Current Advances in Carotenoid Production by Rhodotorula sp.
Abstract
1. Introduction
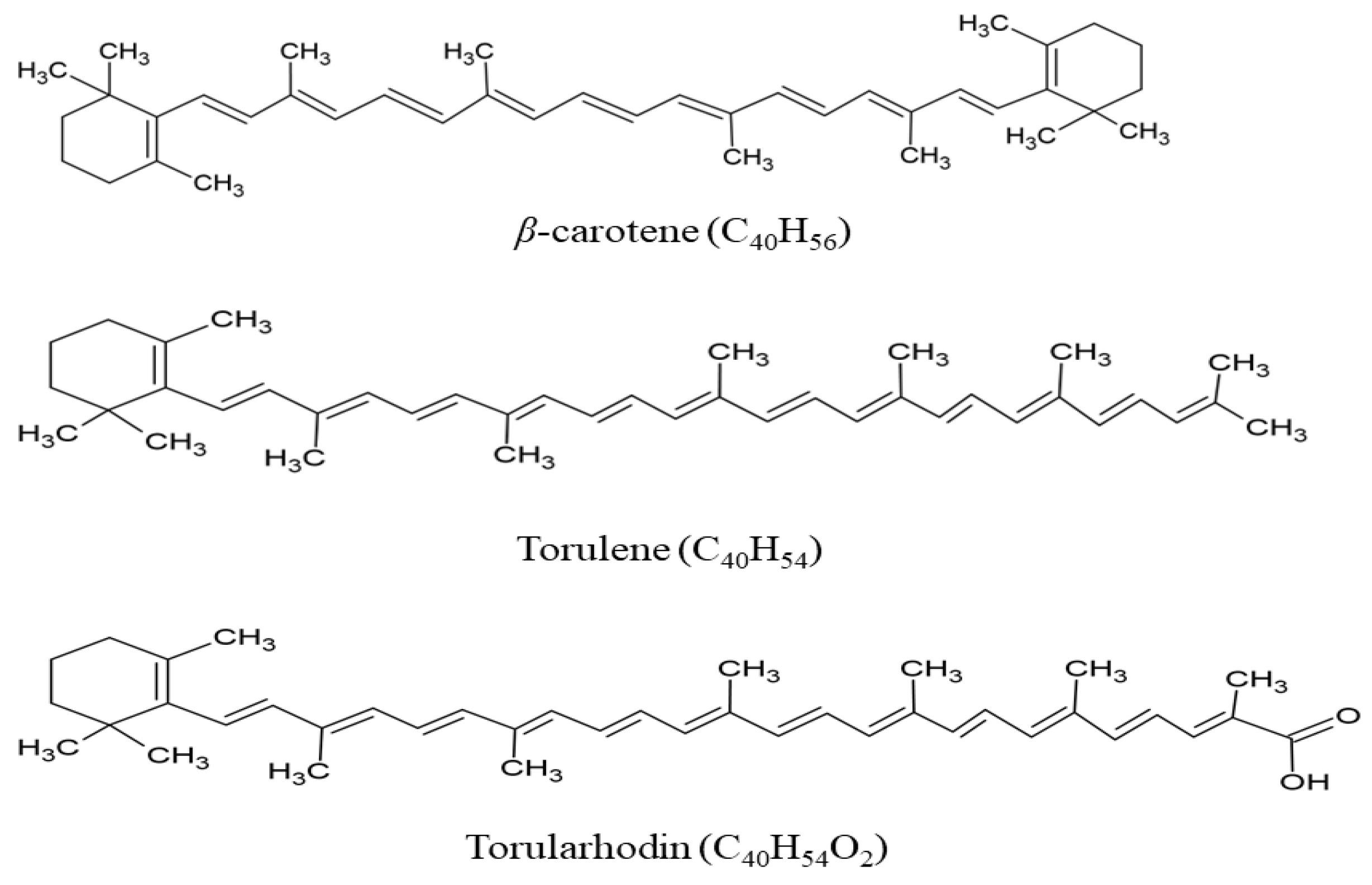
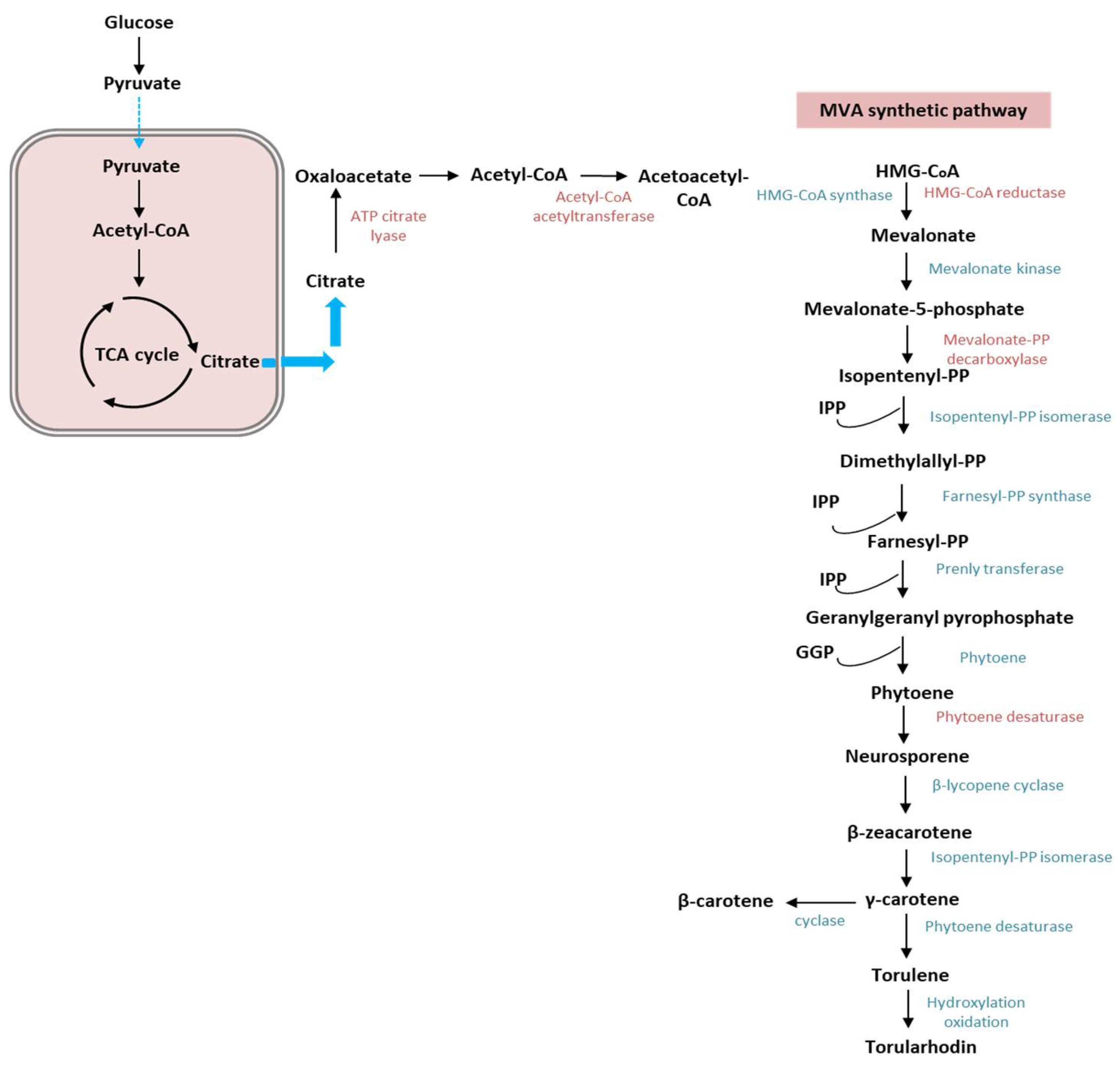
2. Types of Carotenoids and Mechanism of Biosynthesis in Rhodotorula
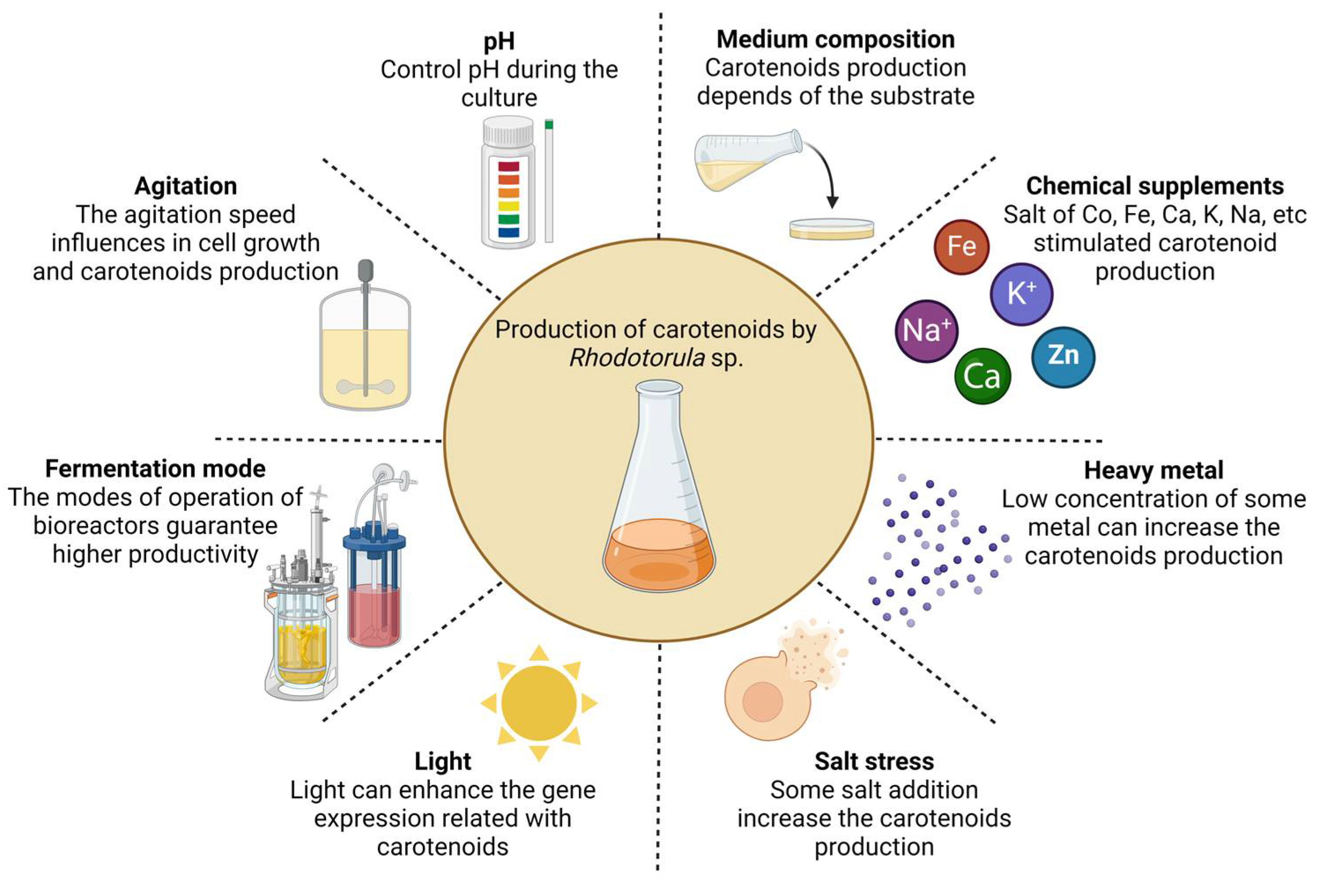
3. Fermentation Factors Influence the Production of Carotenoids
3.1. Medium Composition
3.2. Temperature
3.3. The pH
3.4. Agitation
3.5. Light
3.6. Heavy Metal Ions and Salts
4. Genetic Engineering to Improve Carotenoid Production
| Yeast Strains | Genetic Modification | Carotenoid | Reference |
|---|---|---|---|
| R. gracilis | Genomic mutagenesis (Zeocin as a mutagen) | Carotenoids | [76] |
| R. mucilaginosa C2.5t1 | T-DNA insertional mutagenesis | Carotenoids | [77] |
| R. toruloides | Genetic manipulation methods based on Agrobacterium-mediated transformation (ATMT) | Torularhodin and β-carotene | [78] |
| R. toruloides | Ultraviolet (UV) and gamma irradiation mutagenesis | Astaxanthin | [79] |
| R. glutinis P4-10-9–63Y-14B | Overexpression of truncated HMG1 (tHMG1) from K. marxianus | β-carotene | [80] |
| R. mucilaginosa KC8 | Plasma-induced mutagenesis Metabolic engineering | Carotenoids | [81] |
| R. glutinis | UV irradiation | Carotenoids | [82] |
| R. toruloides M18 | ARTP mutagenesis | β-carotene, Torulene Torularhodin | [40] |
| R. mucilaginosa A734 | UV irradiation | Carotenoids | [83] |
| R. mucilaginosa JH-R23 | New Generation Manned Spacecraft Test Ship | Carotenoids | [84] |
| R. glutinis NCIM 3353 | UV irradiation | β-carotene | [85] |
| R. toruloides CCT 7815 | light irradiation and adaptive laboratory evolution (ALE) | Carotenoids | [61] |
| R. toruloides CCT 0783 | Golden Gate DNA assembly system (RtGGA). | Carotenoids | [86] |
