Eriobotrya japonica Fermentation with Plant-Derived Lactiplantibacillus plantarum MSC-5T Ameliorates Antioxidant Activity in HEK293 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of the LAB
2.2. Fermentation Condition of the MSC-5T Strain in EJ Extract
2.3. Cell Culture and Treatment
2.4. Measurement of Intracellular ROS Levels
2.5. Preparation of the Cell Extract
2.6. Quantification of Intracellular GSSH Levels
2.7. Quantification of Caspase-3 Activity
2.8. Extraction and Identification of an Antioxidant Compound Produced in the Fermented EJ Extract
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Characterization of MSC-5T Strain
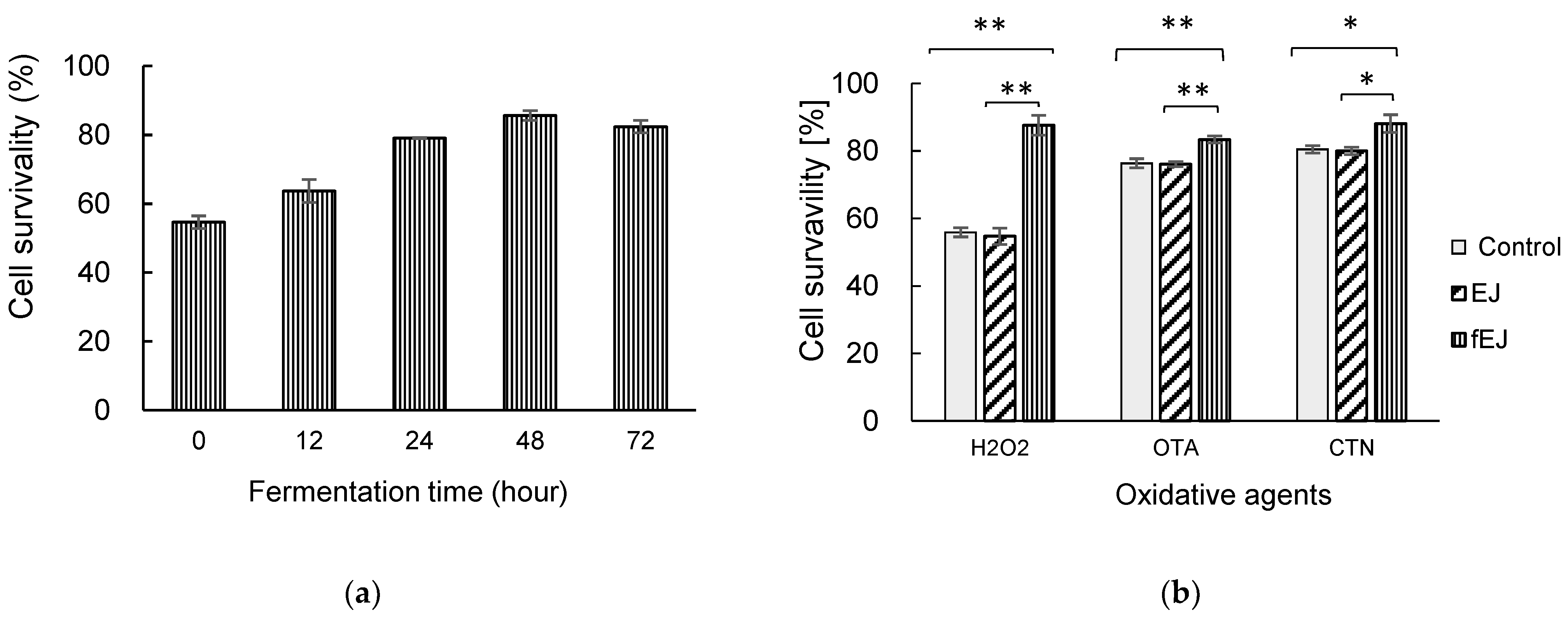
3.2. Effect of Fermented EJ Extracts on the Viability of HEK293 Cells under Oxidative Stress
3.3. Antioxidant and Antiproliferative Activity of Fermented EJ Extract
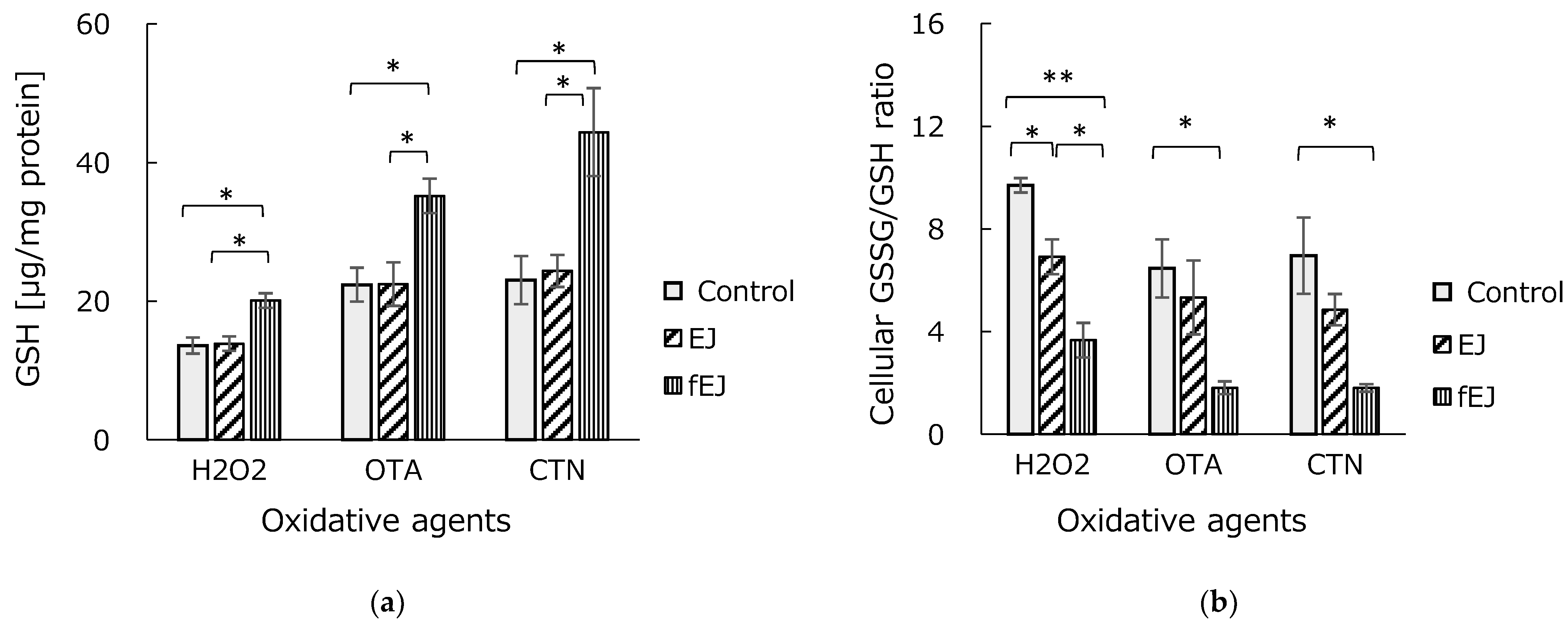
3.4. Effect of Fermented EJ Extract on GSH Homeostasis
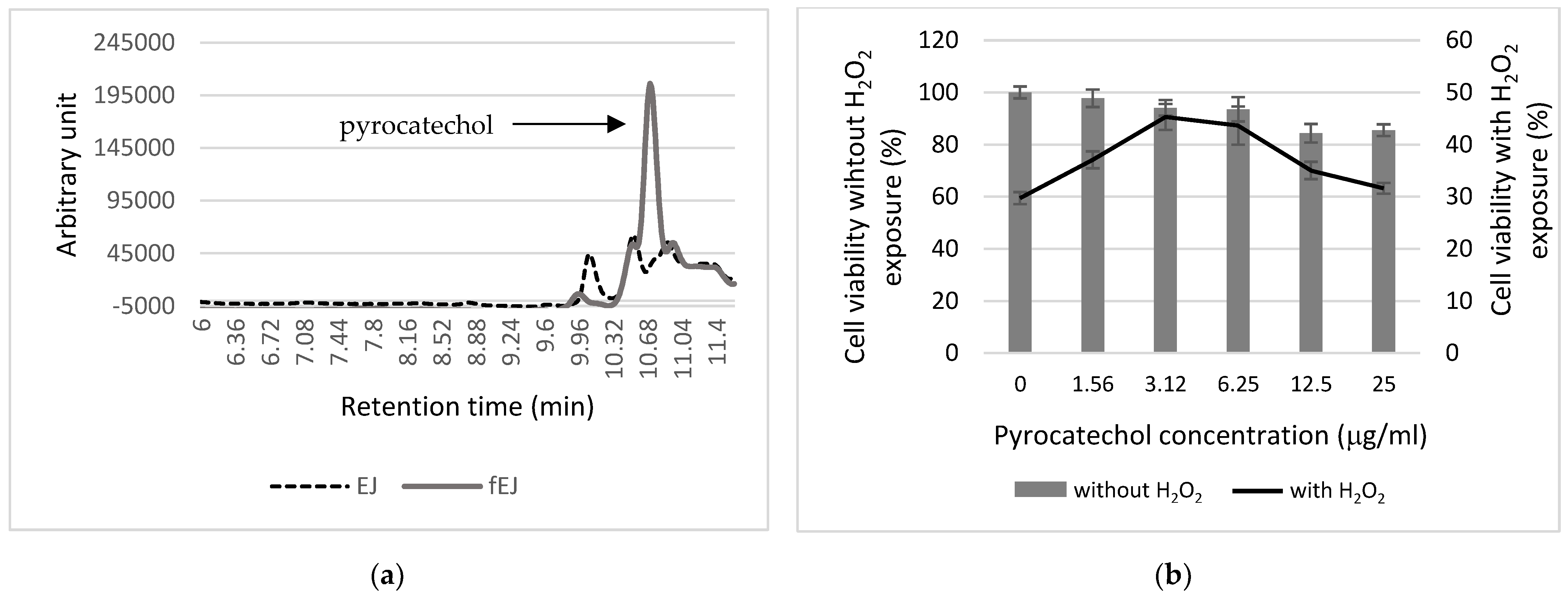
3.5. Analysis of Antioxidant Components
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, R. Probiotics. Indian J. Med. Microbiol. 2009, 27, 202–209. [Google Scholar] [CrossRef]
- Liu, W.; Pang, H.; Zhang, H.; Cai, Y. Biodiversity of lactic acid bacteria. In Lactic Acid Bacteria: Fundamentals and Practice; Springer: Dordrecht, The Netherlands, 2014; pp. 103–203. [Google Scholar]
- Nomura, M.; Kobayashi, M.; Narita, T.; Kimoto-Nira, H.; Okamoto, T. Phenotypic and molecular characterization of Lactococcus lactis from milk and plants. J. Appl. Microbiol. 2006, 101, 396–405. [Google Scholar] [CrossRef]
- Noda, M.; Danshiitsoodol, N.; Kanno, K.; Uchida, T.; Sugiyama, M. The Exopolysaccharide produced by Lactobacillus paracasei IJH-SONE68 prevents and ameliorates inflammatory responses in DSS-induced ulcerative colitis. Microorganisms 2021, 9, 2243. [Google Scholar] [CrossRef] [PubMed]
