The Impact of Microbial Activity on the Chemical Composition and Aroma Profile of Traditional Sparkling Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Commercial Sparkling Wines
2.2. Chemical Analyses
2.3. Aroma Analysis via GC-MS
2.4. Statistical Analysis
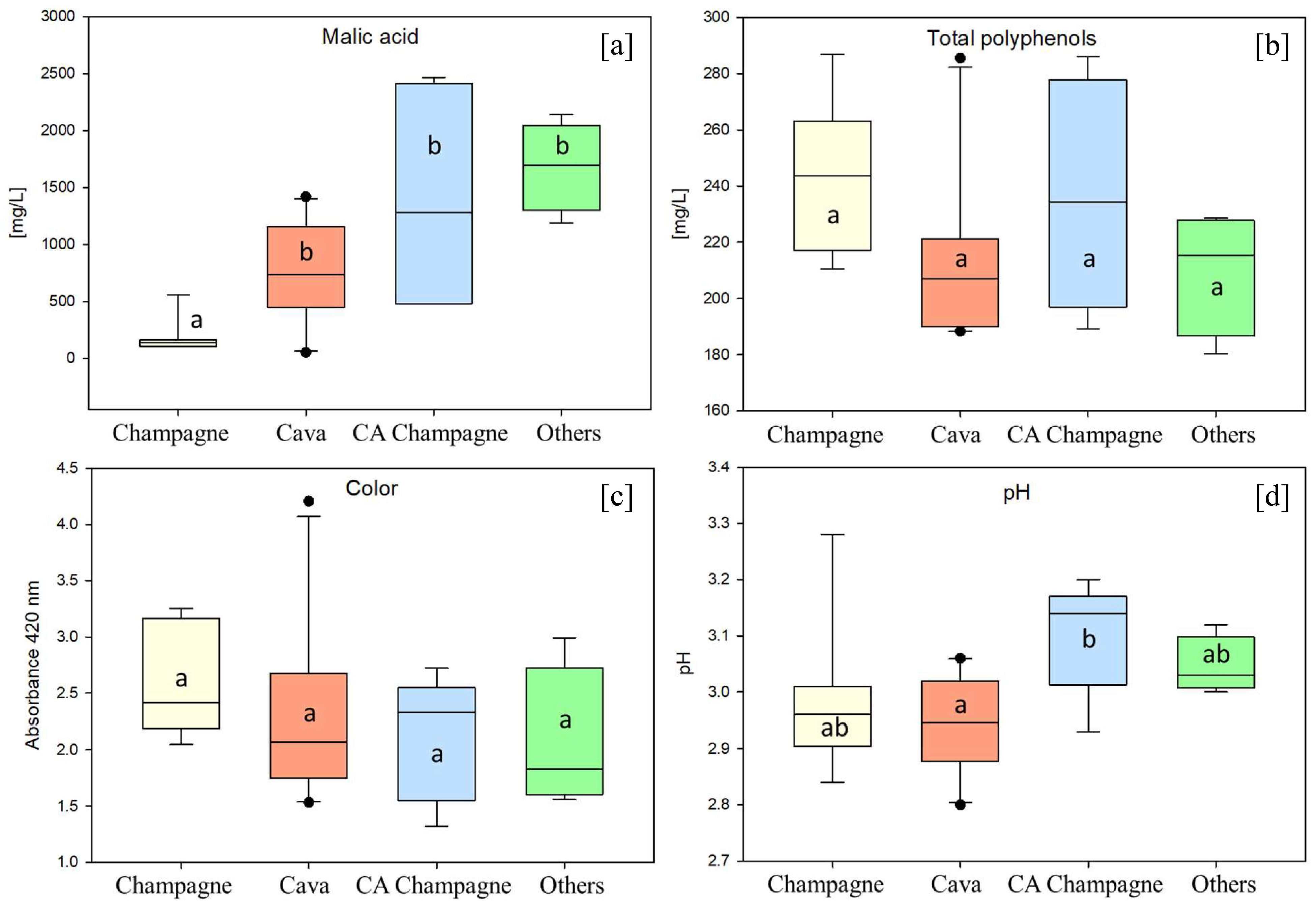
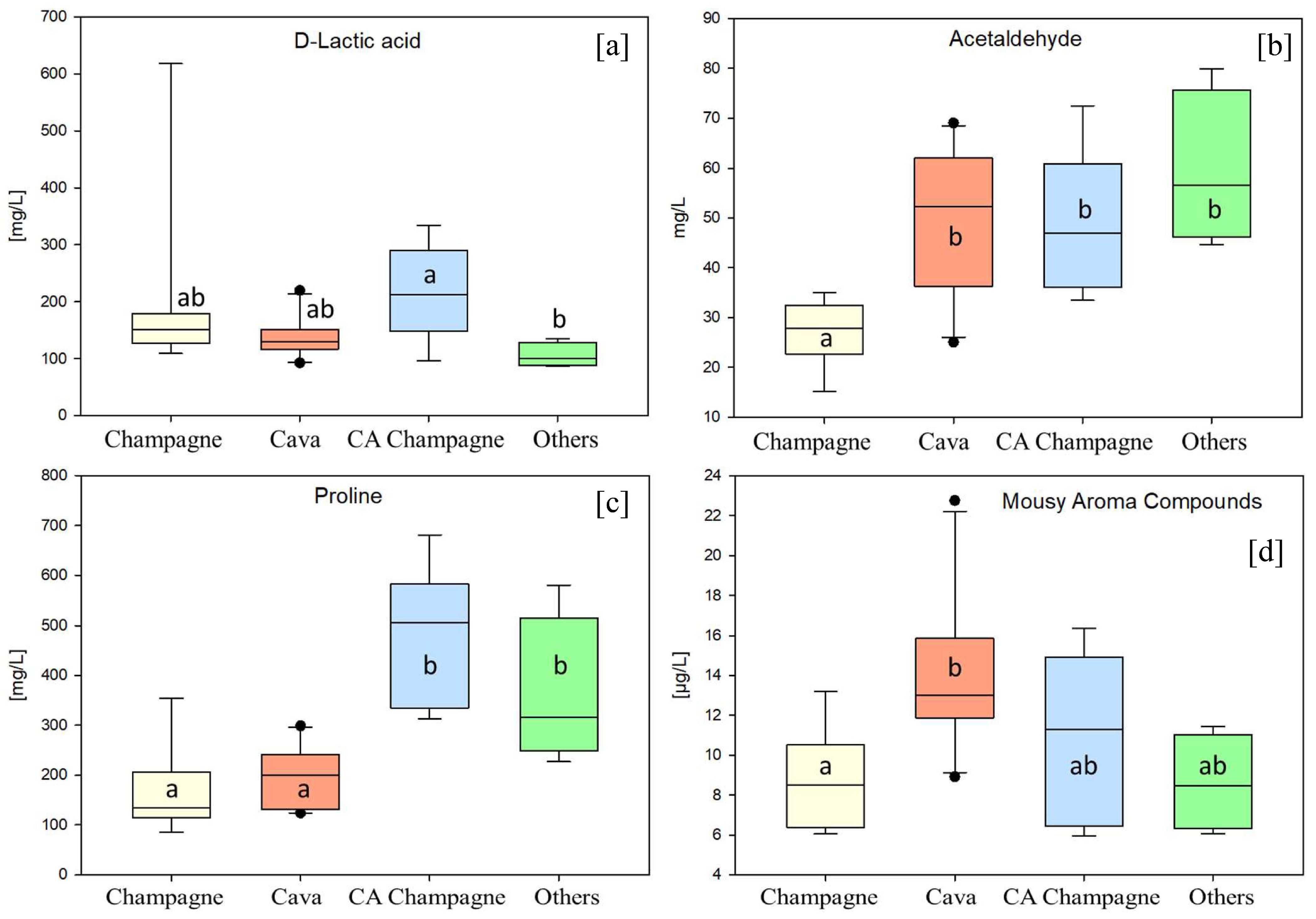
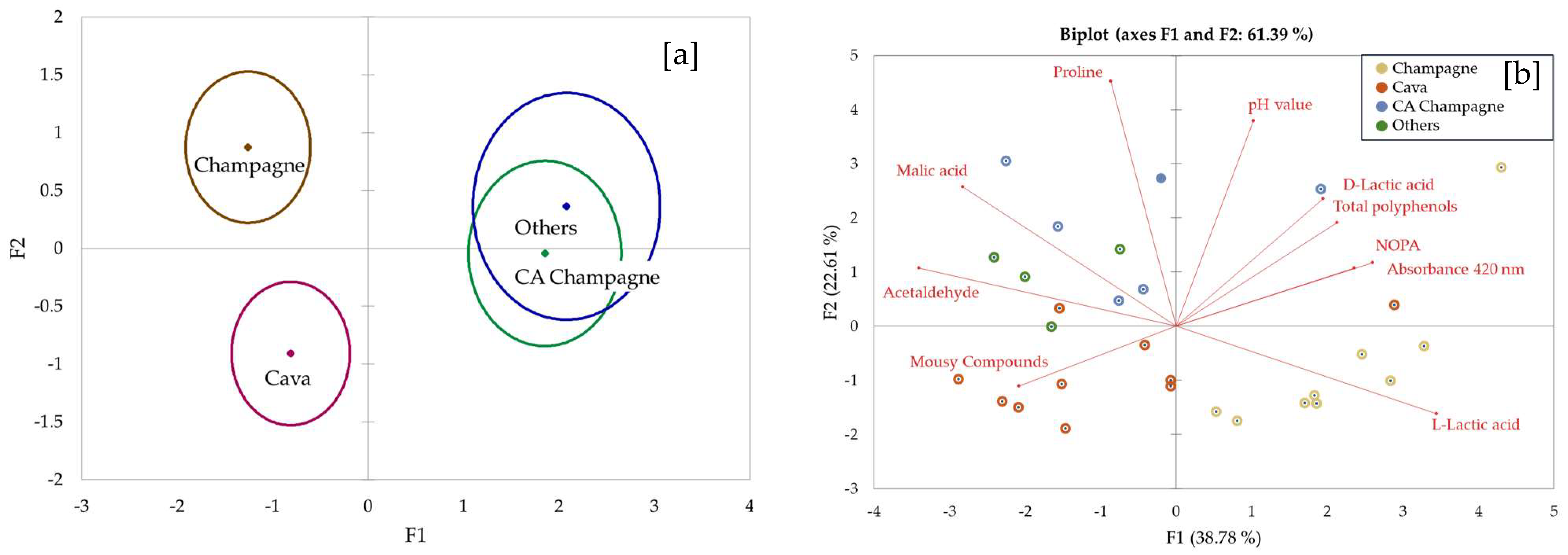
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caliari, V.; Panceri, C.P.; Rosier, J.P.; Bordignon-Luiz, M.T. Effect of the Traditional, Charmat and Asti method production on the volatile composition of Moscato Giallo sparkling wines. LWT-Food Sci. Technol. 2015, 61, 393–400. [Google Scholar] [CrossRef]
- Culbert, J.A.; McRae, J.M.; Condé, B.C.; Schmidtke, L.M.; Nicholson, E.L.; Smith, P.A.; Howell, K.S.; Boss, P.K.; Wilkinson, K.L. Influence of production method on the chemical composition, foaming properties, and quality of Australian carbonated and sparkling white wines. J. Agric. Food Chem. 2017, 65, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Todd, B. Microbiological and Chemical Changes during the Fermentation and Ageing of Sparkling Wines; UNSW Sydney: Kensington, Australia, 1995. [Google Scholar]
- Ubeda, C.; Kania-Zelada, I.; del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, Á. Study of the changes in volatile compounds, aroma and sensory attributes during the production process of sparkling wine by traditional method. Food Res. Int. 2019, 119, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Gnoinski, G.B.; Schmidt, S.A.; Close, D.C.; Goemann, K.; Pinfold, T.L.; Kerslake, F.L. Novel Methods to Manipulate Autolysis in Sparkling Wine: Effects on Yeast. Molecules 2021, 26, 387. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, R.; Mauricio, J.C.; García-Martínez, T.; Peinado, R.A.; Moreno, J. Towards a better understanding of the evolution of odour-active compounds and the aroma perception of sparkling wines during ageing. Food Chem. 2021, 357, 129784. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Guilloux-Benatier, M. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Marcon, Â.R.; Carnieli, G.J.; Vanderlinde, R. Effect of glutathione addition in sparkling wine. Food Chem. 2014, 159, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.; Longo, R.; Solomon, M.; Nicolotti, L.; Westmore, H.; Merry, A.; Gnoinski, G.; Ylia, A.; Dambergs, R.; Kerslake, F. Autolysis and the duration of ageing on lees independently influence the aroma composition of traditional method sparkling wine. Aust. J. Grape Wine Res. 2022, 28, 146–159. [Google Scholar] [CrossRef]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter cultures for sparkling wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Cappello, M.S.; Zapparoli, G.; Logrieco, A.; Bartowsky, E.J. Linking wine lactic acid bacteria diversity with wine aroma and flavour. Int. J. Food Microbiol. 2017, 243, 16–27. [Google Scholar] [CrossRef]