5. Submerged Fermentation Strategies
6. Fermentation by Adhesion to Magnetic Nanoparticles
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Papapostolou, H.; Kachrimanidou, V.; Alexandri, M.; Plessas, S.; Papadaki, A.; Kopsahelis, N. Natural Carotenoids: Recent Advances on Separation from Microbial Biomass and Methods of Analysis. Antioxidants 2023, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj UPHarish Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Brzezińska, R.; Sęk, W.; Kieliszek, M. Effect of Selected Cations and B Vitamins on the Biosynthesis of Carotenoids by Rhodotorula mucilaginosa Yeast in the Media with Agro-Industrial Wastes. Appl. Sci. 2021, 11, 11886. [Google Scholar] [CrossRef]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and Some Other Pigments from Fungi and Yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Kingkaew, E.; Tedsree, N.; Phuengjayaem, S.; Rojsitthisak, P.; Sritularak, B.; Thitikornpong, W.; Thompho, S.; Mhuantong, W.; Tanasupawat, S. Genomic Insight and Optimization of Astaxanthin Production from a New Rhodotorula sp. CP72-2. Fermentation 2023, 9, 501. [Google Scholar] [CrossRef]
- Sereti, F.; Alexandri, M.; Papadaki, A.; Papapostolou, H.; Kopsahelis, N. Natural lycopene and β-carotene synthesis by Rhodosporidium kratochvilovae yeasts: Sustainable production, chemical characterization and antioxidative properties. Food Biosci. 2024, 57, 103425. [Google Scholar] [CrossRef]
- Kot, A.M.; Sęk, W.; Kieliszek, M.; Błażejak, S.; Pobiega, K.; Brzezińska, R. Diversity of Red Yeasts in Various Regions and Environments of Poland and Biotechnological Potential of the Isolated Strains. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Costa, W.A.; Padilha, C.E.A.; Oliveira, J.S.D.; Silva, F.L.H.; Silva, J.; Ancântara, M.A.; Ferrari, M.; dos Santos, E.S. Oil-lipids, carotenoids and fatty acids simultaneous production by Rhodotorula mucilaginosa CCT3892 using sugarcane molasses as carbon source. Braz. J. Food Technol. 2020, 23, e2019064. [Google Scholar] [CrossRef]
- Kot, A.M.; Błazejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of lipids and carotenoids by Rhodotorula gracilis ATCC 10788 yeast in a bioreactor using low—cost wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Keskin, A.; Ünlü, A.E.; Takaç, S. Utilization of olive mill wastewater for selective production of lipids and carotenoids by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2023, 107, 4973–4985. [Google Scholar] [CrossRef]
- Díaz-Ruiz, E.; Balbino, T.R.; dos Santos, J.C.; Kumar, V.; da Silva, S.S.; Chandel, A.K. Fermentative Production of β-Carotene from Sugarcane Bagasse Hydrolysate by Rhodotorula glutinis CCT-2186. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hu, R.; Chen, D.; Chen, J.; He, W.; Huang, L.; Lin, C.; Chen, H.; Chen, Y.; Zhu, J.; et al. Lipid and carotenoid production by the Rhodosporidium toruloides mutant in cane molasses. Bioresour. Technol. 2021, 326, 124816. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Radi, M.; Mohamed, E.T.T.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Adaptive laboratory evolution of Rhodosporidium toruloides to inhibitors derived from lignocellulosic biomass and genetic variations behind evolution. Bioresour. Technol. 2021, 333, 125171. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Ribeiro, H.F.; Santos-Ebinuma, V.C.; Schuur, B.; Pereira JF, B. Rhodotorula sp.–based biorefinery: A source of valuable biomolecules. Appl. Microbiol. Biotechnol. 