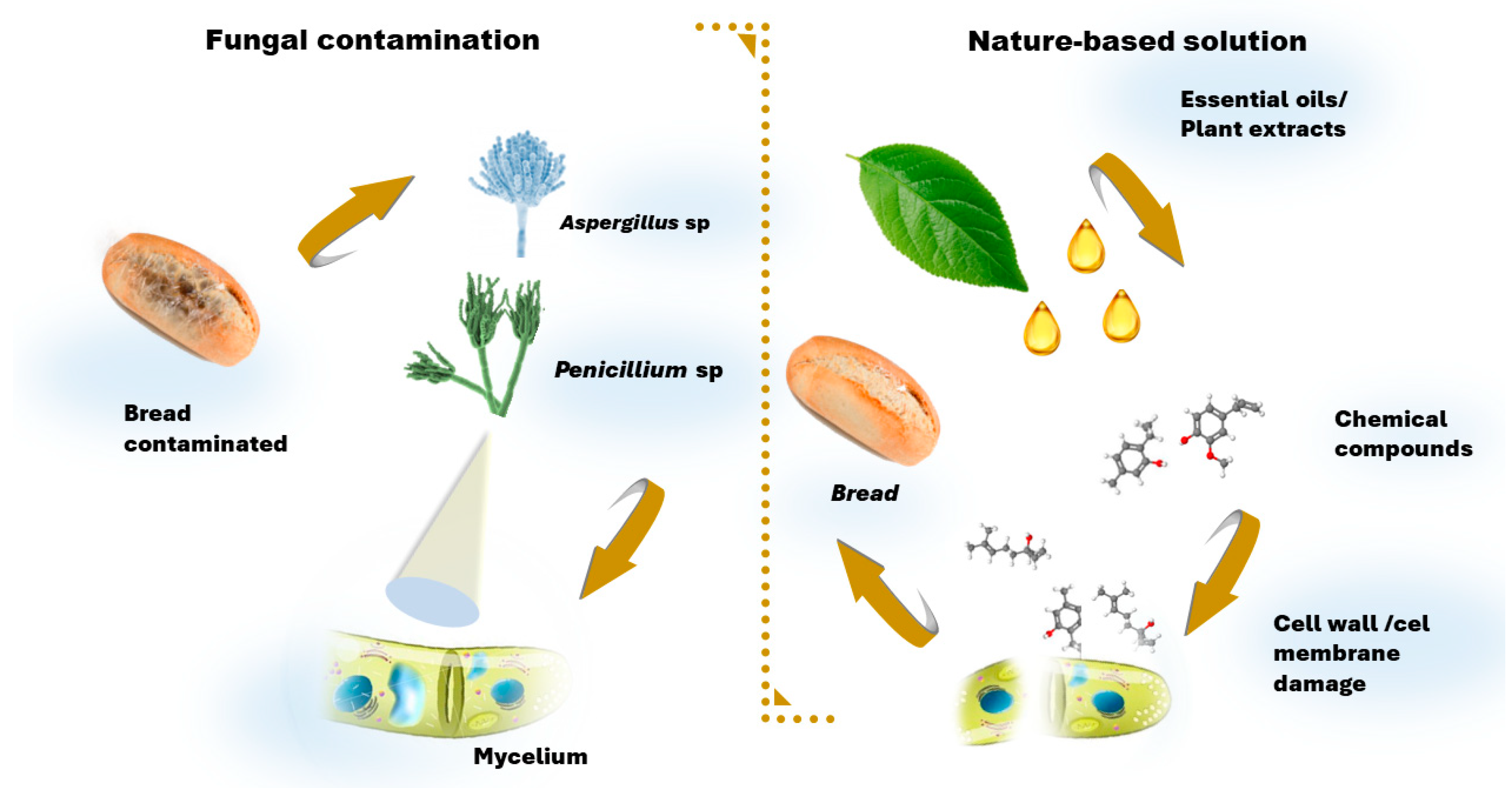
6. Microbial Contamination of Baking: Bread Spoilage
6.1. Molds and Toxins
6.2. Yeasts: Chalk Molds
6.3. Bacillus sp.: Ropiness
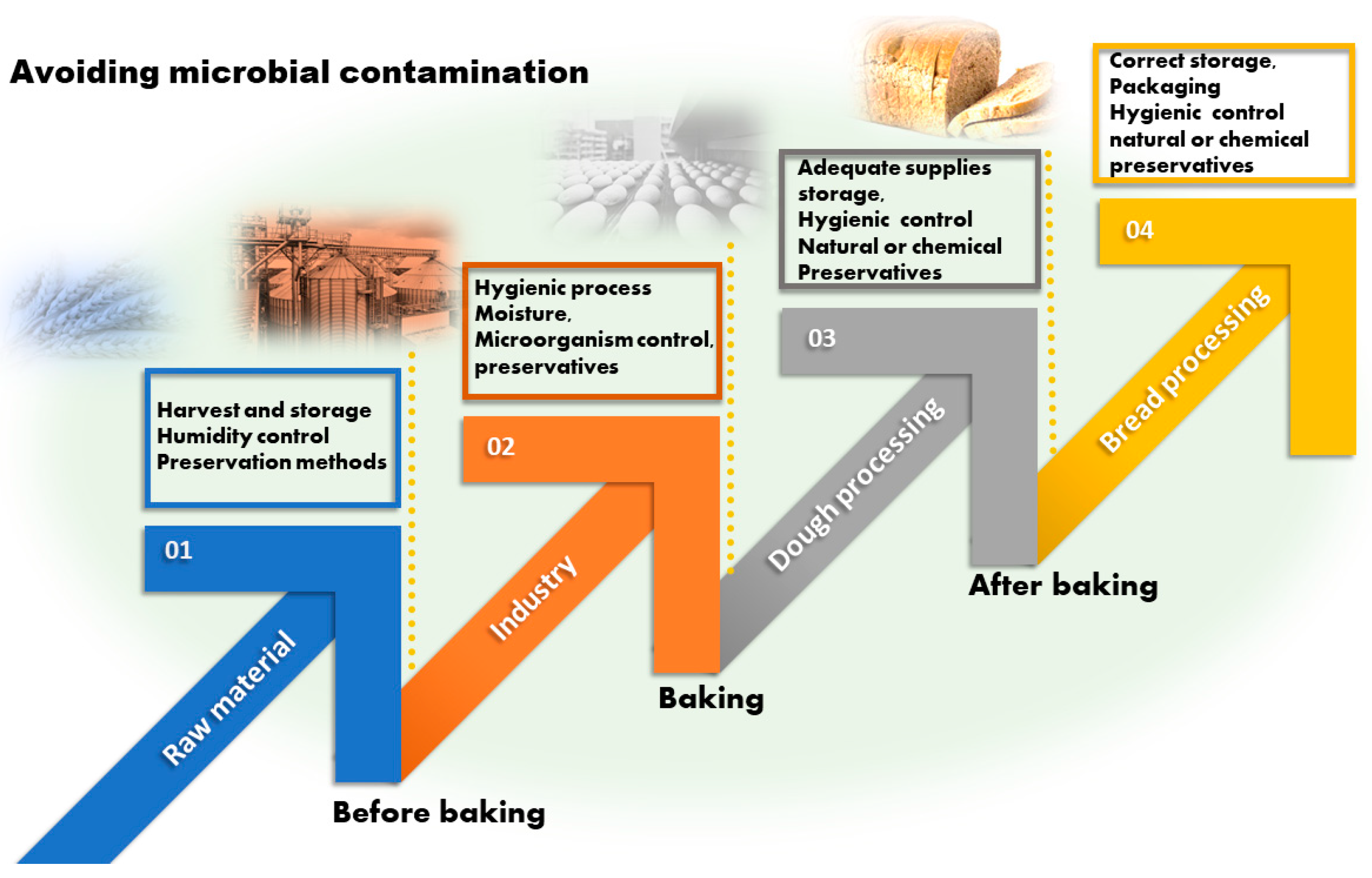
7. Control of Microbial Contamination in the Bread Chain
7.1. Chemical Methods: Organic Acids
7.2. Biological Preservatives
7.2.1. Essential Oils
7.2.2. Plant Extracts
7.2.3. Lactic Acid Bacteria (LAB)
7.3. Physical Methods
7.3.1. Pasteurization and Radio Frequency Heating
7.3.2. Cold Atmospheric Plasma Treatment
7.3.3. Electrolyzed Water
7.4. Packaging Strategies
7.4.1. Modified Atmosphere Packaging (MAP), Active Packaging, and Intelligent Packaging
7.4.2. Coating and Biodegradable Packaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arranz-Otaegui, A.; Gonzalez Carretero, L.; Ramsey, M.N.; Fuller, D.Q.; Richter, T. Archaeobotanical Evidence Reveals the Origins of Bread 14,400 Years Ago in Northeastern Jordan. Proc. Natl. Acad. Sci. USA 2018, 115, 7925–7930. [Google Scholar] [CrossRef]
- Chiron, H. La fermentation du pain: Histoire et modernité. Cah. Fr. Viète 1999, 81–96. [Google Scholar] [CrossRef]
- Dong, Y.; Karboune, S. A Review of Bread Qualities and Current Strategies for Bread Bioprotection: Flavor, Sensory, Rheological, and Textural Attributes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1937–1981. [Google Scholar] [CrossRef] [PubMed]
- Gębski, J.; Jezewska-Zychowicz, M.; Szlachciuk, J.; Kosicka-Gębska, M. Impact of Nutritional Claims on Consumer Preferences for Bread with Varied Fiber and Salt Content. Food Qual. Prefer. 2019, 76, 91–99. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Hasan, S.; Zzaman, W.; Rana, M.R.; Ahmed, S.; Roy, M.; Sayem, A.; Matin, A.; Raposo, A.; et al. A Comprehensive Review on Bio-Preservation of Bread: An Approach to Adopt Wholesome Strategies. Foods 2022, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Ribet, L.; Dessalles, R.; Lesens, C.; Brusselaers, N.; Durand-Dubief, M. Nutritional Benefits of Sourdoughs: A Systematic Review. Adv. Nutr. 2023, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Sandoval, N.; Valencia-Tapia, M.; Calderón De La Barca, A.; Islas-Rubio, A. Microbial Proteases in Baked Goods: Modification of Gluten and Effects on Immunogenicity and Product Quality. Foods 2016, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Akamine, I.T.; Mansoldo, F.R.P.; Cardoso, V.S.; De Souza Dias, E.P.; Vermelho, A.B. Hydrolase Activities of Sourdough Microorganisms. Fermentation 2023, 9, 703. [Google Scholar] [CrossRef]
- Akamine, I.T.; Mansoldo, F.R.P.; Vermelho, A.B. Probiotics in the Sourdough Bread Fermentation: Current Status. Fermentation 2023, 9, 90. [Google Scholar] [CrossRef]
- Marco, I.D.; Silva, C.M.D.; Moraes, J.O.D.; Menezes, L.A.A.; Miotto, M.; Laurindo, J.B.; Lindner, J.D.D. A Systematic Review of Drying Methods and Their Impact on Technological Characteristics of Sourdough Type III. Biotechnol. Res. Innov. 2022, 6, e2022003. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Wang, X.; Guan, X.; Huang, K.; Zhang, Y. Effect of Storage Conditions on the Textural Properties and in Vitro Digestibility of Wheat Bread Containing Whole Quinoa Flour. Food Biosci. 2022, 49, 101921. [Google Scholar] [CrossRef]
- Jideani, V.A. Bread Storage and Preservation. In Encyclopedia of Food Security and Sustainability; Elsevier: Amsterdam, The Netherlands, 2019; pp. 593–604. ISBN 978-0-12-812688-2. [Google Scholar]
- Reese, A.T.; Madden, A.A.; Joossens, M.; Lacaze, G.; Dunn, R.R. Influences of Ingredients and Bakers on the Bacteria and Fungi in Sourdough Starters and Bread. mSphere 2020, 5, e00950-19. [Google Scholar] [CrossRef] [PubMed]
- Gregirchak, N.; Stabnikova, O.; Stabnikov, V. Application of Lactic Acid Bacteria for Coating of Wheat Bread to Protect it from Microbial Spoilage. Plant Foods Hum Nutr 2020, 75, 223. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal Sourdough Lactic Acid Bacteria as Biopreservation Tool in Quinoa and Rice Bread. Int. J. Food Microbiol. 2016, 239, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Saeed, F.; Imran, A.; Shehzadi, U.; Ali, R.; Nosheen, F.; Chauhan, A.; Asghar, A.; Ojukwu, M. Bio-Preservatives and Essential Oils as an Alternative to Chemical Preservatives in the Baking Industry: A Concurrent Review. J. Food Sci. Technol. 2023, 61, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, J.; Mao, Z.; Mah, J.-H.; Jiao, S.; Wang, S. Quality and Mold Control of Enriched White Bread by Combined Radio Frequency and Hot Air Treatment. J. Food Eng. 2011, 104, 492–498. [Google Scholar] [CrossRef]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology-A Traditional Way for Wholesome Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar] [CrossRef]
- Lahue, C.; Madden, A.A.; Dunn, R.R.; Smukowski Heil, C. History and Domestication of Saccharomyces Cerevisiae in Bread Baking. Front. Genet. 2020, 11, 584718. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Zheng, J. Lifestyles of Sourdough Lactobacilli—Do They Matter for Microbial Ecology and Bread Quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- Fekri, A.; Abedinzadeh, S.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Considering Sourdough from a Biochemical, Organoleptic, and Nutritional Perspective. J. Food Compos. Anal. 2024, 125, 105853. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough Production: Fermentation Strategies, Microbial Ecology, and Use of Non-Flour Ingredients. Crit. Rev. Food Sci. Nutr. 2021, 63, 2447–2479. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Maes, D.; De Vuyst, L. Sourdoughs as a Function of Their Species Diversity and Process Conditions, a Meta-Analysis. Trends Food Sci. Technol. 2017, 68, 152–159. [Google Scholar] [CrossRef]