- Davis, C.R.; Wibowo, D.; Fleet, G.H.; Lee, T.H. Properties of Wine Lactic Acid Bacteria: Their Potential Enological Significance. Am. J. Enol. Vitic. 1988, 39, 137–142. [Google Scholar] [CrossRef]
- Elliott, R.J.; Gardner, D.L. Proline determination with isatin, in the presence of amino acids. Anal. Biochem. 1976, 70, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, E. Schaumwein. In Handbuch für die Getränkeindustrie: Ein kaufmännisches Lehr- und Informationswerk für die Getränkewirtschaft; Gabler Verlag: Wiesbaden, Germany, 1968; pp. 80–90. [Google Scholar]
- Ruffner, H.P.; Possner, D.; Brem, S.; Rast, D.M. The physiological role of malic enzyme in grape ripening. Planta 1984, 160, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Fugelsang, K.C. The Lactic Acid Bacteria. In Wine Microbiology; Springer US: Boston, MA, USA, 1997; pp. 3–47. [Google Scholar]
- Durner, D.; Weber, F.; Neddermeyer, J.; Koopmann, K.; Winterhalter, P.; Fischer, U. Sensory and Color Changes Induced by Microoxygenation Treatments of Pinot noir before and after Malolactic Fermentation. Am. J. Enol. Vitic. 2010, 61, 474–485. [Google Scholar] [CrossRef]
- Long, D.; Wilkinson, K.L.; Taylor, D.K.; Jiranek, V. Novel wine yeast for improved utilisation of proline during fermentation. Fermentation 2018, 4, 10. [Google Scholar] [CrossRef]
- Sommer, S.; Wegmann-Herr, P.; Wacker, M.; Fischer, U. Rationale for a stronger disposition of Chardonnay wines for stuck and sluggish fermentation. S. Afr. J. Enol. Vitic. 2015, 36, 180–190. [Google Scholar] [CrossRef]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Presa-Owens, C.D.L.; Lamuela-Raventos, R.M.; Buxaderas, S.; Torre-Boronat, M.C.D.L. Differentiation and Grouping Characteristics of Varietal Grape Musts from Penedès Region (I). Am. J. Enol. Vitic. 1995, 46, 283–291. [Google Scholar] [CrossRef]
- Di Martino, C.; Testa, B.; Letizia, F.; Iorizzo, M.; Lombardi, S.J.; Ianiro, M.; Di Renzo, M.; Strollo, D.; Coppola, R. Effect of exogenous proline on the ethanolic tolerance and malolactic performance of Oenococcus oeni. J. Food Sci. Technol. 2020, 57, 3973–3979. [Google Scholar] [CrossRef]
- Yoshihashi, T.; Huong, N.T.T.; Inatomi, H. Precursors of 2-Acetyl-1-pyrroline, a Potent Flavor Compound of an Aromatic Rice Variety. J. Agric. Food Chem. 2002, 50, 2001–2004. [Google Scholar] [CrossRef]
