Revolutionizing Renewable Resources: Cutting-Edge Trends and Future Prospects in the Valorization of Oligosaccharides
Abstract
1. Introduction
1.1. Global Scenario and Market Potential Delves Commercial Prospects of the Prebiotics Market
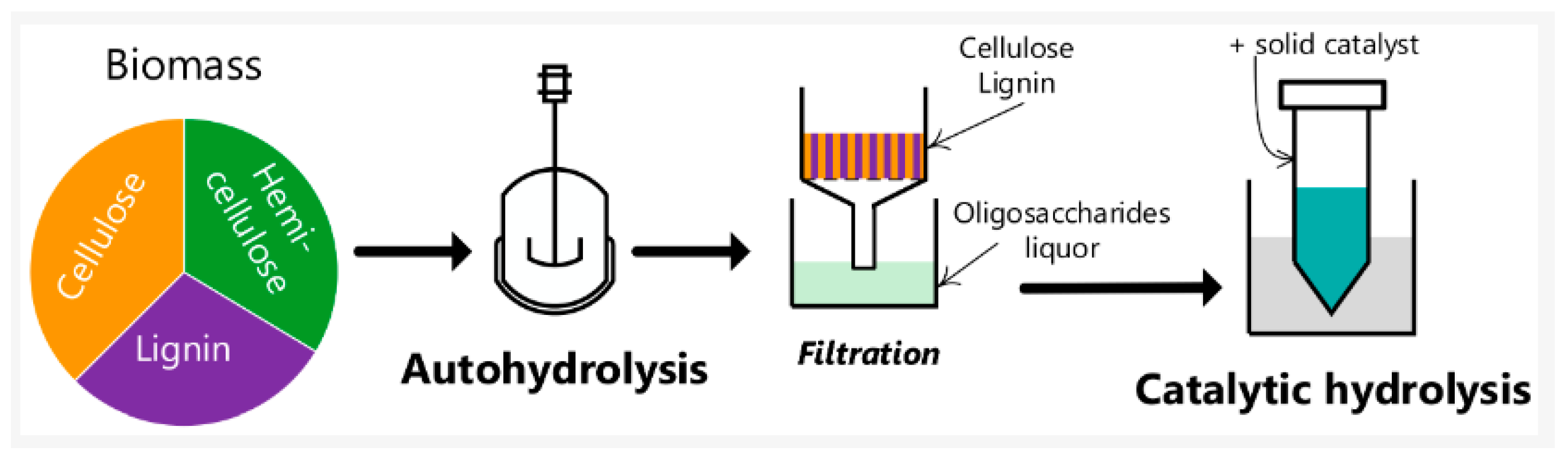
1.2. Autohydrolysis Processes: Principles and Applications
1.3. Utilization of Lignocellulosic Waste Residues: Approaches and Applications
| SNo | Reactor | Condition | Treatment | Product | Residue | Year | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 316 stainless steel pressure reactor of 1 L capacity, featuring water circulation capability and temperature regulation via PID control. | Temperature of 200 °C for 20 min heating time, followed by a 5 min holding period. Liquid to Solid Ratio (LSR) is 10, with a severity factor (H) of 3.94. | Autohydrolysis isothermal. | High degree of polymerization xylooligosaccharides (XOS) at a concentration of 0.077 g per gram, alongside low degree of polymerization XOS at a concentration of 0.033 g per gram. | Almond shells | 2019 | [21] |
| 2 | A 1.5 L capacity reactor, constructed from 5100 series stainless steel, known as a Parr reactor, features temperature regulation via a precision PID-controlled system. | The process involves reaching a temperature of 200 °C, with a Liquid to Solid Ratio (LSR) set at 8, and a severity factor (H) calculated to be 4.01. | Autohydrolysis is performed under isothermal conditions, implying that the process is carried out at a constant temperature throughout. This consistent temperature is a fundamental characteristic of the procedure. | Xylooligosaccharides (XOS) present at a concentration of 0.10 g per gram, and galactooligosaccharides (GOS) at a concentration of 0.069 g per gram. | Vine shoots | 2016 | [22] |
| 3 | A high-pressure reactor, model BR-300, features a 0.6 L capacity stainless steel tank equipped with a heating block for temperature control. It includes a paddle agitator for mixing contents and utilizes tap water for cooling, which is circulated through an internal coil. | The procedure entails heating to 190 °C for a duration of 5 min, followed by maintaining this temperature for an additional 5 min holding period. The Liquid to Solid Ratio (LSR) is set at 10, with the agitator speed at 300 revolutions per min (r.p.m.), and a severity factor (H) calculated at 3.92. | Autohydrolysis is performed under isothermal conditions, indicating that the process is maintained at a constant temperature throughout its duration. | Xylooligosaccharides (XOS) are measured at a concentration of 0.10 g per gram. | Hazelnut shells | 2017 | [23] |
| 4 | A stainless steel Parr reactor, with a volume of 3.75 L, is equipped with two Rushton turbines for mixing. It is heated using external fabric mantles and features cooling through internal stainless steel loops. The temperature within the reactor is regulated using a PID-controlled system for precise thermal management. | The process involves heating to a temperature of 202 °C over a period of 39 min, with the Liquid to Solid Ratio (LSR) established at 8. | The process of autohydrolysis is performed in a nonisothermal manner, which means that it involves changing temperatures throughout the operation. | Xylooligosaccharides (XOS) are found at a concentration of 0.20 g per gram, while arabinooligosaccharides (AROS) are present at a concentration of 0.016 g per gram. | Corncob | 2002 | [23] |
| 5 | A Parr reactor, designed for conducting chemical reactions under controlled conditions. | The procedure involves elevating the temperature to 212 °C and maintaining this heat for a duration of 45 min, with a Liquid to Solid Ratio (LSR) set at 8. | Autohydrolysis is performed using a nonisothermal approach, indicating that the process involves changing temperatures rather than a constant temperature throughout. | Xylooligosaccharides (XOS) are recorded at a concentration of 0.10 g per gram, while galactooligosaccharides (GOS) are observed at a concentration of 0.027 g per gram. | Rice husks | 2004 | [24] |
| 6 | A Parr reactor constructed from stainless steel, designed for performing chemical reactions with precision and durability. | The protocol requires heating to a temperature of 202 °C for a total of 39 min, utilizing a Liquid to Solid Ratio (LSR) of 8. | Autohydrolysis is a non-isothermal process, indicating that it does not maintain a constant temperature throughout the duration of the reaction. Instead, the temperature changes as the reaction progresses. | Xylooligosaccharides (XOS) are detected at a concentration of 0.18 g per gram. | Barley husks | 2004 | [25] |
| 7 | A 1.5 L capacity reactor, crafted from stainless steel and equipped with a Parr PID controller, is utilized for precise temperature regulation during reactions. | The process settings include reaching a temperature of 180 °C, applying a Liquid to Solid Ratio (LSR) of 8, and achieving a severity factor (H) of 3.08. | The method of autohydrolysis is performed under nonisothermal conditions, implying that it involves temperature variations throughout the process. | Xylooligosaccharides (XOS) are present at a concentration of 0.057 g per gram, while galactooligosaccharides (GOS) are measured at a concentration of 0.054 g per gram. | Chestnut shells | 2018 | [26] |
| 8 | A 0.6 L capacity reactor, made of stainless steel and identified as the Parr 4842 model, is designed for conducting various chemical processes. | The experimental setup involves heating to a temperature of 210 °C, maintaining a Liquid to Solid Ratio (LSR) of 8, and achieving a severity factor (H) of 4.09. | Autohydrolysis is carried out under nonisothermal conditions, signifying that the process involves varying temperatures over time. | Xylooligosaccharides (XOS) are identified at a concentration of 0.061 g per gram. | Peanut shells | 2018 | [27] |
| 9 | A stainless steel reactor with a capacity of 0.6 L is employed for the process. The stirring is achieved using two four-blade turbine impellers, and electric heating is utilized to maintain the required temperature. Cooling is facilitated through an internal loop that circulates water, ensuring precise temperature control throughout the reaction. | The experimental conditions involve maintaining a precise temperature of 200 ± 0.2 °C for a duration of 10 min during the holding phase. Additionally, a Liquid to Solid Ratio (LSR) of 10 is applied throughout this process. These specific parameters are critical for ensuring the reproducibility and success of the experiment, allowing for accurate data collection and analysis. | Autohydrolysis Isothermal. | In this context, it’s noteworthy that the concentration of low degree of polymerization xylooligosaccharides (Low DP-XOS) is determined to be 0.12 g per gram. This measurement provides valuable information about the composition of the sample and its suitability for various applications. | Sugarcane bagasse | 2018 | [42] |
| 10 | A stainless steel vessel with a volume of 0.12 L is utilized for the experimental setup. The vessel’s heating is accomplished using an aluminum block heater, which is meticulously regulated through a PID (proportional-integral-derivative) controller to maintain precise temperature conditions. To counteract the generated heat, the vessel is efficiently cooled by a continuous flow of tap water, ensuring the stability and control of the entire system. | The experimental procedure comprises two distinct phases, starting with heating the system to a temperature of 275 °C, followed by a subsequent cooling phase that extends the total duration to 14.5 min. Throughout this process, a Liquid to Solid Ratio (LSR) of 10 is maintained, and a severity factor (H) of 4.52 is calculated. These specific conditions are meticulously selected and are instrumental in achieving the desired results and gaining insights into the behavior of the materials involved in the experiment. | Autohydrolysis nonisothermal. | In the context provided, the concentration of mannooligosaccharides (MANOS) is reported at 0.23 g per gram. This specific measurement is crucial for understanding the composition of the analyzed sample and its potential applications. | Coconut meal | 2014 | [43] |