2022, 106, 7431–7447. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnology advances in β-carotene production by microorganisms. Trends Food Sci. Technol. 2021, 111, 322–332. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “New” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Guo, Y.; Cheng, Y.; Pi, F.; Yao, W.; Xie, Y.; Qian, H. Determination of the Molecular Mechanism of Torularhodin against Hepatic Oxidative Damage by Transcriptome Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 7417263. [Google Scholar] [CrossRef]
- Du, C.; Li, Y.; Guo, Y.; Han, M.; Zhang, W.; Qian, H. The suppression of torulene and torularhodin treatment on the growth of PC-3 xenograft prostate tumors. Biochem. Biophys. Res. Commun. 2016, 469, 1146–1152. [Google Scholar] [CrossRef]
- Fukushima-Sakuno, E. Bioactive small secondary metabolites from the mushrooms Lentinula edodes and Flammulina velutipes. J. Antibiot. 2020, 73, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, M.; Lv, F.; Gao, Y.; Wang, X.; Zhang, X.; Guo, Y.; Cheng, Y.; Qian, H. Study on the Cellular Anti-Inflammatory Effect of Torularhodin Produced by Sporidiobolus pararoseus ZQHL Isolated from Vinegar Fungus. Molecules 2023, 28, 1436. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Das, S.; Saha, B.; Paul, D.; Basu, B. Anti-microbial, anti-oxidant, and anti-breast cancer properties unraveled in yeast carotenoids produced via cost-effective fermentation technique utilizing waste hydrolysate. Front. Microbiol. 2023, 13, 1088477. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Kumari, P.K.; Siddiqui, N. Yeast Carotenoids: Cost-Effective Fermentation Strategies for Health Care Applications. Fermentation 2023, 9, 147. [Google Scholar] [CrossRef]