- Wu, M.-L.; Chou, K.-L.; Wu, C.-R.; Chen, J.-K.; Huang, T.-C. Characterization and the Possible Formation Mechanism of 2-Acetyl-1-Pyrroline in Aromatic Vegetable Soybean (Glycine max L.). J. Food Sci. 2009, 74, S192–S197. [Google Scholar] [CrossRef] [PubMed]
- Herderich, M.; Costello, P.J.; Grbin, P.R.; Henschke, P.A. Occurrence of 2-acetyl-1-pyrroline in mousy wines. Nat. Prod. Lett. 1995, 7, 129–132. [Google Scholar] [CrossRef]
- Cho, I.H.; Peterson, D.G. Chemistry of bread aroma: A review. Food Sci. Biotechnol. 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.-C.; de Revel, G. Characterization of Fruity Aroma Modifications in Red Wines during Malolactic Fermentation. J. Agric. Food Chem. 2012, 60, 12371–12383. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Higher alcohols concentration and its relation with the biological aging evolution. Eur. Food Res. Technol. 2006, 222, 629–635. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Radler, F. Microbial biochemistry. Experientia 1986, 42, 884–893. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Snowdon, E.M.; Bowyer, M.C.; Grbin, P.R.; Bowyer, P.K. Mousy Off-Flavor: A Review. J. Agric. Food Chem. 2006, 54, 6465–6474. [Google Scholar] [CrossRef]
- Arias-Pérez, I.; Sáenz-Navajas, M.P.; de-la-Fuente-Blanco, A.; Ferreira, V.; Escudero, A. Insights on the role of acetaldehyde and other aldehydes in the odour and tactile nasal perception of red wine. Food Chem. 2021, 361, 130081. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape Contribution to Wine Aroma: Production of Hexyl Acetate, Octyl Acetate, and Benzyl Acetate during Yeast Fermentation Is Dependent upon Precursors in the Must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Chávez-Márquez, A.; Gardea, A.A.; González-Rios, H.; Vazquez-Moreno, L. Characterization of Cabernet Sauvignon Wines by Untargeted HS-SPME GC-QTOF-MS. Molecules 2022, 27, 1726. [Google Scholar] [CrossRef] [PubMed]
- Dachery, B.; Hernandes, K.C.; Zini, C.A.; Welke, J.E.; Manfroi, V. Volatile and sensory profile of sparkling wines produced by faster and alternative methods (Ancestral and Single Tank Fermentation) compared to the usual methods (Charmat and Traditional). Eur. Food Res. Technol. 2023, 249, 2363–2376. [Google Scholar] [CrossRef]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory Threshold of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) and Concentrations in Young Riesling and Non-Riesling Wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Schieberle, P. New and Convenient Synthesis of the Important Roasty, Popcorn-like Smelling Food Aroma Compounds 2-Acetyl-1-pyrroline and 2-Acetyltetrahydropyridine from their Corresponding