| 11 | A stainless steel vessel with a volume of 0.125 L is employed for the experimental setup. The vessel’s heating is achieved through the use of an aluminum block heater, while effective cooling is ensured by the continuous flow of tap water, maintaining the desired temperature conditions throughout the process. | The procedure entails heating the system to a specific temperature of 175 °C and maintaining this temperature for a duration of 5.5 min. During this process, a Liquid to Solid Ratio (LSR) of 16 is employed, and a severity factor (H) of 2.21 is calculated, which are key parameters influencing the outcomes of the operation. These precise conditions are crucial for achieving the intended results and understanding the behavior of the materials involved in the experiment. | Autohydrolysis nonisothermal. | In the given context, the concentrations of specific oligosaccharides are observed, with polysaccharides (POS) measured at a concentration of 0.14 g per gram, and galactooligosaccharides (GOS) recorded at a concentration of 0.051 g per gram. These values are significant in characterizing the composition of the analyzed sample. | Passion fruit peel | 2017 | [44] |
| 12 | A Parr reactor constructed from stainless steel, which is widely recognized for its durability and corrosion resistance, is utilized for various chemical processes and experiments. | The experimental setup involves maintaining a temperature of 195 °C, while utilizing a Liquid to Solid Ratio (LSR) of 8. Additionally, a severity factor (H) of 3.65 is calculated for the process. These specific conditions are carefully selected and crucial for achieving the desired outcomes and understanding the behavior of the materials involved in this operation. | Autohydrolysis nonisothermal. | The analysis reveals the presence of specific oligosaccharides, with xylooligosaccharides (XOS) measured at a concentration of 0.12 g per gram, galactooligosaccharides (GOS) at 0.040 g per gram, and arabinooligosaccharides (AROS) at 0.032 g per gram. These concentrations are pivotal in characterizing the composition of the sample and assessing its potential applications. | Brewery’s spent grains | 2015 | [45] |
| 13 | The experimental setup includes a reactor vessel with a capacity of 0.05 L, constructed from SUS316 stainless steel, known for its resistance to corrosion. To achieve the desired temperature, the reactor is heated within a molten salt bath, ensuring precise temperature control during the process. Furthermore, to rapidly cool the reactor down to 50 °C in less than 3 min, a water bath cooling system is employed, allowing for efficient temperature management. | The experimental procedure involves a multi-step process, starting with heating to a temperature of 160 °C for 5 min. Subsequently, there is a 2 min holding period at this temperature, followed by a 3 min cooling phase. Throughout this process, a Liquid to Solid Ratio (LSR) of 8 is maintained. These precise time and temperature intervals, along with the LSR, are critical factors that contribute to the successful execution of the procedure and the desired results. | Autohydrolysis isothermal. | In the context provided, it is crucial to note that the concentration of Arabinooligosaccharides (AROS) is determined to be 0.15 g per gram. This specific measurement play a significant role in characterizing the composition of the analyzed sample and assessing its potential uses or applications. | Beet fiber (beet pulp) | 2013 | [46] |
| 14 | A stainless steel reactor with a substantial capacity of 100 L, known for its durability and versatility in accommodating large-scale chemical processes and reactions. | The operation entails maintaining a temperature of 170 °C for a duration of 15 min while utilizing saturated steam as the heating medium. These specific conditions are carefully chosen and play a significant role in the successful execution of the process at hand. | Steam processing, isothermal. | The analysis includes the identification of polysaccharides (POS), xylooligosaccharides (XOS), and galactooligosaccharides (GOS), although there is no quantitative information available regarding their respective yields in the given context. These components are essential to characterize the composition of the sample, even though the precise quantities are not provided. | Alperujo | 2012 | [47] |
| 15 | The equipment used is an autoclave with a working volume of 0.5 L, specifically designed for conducting controlled experiments and reactions. To monitor the conditions within the reactor, precise measurements of temperature and pressure are obtained using a thermocouple and a pressure gauge, respectively, ensuring accurate data collection and control during the experiments. | The procedure involves maintaining a temperature of 150 °C for a specific holding period of 5 min. During this time, a high Liquid to Solid Ratio (LSR) of 30 is employed, which is a critical factor in the success of the process. These controlled conditions are essential for achieving the desired outcomes in this particular operation. | Autohydrolysis Isothermal. | In the context provided, it is noteworthy that the concentration of Polysaccharides (POS) is quantified at 0.17 g per gram. This specific measurement is crucial for understanding the composition of the analyzed sample and its potential applications in various processes or industries. | Citrus peel, apple pomace | 2014 | [48] |
| 16 | The experimental setup comprises a 3.75 L stainless steel Parr reactor, featuring the integration of two four-blade turbine impellers, which play a crucial role in achieving thorough mixing during chemical processes. The heating system is powered by electricity, allowing for accurate temperature control within the reactor. Furthermore, the system incorporates an internal cooling loop, which effectively dissipates excess heat generated during the reactions, ensuring stable and controlled conditions throughout the experimentation. | The experimental parameters consist of maintaining a temperature of 160 °C, employing a Liquid to Solid Ratio (LSR) of 12. Additionally, a severity factor (H) of 2.46 and an agitator speed of 150 revolutions per min (r.p.m.) are meticulously selected. These precise conditions play a crucial role in ensuring the success and reproducibility of the process being conducted. | Autohydrolysis Nonisothermal. | The analysis indicates that the sample contains polysaccharides (POS) at a concentration of 0.20 g per gram and arabinooligosaccharides (AROS) at a concentration of 0.076 g per gram. These measurements are essential for characterizing the composition of the sample and assessing its suitability for specific applications or processes GALOS (0.066 g/g). | Orange peel | 2010 | [49] |
| 17 | A Parr reactor, crafted from durable stainless steel and boasting a substantial 3.75 L capacity, serves as the primary vessel for conducting various chemical processes and reactions, making it an essential tool in the field of research and experimentation. | The process involves maintaining a temperature of 160 °C while utilizing a Liquid to Solid Ratio (LSR) of 12. Additionally, it incorporates a severity factor (H) of 2.51 and an agitator speed of 150 revolutions per min (r.p.m.). These specific conditions are critical for achieving the desired outcomes in the given procedure. | Autohydrolysis Nonisothermal. | In the context provided, it’s important to note that the sample comprises polysaccharides (POS) with a concentration of 0.25 g per gram, arabinooligosaccharides (AROS) at a concentration of 0.068 g per gram, and galactooligosaccharides (GALOS) measured at 0.026 g per gram. These specific measurements play a pivotal role in characterizing the composition of the sample and evaluating its potential applications in various industries and processes. | Lemon peel | 2013 | [50] |