- Minervini, F.; Celano, G.; Lattanzi, A.; Tedone, L.; de Mastro, G.; Gobbetti, M.; de Angelis, M. Lactic Acid Bacteria in Durum Wheat Flour Are Endophytic Components of the Plant during Its Entire Life Cycle. Appl. Environ. Microbiol. 2015, 81, 6736–6748. [Google Scholar] [CrossRef]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Landis, E.A.; Oliverio, A.M.; McKenney, E.A.; Nichols, L.M.; Kfoury, N.; Biango-Daniels, M.; Shell, L.K.; Madden, A.A.; Shapiro, L.; Sakunala, S.; et al. The Diversity and Function of Sourdough Starter Microbiomes. eLife 2021, 10, e61644. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Malcata, F.X. Microbial Ecology Dynamics in Portuguese Broa Sourdough. J. Food Qual. 2016, 39, 634–648. [Google Scholar] [CrossRef]
- Ercolini, D.; Pontonio, E.; De Filippis, F.; Minervini, F.; Storia, A.L.; Gobbetti, M.; Di Cagno, R. Microbial Ecology Dynamics during Rye and Wheat Sourdough Preparation. Appl. Environ. Microbiol. 2013, 79, 7827–7836. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.A.A.; Sardaro, M.L.S.; Duarte, R.T.D.; Mazzon, R.R.; Neviani, E.; Gatti, M.; De Dea Lindner, J. Sourdough Bacterial Dynamics Revealed by Metagenomic Analysis in Brazil. Food Microbiol. 2020, 85, 103302. [Google Scholar] [CrossRef]
- Ndukwe, J.K.; Aduba, C.C.; Ughamba, K.T.; Chukwu, K.O.; Eze, C.N.; Nwaiwu, O.; Onyeaka, H. Diet Diversification and Priming with Kunu: An Indigenous Probiotic Cereal-Based Non-Alcoholic Beverage in Nigeria. Beverages 2023, 9, 14. [Google Scholar] [CrossRef]
- Ramedani, N.; Sharifan, A.; Nejad, M.R.; Yadegar, A. Influence of a Combination of Three Probiotics on Wheat Dough Fermentation; New Therapeutic Strategy in Celiac Disease. J. Food Meas. Charact. 2024, 18, 2480–2488. [Google Scholar] [CrossRef]
- Kaplan, S.L. Good Bread Is Back: A Contemporary History of French Bread, the Way It Is Made, and the People Who Make It; Duke University Press: Durham, NC, USA, 2006; ISBN 978-0-8223-8828-9. [Google Scholar]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.M.; Hanhineva, K. Metabolic Profiling of Sourdough Fermented Wheat and Rye Bread. Sci. Rep. 2018, 8, 5684. [Google Scholar] [CrossRef]
- Hernández-Parada, N.; González-Ríos, O.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Figueroa-Hernández, C.Y.; Figueroa-Cárdenas, J.D.D.; Rayas-Duarte, P.; Figueroa-Espinoza, M.C. Exploiting the Native Microorganisms from Different Food Matrices to Formulate Starter Cultures for Sourdough Bread Production. Microorganisms 2022, 11, 109. [Google Scholar] [CrossRef]
- De Angelis, M.; Minervini, F.; Siragusa, S.; Rizzello, C.G.; Gobbetti, M. Wholemeal Wheat Flours Drive the Microbiome and Functional Features of Wheat Sourdoughs. Int. J. Food Microbiol. 2019, 302, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.V.C.; Fernandes, Â.; Heleno, S.A.; Rodrigues, P.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Physicochemical Characterization and Microbiology of Wheat and Rye Flours. Food Chem. 2019, 280, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dinardo, F.R.; De Angelis, M.; Gobbetti, M. Tap Water Is One of the Drivers That Establish and Assembly the Lactic Acid Bacterium Biota during Sourdough Preparation. Sci. Rep. 2019, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Fellows, P.J. Dehydration. In Food Processing Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 661–716. ISBN 978-0-08-101907-8. [Google Scholar]
- Dagnas, S.; Gougouli, M.; Onno, B.; Koutsoumanis, K.P.; Membré, J.-M. Quantifying the Effect of Water Activity and Storage Temperature on Single Spore Lag Times of Three Moulds Isolated from Spoiled Bakery Products. Int. J. Food Microbiol. 2017, 240, 75–84. [Google Scholar] [CrossRef]
- Minervini, F.; Lattanzi, A.; Dinardo, F.R.; De Angelis, M.; Gobbetti, M. Wheat Endophytic Lactobacilli Drive the Microbial and Biochemical Features of Sourdoughs. Food Microbiol. 2018, 70, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Lattanzi, A.; De Angelis, M.; Celano, G.; Gobbetti, M. House Microbiotas as Sources of Lactic Acid Bacteria and Yeasts in Traditional Italian Sourdoughs. Food Microbiol. 2015, 52, 66–76. [Google Scholar] [CrossRef]
- Neeharika, B. Leavening Agents for Food Industry. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1812–1817. [Google Scholar] [CrossRef]
- Gaglio, R.; Cirlincione, F.; Di Miceli, G.; Franciosi, E.; Di Gerlando, R.; Francesca, N.; Settanni, L.; Moschetti, G. Microbial Dynamics in Durum Wheat Kernels during Aging. Int. J. Food Microbiol. 2020, 324, 108631. [Google Scholar] [CrossRef]
- Garcia, M.V.; Bernardi, A.O.; Parussolo, G.; Stefanello, A.; Lemos, J.G.; Copetti, M.V. Spoilage Fungi in a Bread Factory in Brazil: Diversity and Incidence through the Bread-Making Process. Food Res. Int. 2019, 126, 108593. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.; Ripari, V. Composition and Function of Sourdough Microbiota: From Ecological Theory to Bread Quality. Int. J. Food Microbiol. 2016, 239, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Duar, R.M.; Lin, X.B.; Zheng, J.; Martino, M.E.; Grenier, T.; Pérez-Muñoz, M.E.; Leulier, F.; Gänzle, M.; Walter, J. Lifestyles in Transition: Evolution and Natural History of the Genus Lactobacillus. FEMS Microbiol. Rev. 2017, 41, S27–S48. [Google Scholar] [CrossRef]
- Santos, J.L.P.D.; Bernardi, A.O.; Pozza Morassi, L.L.; Silva, B.S.; Copetti, M.V.; S. Sant’Ana, A. Incidence, Populations and Diversity of Fungi from Raw Materials, Final Products and Air of Processing Environment of Multigrain Whole Meal Bread. Food Res. Int. 2016, 87, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Sarkar, A.; Hossain, A. Isolation of Pathogenic Microorganisms and Determination of Their Antibiotic Resistance Patterns Collected from Different Bakery Products of Dhaka City. Food Res. 2020, 4. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Muhammad, S.; Ibrahim Galadima, S. Determination of Microbiological Quality of Bread and Sanitation Conditions of Local Bakeries in Aliero Town, Kebbi State. Appl. Sci. Technol. Res. J. 2023, 1, 1–9. [Google Scholar] [CrossRef]