Cyclic α-Amino Acids. J. Agric. Food Chem. 1998, 46, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Which Impact for β-Damascenone on Red Wines Aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Pedastsaar, P.; Vaher, M.; Helmja, K.; Kulp, M.; Kaljurand, M.; Karp, K.; Raal, A.; Karathanos, V.; Püssa, T. Chemical composition of red wines made from hybrid grape and common grape (Vitis vinifera L.) cultivars. Proc. Est. Acad. Sci. 2014, 63, 444. [Google Scholar] [CrossRef]
- Homich, L.J.; Scheinberg, J.A.; Elias, R.J.; Gardner, D.M. Effects of co-inoculation on wine-quality attributes of the high-acid, red hybrid variety chambourcin. Am. J. Enol. Vitic. 2016, 67, 245–250. [Google Scholar] [CrossRef]
- Cadwallader, K.R.; Lopez, J.R.; Menke, S.D.; Surakarnkul, R. Aroma Components of Wines from Chardonel: A French—American Hybrid Grape. In Nutraceutical Beverages; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2003; Volume 871, pp. 365–378. [Google Scholar]
- Boulton, R. The relationships between total acidity, titratable acidity and pH in grape tissue. Vitis 1980, 19, 113–120. [Google Scholar]
| Sample ID | 2-Methyl-1-Butanol | Ethyl Butanoate | Ethyl Lactate | Furfural | Ethyl Isovalerate | Isoamyl Acetate | 2-Ethyltetrahydro Pyridine | ||
|---|---|---|---|---|---|---|---|---|---|
| #1 | 58 ± 23.7 | 31 ± 7.6 | 140 ± 43.9 | 14 ± 4.0 | 6 ± 0.9 | 11 ± 1.4 | 1 ± 0.1 | ||
| #2 | 69 ± 28.7 | 41 ± 8.9 | 98 ± 45.1 | 7 ± 0.6 | 6 ± 0.9 | 15 ± 1.2 | 1 ± 1.2 | ||
| #3 | 92 ± 47.1 | 23 ± 1.8 | 6 ± 1.6 | 9 ± 2.2 | 6 ± 0.4 | 34 ± 4.8 | 1 ± 0.2 | ||
| #4 | 63 ± 7.5 | 28 ± 11.2 | 6 ± 1.3 | 16 ± 0.0 | nd | 45 ± 13.5 | 1 ± 0.1 | ||
| #5 | 55 ± 16.3 | 18 ± 1.2 | 62 ± 5.1 | 7 ± 0.1 | nd | 60 ± 5.7 | 1 ± 0.0 | ||
| #6 | 63 ± 21.5 | 22 ± 0.2 | 8 ± 2.5 | 4 ± 1.6 | nd | 68 ± 3.8 | 1 ± 0.2 | ||
| #7 | 59 ± 7.1 | 17 ± 4.0 | 12 ± 1.6 | 12 ± 4.4 | nd | 41 ± 10.8 | 1 ± 0.1 | ||
| #8 | 58 ± 6.3 | 26 ± 5.9 | 104 ± 4.9 | 26 ± 1.6 | 6 ± 0.9 | 8 ± 1.3 | 1 ± 0.1 | ||
| #9 | 62 ± 15.8 | 24 ± 5.4 | 48 ± 58.0 | 11 ± 4.2 | 4 ± 1.6 | 13 ± 2.6 | nd | ||
| #10 | 52 ± 1.0 | 20 ± 4.1 | 51 ± 66.5 | 33 ± 1.2 | 6 ± 0.7 | 11 ± 2.4 | 1 ± 0.3 | ||
| #11 | 75 ± 33.5 | 30 ± 2.4 | 141 ± 60.4 | 22 ± 9.6 | 7 ± 0.3 | 15 ± 1.8 | 1 ± 0.3 | ||
| #12 | 72 ± 27.6 | 24 ± 3.9 | nd | 15 ± 8.9 | nd | 156 ± 24.5 | 2 ± 1.2 | ||
| #13 | 49 ± 11.7 | 19 ± 4.6 | nd | 11 ± 6.8 | nd | 44 ± 14.2 | 2 ± 1.6 | ||
| #14 | 70 ± 7.3 | 24 ± 0.7 | 11 ± 0.9 | 50 ± 4.4 | nd | 7 ± 2.2 | 1 ± 0.2 | ||
| #15 | 65 ± 16.2 | 28 ± 6.0 | 11 ± 1.7 | 43 ± 10.9 | 7 ± 0.7 | 11 ± 1.9 | 1 ± 0.1 | ||
| #16 | 70 ± 20.0 | 30 ± 10.9 | 44 ± 10.4 | 29 ± 5.2 | 8 ± 1.8 | 10 ± 0.6 | 2 ± 0.0 | ||