2. Functional Properties of Polymeric Oligosaccharides
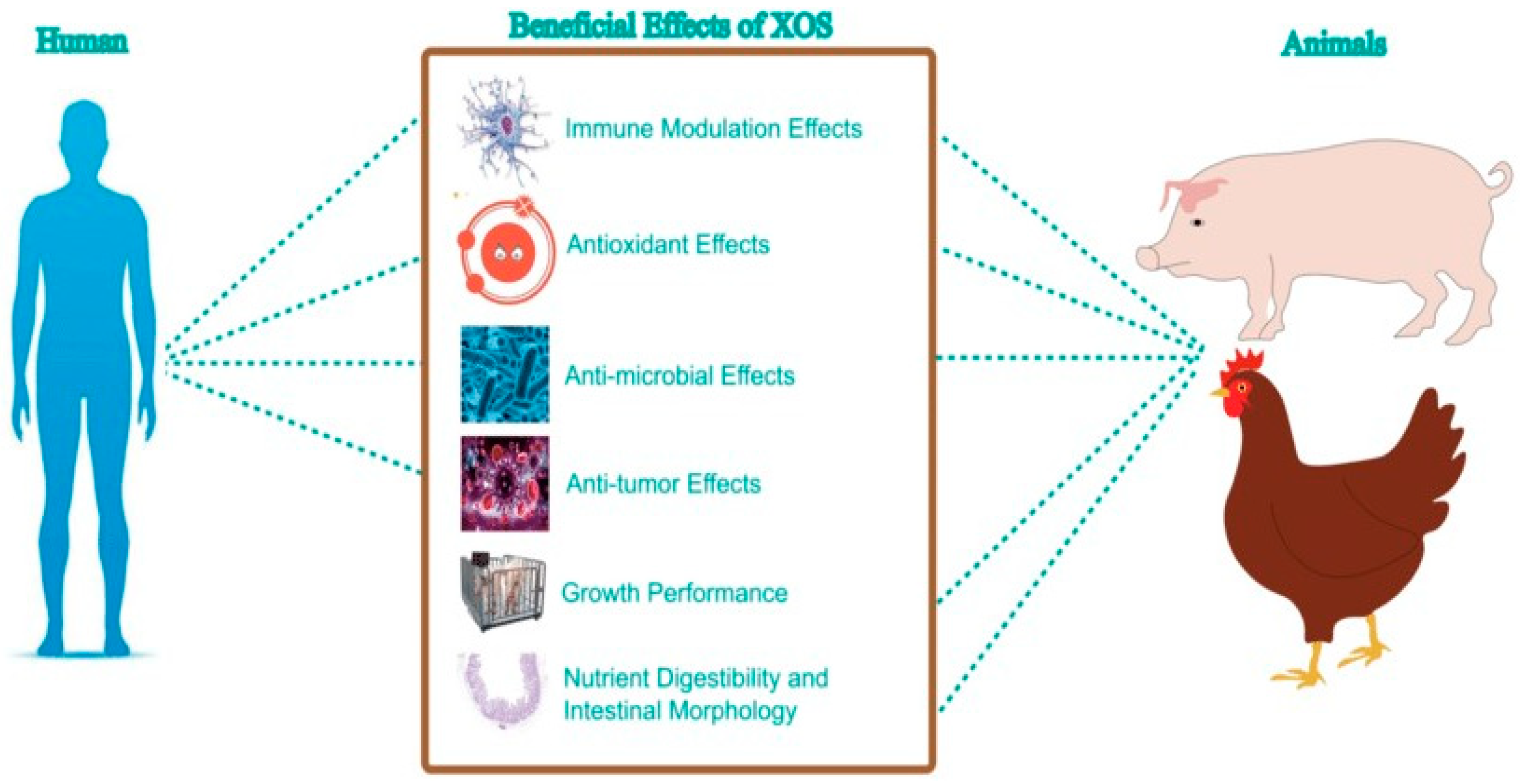
2.1. The Role and Benefits of Galactooligosaccharides (GOS) in Human and Animal Health
2.2. Exploring the Health Impacts of Xylo-Oligosaccharides (XOS) in Human and Animal Nutrition
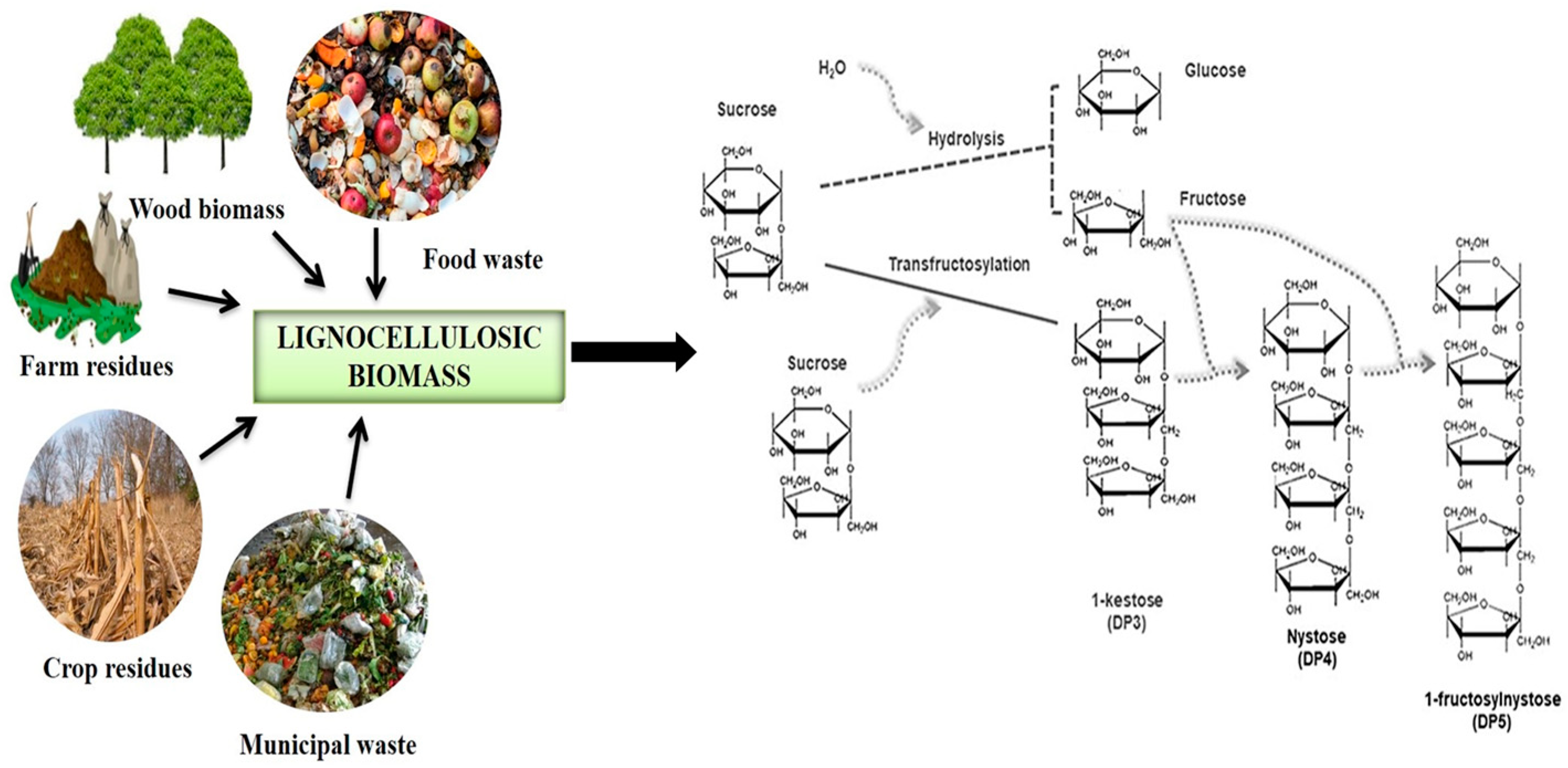
2.3. Investigating the Nutritional and Health Benefits of Fructo-Oligosaccharides (FOS) in Humans and Animals
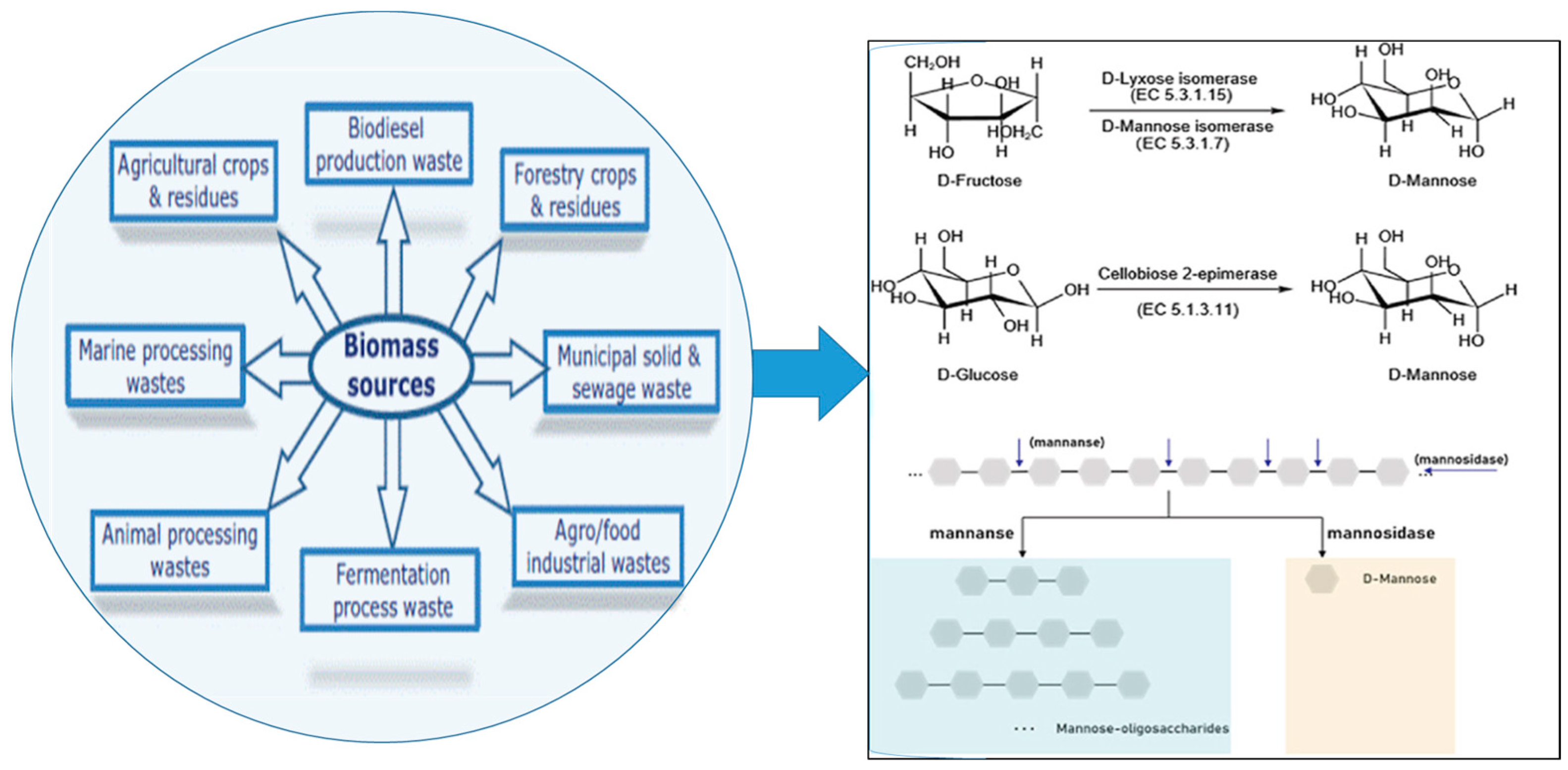
2.4. Assessing the Role of Mannan-Oligosaccharides (MOS) in Enhancing Human and Animal Health and Nutrition
3. Synthesis of Oligosaccharides from Lignocellulosic Biomass
3.1. Approaches to Oligosaccharide Production: Cell-Free and Whole Cell-Mediated Biosynthesis
3.1.1. Production of Galactooligosaccharides (GOS) Using Lignocellulosic Biomass
| SNo | Source of β-Galactosidase | Yield | Process | Reference |
|---|---|---|---|---|
| 1 | L. acidophilus ATCC 4356, a specific strain known for its enzymatic activity. This strain is employed to catalyze various biochemical reactions, including the conversion of lactose into valuable galactooligosaccharides (GOS), making it a crucial component in the production of prebiotic compounds. | 86 g/L | The process involves the immobilization of enzymes on a methacrylic polymer carrier, which serves as a stable and effective support system for the enzymes, facilitating their catalytic activity and enabling various applications in biotechnology and industrial processes. | [82,83] |
| 2 | T. naphthophila RKU-10 | 23.28 g/L/h | Enzyme. | [84,85] |
| 3 | A. niger | 35% | Enzyme. | [86,87] |
| 4 | K. lactis | 21 g/L | In this innovative approach, bead-immobilized β-galactosidase is employed in conjunction with nanofiltration for the fractionation of sugar mixtures. The use of cellulose acetate membranes enhances the efficiency and selectivity of the fractionation process, making it a valuable technique in the production and purification of specific sugar compounds, including oligosaccharides. | [88,89] |
| 5 | A. oryzae | 29 g/100 g of lactose | Fermentation in 50% (w/w) lactose monohydrate. | [90] |
| 6 | T. thermophillus | 34% | Immobilization on to insoluble carrier Eupergit C. | [91] |
| 7 | A. oryzae | 39.30% | Packed bed reactor. | [92] |
| 8 | B. circulans | 44% | Enzyme. | [92] |
3.1.2. Generating Xylooligosaccharides (XOS) from Lignocellulosic Biomass: Techniques and Advancements
3.1.3. Production of Fructooligosaccharides (FOS) from Lignocellulosic Biomass: Methods and Progress
3.1.4. Advancements in Extracting Mannooligosaccharides (MOS) from Lignocellulosic Biomass: Techniques and Developments
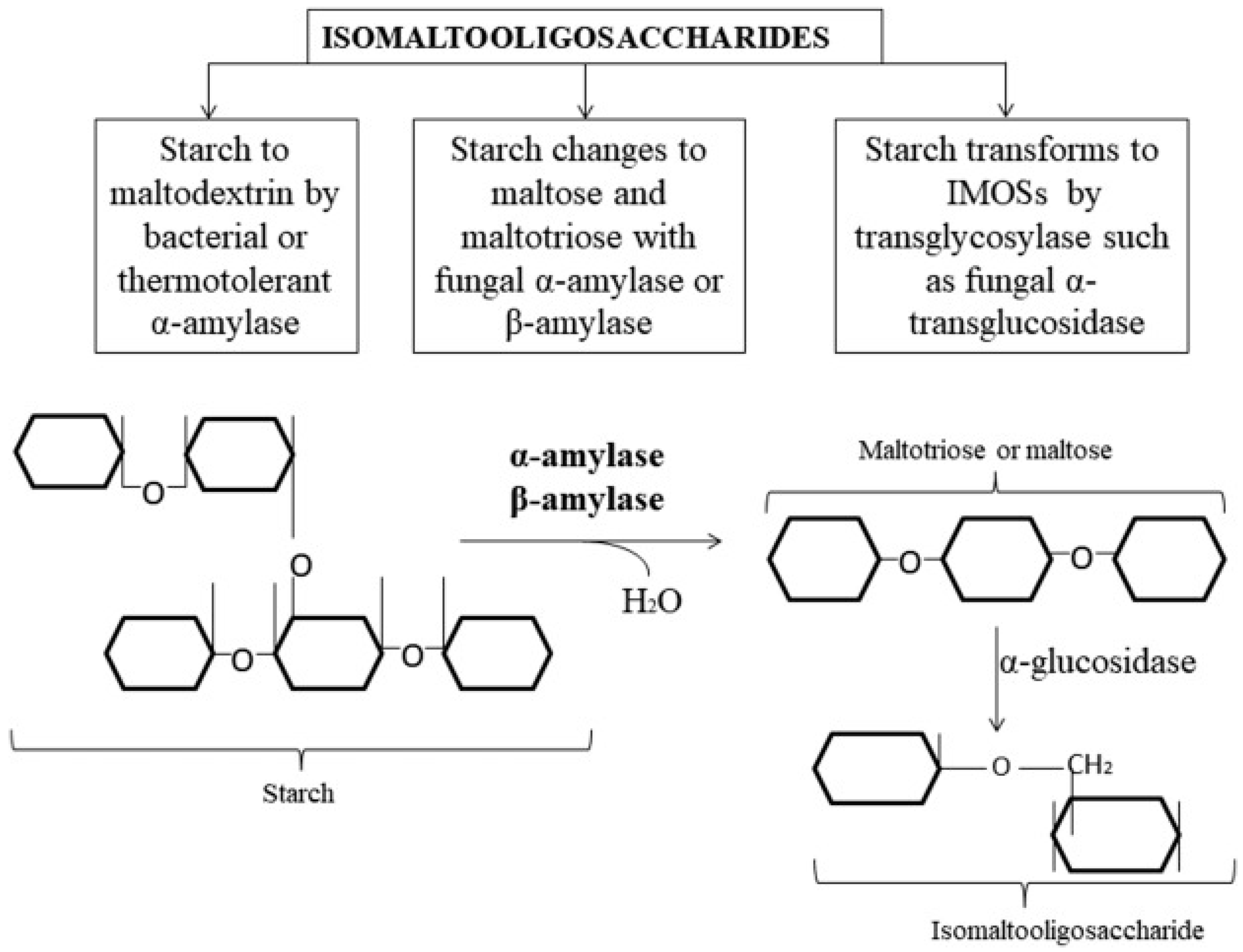
3.1.5. Innovations in Producing Isomaltooligosaccharides (IMOs) from Lignocellulosic Biomass: Processes and Technological Progress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chourasia, R.; Phukon, L.C.; Singh, S.P.; Rai, A.K.; Sahoo, D. Role of enzymatic bioprocesses for the production of functional food and nutraceuticals. In Biomass, Biofuels, Biochemical; Elsevier: Amsterdam, The Netherlands, 2020; pp. 309–334. [Google Scholar]
- Khaneghah, A.M.; Fakhri, Y. Probiotics and prebiotics as functional foods: State of the art. Curr. Nutr. Food Sci. 2019, 15, 20–30. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, S.; Gao, S.; Sun, C.; Tian, Z.; Wen, X.A. Hyperthermophilic anaerobic fermentation platform for highly efficient short chain fatty acids production from thermal hydrolyzed sludge. Water Res. 2023, 243, 120434. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Park, S.J.; Oh, S.; Lee, E.; Daliri, E.B.M.; Elahi, F.; Park, C.R.; Sultan, G.; Madar, I.H.; Oh, D.H. Unveiling the potentials of bioactive oligosaccharide1-kestose (GF2) from Musa paradisiaca Linn peel with an anxiolytic effect based on gut microbiota modulation in stressed mice model. Food Biosci. 2022, 49, 101881. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Polic, I.I.; Matheyambath, A.C.; LaPointe, G. Modulation of human gut microbiota composition and metabolites by arabinogalactan and Bifidobacterium longum subsp. longum BB536 in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). J. Funct. Foods 2021, 87, 104820. [Google Scholar] [CrossRef]
- Patra, R.; Mitra, S.; Das, N.C.; Mukherjee, S. Prebiotics as promising therapeutics for treating gut-related disorders: Biochemical and molecular perspectives. In Prebiotics, Probiotics and Nutraceuticals; Springer Nature: Singapore, 2022; pp. 133–154. [Google Scholar]
- Bamigbade, G.B.; Subhash, A.J.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. An updated review on prebiotics: Insights on potentials of food seeds waste as source of potential prebiotics. Molecules 2022, 27, 5947. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hutkins, R. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From cellulose to cellulose nanofibrils—A comprehensive review of the preparation and modification of cellulose nanofibrils. Materials 2020, 13, 5062. [Google Scholar] [CrossRef]
- Hough, C. Regional Review on Status and Trends in Aquaculture Development in Europe–2020; Food & Agriculture Org: Rome, Italy, 2022. [Google Scholar]
- Panwar, D.; Panesar, P.S.; Saini, A. Prebiotics and their Role in Functional Food Product Development. In Probiotics, Prebiotics and Synbiotics: Technological Advancements Towards Safety and Industrial Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 233–271. [Google Scholar]
- Barclay, A.W.; Augustin, L.S.; Brighenti, F.; Delport, E.; Henry, C.J.; Sievenpiper, J.L.; Usic, K.; Yuexin, Y.; Zurbau, A.; Wolever, T.M.; et al. Dietary glycaemic index labelling: A global perspective. Nutrients 2021, 13, 3244. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.; Aas, A.M.; Astrup, A.; Atkinson, F.S.; Baer-Sinnott, S.; Barclay, A.W.; Jenkins, D.J. Dietary fibre consensus from the international carbohydrate quality consortium (ICQC). Nutrients 2020, 12, 2553. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Ruiz-Pulido, G.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M. Insight of nanotechnological processing for nano-fortified functional foods and nutraceutical—Opportunities, challenges, and future scope in food for better health. Crit. Rev. Food Sci. Nutr. 2023, 63, 4618–4635. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, T.; De Carolis, C.; Cecchi, T.; de Carolis, C. Biocascading: General Strategy for the Recovery of Valuable Substances from Food Waste. In Biobased Products from Food Sector Waste: Bioplastics, Biocomposites, and Biocascading; Springer: Cham, Switzerland, 2021; pp. 109–167. [Google Scholar]
- Chatterjee, B.; Mazumder, D. Enzyme mediated hydrolysis of organic fraction of municipal solid waste (OFMSW): A short review. J. Indian Chem. Soc. 2018, 95, 295–312. [Google Scholar]
- Bekhit, A.E.A.; Cheng, V.J.; Harrison, R.; Ye, Z.J.; Bekhit, A.A.; Ng, T.B.; Kong, L.M. Technological aspects of by-product utilization. In Valorization of Wine Making By-Products; CRC Press: Boca Raton, FL, USA, 2016; Volume 4, p. 117. [Google Scholar]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6. [Google Scholar] [CrossRef]
- Cantero, D.; Jara, R.; Navarrete, A.; Pelaz, L.; Queiroz, J.; Rodríguez-Rojo, S.; Cocero, M.J. Pretreatment processes of biomass for biorefineries: Current status and prospects. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Sing, R.D.; Nadar, C.G.; Muir, J.; Arora, A. Green and clean process to obtain low degree of polymerization xylooligosaccharides from almond shell. J. Clean. Prod. 2019, 241, 118237. [Google Scholar] [CrossRef]
- Dávila, I.; Gordobil, O.; Labidi, J.; Gullón, P. Assessment of suitability of vine shoots for hemicellulosic oligosaccharides production through aqueous processing. Bioresour. Technol. 2016, 211, 636–644. [Google Scholar] [CrossRef]