- Ali, M.A.; Hashish, M.H.; Fekry, M.M. Microbiological Quality of Some Packed and Unpacked Bread Products in Alexandria, Egypt. J. Egypt. Public Health Assoc. 2023, 98, 16. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Succi, M.; Tremonte, P.; Coppola, R.; Sorrentino, E. Microbiological and Fermentative Properties of Baker’s Yeast Starter Used in Breadmaking. J. Food Sci. 2013, 78, M1224–M1231. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces Cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Türker, M.; Kanarya, A.; Yüzgeç, U.; Kapucu, H.; Şenalp, Z. Drying of Baker’s Yeast in Batch Fluidized Bed. Chem. Eng. Process. Process Intensif. 2006, 45, 1019–1028. [Google Scholar] [CrossRef]
- Akbari, H.; Karimi, K.; Lundin, M.; Taherzadeh, M.J. Optimization of Baker’s Yeast Drying in Industrial Continuous Fluidized Bed Dryer. Food Bioprod. Process. 2012, 90, 52–57. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Preziuso, M.; Panfili, G.; Cipriano, L.; Messia, M.C. Stabilization of Sourdough Starter by Spray Drying Technique: New Breadmaking Perspective. LWT 2019, 99, 468–475. [Google Scholar] [CrossRef]
- Blaiotta, G.; Romano, R.; Trifuoggi, M.; Aponte, M.; Miro, A. Development of a Wet-Granulated Sourdough Multiple Starter for Direct Use. Foods 2022, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Coorey, R. A Review on Probiotic Microencapsulation and Recent Advances of Their Application in Bakery Products. Food Bioprocess Technol. 2022, 15, 1677–1699. [Google Scholar] [CrossRef]
- Gélinas, P. Active Dry Yeast: Lessons from Patents and Science. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1227–1255. [Google Scholar] [CrossRef] [PubMed]
- Mohd Roby, B.H.; Muhialdin, B.J.; Abadl, M.M.T.; Mat Nor, N.A.; Marzlan, A.A.; Lim, S.A.H.; Mustapha, N.A.; Meor Hussin, A.S. Physical Properties, Storage Stability, and Consumer Acceptability for Sourdough Bread Produced Using Encapsulated Kombucha Sourdough Starter Culture. J. Food Sci. 2020, 85, 2286–2295. [Google Scholar] [CrossRef]
- Caglar, N.; Ermis, E.; Durak, M.Z. Spray-Dried and Freeze-Dried Sourdough Powders: Properties and Evaluation of Their Use in Breadmaking. J. Food Eng. 2021, 292, 110355. [Google Scholar] [CrossRef]
- Penhasi, A.; Reuveni, A.; Baluashvili, I. Microencapsulation May Preserve the Viability of Probiotic Bacteria During a Baking Process and Digestion: A Case Study with Bifidobacterium animalis Subsp. lactis in Bread. Curr. Microbiol. 2021, 78, 576–589. [Google Scholar] [CrossRef]
- Sbehat, M.; Mauriello, G.; Altamimi, M. Microencapsulation of Probiotics for Food Functionalization: An Update on Literature Reviews. Microorganisms 2022, 10, 1948. [Google Scholar] [CrossRef]
- Guha, S.; Chakraborty, A.; Chakraborty, D. Application of Nanotechnology in Food Microbiology: Implication on Public Health. In Applications of Nanotechnology in Microbiology; Chaughule, R.S., Lokur, A.S., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 135–156. ISBN 978-3-031-49932-6. [Google Scholar]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel Approaches for Co-Encapsulation of Probiotic Bacteria with Bioactive Compounds, Their Health Benefits and Functional Food Product Development: A Review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Afzal, A.; Afzaal, M.; Saeed, F.; Shah, Y.A.; Raza, M.A.; Khan, M.H.; Asghar, A.; Akram, N.; Ateeq, H.; Teferi Asres, D. Milk Protein Based Encapsulation of Probiotics and Other Food Material: Comprehensive Review. Int. J. Food Prop. 2024, 27, 245–262. [Google Scholar] [CrossRef]
- Rajam, R.; Subramanian, P. Encapsulation of Probiotics: Past, Present and Future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Panigrahi, C.; Mishra, H.N. A Comparative Approach on the Spray and Freeze Drying of Probiotic and Gamma-Aminobutyric Acid as a Single Entity: Characterization and Evaluation of Stability in Simulated Gastrointestinal Conditions. Food Chem. Adv. 2023, 3, 100385. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective Approaches and Mechanisms of Microencapsulation to the Survival of Probiotic Bacteria during Processing, Storage and Gastrointestinal Digestion: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2863–2878. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, C.-M.; Gong, J. Issues Deserve Attention in Encapsulating Probiotics: Critical Review of Existing Literature. Crit. Rev. Food Sci. Nutr. 2017, 57, 1228–1238. [Google Scholar] [CrossRef]
- Liu, A.; Su, S.; Sun, Y.; Li, Q.; Li, J.; Hu, K.; Zhao, N.; He, L.; Chen, S.; Liu, S. Enhancing the Highland Barley-Wheat Dough Network Structure and Bread Quality Using Freeze-Dried Sourdough Powder with Inulin as a Protectant. LWT 2024, 191, 115599. [Google Scholar] [CrossRef]
- Gu, Y.; Luo, X.; Qian, H.; Li, Y.; Fan, M.; Wang, L. Effects of Freeze-Dried Pure Strains to Replace Type II Sourdough in Bread Production. Food Biosci. 2023, 53, 102752. [Google Scholar] [CrossRef]
- Gul, L.B.; Gul, O.; Yilmaz, M.T.; Dertli, E.; Con, A.H. Optimization of Cryoprotectant Formulation to Enhance the Viability of Lactobacillus Brevis ED25: Determination of Storage Stability and Acidification Kinetics in Sourdough. J. Food Process. Preserv. 2020, 44, e14400. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Lee, S.; Yu, H.H.; Yang, S.J.; Kim, T.-W. Combinatorial Effects of Protective Agents on Survival Rate of the Yeast Starter, Saccharomyces Cerevisiae 88-4, after Freeze-Drying. Microorganisms 2021, 9, 613. [Google Scholar] [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of Whey Protein—Alginate Wall Systems on Survival of Microencapsulated Lactobacillus Plantarum in Simulated Gastrointestinal Conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, R.; Chawla, S.; Gauba, P.; Singh, M.; Tiwari, R.K.; Upadhyay, S.; Sharma, S.; Chanda, S.; Gaur, S. Natural Sources and Encapsulating Materials for Probiotics Delivery Systems: Recent Applications and Challenges in Functional Food Development. Front. Nutr. 2022, 9, 971784. [Google Scholar] [CrossRef] [PubMed]
- Poozesh, S.; Akafuah, N.K.; Campbell, H.R.; Bashiri, F.; Saito, K. Experimental and Mathematical Tools to Predict Droplet Size and Velocity Distribution for a Two-Fluid Nozzle. Fluids 2020, 5, 231. [Google Scholar] [CrossRef]
- Haron, N.S.; Zakaria, J.H.; Mohideen Batcha, M.F. Recent Advances in Fluidized Bed Drying. IOP Conf. Ser. Mater. Sci. Eng. 2017, 243, 012038. [Google Scholar] [CrossRef]