| #17 | 53 ± 13.1 | 24 ± 7.7 | 16 ± 1.4 | nd | nd | 194 ± 45.2 | 3 ± 0.5 | ||
| #18 | 62 ± 0.9 | 27 ± 7.9 | 16 ± 1.7 | nd | nd | 177 ± 18.7 | 3 ± 0.3 | ||
| #19 | 65 ± 13.7 | 25 ± 3.9 | nd | 18 ± 2.7 | 6 ± 1.3 | 18 ± 2.9 | 1 ± 0.1 | ||
| #20 | 62 ± 11.9 | 28 ± 7.7 | 24 ± 2.7 | 41 ± 16.3 | 8 ± 1.9 | 8 ± 1.8 | 1 ± 0.0 | ||
| #21 | 66 ± 17.3 | 23 ± 7.8 | 121 ± 10.0 | 90 ± 4.1 | 8 ± 2.8 | 11 ± 2.9 | 2 ± 0.3 | ||
| #22 | 72 ± 27.5 | 25 ± 6.5 | 115 ± 7.9 | 74 ± 0.9 | 7 ± 2.4 | 9 ± 2.2 | 1 ± 0.0 | ||
| #23 | 65 ± 10.5 | 31 ± 6.1 | 34 ± 37.9 | 29 ± 9.2 | nd | 10 ± 1.9 | 1 ± 0.3 | ||
| #24 | 84 ± 23.2 | 35 ± 12.2 | 150 ± 0.2 | 17 ± 0.8 | 6 ± 1.0 | 9 ± 2.7 | 1 ± 0.3 | ||
| #25 | 72 ± 9.2 | 27 ± 5.4 | 102 ± 25.8 | 57 ± 11.3 | 5 ± 1.0 | 10 ± 2.0 | 1 ± 0.2 | ||
| #26 | 106 ± 1.0 | 23 ± 4.7 | 86 ± 20.1 | 136 ± 12.3 | 10 ± 3.2 | 20 ± 6.0 | 1 ± 0.7 | ||
| #27 | 129 ± 52.3 | 37 ± 14.8 | nd | nd | 10 ± 3.3 | 12 ± 3.3 | 2 ± 0.1 | ||
| #28 | 89 ± 1.9 | 33 ± 7.9 | 58 ± 6.7 | 23 ± 15.9 | nd | 21 ± 5.6 | 1 ± 0.1 | ||
| #29 | 128 ± 78.5 | 29 ± 0.9 | 4 ± 5.2 | 25 ± 5.3 | nd | 10 ± 0.3 | 3 ± 0.8 | ||
| Champagne | 66 ± 10.2 a | 27 ± 4.5 ab | 108 ± 37.1 a | 38 ± 28.6 a | 6 ± 1.4 a | 11 ± 2.2 a | 1 ± 0.4 a | ||
| Cava | 85 ± 26.5 a | 30 ± 5.5 b | 37 ± 34.4 b | 38 ± 37.5 ab | 5 ± 4.4 a | 12 ± 4.8 a | 1 ± 0.7 a | ||
| CA Champagne | 59 ± 8.3 a | 23 ± 4.2 a | 17 ± 23.4 b | 8 ± 7.0 b | nd | 113 ± 70.3 b | 2 ± 1.0 a | ||
| Others | 70 ± 15.2 a | 22 ± 3.2 a | 7 ± 5.0 b | 11 ± 5.9 ab | 3 ± 3.5 a | 40 ± 20.7 ab | 1 ± 0.2 a | ||
| Sample ID | Benzaldehyde | 2-Acetyl-1-pyrroline | Ethyl hexanoate | Hexyl acetate | Ethyl furoate | Methyl benzaldehyde | Phenylethanol | ||
| #1 | 5 ± 1.6 | 2 ± 0.1 | 351 ± 9.3 | nd | 6 ± 2.0 | 143 ± 27.6 | 89 ± 22.2 | ||
| #2 | nd | 3 ± 0.0 | nd | nd | 7 ± 1.5 | 273 ± 25.8 | 104 ± 17.8 | ||
| #3 | nd | 2 ± 0.1 | 321 ± 13.8 | nd | 6 ± 1.8 | 208 ± 44.1 | 132 ± 38.6 | ||
| #4 | nd | 2 ± 0.6 | 270 ± 8.1 | 6 ± 1.7 | 4 ± 1.1 | 199 ± 30.1 | 98 ± 13.4 | ||
| #5 | nd | 2 ± 0.1 | 212 ± 29.0 | 8 ± 1.0 | nd | 245 ± 1.9 | 86 ± 8.6 | ||
| #6 | 4 ± 1.3 | 3 ± 1.3 | 312 ± 17.4 | 9 ± 4.0 | nd | 365 ± 75.3 | 193 ± 55.6 | ||
| #7 | nd | 2 ± 0.5 | 282 ± 7.1 | nd | nd | 182 ± 6.2 | 171 ± 8.5 | ||
| #8 | 37 ± 4.8 | 3 ± 0.2 | 316 ± 20.9 | nd | 7 ± 1.4 | 145 ± 15.4 | 125 ± 6.4 | ||
| #9 | 15 ± 0.4 | 2 ± 0.2 | 355 ± 37.2 | nd | 6 ± 0.6 | 171 ± 5.5 | 141 ± 2.3 | ||
| #10 | 60 ± 2.7 | 2 ± 0.5 | 267 ± 6.3 | nd | 7 ± 0.3 | 96 ± 4.8 | 104 ± 0.2 | ||
| #11 | 10 ± 4.9 | 3 ± 1.1 | 410 ± 64.4 | nd | 10 ± 4.8 | 155 ± 41.2 | 211 ± 63.1 | ||
| #12 | 6 ± 2.1 | 3 ± 1.4 | 330 ± 8.8 | 25 ± 4.1 | nd | 322 ± 99.1 | 153 ± 48.2 | ||
| #13 | 6 ± 0.9 | 2 ± 0.8 | 284 ± 105.4 | 19 ± 12.6 | nd | 345 ± 59.3 | 140 ± 38.2 | ||