- Surek, E.; Buyukkileci, A.O. Production of xylooligosaccharides by autohydrolysis of hazelnut (Corylus avellana L.) shell. Carbohydr. Polym. 2017, 174, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Production of substituted oligosaccharides by hydrolytic processing of barley husks. Ind. Eng. Chem. Res. 2004, 43, 1608–1614. [Google Scholar] [CrossRef]
- Vegas, R.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Processing of rice husk autohydrolysis liquors for obtaining food ingredients. J. Agric. Food Chem. 2004, 52, 7311–7317. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Dávila, I.; Moreira, M.T.; Labidi, J.; Gullón, P. Hydrothermal treatment of chestnut shells (Castanea sativa) to produce oligosaccharides and antioxidant compounds. Carbohydr. Polym. 2018, 192, 75–83. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic biomass valorization for bioethanol production: A circular bioeconomy approach. Bioenergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef]
- Šekuljica, N.; Jakovetić Tanasković, S.; Mijalković, J.; Simović, M.; Pavlović, N.; Đorđević, N.; Knežević-Jugović, Z. Xylanase Production by Solid-State Fermentation for the Extraction of Xylooligosaccharides from Soybean Hulls §. Food Technol. Biotechnol. 2023, 61, 439–450. [Google Scholar] [CrossRef]
- Dai, X.J.; Liu, M.Q.; Jin, H.X.; Jing, M.Y. Optimisation of solid-state fermentation of Aspergillus niger JL-15 for xylanase production and xylooligosaccharides preparation. Czech J. Food Sci. 2011, 29, 557–567. [Google Scholar] [CrossRef]
- Cano, M.E.; García-Martin, A.; Comendador Morales, P.; Wojtusik, M.; Santos, V.E.; Kovensky, J.; Ladero, M. Production of oligosaccharides from agrofood wastes. Fermentation 2020, 6, 31. [Google Scholar] [CrossRef]
- Guo, J.; Cao, R.; Huang, K.; Xu, Y. Comparison of selective acidolysis of xylan and enzymatic hydrolysability of cellulose in various lignocellulosic materials by a novel xylonic acid catalysis method. Bioresour. Technol. 2020, 304, 122943. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Mahmood, S.; Liaqat, H.; Mushtaq, A.; Khan, S.; Qi, X. Recent advances in the production, analysis, and application of galacto-oligosaccharides. Food Rev. Int. 2023, 39, 5814–5843. [Google Scholar] [CrossRef]
- Cao, R.; Liu, X.; Guo, J.; Xu, Y. Comparison of various organic acids for xylo-oligosaccharide productions in terms of pKa values and combined severity. Biotechnol. Biofuels 2021, 14, 69. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Muñiz-Mouro, A.; Lú-Chau, T.A.; Eibes, G. Valorization of horse chestnut burs to produce simultaneously valuable compounds under a green integrated biorefinery approach. Sci. Total Environ. 2020, 730, 139143. [Google Scholar] [CrossRef]
- Sulaiman, S.H.A.S. Determination of Oxidative Stress Levels and Some Antioxidant Activities in Acute and Chronic Renal Failure Patients. Master’s Thesis, Fen Bilimleri Enstitüsü, Mersin, Turkey, 2021. [Google Scholar]
- Pinales-Márquez, C.D.; Rodríguez-Jasso, R.M.; Araújo, R.G.; Loredo-Trevino, A.; Nabarlatz, D.; Gullón, B.; Ruiz, H.A. Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: A review. Ind. Crops Prod. 2021, 162, 113274. [Google Scholar] [CrossRef]
- Santibáñez, L.; Henríquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef] [PubMed]
- Dikkala, P.K.; Mummaleti, G.; Rajput, R.; Modi, R.; Sarma, C.; Himabindu, G.; Srinivas, Y.; Sharma, M.; Gupta, V.K.; Usmani, Z.; et al. Microbial Fructo-Oligosaccharides Derived from Agri-Food Waste. In Microbial Bioprocessing of Agri-food Wastes; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Antia, U.E.; Stephen, N.U.; Onilude, A.A.; Udo, I.O.M.; Amande, T.J. Bioconvertibility of mannan-containing polysaccharides to bioethanol: A comparative study of palm kernel cake and copra meal feedstocks. Biomass Convers. Biorefin. 2023, 13, 5175–5186. [Google Scholar] [CrossRef]
- Dwivedi, S.; Tanveer, A.; Yadav, S.; Anand, G.; Yadav, D. Agro-Wastes for Cost Effective Production of Industrially Important Microbial Enzymes: An Overview. In Microbial Biotechnology: Role in Ecological Sustainability and Research; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 435–460. [Google Scholar]
- Banu, R.; Kumar, G.; Gunasekaran, M.; Kavitha, S. (Eds.) Food Waste to Valuable Resources: Applications and Management; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Zhang, W.; You, Y.; Lei, F.; Li, P.; Jiang, J. Acetyl-assisted autohydrolysis of sugarcane bagasse for the production of xylo-oligosaccharides without additional chemicals. Bioresour. Technol. 2018, 265, 387–393. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Pokpong, A.; Klinchongkon, K.; Adachi, S. Production of oligosaccharides from coconut meal by subcritical water treatment. Food Sci. Technol. 2014, 49, 1946–1952. [Google Scholar] [CrossRef]
- Klinchongkon, K.; Khuwijitjaru, P.; Wiboonsirikul, J.; Adachi, S. Extraction of oligosaccharides from passion fruit peel by subcritical water treatment. J. Food Process Eng. 2017, 40, e12269. [Google Scholar] [CrossRef]
- Gómez, B.; Míguez, B.; Veiga, A.; Parajó, J.C.; Alonso, J.L. Production, purification, and in vitro evaluation of the prebiotic potential of arabinoxylooligosaccharides from brewer’s spent grain. J. Agric. Food Chem. 2015, 63, 8429–8438. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Takano, Y.; Mizuno, M.; Nozaki, K.; Umemura, S.; Matsuzawa, T.; Amano, Y.; Makishima, S. Production of feruloylated arabino-oligosaccharides (FA-AOs) from beet fiber by hydrothermal treatment. J. Supercrit. Fluids 2013, 79, 84–91. [Google Scholar] [CrossRef]
- Lama, A.; Rodríguez, G.; Rubio, F.; Fernández, J. Production, characterization and isolation of neutral and pectic oligosaccharides with low molecular weights from olive by-products thermally treated. Food Hydrocoll. 2012, 28, 92–104. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Martínez, M.; Yáñez, R.; Alonso, J.L.; Parajó, J.C. Chemical production of pectic oligosaccharides from orange peel wastes. Ind. Eng. Chem. Res. 2010, 49, 8470–8476. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic oligosacharides from lemon peel wastes: Production, Purification, and Chemical Characterization. J. Agric. Food Chem. 2013, 61, 10043–10053. [Google Scholar] [CrossRef]
- Meyer, T.S.M.; Miguel, Â.S.M.; Fernández, D.E.R.; Ortiz, G.M.D. Biotechnological production of oligosaccharides—Applications in the food industry. Food Prod. Ind. 2015, 2, 25–78. [Google Scholar]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.C.; Gomez-Zavaglia, A. Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.; Brown, L.L.; Costello, R.B.; Deuster, P.A. Select dietary supplement ingredients for preserving and protecting the immune system in healthy individuals: A systematic review. Nutrients 2022, 14, 4604. [Google Scholar] [CrossRef]
- Balzano, T. Active Clinical Trials in Hepatic Encephalopathy: Something Old, Something New and Something Borrowed. Neurochem. Res. 2023, 48, 2309–2319. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Selvakumar, S.; Vasanth, K. Unraveling the role of medicinal plants and Gut microbiota in colon cancer: Towards microbiota-based strategies for prevention and treatment. Health Sci. Rev. 2023, 9, 100115. [Google Scholar] [CrossRef]
- Song, H.; Guo, R.; Sun, X.; Kou, Y.; Ma, X.; Chen, Y.; Wu, Y. Xylooligosaccharides from corn cobs alleviate loperamide-induced constipation in mice via modulation of gut microbiota and SCFA metabolism. Food Funct. 2023, 14, 8734–8746. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef]
- Mojikon, F.D.; Kasimin, M.E.; Molujin, A.M.; Gansau, J.A.; Jawan, R. Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products. Nutrients 2022, 14, 3457. [Google Scholar] [CrossRef]
- Ajibola, O.O.; Thomas, R.; Bakare, B.F. Selected fermented indigenous vegetables and fruits from Malaysia as potential sources of natural probiotics for improving gut health. Food Sci. Hum. Wellness 2023, 12, 1493–1509. [Google Scholar] [CrossRef]