- Vorländer, K.; Bahlmann, L.; Kwade, A.; Finke, J.H.; Kampen, I. Effect of Process Parameters, Protectants and Carrier Materials on the Survival of Yeast Cells during Fluidized Bed Granulation for Tableting. Pharmaceutics 2023, 15, 884. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.Y.; Lim, X.X.; Tan, T.-C.; Kobun, R.; Rasti, B. Encapsulated Probiotics: Potential Techniques and Coating Materials for Non-Dairy Food Applications. Appl. Sci. 2022, 12, 10005. [Google Scholar] [CrossRef]
- Sánchez-Portilla, Z.; Melgoza-Contreras, L.M.; Reynoso-Camacho, R.; Pérez-Carreón, J.I.; Gutiérrez-Nava, A. Incorporation of Bifidobacterium Sp. into Powder Products through a Fluidized Bed Process for Enteric Targeted Release. J. Dairy Sci. 2020, 103, 11129–11137. [Google Scholar] [CrossRef] [PubMed]
- Stummer, S.; Toegel, S.; Rabenreither, M.-C.; Unger, F.M.; Wirth, M.; Viernstein, H.; Salar-Behzadi, S. Fluidized-Bed Drying as a Feasible Method for Dehydration of Enterococcus Faecium M74. J. Food Eng. 2012, 111, 156–165. [Google Scholar] [CrossRef]
- Altay, Ö.; Bozkurt, S.; Alemdar, F.; Türker, M.; Koç, M.; Kaymak-Ertekin, F. Coating of Baker’s Yeast with a Fluidized Bed System: Effects of Process Parameters and Storage Stability of Coated Yeast in Flour Mixtures. Dry. Technol. 2024, 42, 253–268. [Google Scholar] [CrossRef]
- Wirunpan, M.; Savedboworn, W.; Wanchaitanawong, P. Survival and Shelf Life of Lactobacillus Lactis 1464 in Shrimp Feed Pellet after Fluidized Bed Drying. Agric. Nat. Resour. 2016, 50, 1–7. [Google Scholar] [CrossRef][Green Version]
- Wu, C.-H.; Liu, Y.-C.; Ou, S.-F.; Chen, S.-T.; Kuo, J.-M.; Hsueh, Y.-H. Improving Acid Resistance and Characteristics of Microencapsulated Lactobacillus Brevis RK03 Using Top Fluid Bed Drying Technology. Process Biochem. 2021, 110, 1–8. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging Technologies and Coating Materials for Improved Probiotication in Food Products: A Review. Food Bioprocess Technol. 2022, 15, 998–1039. [Google Scholar] [CrossRef]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying Techniques of Probiotic Bacteria as an Important Step towards the Development of Novel Pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Haldar, L.; Gandhi, D.N. Development of Vacuum-dried Probiotic Milk Powder with Bacillus coagulans. Int. J. Dairy Technol. 2020, 73, 283–291. [Google Scholar] [CrossRef]
- Ermis, E. A Review of Drying Methods for Improving the Quality of Probiotic Powders and Characterization. Dry. Technol. 2022, 40, 2199–2216. [Google Scholar] [CrossRef]
- Ambros, S.; Foerst, P.; Kulozik, U. Temperature-Controlled Microwave-Vacuum Drying of Lactic Acid Bacteria: Impact of Drying Conditions on Process and Product Characteristics. J. Food Eng. 2018, 224, 80–87. [Google Scholar] [CrossRef]
- Foerst, P.; Kulozik, U.; Schmitt, M.; Bauer, S.; Santivarangkna, C. Storage Stability of Vacuum-Dried Probiotic Bacterium Lactobacillus Paracasei F19. Food Bioprod. Process. 2012, 90, 295–300. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Effect of Carbohydrates on the Survival of Lactobacillus Helveticus during Vacuum Drying. Lett. Appl. Microbiol. 2006, 42, 271–276. [Google Scholar] [CrossRef]
- Conrad, P.B.; Miller, D.P.; Cielenski, P.R.; De Pablo, J.J. Stabilization and Preservation of Lactobacillus Acidophilus in Saccharide Matrices. Cryobiology 2000, 41, 17–24. [Google Scholar] [CrossRef]
- Pareyt, B.; Finnie, S.M.; Putseys, J.A.; Delcour, J.A. Lipids in Bread Making: Sources, Interactions, and Impact on Bread Quality. J. Cereal Sci. 2011, 54, 266–279. [Google Scholar] [CrossRef]
- Chou, K.; Yan, C.-T.; Hsiao, H.-I. Identification of Postbaking Mold Contamination through Onsite Monitoring of Baking Factory Environment: A Case Study of Bakery Company in Taiwan. Food Control 2023, 145, 109495. [Google Scholar] [CrossRef]
- Raters, M.; Matissek, R. Thermal Stability of Aflatoxin B1 and Ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Authority (EFSA), E.F.S. Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] Related to the Potential Increase of Consumer Health Risk by a Possible Increase of the Existing Maximum Levels for Aflatoxins in Almonds, Hazelnuts and Pistachios and Derived Products. EFSA J. 2007, 5, 446. [Google Scholar] [CrossRef]
- Trombete, F.; Moraes, D.; Porto, Y.; Santos, T.; Direito, G.; Fraga, M.; Saldanha, T. Determination of Aflatoxins in Wheat and Wheat By-Products Intended for Human Consumption, Marketed in Rio de Janeiro, Brazil. J. Food Nutr. Res. 2014, 2, 671–674. [Google Scholar] [CrossRef]
- Coton, E.; Coton, M.; Hymery, N.; Mounier, J.; Jany, J.-L. Penicillium roqueforti: An Overview of Its Genetics, Physiology, Metabolism and Biotechnological Applications. Fungal Biol. Rev. 2020, 34, 59–73. [Google Scholar] [CrossRef]
- Dumas, E.; Feurtey, A.; Rodríguez de la Vega, R.C.; Le Prieur, S.; Snirc, A.; Coton, M.; Thierry, A.; Coton, E.; Le Piver, M.; Roueyre, D.; et al. Independent Domestication Events in the Blue-cheese Fungus Penicillium roqueforti. Mol. Ecol. 2020, 29, 2639–2660. [Google Scholar] [CrossRef] [PubMed]
- Crequer, E.; Ropars, J.; Jany, J.; Caron, T.; Coton, M.; Snirc, A.; Vernadet, J.; Branca, A.; Giraud, T.; Coton, E. A New Cheese Population in Penicillium roqueforti and Adaptation of the Five Populations to Their Ecological Niche. Evol. Appl. 2023, 16, 1438–1457. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, L.; Calpe, J.; Lafuente, C.; Moreno, A.; Musto, L.; Meca, G.; Luz, C. Aflatoxin B1 and Ochratoxin A Reduction by Lactobacillus Spp. during Bread Making. J. Sci. Food Agric. 2023, 103, 7095–7103. [Google Scholar] [CrossRef] [PubMed]
- Debonne, E.; Meuninck, V.; Vroman, A.; Eeckhout, M. Influence of Environmental Growth Conditions on Chalk Yeasts Causing Bread Spoilage. LWT 2021, 148, 111756. [Google Scholar] [CrossRef]
- Cremonesi, P.; Garofalo, C.; Picozzi, C.; Castiglioni, B.; Mangieri, N.; Milanović, V.; Osimani, A.; Aquilanti, L. Development of Quantitative Real-Time PCR and Digital Droplet-PCR Assays for Rapid and Early Detection of the Spoilage Yeasts Saccharomycopsis fibuligera and Wickerhamomyces anomalus in Bread. Food Microbiol. 2022, 101, 103894. [Google Scholar] [CrossRef]