- Danshiitsoodol, N.; Noda, M.; Kanno, K.; Uchida, T.; Sugiyama, M. Plant-derived Lactobacillus paracasei IJH-SONE68 improves the gut microbiota associated with hepatic disorders: A randomized, double-blind, and placebo-controlled clinical trial. Nutrients 2022, 14, 4492. [Google Scholar] [CrossRef] [PubMed]
- Higashikawa, F.; Danshiitsoodol, N.; Kanno, K.; Ishida, R.; Tazuma, T.; Sugiyama, M. Lactobacillus plantarum SN13T cells improve hepatic dysfunction and fecal microbiota: A randomized pilot study. Arch. Clin. Biomed. Res. 2020, 4, 605–625. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, 13659. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Okamoto, T.; Sugimoto, S.; Noda, M.; Yokooji, T.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Interleukin-8 release inhibitors generated by fermentation of artemisia princeps pampanini herb extract with Lactobacillus plantarum SN13T. Front. Microbiol. 2020, 11, 1159. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołebiewska, E.; Zawadzka, M.; Choińska, R.; Koronkiewicz, K.; Piasecka-Jóźwiak, K.; Bujak, M. Sustainable extraction of bioactive compound from apple pomace through lactic acid bacteria (LAB) fermentation. Sci. Rep. 2023, 13, 19310. [Google Scholar] [CrossRef]
- Pham, T.N.A.; Kim, H.L.; Lee, D.R.; Choi, B.K.; Yang, S.H. Anti-inflammatory effects of Scrophularia buergeriana extract mixture fermented with Lactic Acid Bacteria. Biotechnol. Bioproc. Eng. 2022, E27, 370–378. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Xu, C.; Li, X. Biological activities of extracts from loquat (Eriobotrya japonica Lindl.): A Review. Int. J. Mol. Sci. 2016, 17, 1983. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Zhao, T.; Jiang, Q. Traditional uses, phytochemistry, pharmacology, and toxicity of Eriobotrya japonica leaves A summary. J. Ethnopharmacol. 2022, 298, 115566. [Google Scholar] [CrossRef]
- Khouya, T.; Ramchoun, M.; Elbouny, H.; Hmidani, A.; Bouhlali, E.D.T.; Alem, C. Loquat (Eriobotrya japonica (Thunb) Lindl.): Evaluation of nutritional value, polyphenol composition, antidiabetic effect, and toxicity of leaf aqueous extract. J. Ethnopharmacol. 2022, 296, 115473. [Google Scholar]