- Kherade, M.; Solanke, S.; Tawar, M.; Wankhede, S. Fructooligosaccharides: A comprehensive review. J. Ayurvedic Herb. Med. 2021, 7, 193–200. [Google Scholar] [CrossRef]
- Steinbach, E. Investigation of the Duodenojejunal Microbiome in Human Obesity. Ph.D. Dissertation, Sorbonne Université, Paris, France, 2023. [Google Scholar]
- Basavaiah, R.; Gurudutt, P.S. Prebiotic Carbohydrates for Therapeutics. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2021, 21, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.B.; Amorim, C.; Silvério, S.C.; Rodrigues, L.R. Novel and emerging prebiotics: Advances and opportunities. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2021; Volume 95, pp. 41–95. [Google Scholar]
- Salinas, E.; Reyes-Pavón, D.; Cortes-Perez, N.G.; Torres-Maravilla, E.; Bitzer-Quintero, O.K.; Langella, P.; Bermúdez-Humarán, L.G. Bioactive compounds in food as a current therapeutic approach to maintain a healthy intestinal epithelium. Microorganisms 2021, 9, 1634. [Google Scholar] [CrossRef]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, T.; Meng, Y.; Hu, M.; Shu, L.; Jiang, H.; Zhou, X. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism 2021, 119, 154767. [Google Scholar] [CrossRef]
- Barbalho, R.L.D.C.; Castaneda, C.; Araújo, L.F.; Kiess, A.S.; Carvalho, R.S.; Barbalho, C.B.; Bonato, M.A. Β-glucans and MOS, essential oil, and probiotics in diets of broilers challenged with Eimeria spp. and Clostridium perfringens. Poult. Sci. 2023, 102, 102541. [Google Scholar] [CrossRef]
- Hazrati, S.; Rezaeipour, V.; Asadzadeh, S. Effects of phytogenic feed additives, probiotic and mannan-oligosaccharides on performance, blood metabolites, meat quality, intestinal morphology, and microbial population of Japanese quail. Br. Poult. Sci. 2020, 61, 132–139. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R. Ability of a Propionate-Producing Synthetic Microbial Consortium to Restore Functionality in a Dysbiosed Human gut Microbiome. Ph.D. Dissertation, Ghent University, Ghent, Belgium, 2019. [Google Scholar]
- Ojwach, J.; Adetunji, A.I.; Mutanda, T.; Mukaratirwa, S. Oligosaccharides production from coprophilous fungi: An emerging functional food with potential health-promoting properties. Biotechnol. Rep. 2022, 33, e00702. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Mohapatra, S.; Oh, J.H.; Astmann, T.; van Pijkeren, J.P.; Pan, X. Novel galacto-oligosaccharides from lactose: Chemical synthesis, structural characterization, and in vitro assessment of prebiotic activity. ACS Sustain. Chem. Eng. 2023, 11, 14031–14045. [Google Scholar] [CrossRef]
- Vera, C.; Córdova, A.; Aburto, C.; Guerrero, C.; Suárez, S.; Illanes, A. Synthesis and purification of galacto-oligosaccharides: State of the art. World J. Microbiol. Biotechnol. 2016, 32, 197. [Google Scholar] [CrossRef]
- Rico-Rodríguez, F.; Villamiel, M.; Ruiz-Aceituno, L.; Serrato, J.C.; Montilla, A. Effect of the lactose source on the ultrasound-assisted enzymatic production of galactooligosaccharides and gluconic acid. Ultrason. Sonochem. 2020, 67, 104945. [Google Scholar] [CrossRef] [PubMed]
- Perotti, M.C.; Vénica, C.I.; Wolf, I.V.; Vélez, M.A.; Peralta, G.H.; Quiberoni, A.; Bergamini, C.V. Biomolecules Derived from Whey: Strategies for Production and Biological Properties. In Biomolecules from Natural Sources: Advances and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 390–432. [Google Scholar]
- Ureta, M.M.; Martins, G.N.; Figueira, O.; Pires, P.F.; Castilho, P.C.; Gomez-Zavaglia, A. Recent advances in β-galactosidase and fructosyltransferase immobilization technology. Crit. Rev. Food Sci. Nutr. 2021, 61, 2659–2690. [Google Scholar] [CrossRef] [PubMed]
- Todea, A.; Boeriu, C.G.; Peter, F.; Biró, E. Immobilized β-d-galactosidases for improved synthesis of short-chain galacto-oligosaccharides. In Biotechnological Progress and Beverage Consumption; Academic Press: Cambridge, MA, USA, 2020; pp. 71–110. [Google Scholar]
- Yang, X.D.; Wang, L.K.; Wu, H.Y.; Jiao, L. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiol. 2018, 18, 177. [Google Scholar] [CrossRef]
- Hackenhaar, C.R.; Spolidoro, L.S.; Flores, E.E.E.; Klein, M.P.; Hertz, P.F. Batch synthesis of galactooligosaccharides from co-products of milk processing using immobilized β-galactosidase from Bacillus circulans. Biocatal. Agric. Biotechnol. 2021, 36, 102136. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Pham, M.L.; Tran, A.M.; Nguyen, T.H. β-Galactosidase from Lactobacillus helveticus DSM 20075: Biochemical characterization and recombinant expression for applications in dairy industry. Int. J. Mol. Sci. 2019, 20, 947. [Google Scholar] [CrossRef] [PubMed]
- Arsov, A.; Ivanov, I.; Tsigoriyna, L.; Petrov, K.; Petrova, P. In Vitro Production of Galactooligosaccharides by a Novel β-Galactosidase of Lactobacillus bulgaricus. Int. J. Mol. Sci. 2022, 23, 14308. [Google Scholar] [CrossRef]
- Delgado-Fernandez, P.; Plaza-Vinuesa, L.; Hernandez-Hernandez, O.; de Las Rivas, B.; Corzo, N.; Muñoz, R.; Moreno, F.J. Unravelling the carbohydrate specificity of MelA from Lactobacillus plantarum WCFS1: An α-galactosidase displaying regioselective transgalactosylation. Int. J. Biol. Macromol. 2020, 153, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Akyon, Y.; Aydin-Kose, F.; Karagozlu, C.; Ozgen, A.G.; Buyuktuncer, Z. Effects of regular kefir consumption on gut microbiota in patients with metabolic syndrome: A parallel-group, randomized, controlled study. Nutrients 2019, 11, 2089. [Google Scholar] [CrossRef]
- Fai, A.E.C.; da Silva, J.B.; de Andrade, C.J.; Bution, M.L.; Pastore, G.M. Production of prebiotic galactooligosaccharides from lactose by Pseudozyma tsukubaensis and Pichia kluyveri. Biocatal. Agric. Biotechnol. 2014, 3, 343–350. [Google Scholar] [CrossRef]
- Usvalampi, A.; Maaheimo, H.; Tossavainen, O.; Frey, A.D. Enzymatic synthesis of fucose-containing galacto-oligosaccharides using β-galactosidase and identification of novel disaccharide structures. Glycoconj. J. 2018, 35, 31–40. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Abbaspourrad, A. Production of galacto-oligosaccharides from whey permeate using β-galactosidase immobilized on functionalized glass beads. Food Chem. 2018, 251, 115–124. [Google Scholar] [CrossRef]
- Carević, M.; Vukašinović-Sekulić, M.; Ćorović, M.; Rogniaux, H.; Ropartz, D.; Veličković, D.; Bezbradica, D. Evaluation of β-galactosidase from Lactobacillus acidophilus as biocatalyst for galacto-oligosaccharides synthesis: Product structural characterization and enzyme immobilization. J. Biosci. Bioeng. 2018, 126, 697–704. [Google Scholar] [CrossRef]
- Yang, J.; Gao, R.; Zhou, Y.; Anankanbil, S.; Li, J.; Xie, G.; Guo, Z. β-Glucosidase from Thermotoga naphthophila RKU-10 for exclusive synthesis of galactotrisaccharides: Kinetics and thermodynamics insight into reaction mechanism. Food Chem. 2018, 240, 422–429. [Google Scholar] [CrossRef]
- Botelho-Cunha, V.A.; Mateus, M.; Petrus, J.C.C.; de Pinho, M.N. Tailoring the enzymatic synthesis and nanofiltration fractionation of galacto-oligosaccharides. Biochem. Eng. J. 2010, 50, 29–36. [Google Scholar] [CrossRef]
- Vera, C.; Guerrero, C.; Conejeros, R.; Illanes, A. Synthesis of galactooligosaccharides by β-galactosidase from Aspergillus oryzae using partially dissolved and supersaturated solution of lactose. Enzym. Microb. Technol. 2012, 50, 188–194. [Google Scholar] [CrossRef]
- Jovanovic-Malinovska, R.; Fernandes, P.; Winkelhausen, E.; Fonseca, L. Galacto-oligosaccharides synthesis from lactose and whey by β-galactosidase immobilized in PVA. Appl. Biochem. Biotechnol. 2012, 168, 1197–1211. [Google Scholar] [CrossRef]
- Oh, S.Y.; Park, M.S.; Lee, Y.G.; Thi, N.N.; Baek, N.I.; Ji, G.E. Enzymatic synthesis of β-galactosyl fucose using recombinant bifidobacterial β-galactosidase and its prebiotic effect. Glycoconj. J. 2019, 36, 199–209. [Google Scholar] [CrossRef]