- Thompson, J.M.; Waites, W.M.; Dodd, C.E.R. Detection of Rope Spoilage in Bread Caused by Bacillus Species. J. Appl. Microbiol. 1998, 85, 481–486. [Google Scholar] [CrossRef]
- Thompson, J.M.; Dodd, C.E.R.; Waites, W.M. Spoilage of Bread by Bacillus. Int. Biodeterior. Biodegrad. 1993, 32, 55–66. [Google Scholar] [CrossRef]
- Pacher, N.; Burtscher, J.; Johler, S.; Etter, D.; Bender, D.; Fieseler, L.; Domig, K.J. Ropiness in Bread—A Re-Emerging Spoilage Phenomenon. Foods 2022, 11, 3021. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, A. Role of Microorganisms in the Food Industry. In Food Microbial Sustainability; Karnwal, A., Mohammad Said Al-Tawaha, A.R., Eds.; Springer Nature: Singapore, 2023; pp. 1–23. ISBN 978-981-9947-83-6. [Google Scholar]
- Zavorohina, N.V.; Pankratyeva, N.A.; Goncharova, N.A. Development of an Express Method for the Quantitative Assessment of the Contamination of Wheat Flour with Bac. Spores. Subtilis. E3S Web Conf. 2020, 222, 06029. [Google Scholar] [CrossRef]
- Pepe, O.; Blaiotta, G.; Moschetti, G.; Greco, T.; Villani, F. Rope-Producing Strains of Bacillus Spp. from Wheat Bread and Strategy for Their Control by Lactic Acid Bacteria. Appl. Environ. Microbiol. 2003, 69, 2321–2329. [Google Scholar] [CrossRef]
- Rumeus, I. Bread Rope Spoilage Development. J. Eng. Sci. 2023, 30, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Malcata, F.X. On the Microbiological Profile of Traditional Portuguese Sourdough. J. Food Prot. 1999, 62, 1416–1429. [Google Scholar] [CrossRef]
- Meruvu, H.; Harsa, S.T. Lactic Acid Bacteria: Isolation–Characterization Approaches and Industrial Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 8337–8356. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; De Bellis, P.; Di Biase, M.; Lonigro, S.L.; Giussani, B.; Visconti, A.; Lavermicocca, P.; Sisto, A. Diversity of Spore-Forming Bacteria and Identification of Bacillus amyloliquefaciens as a Species Frequently Associated with the Ropy Spoilage of Bread. Int. J. Food Microbiol. 2012, 156, 278–285. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Zheng, J.; Gänzle, M.G. Bacillus Species in Food Fermentations: An Underappreciated Group of Organisms for Safe Use in Food Fermentations. Curr. Opin. Food Sci. 2023, 50, 101007. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Bourke, P. Current and Future Technologies for Microbiological Decontamination of Cereal Grains. J. Food Sci. 2018, 83, 1484–1493. [Google Scholar] [CrossRef]
- Berghofer, L.K.; Hocking, A.D.; Miskelly, D.; Jansson, E. Microbiology of Wheat and Flour Milling in Australia. Int. J. Food Microbiol. 2003, 85, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Hemery, Y.M.; Fontan, L.; Laillou, A.; Jallier, V.; Moench-Pfanner, R.; Avallone, S.; Berger, J. Influence of Storage Conditions and Packaging of Fortified Wheat Flour on Microbial Load and Stability of Folate and Vitamin B12. Food Chem. X 2020, 5, 100076. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold Spoilage of Bread and Its Biopreservation: A Review of Current Strategies for Bread Shelf Life Extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef]
- Saranraj, P.; Geetha, M. Microbial Spoilage of Bakery Products and Its Control by Preservatives. Int. J. Pharm. Biol. Arch. 2012, 3, 38–48. [Google Scholar]
- Debonne, E.; Vermeulen, A.; Van Bockstaele, F.; Soljic, I.; Eeckhout, M.; Devlieghere, F. Growth/No-Growth Models of in-Vitro Growth of Penicillium paneum as a Function of Thyme Essential Oil, pH, a, Temperature. Food Microbiol. 2019, 83, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.V.; Garcia-Cela, E.; Magan, N.; Copetti, M.V.; Medina, A. Comparative Growth Inhibition of Bread Spoilage Fungi by Different Preservative Concentrations Using a Rapid Turbidimetric Assay System. Front. Microbiol. 2021, 12, 678406. [Google Scholar] [CrossRef]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting Synergies of Sourdough and Antifungal Organic Acids to Delay Fungal Spoilage of Bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef]
- Debonne, E.; Van Bockstaele, F.; Van Driessche, M.; De Leyn, I.; Eeckhout, M.; Devlieghere, F. Impact of Par-Baking and Packaging on the Microbial Quality of Par-Baked Wheat and Sourdough Bread. Food Control 2018, 91, 12–19. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic Acids Production from Lactic Acid Bacteria: A Preservation Approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Konfo, T.R.C.; Djouhou, F.M.C.; Koudoro, Y.A.; Dahouenon-Ahoussi, E.; Avlessi, F.; Sohounhloue, C.K.D.; Simal-Gandara, J. Essential Oils as Natural Antioxidants for the Control of Food Preservation. Food Chem. Adv. 2023, 2, 100312. [Google Scholar] [CrossRef]
- -ur-Rehman, S.; Hussain, S.; Nawaz, H.; Mushtaq Ah, M.; Anjum Murt, M.; Jaffar Riz, A. Inhibitory Effect of Citrus Peel Essential Oils on the Microbial Growth of Bread. Pak. J. Nutr. 2007, 6, 558–561. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Muir, B.W.; Jadhav, S.R. The Antimicrobial Efficacy of Native Australian Essential Oils in Liquid and Vapour Phase against Foodborne Pathogens and Spoilage Microorganisms. Food Control 2023, 151, 109774. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef]
- Almeida, N.A.; Freire, L.; Carnielli-Queiroz, L.; Bragotto, A.P.A.; Silva, N.C.C.; Rocha, L.O. Essential Oils: An Eco-friendly Alternative for Controlling Toxigenic Fungi in Cereal Grains. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13251. [Google Scholar] [CrossRef]
- Debonne, E.; Van Bockstaele, F.; Samapundo, S.; Eeckhout, M.; Devlieghere, F. The Use of Essential Oils as Natural Antifungal Preservatives in Bread Products. J. Essent. Oil Res. 2018, 30, 309–318. [Google Scholar] [CrossRef]