| #14 | nd | 4 ± 0.7 | 384 ± 55.0 | nd | 13 ± 2.1 | 414 ± 57.9 | 221 ± 1.8 | ||
| #15 | 5 ± 0.1 | 4 ± 1.0 | 325 ± 19.3 | nd | 18 ± 3.8 | 353 ± 56.0 | 191 ± 7.9 | ||
| #16 | nd | 6 ± 0.9 | 338 ± 26.3 | nd | 12 ± 2.6 | 398 ± 71.2 | 197 ± 12.5 | ||
| #17 | nd | 2 ± 0.3 | 309 ± 92.1 | 19 ± 7.5 | nd | 455 ± 96.9 | 151 ± 61.1 | ||
| #18 | 6 ± 2.0 | 2 ± 0.1 | 323 ± 23.7 | 16 ± 2.3 | nd | 371 ± 25.1 | 176 ± 50.2 | ||
| #19 | nd | 2 ± 0.5 | 344 ± 22.5 | nd | 10 ± 1.5 | 297 ± 15.6 | 183 ± 11.8 | ||
| #20 | nd | 3 ± 0.7 | 299 ± 30.4 | nd | 15 ± 1.7 | 215 ± 6.5 | 198 ± 13.1 | ||
| #21 | 12 ± 0.6 | 5 ± 1.0 | 332 ± 33.6 | nd | 11 ± 1.3 | 219 ± 9.2 | 216 ± 24.7 | ||
| #22 | 22 ± 1.2 | 3 ± 0.1 | 289 ± 20.6 | nd | 9 ± 0.5 | 182 ± 23.1 | 174 ± 38.9 | ||
| #23 | 8 ± 1.1 | 3 ± 0.2 | 355 ± 7.2 | nd | 14 ± 0.1 | 290 ± 21.9 | 221 ± 72.1 | ||
| #24 | nd | 4 ± 0.2 | 454 ± 55.7 | nd | 8 ± 0.0 | 219 ± 17.6 | 293 ± 63.8 | ||
| #25 | 13 ± 0.8 | 3 ± 0.2 | 306 ± 14.9 | nd | 9 ± 1.2 | 138 ± 34.5 | 191 ± 81.0 | ||
| #26 | 18 ± 2.2 | 5 ± 1.1 | 274 ± 21.5 | nd | 15 ± 2.3 | 215 ± 36.2 | 252 ± 89.9 | ||
| #27 | nd | 5 ± 1.6 | 376 ± 1.0 | nd | 24 ± 3.0 | 358 ± 46.3 | 327 ± 103.7 | ||
| #28 | 16 ± 0.2 | 4 ± 0.6 | 398 ± 31.2 | nd | 11 ± 1.1 | 492 ± 29.4 | 264 ± 41.4 | ||
| #29 | 7 ± 2.4 | 8 ± 2.3 | 375 ± 49.8 | nd | 13 ± 0.6 | 383 ± 60.5 | 344 ± 43.7 | ||
| Champagne | 19 ± 18.6 a | 3 ± 1.0 ab | 342 ± 59.1 a | nd | 8 ± 1.9 a | 163 ± 39.6 a | 172 ± 64.3 ab | ||
| Cava | 5 ± 6.8 ab | 5 ± 1.6 b | 312 ± 116.6 a | nd | 14 ± 4.4 b | 339 ± 89.7 b | 232 ± 69.7 a | ||
| CA Champagne | 3 ± 3.4 a | 2 ± 0.5 a | 288 ± 43.7 a | 15 ± 7.3 a | 1 ± 1.7 c | 323 ± 91.3 b | 134 ± 34.7 b | ||
| Others | 1 ± 2.1 b | 2 ± 0.6 a | 315 ± 25.4 a | 2 ± 4.3 b | 4 ± 5.0 ac | 263 ± 84.0 ab | 170 ± 26.7 ab | ||
| Sample ID | 2-Acetyl-3,4,5,6-tetrahydropyridine | Ethyl succinate | Ethyl octanoate | 2-Acetyl-1,4,5,6-tetrahydropyridine | Phenylethyl acetate | TDN (1,1,6,-trimethyl-1,2-dihydronapthalene) | β-Damascenone | Ethyl decanoate | |
| #1 | 3 ± 0.1 | 126 ± 21.7 | 194 ± 11.2 | 1 ± 0.1 | nd | 42 ± 50.9 | 6 ± 2.6 | 5 ± 0.4 | |
| #2 | 5 ± 0.7 | 132 ± 23.0 | 388 ± 21.3 | 2 ± 0.2 | nd | 201 ± 152.9 | 5 ± 1.9 | 13 ± 2.4 | |
| #3 | 3 ± 0.1 | 164 ± 32.3 | 212 ± 29.9 | 1 ± 0.0 | nd | 32 ± 25.9 | 7 ± 3.0 | 5 ± 0.3 | |
| #4 | 3 ± 0.6 | 90 ± 15.4 | 192 ± 42.2 | 1 ± 0.1 | nd | 36 ± 19.3 | 10 ± 4.3 | 6 ± 1.1 | |
| #5 | 3 ± 0.4 | 123 ± 3.8 | 201 ± 30.9 | 1 ± 0.1 | 4 ± 0.9 | 18 ± 7.4 | 6 ± 2.7 | 4 ± 0.1 | |
| #6 | 6 ± 2.6 | 90 ± 26.2 | 404 ± 141.7 | 2 ± 0.8 | 8 ± 5.5 | 66 ± 45.9 | 3 ± 1.8 | 6 ± 3.3 | |
| #7 | 4 ± 1.2 | 158 ± 15.0 | 247 ± 39.8 | 1 ± 0.3 | 6 ± 1.4 | 56 ± 29.3 | 6 ± 2.0 | 5 ± 2.6 | |