- Kumar, N.V.; Sawargaonkar, G.L.; Rani, C.S.; Singh, A.; Prakash, T.R.; Triveni, S.; Venkatesh, B. Comparative Analysis of Pigeonpea Stalk Biochar Characteristics and Energy Use under Different Biochar Production Methods. Sustainability 2023, 15, 14394. [Google Scholar] [CrossRef]
- Dahiya, S.; Rapoport, A.; Singh, B. Biotechnological Potential of Lignocellulosic Biomass as Substrates for Fungal Xylanases and Its Bioconversion into Useful Products: A Review. Fermentation 2024, 10, 82. [Google Scholar] [CrossRef]
- Peng, P.; Peng, F.; Bian, J.; Xu, F.; Sun, R. Studies on the starch and hemicelluloses fractionated by graded ethanol precipitation from bamboo Phyllostachys bambusoides f. shouzhu Yi. J. Agric. Food Chem. 2011, 59, 2680–2688. [Google Scholar] [CrossRef]
- Samanta, A.K.; Kolte, A.P.; Elangovan, A.V.; Dhali, A.; Senani, S.; Sridhar, M.; Roy, S. Value addition of corn husks through enzymatic production of xylooligosaccharides. Braz. Arch. Biol. Technol. 2016, 59, e16160078. [Google Scholar] [CrossRef]
- Jnawali, P.; Kumar, V.; Tanwar, B.; Hirdyani, H.; Gupta, P. Enzymatic production of xylooligosaccharides from brown coconut husk treated with sodium hydroxide. Waste Biomass Valorization 2018, 9, 1757–1766. [Google Scholar] [CrossRef]
- Miao, Y.; Li, P.; Li, G.; Liu, D.; Druzhinina, I.S.; Kubicek, C.P.; Zhang, R. Two degradation strategies for overcoming the recalcitrance of natural lignocellulosic xylan by polysaccharides-binding GH 10 and GH 11 xylanases of filamentous fungi. Environ. Microbiol. 2017, 19, 1054–1064. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mousavi, S.M. RSM based optimization of PEG assisted ionic liquid pretreatment of sugarcane bagasse for enhanced bioethanol production: Effect of process parameters. Biomass Bioenergy 2018, 116, 89–98. [Google Scholar] [CrossRef]
- Pu, J.; Zhao, X.; Wang, Q.; Wang, Y.; Zhou, H. Development and validation of a HPLC method for determination of degree of polymerization of xylo-oligosaccharides. Food Chem. 2016, 213, 654–659. [Google Scholar] [CrossRef]
- Chapla, D.; Pandit, P.; Shah, A. Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour. Technol. 2012, 115, 215–221. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.; Gao, J.; Li, X.; Zhao, J. Xylo-oligosaccharides inhibit enzymatic hydrolysis by influencing enzymatic activity of cellulase from Penicillium oxalicum. Energy Fuels 2018, 32, 9427–9437. [Google Scholar] [CrossRef]
- Dhiman, S.; Mukherjee, G. Recent advances and industrial applications of microbial xylanases: A review. In Fungi and Their Role in Sustainable Development: Current Perspectives; Springer: Singapore, 2018; pp. 329–348. [Google Scholar]
- da Silva Menezes, B.; Rossi, D.M.; Squina, F.; Ayub, M.A.Z. Comparative production of xylanase and the liberation of xylooligosaccharides from lignocellulosic biomass by Aspergillus brasiliensis BLf1 and recombinant Aspergillus nidulans XynC A773. Int. J. Food Sci. Technol. 2018, 53, 2110–2118. [Google Scholar] [CrossRef]
- Adsul, M.G.; Bastawde, K.B.; Gokhale, D.V. Biochemical characterization of two xylanases from yeast Pseudozyma hubeiensis producing only xylooligosaccharides. Bioresour. Technol. 2009, 100, 6488–6495. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Tian, R.; Bian, J.; Peng, F. Hydrothermal pretreatment for the production of oligosaccharides: A review. Bioresour. Technol. 2022, 343, 126075. [Google Scholar] [CrossRef]
- Alawiye, T.T.; Babalola, O.O. Metagenomic insight into the community structure and functional genes in the sunflower rhizosphere microbiome. Agriculture 2021, 11, 167. [Google Scholar] [CrossRef]
- Reddy, S.S.; Krishnan, C. Production of xylooligosaccharides in SSF by Bacillus subtilis KCX006 producing β-xylosidase-free endo-xylanase and multiple xylan debranching enzymes. Prep. Biochem. Biotechnol. 2016, 46, 49–55. [Google Scholar] [CrossRef]
- Boonchuay, P.; Takenaka, S.; Kuntiya, A.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T. Purification, characterization, and molecular cloning of the xylanase from Streptomyces thermovulgaris TISTR1948 and its application to xylooligosaccharide production. J. Mol. Catal. B Enzym. 2016, 129, 61–68. [Google Scholar] [CrossRef]
- Sun, M.-Z.; Zheng, H.-C.; Meng, L.-C.; Sun, J.-S.; Song, H.; Bao, Y.-J.; Pei, H.-S.; Yan, Z.; Zhang, X.-Q.; Zhang, J.-S.; et al. Direct cloning, expression of a thermostable xylanase gene from the metagenomic DNA of cow dung compost and enzymatic production of xylooligosaccharides from corncob. Biotechnol. Lett. 2015, 37, 1877–1886. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Seesuriyachan, P.; Chaiyaso, T. Production of xylooligosaccharides from corncob using a crude thermostable endo-xylanase from Streptomyces thermovulgaris TISTR1948 and prebiotic properties. Food Sci. Biotechnol. 2014, 23, 1515–1523. [Google Scholar] [CrossRef]
- Bian, J.; Peng, F.; Peng, X.-P.; Peng, P.; Xu, F.; Sun, R.-C. Structural features and antioxidant activity of xylooligosaccharides enzymatically produced from sugarcane bagasse. Bioresour. Technol. 2013, 127, 236–241. [Google Scholar] [CrossRef]
- Wang, T.H.; Lu, S. Production of xylooligosaccharide from wheat bran by microwave assisted enzymatic hydrolysis. Food Chem. 2013, 138, 1531–1535. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Kolte, A.P.; Senani, S.; Sridhar, M.; Mishra, S.; Prasad, C.S.; Suresh, K.P. Application of pigeon pea (Cajanus cajan) stalks as raw material for xylooligosaccharides production. Appl. Biochem. Biotechnol. 2013, 169, 2392–2404. [Google Scholar] [CrossRef]
- Jayapal, N.; Samanta, A.K.; Kolte, A.P.; Senani, S.; Sridhar, M.; Suresh, K.P.; Sampath, K.T. Value addition to sugarcane bagasse: Xylan extraction and its process optimization for xylooligosaccharides production. Ind. Crops Prod. 2013, 42, 14–24. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Kolte, A.P.; Senani, S.; Sridhar, M.; Dhali, A.; Suresh, K.P.; Jayaram, C.; Prasad, C.S. Process for enzymatic production of xylooligosaccharides from the xylan of corn cobs. J. Food Process. Preserv. 2015, 39, 729–736. [Google Scholar] [CrossRef]
- Khat-Udomkiri, N.; Sivamaruthi, B.S.; Sirilun, S.; Lailerd, N.; Peerajan, S.; Chaiyasut, C. Optimization of alkaline pretreatment and enzymatic hydrolysis for the extraction of xylooligosaccharide from rice husk. AMB Express 2018, 8, 115. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, R.P.; Kennedy, J.F. Recent insights in enzymatic synthesis of fructooligosaccharides from inulin. Int. J. Biol. Macromol. 2016, 85, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Narisetty, V.; Parhi, P.; Mohan, B.; Hazeena, S.H.; Kumar, A.N.; Gullón, B.; Binod, P. Valorization of renewable resources to functional oligosaccharides: Recent trends and future prospective. Bioresour. Technol. 2022, 346, 126590. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, T.; Kennedy, J.F. Enzymatic synthesis of fructooligosaccharides from inulin in a batch system. Carbohydr. Polym. Technol. Appl. 2020, 1, 100009. [Google Scholar] [CrossRef]
- Mohan, C.O.; Carvajal-Millan, E.; Ravishankar, C.N. Current Trends in the Biotechnological Production of Fructooligosaccharides. In Research Methodology in Food Sciences; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 199–220. [Google Scholar]
- Smaali, I.; Jazzar, S.; Soussi, A.; Muzard, M.; Aubry, N.; Marzouki, M.N. Enzymatic synthesis of fructooligosaccharides from date by-products using an immobilized crude enzyme preparation of β-D-fructofuranosidase from Aspergillus awamori NBRC 4033. Biotechnol. Bioprocess Eng. 2012, 17, 385–392. [Google Scholar] [CrossRef]
- Bahlawan, R.; Karboune, S.; Liu, L.; Sahyoun, A.M. Investigation of Biocatalytic Production of Lactosucrose and Fructooligosaccharides Using Levansucrases and Dairy By-products as Starting Materials. Enzym. Microb. Technol. 2023, 169, 110279. [Google Scholar] [CrossRef]