- Tabarraei, H.; Hassan, J.; Parvizi, M.R.; Golshahi, H.; keshavarz-Tarikhi, H. Evaluation of the Acute and Sub-Acute Toxicity of the Black Caraway Seed Essential Oil in Wistar Rats. Toxicol. Rep. 2019, 6, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical Composition, Extraction Sources and Action Mechanisms of Essential Oils: Natural Preservative and Limitations of Use in Meat Products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xu, X.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Inhibitory Effects of Cinnamon and Clove Essential Oils on Mold Growth on Baked Foods. Food Chem. 2018, 240, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Soudani, S.; Poza-Carrión, C.; De La Cruz Gómez, N.; González-Coloma, A.; Andrés, M.F.; Berrocal-Lobo, M. Essential Oils Prime Epigenetic and Metabolomic Changes in Tomato Defense Against Fusarium Oxysporum. Front. Plant Sci. 2022, 13, 804104. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yuan, K.; Zhou, Q.; Lu, C.; Du, L.; Liu, F. Mechanism of Antifungal Activity of Perilla Frutescens Essential Oil against Aspergillus Flavus by Transcriptomic Analysis. Food Control 2021, 123, 107703. [Google Scholar] [CrossRef]
- Guynot, M.E.; Ramos, A.J.; Seto, L.; Purroy, P.; Sanchis, V.; Marin, S. Antifungal Activity of Volatile Compounds Generated by Essential Oils against Fungi Commonly Causing Deterioration of Bakery Products. J. Appl. Microbiol. 2003, 94, 893–899. [Google Scholar] [CrossRef]
- Nielsen, P.V.; Rios, R. Inhibition of Fungal Growth on Bread by Volatile Components from Spices and Herbs, and the Possible Application in Active Packaging, with Special Emphasis on Mustard Essential Oil. Int. J. Food Microbiol. 2000, 60, 219–229. [Google Scholar] [CrossRef]
- Passone, M.A.; Girardi, N.S.; Ferrand, C.A.; Etcheverry, M. Invitro Evaluation of Five Essential Oils as Botanical Fungitoxicants for the Protection of Stored Peanuts from Aspergillus Flavus and A. Parasiticus Contamination. Int. Biodeterior. Biodegrad. 2012, 70, 82–88. [Google Scholar] [CrossRef]
- Otoni, C.G.; Pontes, S.F.O.; Medeiros, E.A.A.; Soares, N.D.F.F. Edible Films from Methylcellulose and Nanoemulsions of Clove Bud (Syzygium aromaticum) and Oregano (Origanum Vulgare) Essential Oils as Shelf Life Extenders for Sliced Bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [Google Scholar] [CrossRef]
- Aljabeili, H.S.; Barakat, H.; Abdel-Rahman, H.A. Chemical Composition, Antibacterial and Antioxidant Activities of Thyme Essential Oil (Thymus vulgaris). Food Nutr. Sci. 2018, 9, 433–446. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus vulgaris L. Essential Oil as a Safe Botanical Preservative against Post Harvest Fungal Infestation of Food Commodities. Innov. Food Sci. Emerg. Technol. 2008, 9, 575–580. [Google Scholar] [CrossRef]
- Askarne, L.; Talibi, I.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Serghini, M.A.; Ait Ben Aoumar, A. In Vitro and in Vivo Antifungal Activity of Several Moroccan Plants against Penicillium Italicum, the Causal Agent of Citrus Blue Mold. Crop Prot. 2012, 40, 53–58. [Google Scholar] [CrossRef]
- Mani López, E.; Valle Vargas, G.P.; Palou, E.; López Malo, A. Penicillium Expansum Inhibition on Bread by Lemongrass Essential Oil in Vapor Phase. J. Food Prot. 2018, 81, 467–471. [Google Scholar] [CrossRef]
- Teodoro, R.A.R.; De Barros Fernandes, R.V.; Botrel, D.A.; Borges, S.V.; De Souza, A.U. Characterization of Microencapsulated Rosemary Essential Oil and Its Antimicrobial Effect on Fresh Dough. Food Bioprocess Technol. 2014, 7, 2560–2569. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Karim, A.A.; Ariffin, F. Enhancing the Functional Properties of Fish Gelatin Mats by Dual Encapsulation of Essential Oils in β-Cyclodextrins/Fish Gelatin Matrix via Coaxial Electrospinning. Food Hydrocoll. 2023, 137, 108324. [Google Scholar] [CrossRef]
- Friedlein, U.; Dorn-In, S.; Schwaiger, K. Antimicrobial Effects of Plant Extracts against Clostridium Perfringens with Respect to Food-Relevant Influencing Factors. J. Food Prot. 2021, 84, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Maurya, S.; deLampasona, M.P.; Catalan, C.A.N. A Comparison of Chemical, Antioxidant and Antimicrobial Studies of Cinnamon Leaf and Bark Volatile Oils, Oleoresins and Their Constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M. Effect of Plant Extracts against Alzheimer’s Disease. In Plant Extracts in Neurodegenerative Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–15. ISBN 978-0-323-95762-5. [Google Scholar]
- Jiménez, M.C.; Prieto, K.; Lasso, P.; Gutiérrez, M.; Rodriguez-Pardo, V.; Fiorentino, S.; Barreto, A. Plant Extract from Caesalpinia spinosa Inhibits Cancer-Associated Fibroblast-like Cells Generation and Function in a Tumor Microenvironment Model. Heliyon 2023, 9, e14148. [Google Scholar] [CrossRef] [PubMed]
- Kola, V.; Carvalho, I.S. Plant Extracts as Additives in Biodegradable Films and Coatings in Active Food Packaging. Food Biosci. 2023, 54, 102860. [Google Scholar] [CrossRef]
- Czubaszek, A.; Czaja, A.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kucharska, A.Z. Quality of Bread Enriched with Microencapsulated Anthocyanin Extracts during in Vitro Simulated Digestion. J. Cereal Sci. 2023, 113, 103724. [Google Scholar] [CrossRef]
- Maibam, B.D.; Chakraborty, S.; Nickhil, C.; Deka, S.C. Effect of Euryale Ferox Seed Shell Extract Addition on the in Vitro Starch Digestibility and Predicted Glycemic Index of Wheat-Based Bread. Int. J. Biol. Macromol. 2023, 226, 1066–1078. [Google Scholar] [CrossRef]
- Balasubramaniam, V.G.; Ramakrishnan, S.R.; Antony, U. Opportunities and Challenges of Plant Extracts in Food Industry. In Plant Extracts: Applications in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 295–315. ISBN 978-0-12-822475-5. [Google Scholar]
- Negi, P.S. Plant Extracts for the Control of Bacterial Growth: Efficacy, Stability and Safety Issues for Food Application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Bhalla, R.; Bhalla, N.; Norton, L. Bread Preservation Efficacy and Antifungal Activity of Six Wood and Leaf Essential Oils. Editors’ Remarks. Available online: https://journalhosting.ucalgary.ca/index.php/jura/issue/view/5380/71#page=17 (accessed on 22 April 2024).