| #8 | 2 ± 0.6 | 171 ± 16.5 | 173 ± 19.5 | 1 ± 0.2 | nd | 16 ± 8.2 | 5 ± 2.4 | 3 ± 0.6 | |
| #9 | 5 ± 2.2 | 175 ± 10.4 | 373 ± 133.4 | 1 ± 0.5 | nd | 25 ± 9.5 | 9 ± 2.5 | 11 ± 7.8 | |
| #10 | 2 ± 0.8 | 160 ± 1.3 | 172 ± 22.4 | 1 ± 0.2 | nd | 37 ± 16.5 | 10 ± 3.4 | 6 ± 0.8 | |
| #11 | 5 ± 1.2 | 217 ± 38.6 | 310 ± 44.9 | 1 ± 0.2 | nd | 40 ± 25.3 | 11 ± 6.7 | 8 ± 3.0 | |
| #12 | 7 ± 1.1 | 62 ± 25.6 | 439 ± 46.4 | 2 ± 0.3 | 11 ± 3.8 | 217 ± 190.2 | 33 ± 17.3 | 16 ± 6.8 | |
| #13 | 4 ± 1.4 | 61 ± 8.7 | 346 ± 178.4 | 1 ± 0.4 | 5 ± 0.4 | 202 ± 123.7 | 26 ± 10.0 | 16 ± 5.9 | |
| #14 | 6 ± 1.6 | 203 ± 40.8 | 416 ± 58.9 | 2 ± 0.4 | nd | 509 ± 416.9 | 5 ± 1.6 | 15 ± 4.6 | |
| #15 | 6 ± 0.1 | 144 ± 118.3 | 434 ± 6.0 | 1 ± 0.2 | nd | 610 ± 493.0 | 7 ± 1.8 | 15 ± 2.1 | |
| #16 | 6 ± 1.5 | 241 ± 34.9 | 432 ± 10.5 | 1 ± 0.1 | nd | 937 ± 714.4 | 3 ± 0.9 | 12 ± 2.7 | |
| #17 | 7 ± 1.8 | 128 ± 28.6 | 468 ± 177.6 | 2 ± 0.5 | 38 ± 3.3 | 215 ± 119.9 | 5 ± 1.8 | 19 ± 0.6 | |
| #18 | 8 ± 0.3 | 131 ± 11.6 | 530 ± 40.0 | 2 ± 0.1 | 43 ± 2.3 | 241 ± 138.2 | 16 ± 4.7 | 23 ± 6.9 | |
| #19 | 5 ± 0.9 | 221 ± 11.7 | 349 ± 41.3 | 1 ± 0.4 | 6 ± 1.6 | 90 ± 68.0 | 4 ± 1.4 | 17 ± 6.5 | |
| #20 | 4 ± 0.6 | 225 ± 17.5 | 263 ± 32.9 | 1 ± 0.1 | nd | 345 ± 214.5 | 8 ± 1.9 | 11 ± 3.6 | |
| #21 | 3 ± 0.5 | 271 ± 2.2 | 249 ± 14.0 | 1 ± 0.1 | nd | 63 ± 31.6 | 8 ± 2.4 | 6 ± 2.4 | |
| #22 | 3 ± 0.4 | 198 ± 23.3 | 201 ± 33.5 | 1 ± 0.0 | nd | 54 ± 22.9 | 8 ± 1.2 | 5 ± 1.8 | |
| #23 | 8 ± 1.3 | 208 ± 27.3 | 541 ± 58.8 | 2 ± 0.2 | nd | 1023 ± 529.8 | 9 ± 0.6 | 24 ± 4.9 | |
| #24 | 7 ± 1.3 | 330 ± 26.0 | 534 ± 97.2 | 2 ± 0.4 | nd | 127 ± 76.2 | 11 ± 1.2 | 18 ± 6.3 | |
| #25 | 4 ± 0.8 | 167 ± 40.9 | 329 ± 49.5 | 1 ± 0.2 | nd | 60 ± 5.3 | 13 ± 1.7 | 15 ± 6.4 | |
| #26 | 5 ± 2.2 | 282 ± 62.0 | 271 ± 34.6 | 1 ± 0.0 | nd | 546 ± 284.0 | 6 ± 0.2 | 8 ± 3.9 | |
| #27 | 7 ± 0.0 | 318 ± 38.4 | 549 ± 16.8 | 2 ± 0.1 | nd | 1130 ± 325.5 | 11 ± 0.4 | 23 ± 5.2 | |
| #28 | 8 ± 1.7 | 224 ± 11.5 | 601 ± 148.4 | 2 ± 0.4 | nd | 907 ± 521.5 | 3 ± 0.7 | 24 ± 9.6 | |
| #29 | 9 ± 2.7 | 286 ± 9.4 | 592 ± 9.3 | 2 ± 0.5 | nd | 1577 ± 994.3 | 10 ± 1.8 | 25 ± 3.1 | |
| Champagne | 4 ± 1.5 a | 202 ± 63.1 a | 282 ± 119.3 a | 1 ± 0.4 a | nd | 51 ± 32.1 ab | 9 ± 2.5 a | 8 ± 5.2 a | |
| Cava | 7 ± 1.7 b | 226 ± 59.4 a | 449 ± 121.9 b | 2 ± 0.5 a | nd | 779 ± 413.5 b | 7 ± 2.7 a | 17 ± 6.3 b | |
| CA Champagne | 5 ± 2.5 ab | 99 ± 32.5 b | 363 ± 141.8 ab | 1 ± 0.7 a | 17 ± 18.7 a | 155 ± 100.0 ab | 16 ± 11.6 a | 14 ± 7.5 ab | |
| Others | 4 ± 1.4 ab | 158 ± 53.6 ab | 303 ± 88.9 ab | 1 ± 0.4 a | 5 ± 3.3 a | 61 ± 24.3 a | 5 ± 2.0 a | 8 ± 5.7 ab | |
| Compound | Threshold [µg/L] | Sensory Impression | References |