- Díez-Municio, M.; de las Rivas, B.; Jimeno, M.L.; Muñoz, R.; Moreno, F.J.; Herrero, M. Enzymatic synthesis and characterization of fructooligosaccharides and novel maltosylfructosides by inulosucrase from Lactobacillus gasseri DSM 20604. Appl. Environ. Microbiol. 2013, 79, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Karboune, S.; Hill, A. Synthesis of fructooligosaccharides and oligolevans by the combined use of levansucrase and endo-inulinase in one-step bi-enzymatic system. Innov. Food Sci. Emerg. Technol. 2014, 22, 230–238. [Google Scholar] [CrossRef]
- Soliman, A.B.; Hassan, M.H.; Huan, T.N.; Abugable, A.A.; Elmehalmey, W.A.; Karakalos, S.G.; Alkordi, M.H. Pt Immobilization within a Tailored Porous-Organic Polymer–Graphene Composite: Opportunities in the Hydrogen Evolving Reaction. ACS Catal. 2017, 7, 7847–7854. [Google Scholar] [CrossRef]
- de Siqueira, E.C.; Öner, E.T. Co-production of levan with other high-value bioproducts: A review. Int. J. Biol. Macromol. 2023, 235, 123800. [Google Scholar] [CrossRef]
- Huang, M.P.; Wu, M.; Xu, Q.S.; Mo, D.J.; Feng, J.X. Highly efficient synthesis of fructooligosaccharides by extracellular fructooligosaccharide-producing enzymes and immobilized cells of Aspergillus aculeatus M105 and purification and biochemical characterization of a fructosyltransferase from the fungus. J. Agric. Food Chem. 2016, 64, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cardeña, A.; Rodríguez-Alegría, M.E.; Olvera, C.; Munguía, A.L. Synthesis of Fructooligosaccharides by IslA4, a truncated inulosucrase from Leuconostoc citreum. BMC Biotechnol. 2015, 15, 2. [Google Scholar] [CrossRef][Green Version]
- Kralj, S.; Leeflang, C.; Sierra, E.I.; Kempiński, B.; Alkan, V.; Kolkman, M. Synthesis of fructooligosaccharides (FosA) and inulin (InuO) by GH68 fructosyltransferases from Bacillus agaradhaerens strain WDG185. Carbohydr. Polym. 2018, 179, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C. Medium improvement for β-fructofuranosidase production by Aspergillus japonicus. Process Biochem. 1998, 33, 267–271. [Google Scholar] [CrossRef]
- Hirayama, M.; Sumi, N.; Hidaka, H. Purification and properties of a fructooligosaccharide-producing β-fructofuranosidase from Aspergillus niger ATCC 20611. Agric. Biol. Chem. 1989, 53, 667–673. [Google Scholar] [CrossRef][Green Version]
- Patil, M.; Ashwin, B. Fructosyltransferase production by indigenously isolated Syncephalastrum racemosum Cohn. J. Glob. Biosci. 2014, 3, 597–603. [Google Scholar]
- Bhatia, L.; Sharma, A.; Bachheti, R.K.; Chandel, A.K. Lignocellulose derived functional oligosaccharides: Production, properties, and health benefits. Prep. Biochem. Biotechnol. 2019, 49, 744–758. [Google Scholar] [CrossRef]
- Usami, S.; Ishii, T.; Kirimura, K.; Uehara, K.I.; Chen, J. Production of β-fructofuranosidase showing fructose-transferring activity by Penicillium frequentans (P. glabrum). J. Ferment. Bioeng. 1991, 72, 303–305. [Google Scholar] [CrossRef]
- Barthomeuf, C.; Pourrat, H. Production of high-content fructo-oligosaccharides by an enzymatic system from Penicillium rugulosum. Biotechnol. Lett. 1995, 17, 911–916. [Google Scholar] [CrossRef]
- Yun, J.W.; Kim, D.H.; Moon, H.Y.; Song, C.H.; Song, S.K. Simultaneous formation of fructosyltransferase and glucosyltransferase in Aureobasidium pullulans. J. Microbiol. Biotechnol. 1997, 7, 204–208. [Google Scholar]
- Hayashi, S.; Ito, K.; Nonoguchi, M.; Takasaki, Y.; Imada, K. Immobilization of a fructosyl-transferring enzyme from Aureobasidium sp. on shirasu porous glass. J. Ferment. Bioeng. 1991, 72, 68–70. [Google Scholar] [CrossRef]
- Fujita, K.; Hara, K.; Hashimoto, H.; Kitahata, S. Transfructosylation catalyzed by β-fructofuranosidase I from Arthrobacter sp. K-1. Agric. Biol. Chem. 1990, 54, 2655–2661. [Google Scholar] [CrossRef]
- de Menezes, C.R.; Silva, Í.S.; Pavarina, É.C.; Guímaro Dias, E.F.; Guímaro Dias, F.; Grossman, M.J.; Durrant, L.R. Production of xylooligosaccharides from enzymatic hydrolysis of xylan by the white-rot fungi Pleurotus. Int. Biodeterior. Biodegrad. 2009, 63, 673–678. [Google Scholar] [CrossRef]
- Bersaneti, G.T.; Pan, N.C.; Baldo, C.; Celligoi, M.A.P.C. Co-production of Fructooligosaccharides and Levan by Levansucrase from Bacillus subtilis natto with Potential Application in the Food Industry. Appl. Biochem. Biotechnol. 2018, 184, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Rigo, D.; Mossi, V.; Golunski, S.; de Oliveira Kuhn, G.; Di Luccio, M.; Dallago, R.; de Oliveira, D.; Oliveira, J.V.; Treichel, H. Enzymatic synthesis of fructooligosaccharides by inulinases from Aspergillus niger and Kluyveromyces marxianus NRRL Y-7571 in aqueous–organic medium. Food Chem. 2013, 138, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.K.C.; Nobre, C.; Cavalcanti, M.T.H.; Teixeira, J.A.; Porto, A.L.F. Screening of fungi from the genus Penicillium for production of β-fructofuranosidase and enzymatic synthesis of fructooligosaccharides. J. Mol. Catal. B Enzym. 2016, 134, 70–78. [Google Scholar] [CrossRef][Green Version]
- Karboune, S.; Appanah, N.; Khodaei, N.; Tian, F. Enzymatic synthesis of fructooligosaccharides from sucrose by endo-inulinase-catalyzed transfructosylation reaction in biphasic systems. Process Biochem. 2018, 69, 82–91. [Google Scholar] [CrossRef]
- Kuhn, G.d.O.; Silva, M.F.; Mulinari, J.; Golunski, S.; Dallago, R.M.; Dalla Rosa, C.; Val´erio, A.; Oliveira, D.d.; Oliveira, J.V.; Mossi, A.J.; et al. Aspergillus niger inulinase immobilized in polyurethane foam and treated in pressurized LPG: A potential catalyst for enzymatic synthesis of fructooligosaccharides. Biocatal. Biotransform. 2016, 34, 291–294. [Google Scholar] [CrossRef]
- Aung, T.; Jiang, H.; Liu, G.L.; Chi, Z.; Hu, Z.; Chi, Z.M. Overproduction of a β-fructofuranosidase1 with a high FOS synthesis activity for efficient biosynthesis of fructooligosaccharides. Int. J. Biol. Macromol. 2019, 130, 988–996. [Google Scholar] [CrossRef]
- Sathitkowitchai, W.; Sathapondecha, P.; Angthong, P.; Srimarut, Y.; Malila, Y.; Nakkongkam, W.; Rungrassamee, W. Isolation and Characterization of Mannanase-Producing Bacteria for Potential Synbiotic Application in Shrimp Farming. Animals 2022, 12, 2583. [Google Scholar] [CrossRef]
- Ghosh, K.; Ray, M.; Adak, A.; Halder, S.K.; Das, A.; Jana, A.; Mondal, K.C. Role of probiotic Lactobacillus fermentum KKL1 in the preparation of a rice based fermented beverage. Bioresour. Technol. 2015, 188, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jana, U.K.; Suryawanshi, R.K.; Prajapati, B.P.; Soni, H.; Kango, N. Production optimization and characterization of mannooligosaccharide generating β-mannanase from Aspergillus oryzae. Bioresour. Technol. 2018, 268, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Cescutti, P.; Campa, C.; Delben, F.; Rizzo, R. Structure of the oligomers obtained by enzymatic hydrolysis of the glucomannan produced by the plant Amorphophallus konjac. Carbohydr. Res. 2002, 337, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, H.; Virkki, L.; Tuomainen, P.; Kabel, M.; Schols, H.; Tenkanen, M. Preparation of arabinoxylobiose from rye xylan using family 10 Aspergillus aculeatus endo-1, 4-β-D-xylanase. Carbohydr. Polym. 2007, 68, 350–359. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.; Ling, N.; Yuan, Y.; Zheng, X.; Shen, B.; Shen, Q. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils 2011, 47, 495–506. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.L.; Zhang, Z.H.; Sun, C.Y.; Chen, L.L.; He, H.L.; Zhou, B.C.; Zhang, Y.Z. Purification and functional characterization of endo-β-mannanase MAN5 and its application in oligosaccharide production from konjac flour. Appl. Microbiol. Biotechnol. 2009, 83, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Blibech, M.; Chaari, F.; Bhiri, F.; Dammak, I.; Ghorbel, R.E.; Chaabouni, S.E. Production of manno-oligosaccharides from locust bean gum using immobilized Penicillium occitanis mannanase. J. Mol. Catal. B Enzym. 2011, 73, 111–115. [Google Scholar] [CrossRef]