- Ito, H.; Kobayashi, E.; Takamatsu, Y.; Li, S.H.; Hatano, T.; Sakagami, H.; Kusama, K.; Satoh, K.; Sugita, D.; Shimura, S.; et al. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem. Pharm. Bull. 2000, 48, 687–693. [Google Scholar] [CrossRef]
- Hiraishi, A. Direct automated sequencing of 16S rDNA amplified by a polymerase chain reaction from bacterial cultures without DNA purification. Lett. Appl. Microbiol. 1992, 15, 210–213. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Jang, J.Y.; Lee, J.H.; Choi, Y.W.; Choi, Y.H.; Kim, N.D. Loquat leaf extract inhibits oxidative stress-induced DNA damage and apoptosis via AMPK and Nrf2/HO-1 signaling pathways in C2C12 cells. Appl. Sci. 2023, 13, 572. [Google Scholar] [CrossRef]
- Bae, D.; You, Y.; Yoon, H.G.; Kim, K.; Lee, Y.H.; Kim, Y.; Baek, H.; Kim, S.; Lee, J.; Jun, W. Protective effects of loquat (Eriobotrya japonica) leaves against ethanol-induced toxicity in HepG2 cells transfected with CYP2E1. Food Sci. Biotechnol. 2010, 19, 1093–1096. [Google Scholar] [CrossRef]
- Mun, J.; Park, J.; Yoon, H.J.; You, Y.; Choi, K.C.; Lee, Y.H.; Kim, K.; Lee, J.; Kim, O.K.; Jun, W. Effects of Eriobotrya japonica water extract on alcoholic and nonalcoholic fatty liver impairment. J. Med. Food 2019, 22, 1262–1270. [Google Scholar] [CrossRef]
- Klarić, M.Š.; Želježić, D.; Rumora, L.; Peraica, M.; Pepeljnjak, S.; Domijan, A.M. A potential role of calcium in apoptosis and aberrant chromatin forms in porcine kidney PK15 cells induced by individual and combined ochratoxin A and citrinin. Arch. Toxicol. 2012, 86, 97–107. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, F.; Tian, J.; Guo, X.; An, R. Protective effects of compound ammonium glycyrrhizin, L-arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes. Mol. Med. Rep. 2018, 18, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Eraso, A.J.; Albesa, I. Eriobotrya japonica counteracts reactive oxygen species and nitric oxide stimulated by chloramphenicol. Am. J. Chin. Med. 2007, 35, 875–885. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.; Seong, A.R.; Lee, Y.H.; Kim, Y.J.; Baek, H.Y.; Kim, Y.J.; Jun, W.J.; Yoon, H.J. Neuroprotective effects of Eriobotrya japonica against β-amyloid-induced oxidative stress and memory impairment. Food Chem. Toxicol. 2011, 49, 780–784. [Google Scholar] [CrossRef]
- Kikuchi, T.; Akazawa, H.; Tabata, K.; Manosroi, A.; Manosroi, J.; Suzuki, T.; Akihisa, T. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem. Pharm. Bull. 2011, 59, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Sakamoto, A.; Tung, N.H.; Fujiki, T.; Kishihara, K.; Oiso, S.; Kariyazono, H.; Morinaga, O.; Shoyama, Y. Anti-proliferative activities and apoptosis induction by triterpenes derived from Eriobotrya japonica in human leukemia cell lines. Int. J. Mol. Sci. 2013, 14, 4106–4120. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chen, S.S.; Zhang, L.; Wang, J.; Yang, Y.; Zhang, S.; Pan, Y.; Wang, Y.; Yang, L. Systems pharmacology dissection of the anti-inflammatory mechanism for the medicinal herb Folium eriobotryae. Int. J. Mol. Sci. 2015, 16, 2913–2941. [Google Scholar] [CrossRef]
- Pawłowska, A.M.; Żurek, N.; Kapusta, I.; De Leo, M.; Braca, A. Antioxidant and antiproliferative activities of phenolic extracts of Eriobotrya japonica (Thunb.) Lindl. fruits and leaves. Plants 2023, 12, 3221. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Danshiitsoodol, N.; Noda, M.; Sugiyama, M. Role of phenolic acid metabolism in enhancing bioactivity of Mentha extract fermented with plant-derived Lactobacillus plantarum SN13T. Probiotics Antimicrob. Proteins 2023, 6. [Google Scholar] [CrossRef]
- Kosobutskii, V.S. Pyrocatechol and its derivatives as antioxidants and prooxidants. Russ. J. Gen. Chem. 2014, 84, 839–842. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nonaka, Y.; Tago, K.; Takeda, M.; Ishihara, Y.; Sakai, A.; Matsutaka, M.; Kobata, K.; Tamura, H. Pyrocatechol, a component of coffee, suppresses LPS-induced inflammatory responses by inhibiting NF-κB and activating Nrf2. Sci. Rep. 2020, 10, 2584. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wu, F.; Tian, J.; Liu, Z.; He, H.; Bao, D.; Li, G.; Li, H.; Chen, J.; Lai, Y.; et al. Pyrocatechol alleviates cisplatin-induced acute kidney injury by inhibiting ROS production. Oxid. Med. Cell. Longev. 2022, 9, 2158644. [Google Scholar] [CrossRef] [PubMed]
| Unfermented EJ Extract | Fermented EJ Extract | ||
|---|---|---|---|
| Intracellular ROS (%) | H2O2 | 81 ± 4.1 | 70.5 ± 3.7 |
| OTA | 93.3 ± 4.1 | 82.5 ± 3.7 | |
| CTN | 80.3 ± 2.5 | 68.1 ± 5.2 | |
| Intracellular Caspase-3 (%) | H2O2 | 103.1 ± 6.2 | 42.6 ± 3.0 |
| OTA | 95 ± 5.1 | 68.9 ± 5.1 | |
| CTN | 92.8 ± 2.3 | 83.6 ± 1.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danshiitsoodol, N.; Inoue, Y.; Sugimoto, S.; Shakya, S.; Noda, M.; Sugiyama, M. Eriobotrya japonica Fermentation with Plant-Derived Lactiplantibacillus plantarum MSC-5T Ameliorates Antioxidant Activity in HEK293 Cells. Fermentation 2024, 10, 197. https://doi.org/10.3390/fermentation10040197
Danshiitsoodol N, Inoue Y, Sugimoto S, Shakya S, Noda M, Sugiyama M. Eriobotrya japonica Fermentation with Plant-Derived Lactiplantibacillus plantarum MSC-5T Ameliorates Antioxidant Activity in HEK293 Cells. Fermentation. 2024; 10(4):197. https://doi.org/10.3390/fermentation10040197
Chicago/Turabian StyleDanshiitsoodol, Narandalai, Yusuke Inoue, Sachiko Sugimoto, Shrijana Shakya, Masafumi Noda, and Masanori Sugiyama. 2024. "Eriobotrya japonica Fermentation with Plant-Derived Lactiplantibacillus plantarum MSC-5T Ameliorates Antioxidant Activity in HEK293 Cells" Fermentation 10, no. 4: 197. https://doi.org/10.3390/fermentation10040197
APA StyleDanshiitsoodol, N., Inoue, Y., Sugimoto, S., Shakya, S., Noda, M., & Sugiyama, M. (2024). Eriobotrya japonica Fermentation with Plant-Derived Lactiplantibacillus plantarum MSC-5T Ameliorates Antioxidant Activity in HEK293 Cells. Fermentation, 10(4), 197. https://doi.org/10.3390/fermentation10040197