|---|---|---|---|
| 2-Methyl-1-butanol | not reported | Sherry | Muños et al., 2006 [28] |
| Ethyl butanoate | 20 | Pineapple | Guth 1997 [29] |
| Ethyl lactate | 110,000 | Sweet, creamy | Radler 1986 [30] |
| Furfural | 14,100 | Almond | Ferreira et al., 2000 [31] |
| Ethyl isovalerate | 3 | Pineapple | Ferreira et al., 2000 [31] |
| Isoamyl acetate | 30 | Banana | Guth 1997 [29] |
| 2-Ethyltetrahydropyridine | 150 | Brioche, mousy | Snowdon et al., 2006 [32] |
| Benzaldehyde | 2000 | Honey | Arias-Perez et al., 2021 [33] |
| 2-Acetyl-1-pyrroline | 0.1 | Bread crust, mousy | Herderich et al., 1995 [25] |
| Ethyl hexanoate | 14 | Pineapple | Ferreira et al., 2000 [31] |
| Hexyl acetate | not reported | Pear | Dennis et al., 2012 [34] |
| Ethyl furoate | 16,000 | Balsamic | Ferreira et al., 2000 [31] |
| Methylbenzaldehyde | not reported | Cherry | Chávez-Márquez et al., 2022 [35] |
| Phenylethanol | 14,000 | Rose | Ferreira et al., 2000 [31] |
| 2-Acetyl-3,4,5,6-tetrahydropyridine | 1.6 | Popcorn, mousy | Snowdon et al., 2006 [32] |
| Ethyl succinate | not reported | Cooked apple | Dachery et al., 2023 [36] |
| Ethyl octanoate | 5 | Waxy, musty | Ferreira et al., 2000 [31] |
| 2-Acetyl-1,4,5,6-tetrahydropyridine | 1.6 | Popcorn, mousy | Snowdon et al., 2006 [32] |
| Phenylethyl acetate | 250 | Rose | Guth 1997 [29] |
| TDN (1,1,6,-trimethyl-1,2-dihydronapthalene) | 2 | Kerosene | Sacks et al., 2012 [37] |
| β-Damascenone | 0.05 | Floral | Guth 1997 [29] |
| Ethyl decanoate | 200 | waxy, Brandy | Ferreira et al., 2000 [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, S.; Sommer, S.J.; Liu, C.; Burken, O.; Anderson, A.F. The Impact of Microbial Activity on the Chemical Composition and Aroma Profile of Traditional Sparkling Wines. Fermentation 2024, 10, 212. https://doi.org/10.3390/fermentation10040212
Sommer S, Sommer SJ, Liu C, Burken O, Anderson AF. The Impact of Microbial Activity on the Chemical Composition and Aroma Profile of Traditional Sparkling Wines. Fermentation. 2024; 10(4):212. https://doi.org/10.3390/fermentation10040212
Chicago/Turabian StyleSommer, Stephan, Stella J. Sommer, Connie Liu, Olivia Burken, and Andrea Faeth Anderson. 2024. "The Impact of Microbial Activity on the Chemical Composition and Aroma Profile of Traditional Sparkling Wines" Fermentation 10, no. 4: 212. https://doi.org/10.3390/fermentation10040212
APA StyleSommer, S., Sommer, S. J., Liu, C., Burken, O., & Anderson, A. F. (2024). The Impact of Microbial Activity on the Chemical Composition and Aroma Profile of Traditional Sparkling Wines. Fermentation, 10(4), 212. https://doi.org/10.3390/fermentation10040212