- Oda, Y.; Tonomura, K. Characterization of β-mannanase and β-mannosidase secreted from the yeast Trichosporon cutaneum JCM 2947. Lett. Appl. Microbiol. 1996, 22, 173–178. [Google Scholar] [CrossRef]
- Ghosh, A.; Verma, A.K.; Tingirikari, J.R.; Shukla, R.; Goyal, A. Recovery and purification of oligosaccharides from copra meal by recombinant endo-β-mannanase and deciphering molecular mechanism involved and its role as potent therapeutic agent. Mol. Biotechnol. 2015, 57, 111–127. [Google Scholar] [CrossRef]
- Jian, H.-L.; Zhu, L.-W.; Zhang, W.-M.; Sun, D.-F.; Jiang, J.-X. Enzymatic production and characterization of manno-oligosaccharides from Gleditsia sinensis galactomannan gum. Int. J. Biol. Macromol. 2013, 55, 282–288. [Google Scholar] [CrossRef]
- Albrecht, S.; van Muiswinkel, G.C.J.; Xu, J.; Schols, H.A.; Voragen, A.G.J.; Gruppen, H. Enzymatic production and characterization of konjac glucomannan oligosaccharides. J. Agric. Food Chem. 2011, 59, 12658–12666. [Google Scholar] [CrossRef]
- Yang, J.K.; Chen, Q.C.; Zhou, B.; Wang, X.J.; Liu, S.Q. Manno-oligosaccharide preparation by the hydrolysis of konjac flour with a thermostable endo-mannanase from Talaromyces cellulolyticus. J. Appl. Microbiol. 2019, 127, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Nopvichai, C.; Charoenwongpaiboon, T.; Luengluepunya, N.; Ito, K.; Muanprasat, C.; Pichyangkura, R. Production and purification of mannan oligosaccharide with epithelial tight junction enhancing activity. Peer J. 2019, 7, e7206. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, M.C.; Honorato, T.L.; Gonçalves, L.R.B.; Pinto, G.A.S.; Rodrigues, S. Optimization of enzymatic synthesis of isomalto-oligosaccharides production. J. Food Biochem. 2009, 33, 342–354. [Google Scholar] [CrossRef][Green Version]
- Sorndech, W.; Nakorn, K.N.; Tongta, S.; Blennow, A. Isomalto-oligosaccharides: Recent insights in production technology and their use for food and medical applications. LWT 2018, 95, 135–142. [Google Scholar] [CrossRef]
- Saman, P.; Chaiongkarn, A.; Moonmangmee, S.; Artjariyasripong, S. Prebiotic isomalto-oligosaccharide production from economic crops of Thailand. Asia-Pac. J. Sci. Technol. 2012, 17, 794–799. [Google Scholar]
- Basu, A.; Mutturi, S.; Prapulla, S.G. Production of isomaltooligosaccharides (IMO) using simultaneous saccharification and transglucosylation from starch and sustainable sources. Process Biochem. 2016, 51, 1464–1471. [Google Scholar] [CrossRef]
- Basu, A.; Mutturi, S.; Prapulla, S.G. Modeling of enzymatic production of isomaltooligosaccharides: A mechanistic approach. Catal. Sci. Technol. 2015, 5, 2945–2958. [Google Scholar] [CrossRef]
- Shinde, V.K.; Vamkudoth, K.R. Maltooligosaccharide forming amylases and their applications in food and pharma industry. J. Food Sci. Technol. 2022, 59, 3733–3744. [Google Scholar] [CrossRef]
- Plongbunjong, V.; Graidist, P.; Knudsen, K.E.; Wichienchot, S. Isomaltooligosaccharide synthesised from rice starch and its prebiotic properties in vitro. Int. J. Food Sci. Technol. 2017, 52, 2589–2595. [Google Scholar] [CrossRef]
- Cui, J.; Li, Y.; Wang, Q.; Li, J.; Ou, Y.; Wang, J.; Wang, W. Production, purification and analysis of the isomalto-oligosaccharides from Chinese chestnut (Castanea mollissima Blume) and the prebiotics effects of them on proliferation of Lactobacillus. Food Bioprod. Process. 2017, 106, 75–81. [Google Scholar] [CrossRef]
- Prapulla, S.G.; Subhaprada, V.; Karanth, N.G. Microbial production of oligosaccharides: A review. Adv. Appl. Microbiol. 2000, 47, 299–343. [Google Scholar] [PubMed]
- Casa-Villegas, M.; Marín-Navarro, J.; Polaina, J. Synergies in coupled hydrolysis and fermentation of cellulose using a Trichoderma reesei enzyme preparation and a recombinant Saccharomyces cerevisiae strain. World J. Microbiol. Biotechnol. 2017, 33, 140. [Google Scholar] [CrossRef] [PubMed]
- Saman, P.; Kuancha, C.; Chaiongkarn, A.; Moonmangmee, S.; Fungsin, B. Isomalto-oligosaccharides production from rice flour and cassava starch. J. Food Sci. Agric. Technol. (JFAT) 2019, 5, 188–192. [Google Scholar]
- Kaulpiboon, J.; Rudeekulthamrong, P.; Watanasatitarpa, S.; Ito, K.; Pongsawasdi, P. Synthesis of long-chain isomaltooligosaccharides from tapioca starch and an in vitro investigation of their prebiotic properties. J. Mol. Catal. B Enzym. 2015, 120, 127–135. [Google Scholar] [CrossRef]
- Sorndech, W. Isomaltooligosaccharides as prebiotics and their health benefits. In Probiotics, Prebiotics and Synbiotics: Technological Advancements Towards Safety and Industrial Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 361–377. [Google Scholar]
- Chockchaisawasdee, S.; Poosaran, N. Production of isomaltooligosaccharides from banana flour. J. Sci. Food Agric. 2013, 93, 180–186. [Google Scholar] [CrossRef]


| SNo | Source of Enzyme | Yield | Source of Xylan | Reference |
|---|---|---|---|---|
| 1 | B. megaterium MS941 | Enhancement 80% | Corn cob | [110] |
| 2 | S. thermovulgaris | 162.97 mg/g | Corn cob | [111] |
| 3 | P. stipites | 31.80% | Sugarcane bagasse | [112,113] |
| 4 | Enzyme applied in the production of xylooligosaccharides (XOS) was sourced from B. subtilis | A yield of 3.2 g of xylooligosaccharides (XOS) was obtained from 50 g of wheat bran | Wheat bran | [114] |
| 5 | B. subtilis | xylobiose 68.48 mg/g | Sugarcane bagasse | [108] |
| 6 | T. viridae | xylobiose 96% | Pigeon pea | [114] |
| 7 | T.a viridae | xylobiose 0.502 mg/mL | Pigeon pea | [115] |
| 8 | T. viridae | xylobiose 1.208 mg/mL | Corn cob | [116,117] |
| Sno | Source of Enzyme | Yield | Process | Reference |
|---|---|---|---|---|
| 1 | L. gasseri | 45% | Inulosucrase enzyme | [140] |
| 2 | A. awamori | 123 g/L | β-fructofuranosidase enzyme immobilized on chitosan | [140] |
| 3 | B. subtilis | 41 g/L | Levansucrase enzyme | [141] |
| 4 | K. mycesmarxianus | kestose (12%), nystose (21%) | Inulinase enzyme | [142] |
| 5 | P. citreonigrum | 59 g/L | β-fructofuranosidase enzyme | [143] |
| 6 | A. niger | 60% | Endoinulinase Enzyme | [144] |
| 7 | A. niger | 31% | Inulinase enzyme | [145,146] |
| SNo | Source of Enzyme | Yield | Source of Mannan | Reference |
|---|---|---|---|---|
| 1 | C. thermocellum | 40% Mannobiose and 18% Mannotriose | Copra meal | [156] |
| 2 | G. sinensis | 29.1 g/L | Galactomannan gum | [157] |
| 3 | T. viridae | Trimers (27%), Tetramers (6%), and Pentamers (3%) | Konjac glucomannan | [158] |
| 4 | T. cellulolyticus | 71.2% | Konjac flour | [159] |
| 5 | B. subtilis | 8.25% | Copra meal | [160] |
| SNo | Substrate | Yield | Reference |
|---|---|---|---|
| 1 | Nonglutinous rice flour | 169 g/L | [171] |
| 2 | Potato processing waste | 93 g/L | [172] |
| 3 | Tapioca starch | 68 g/L | [173] |
| 4 | Banana flour | 77 g/L | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelliah, R.; Kim, N.H.; Park, S.; Park, Y.; Yeon, S.-J.; Barathikannan, K.; Vijayalakshmi, S.; Oh, D.-H. Revolutionizing Renewable Resources: Cutting-Edge Trends and Future Prospects in the Valorization of Oligosaccharides. Fermentation 2024, 10, 195. https://doi.org/10.3390/fermentation10040195
Chelliah R, Kim NH, Park S, Park Y, Yeon S-J, Barathikannan K, Vijayalakshmi S, Oh D-H. Revolutionizing Renewable Resources: Cutting-Edge Trends and Future Prospects in the Valorization of Oligosaccharides. Fermentation. 2024; 10(4):195. https://doi.org/10.3390/fermentation10040195
Chicago/Turabian StyleChelliah, Ramachandran, Nam Hyeon Kim, SeonJu Park, Younseo Park, Su-Jung Yeon, Kaliyan Barathikannan, Selvakumar Vijayalakshmi, and Deog-Hwan Oh. 2024. "Revolutionizing Renewable Resources: Cutting-Edge Trends and Future Prospects in the Valorization of Oligosaccharides" Fermentation 10, no. 4: 195. https://doi.org/10.3390/fermentation10040195
APA StyleChelliah, R., Kim, N. H., Park, S., Park, Y., Yeon, S.-J., Barathikannan, K., Vijayalakshmi, S., & Oh, D.-H. (2024). Revolutionizing Renewable Resources: Cutting-Edge Trends and Future Prospects in the Valorization of Oligosaccharides. Fermentation, 10(4), 195. https://doi.org/10.3390/fermentation10040195