- Bao, Z.; Fan, M.; Hannachi, K.; Li, T.; Zhao, J.; Li, Y.; Qian, H.; Wang, L. Antifungal Activity of Star Anise Extract against Penicillium roqueforti and Aspergillus niger for Bread Shelf Life. Food Res. Int. 2023, 172, 113225. [Google Scholar] [CrossRef] [PubMed]
- Torgbo, S.; Sukatta, U.; Kamonpatana, P.; Sukyai, P. Ohmic Heating Extraction and Characterization of Rambutan (Nephelium lappaceum L.) Peel Extract with Enhanced Antioxidant and Antifungal Activity as a Bioactive and Functional Ingredient in White Bread Preparation. Food Chem. 2022, 382, 132332. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Strategies to Extend Bread and GF Bread Shelf-Life: From Sourdough to Antimicrobial Active Packaging and Nanotechnology. Fermentation 2018, 4, 9. [Google Scholar] [CrossRef]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; Van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the Quality and Shelf Life of Wheat Bread by Fermentation with the Antifungal Strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Gerez, C.L.; Dallagnol, A.; Ponsone, L.; Chulze, S.; Font De Valdez, G. Ochratoxin A Production by Aspergillus Niger: Effect of Water Activity and a Biopreserver Formulated with Lactobacillus plantarum CRL 778. Food Control 2014, 45, 115–119. [Google Scholar] [CrossRef]
- Ryan, L.A.M.; Zannini, E.; Dal Bello, F.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus amylovorus DSM 19280 as a Novel Food-Grade Antifungal Agent for Bakery Products. Int. J. Food Microbiol. 2011, 146, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.A.M.; Dal Bello, F.; Arendt, E.K. The Use of Sourdough Fermented by Antifungal LAB to Reduce the Amount of Calcium Propionate in Bread. Int. J. Food Microbiol. 2008, 125, 274–278. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Cassone, A.; Coda, R.; Gobbetti, M. Antifungal Activity of Sourdough Fermented Wheat Germ Used as an Ingredient for Bread Making. Food Chem. 2011, 127, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Zannini, E.; Aquilanti, L.; Silvestri, G.; Fierro, O.; Picariello, G.; Clementi, F. Selection of Sourdough Lactobacilli with Antifungal Activity for Use as Biopreservatives in Bakery Products. J. Agric. Food Chem. 2012, 60, 7719–7728. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial Activity of Lactic Acid Bacteria against Pathogenic and Spoilage Microorganism Isolated from Food and Their Control in Wheat Bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Thierry, A.; Lemaître, M.; Garric, G.; Harel-Oger, M.; Chatel, M.; Lê, S.; Mounier, J.; Valence, F.; Coton, E. Antifungal Activity of Lactic Acid Bacteria Combinations in Dairy Mimicking Models and Their Potential as Bioprotective Cultures in Pilot Scale Applications. Front. Microbiol. 2018, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- Ouiddir, M.; Bettache, G.; Leyva Salas, M.; Pawtowski, A.; Donot, C.; Brahimi, S.; Mabrouk, K.; Coton, E.; Mounier, J. Selection of Algerian Lactic Acid Bacteria for Use as Antifungal Bioprotective Cultures and Application in Dairy and Bakery Products. Food Microbiol. 2019, 82, 160–170. [Google Scholar] [CrossRef]
- Iosca, G.; Turetta, M.; De Vero, L.; Bang-Berthelsen, C.H.; Gullo, M.; Pulvirenti, A. Valorization of Wheat Bread Waste and Cheese Whey through Cultivation of Lactic Acid Bacteria for Bio-Preservation of Bakery Products. LWT 2023, 176, 114524. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lavecchia, A.; Gramaglia, V.; Gobbetti, M. Long-Term Fungal Inhibition by Pisum Sativum Flour Hydrolysate during Storage of Wheat Flour Bread. Appl. Environ. Microbiol. 2015, 81, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Fundamental Study on the Improvement of the Antifungal Activity of Lactobacillus reuteri R29 through Increased Production of Phenyllactic Acid and Reuterin. Food Control 2018, 88, 139–148. [Google Scholar] [CrossRef]
- El Houssni, I.; Khedid, K.; Zahidi, A.; Hassikou, R. The Inhibitory Effects of Lactic Acid Bacteria Isolated from Sourdough on the Mycotoxigenic Fungi Growth and Mycotoxins from Wheat Bread. Biocatal. Agric. Biotechnol. 2023, 50, 102702. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Mounier, J.; Maillard, M.-B.; Valence, F.; Coton, E.; Thierry, A. Identification and Quantification of Natural Compounds Produced by Antifungal Bioprotective Cultures in Dairy Products. Food Chem. 2019, 301, 125260. [Google Scholar] [CrossRef]
- Illueca, F.; Moreno, A.; Calpe, J.; Nazareth, T.D.M.; Dopazo, V.; Meca, G.; Quiles, J.M.; Luz, C. Bread Biopreservation through the Addition of Lactic Acid Bacteria in Sourdough. Foods 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Day, L. Cereal Food Production with Low Salt. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Netherlands, 2016; pp. 396–402. ISBN 978-0-12-394786-4. [Google Scholar]
- Sun, Q.; Gong, M.; Li, Y.; Xiong, L. Effect of Dry Heat Treatment on the Physicochemical Properties and Structure of Proso Millet Flour and Starch. Carbohydr. Polym. 2014, 110, 128–134. [Google Scholar] [CrossRef]
- Neill, G.; Al-Muhtaseb, A.H.; Magee, T.R.A. Optimisation of Time/Temperature Treatment, for Heat Treated Soft Wheat Flour. J. Food Eng. 2012, 113, 422–426. [Google Scholar] [CrossRef]
- Saka, I.; Topcam, H.; Son, E.; Ozkaya, B.; Erdogdu, F. Effect of Radio Frequency Processing on Physical, Chemical, Rheological and Bread-Baking Properties of White and Whole Wheat Flour. LWT 2021, 147, 111563. [Google Scholar] [CrossRef]
- Starek-Wójcicka, A.; Różyło, R.; Niedźwiedź, I.; Kwiatkowski, M.; Terebun, P.; Polak-Berecka, M.; Pawłat, J. Pilot Study on the Use of Cold Atmospheric Plasma for Preservation of Bread. Sci. Rep. 2022, 12, 22003. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.G.; Stefanello, A.; Garcia, M.V.; Furian, A.F.; Cichoski, A.J.; Copetti, M.V. Potential of Electrolyzed Water to Inactivate Bread and Cheese Spoilage Fungi. Food Res. Int. 2022, 162, 111931. [Google Scholar] [CrossRef] [PubMed]
- Bölek, S.; Tosya, F.; Dinç, Ö. Effects of Different Types of Electrolyzed Waters on Rheological Characteristics of Dough and Quality Properties of Bread. Food Sci. Technol. Int. 2023, 108201322311702. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A. Bread Packaging: Features and Functions. In Flour and Breads and Their Fortification in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–222. ISBN 978-0-12-814639-2. [Google Scholar]
- Jiang, Q.; Zhao, W.; Zhao, S.; Wang, P.; Wang, Y.; Zhao, Y.; Zhao, X.; Wang, D. Comparison between Vacuum and Modified-Atmosphere Packaging on Dynamic Analysis of Flavor Properties and Microbial Communities in Fresh-Cut Potatoes (Solanum tuberosum L.). Food Packag. Shelf Life 2023, 39, 101149. [Google Scholar] [CrossRef]
- Hou, X.; Zhao, H.; Yan, L.; Li, S.; Chen, X.; Fan, J. Effect of CO2 on the Preservation Effectiveness of Chilled Fresh Boneless Beef Knuckle in Modified Atmosphere Packaging and Microbial Diversity Analysis. LWT 2023, 187, 115262. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, Y.; Cai, C.; Lin, W.; Li, L.; Shen, W. Hydrogen-Based Modified Atmosphere Packaging Delays the Deterioration of Dried Shrimp (Fenneropenaeus chinensis) during Accelerated Storage. Food Control 2023, 152, 109897. [Google Scholar] [CrossRef]
- Giannone, V.; Pitino, I.; Pecorino, B.; Todaro, A.; Spina, A.; Lauro, M.R.; Tomaselli, F.; Restuccia, C. Effects of Innovative and Conventional Sanitizing Treatments on the Reduction of Saccharomycopsis fibuligera Defects on Industrial Durum Wheat Bread. Int. J. Food Microbiol. 2016, 235, 71–76. [Google Scholar] [CrossRef]
- Fik, M.; Surówka, K.; Maciejaszek, I.; Macura, M.; Michalczyk, M. Quality and Shelf Life of Calcium-Enriched Wholemeal Bread Stored in a Modified Atmosphere. J. Cereal Sci. 2012, 56, 418–424. [Google Scholar] [CrossRef]
- Fernandez, U.; Vodovotz, Y.; Courtney, P.; Pascall, M.A. Extended Shelf Life of Soy Bread Using Modified Atmosphere Packaging. J. Food Prot. 2006, 69, 693–698. [Google Scholar] [CrossRef]
- Selvam, P.; Subramaniyan, V.; Subramanian, S.; Sathiavelu, M. Bread Packaging Techniques and Trends. Ital. J. Food Saf. 2022, 11, 10771. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H.; Lorenzo, J.M.; Mousavi Khaneghah, A.; Barba, F.J. Essential Oils as Natural Preservatives for Bakery Products: Understanding the Mechanisms of Action, Recent Findings, and Applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 310–321. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Ajji, A. Moisture Absorbers for Food Packaging Applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Upasen, S.; Wattanachai, P. Packaging to Prolong Shelf Life of Preservative-Free White Bread. Heliyon 2018, 4, e00802. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A Review of Active Packaging in Bakery Products: Applications and Future Trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Thirupathi Vasuki, M.; Kadirvel, V.; Pejavara Narayana, G. Smart Packaging—An Overview of Concepts and Applications in Various Food Industries. Food Bioeng. 2023, 2, 25–41. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Wong, D.; Roman, M.; Lin, Z.; Goddard, J. Active Packaging Coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef]
- Viscusi, G.; Bugatti, V.; Vittoria, V.; Gorrasi, G. Antimicrobial Sorbate Anchored to Layered Double Hydroxide (LDH) Nano-Carrier Employed as Active Coating on Polypropylene (PP) Packaging: Application to Bread Stored at Ambient Temperature. Future Foods 2021, 4, 100063. [Google Scholar] [CrossRef]
- Mittal, M.; Ahuja, S.; Yadav, A.; Aggarwal, N.K. Development of Poly(Hydroxybutyrate) Film Incorporated with Nano Silica and Clove Essential Oil Intended for Active Packaging of Brown Bread. Int. J. Biol. Macromol. 2023, 233, 123512. [Google Scholar] [CrossRef]
- Moe, N.C.; Basbasan, A.J.; Winotapun, C.; Hararak, B.; Wanmolee, W.; Suwanamornlert, P.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Application of Lignin Nanoparticles in Polybutylene Succinate Based Antifungal Packaging for Extending the Shelf Life of Bread. Food Packag. Shelf Life 2023, 39, 101127. [Google Scholar] [CrossRef]
- Basbasan, A.J.; Hararak, B.; Winotapun, C.; Wanmolee, W.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Emerging Challenges on Viability and Commercialization of Lignin in Biobased Polymers for Food Packaging: A Review. Food Packag. Shelf Life 2022, 34, 100969. [Google Scholar] [CrossRef]
- Esakkimuthu, E.S.; DeVallance, D.; Pylypchuk, I.; Moreno, A.; Sipponen, M.H. Multifunctional Lignin-Poly (Lactic Acid) Biocomposites for Packaging Applications. Front. Bioeng. Biotechnol. 2022, 10, 1025076. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of Biodegradable Materials in Food Packaging: A Review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Handayani, W.; Anggraeni, D.; Susetyo, I.; Soraya, M.; Triputranto, A.; Hadipernata, M. Development of Palm Oil Derivative Based Coating to Extend the Gedong Gincu Mangoes Freshness. In Proceedings of the 4th International Conference on Social Science, Humanity and Public Health, ICoSHIP 2023, Surabaya, East Java, Indonesia, 18–19 November 2023; EAI: Surabaya, Indonesia, 2024. [Google Scholar]
- Mohammadi, M.; Zoghi, A.; Azizi, M.H. Assessment of Properties of Gluten-based Edible Film Formulated with Beeswax and DATEM for Hamburger Bread Coating. Food Sci. Nutr. 2023, 11, 2061–2068. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sharma, N.; Sharma, V.; Alam, T.; Sahu, J.K.; Hamid, H. Assessing the Consumer Acceptance and Storability of Chitosan and Beeswax Coated Cellulose Packaging for Whole Wheat Bread. Food Control 2022, 133, 108682. [Google Scholar] [CrossRef]

| Starter | Mo | Before Spray-Drying (Log CFU/g) | After Spray-Drying (Log CFU/g) | Survival Rate (%) | Reference |
|---|---|---|---|---|---|
| Kombucha sourdough type I | LAB | 11.00 ± 0.05 | 9.93 ± 0.10 | 90.27 | [60] |
| Yeast | 10.50 ± 0.46 | 9.40 ± 0.15 | 89.52 | ||
| Sourdough type I | LAB | 8.7 ± 0.0 | 5.0 ± 0.0 | 57.47 | [61] |
| Yeast | 8.6 ± 0.0 | 4.9 ± 0.1 | 56.97 | ||
| Sourdough type I | LAB | 9.17 ± 0.17 | 7.9 ± 0.1 | 86.15 | [56] |
| Yeast | 7.53 ± 0.12 | 5.7 | 75.69 |
| Essential Oils | Major Compounds | Targeted Molds | Action | Reference |
|---|---|---|---|---|
| Clove (Syzygium aromaticum L.) | Eugenol, acetyleugenol, caryophyllene, gallic acid, kaempferol, quercetin, tannins | Aspergillus flavus, A. niger, Aspergillus parasiticus, Eurotium amstelodami, Eurotium herbariorum, Eurotium repens, Eurotium rubrum, Penicillium corylophilum, Penicillium commune, P. roqueforti, Penicillium citrinum, Endomyces fibuliger, Rhizopus nigricans, Penicillium sp. | Reduced yeast and mold growth | [139,140,141,142] |
| Thyme (Thymus vugaris L.) | Thymol, carvacrol, linalool, p-Cymene, camphene, myrcene, caryophyllene, rosmarinic acid | A. flavus, A. niger, Aspergillus terreus, Alternaria alternata, E. amstelodami, E. herbariorum, E. repens, E. rubrum, Fusarium oxysporum, P. corylophilum, Penicillium italicum, P. paneum | Bread shelf life | [121,133,139,143,144,145] |
| Lemongrass (Cymbopogan citratus) | Citral, geraniol, limonen, neral, nerol, myrcene, citronellal | A. flavus, A. niger, E. amstelodami, E. Herbariorum, E. repens, E. rubrum, P. corylophilum, Penicillium expansum | Mold growth inhibited | [139,146] |
| Rosemary (Rosemary officinalis) | Carnosic acid, carnosol, rosmarinic acid, hesperidin | Penicillium sp. Aspergillus sp. | Fungal generation reduced | [133,147] |
| Oregano (Origanum vulgare L.) | Carvacrol, thymol, rosmarinic acid, p-Cymene, terpinene, linalool, naringin, β-caryophyllene | A. flavus, A. niger, Aspergillus fumigatus, Aspergillus ochraceus, A. parasiticus, A. terreus, Eurotium fibuliger, P. commune, P. roqueforti | Mold growth inhibited | [140] |
| Cinnamon (Cinnamomum jersenianum Hand.-Mazz) | Cinnamaldehyde, eugenol, cinnamyl acetate, coumarin, proanthocyanidins | A. flavus, A. niger, A. ochraceus, A. terreus, E. fibuliger, E. amstelodami, E. Herbariorum, E. repens, E. rubrum, P. corylophilum, P. citrinum; P. commune, Penicillium viridicatum, P. roqueforti | Reduction of the targeted mold growth | [139,140] |
| Antifungal Lactic Acid Bacteria | Microorganisms | Reference |
|---|---|---|
| L. plantarum FST 1.7 | Fusarium culmorum and Fusarium graminearum | [162] |
| L. plantarum CRL 778, Lactobacillus reuteri CRL 1100, L. brevis CRL 772 and CRL 796 | Aspergillus, Fusarium, and Penicillium species | [163] |
| Lactobacillus amylovorus DSM 19280 | A. niger FST4.21, P. expansum FST 4.22, P. roqueforti FST 4.11, F. culmorum FST 4.05 | [164] |
| L. plantarum | A. niger FST4.21, F. culmorum TMW 4.0754, P. expansum LTH S46 | [165] |
| L. plantarum LB1, F. rossiae LB5 | P. roqueforti DPPMAF1 | [166] |
| F. rossiae LD108, Companilactobacillus paralimentarius PB12 (formerly Lactobacillus paralimentarius) | Aspergillus japonicus, E. repens and Penicillium roseopurpureum | [167] |
| Latilactobacillus sakei (formerly Lactobacillus sakei) KTU05-6, P. acidilactici KTU05-7, P. pentosaceus KTU05-8, P. pentosaceus KTU05-9, P. pentosaceus KTU05-10 | Molds | [168] |
| L. amylovorus DSM19280 | Molds | [119] |
| L. plantarum L244 with Schleiferilactobacillus harbinensis L172 (formerly Lactobacillus harbinensis) | P. commune, Mucor racemosus, and R. mucilaginosa | [169] |
| L. plantarum CH1, L. paracasei B20, L. mesenteroides L1 | M. racemosus UBOCC-A-109155, P. commune UBOCC-A-116003, Yarrowia lipolytica UBOCC-A-216006, Aspergillus tubingensis AN, A. flavus T5, Paecilomyces formosus AT | [170] |
| L. plantarum UMCC 2996, F. rossiae UMCC 3002, P. pentosaceus UMCC 3010 | A. flavus ITEM 7828, P. paneum ITEM 1381, A. niger ITEM 7090 | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermelho, A.B.; Moreira, J.V.; Junior, A.N.; da Silva, C.R.; Cardoso, V.d.S.; Akamine, I.T. Microbial Preservation and Contamination Control in the Baking Industry. Fermentation 2024, 10, 231. https://doi.org/10.3390/fermentation10050231
Vermelho AB, Moreira JV, Junior AN, da Silva CR, Cardoso VdS, Akamine IT. Microbial Preservation and Contamination Control in the Baking Industry. Fermentation. 2024; 10(5):231. https://doi.org/10.3390/fermentation10050231
Chicago/Turabian StyleVermelho, Alane Beatriz, Jean Vinícius Moreira, Athayde Neves Junior, Claudia Ramos da Silva, Veronica da Silva Cardoso, and Ingrid Teixeira Akamine. 2024. "Microbial Preservation and Contamination Control in the Baking Industry" Fermentation 10, no. 5: 231. https://doi.org/10.3390/fermentation10050231
APA StyleVermelho, A. B., Moreira, J. V., Junior, A. N., da Silva, C. R., Cardoso, V. d. S., & Akamine, I. T. (2024). Microbial Preservation and Contamination Control in the Baking Industry. Fermentation, 10(5), 231. https://doi.org/10.3390/fermentation10050231