- Naz, T.; Ullah, S.; Nazir, Y.; Li, S.; Iqbal, B.; Liu, Q.; Mohamed, H.; Song, Y. Industrially Important Fungal Carotenoids: Advancements in Biotechnological Production and Extraction. J. Fungi 2023, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.T.; Mi, B.Q.; Lu, Y.J.; Chen, M.T.; Ye, W.Z. Research progress on carotenoid production by Rhodosporidium toruloides. Appl. Microbiol. Biotechnol. 2024, 108, 7. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Gao, N.; Lv, J.; Ji Chaofan Liang, H.; Li, S.; Yu, C.; Wang, Z.; Lin, X. Enhancement of Torularhodin Production in Rhodosporidium toruloides by Agrobacterium tumefaciens-Mediated Transformation and Culture Condition Optimization. J. Agric. Food Chem. 2019, 67, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.J. C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef]
- Sereti, F.; Papadaki, A.; Alexandri, M.; Kachrimanidou, V.; Kopsahelis, N. Exploring the potential of novel R. kratochvilovae red yeasts towards the sustainable synthesis of natural carotenoids. Sustain. Chem. Pharm. 2023, 31, 100927. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, R.; Liang, J.; Zhang, H.; Yi, J.; Liu, Z. Strategies for Recovery, Purification and Quantification of Torularhodin Produced by Rhodotorula mucilaginosa Using Different Carbon Sources. Fermentation 2023, 9, 846. [Google Scholar] [CrossRef]
- Elfeky, N.; Elmahmoudy, M.; Zhang, Y.; Guo, J.; Bao, Y. Lipid and Carotenoid Production by Rhodotorula glutinis with a Combined Cultivation Mode of Nitrogen, Sulfur, and Aluminium Stress. Appl. Sci. 2019, 9, 2444. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods 2023, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sinha, S.; Bandyopadhyay, K.K.; Lawrence, M.; Prasad, R.; Paul, D. Triauxic growth of an oleaginous red yeast Rhodosporidium toruloides on waste ‘extract’ for enhanced and concomitant lipid and β-carotene production. Microb. Cell Fact. 2018, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, C.; Sanmartin Negrete, P.; Carelli, A.A.; Borroni, V. Evaluation of olive mill waste as substrate for carotenoid production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020, 7, 52. [Google Scholar] [CrossRef]
- Días, R.T.V.; Amore, T.D.; Teixeira, E.C.; de Medeiros, B.J.F. Carotenoid Production by Rhodotorula mucilaginosa in Batch and Fed-Batch Fermentation Using Agroindustrial Byproducts. Food Technol. Biotechnol. 2019, 57, 388–398. [Google Scholar]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Bryś, J.; Gientka, I.; Bzducha-Wróbel, A.; Maliszewska, M.; Reczek, L. Effect of initial pH of medium with potato wastewater and glycerol on protein, lipid and carotenoid biosynthesis by Rhodotorula glutinis. Electron. J. Biotechnol. 2017, 27, 25–31. [Google Scholar] [CrossRef]
- Martins, V.; Días, C.; Caldeira, J.; Duarte, L.C.; Reis, A.; da Silva, T.L. Carob pulp syrup: A potential Mediterranean carbon source for carotenoids production by Rhodosporidium toruloides NCYC 921. Bioresour. Technol. Rep. 2018, 3, 177–184. [Google Scholar] [CrossRef]
- Ribeiro, J.E.S.; Sant’Ana, A.M.S.; Martini, M.; Sorce, C.; Andreucci, A.; Melo, D.J.N.; Silva, F.L.H. Rhodotorula glutinis cultivation on cassava wastewater for carotenoids and fatty acids generation. Biocatal. Agric. Biotechnol. 2019, 22, 101419. [Google Scholar] [CrossRef]
- da Silva, J.; da Silva, F.L.H.; Ribeiro, J.E.S.; Melo, D.J.N.; Santos, F.A.; Medeiros, L.L. Effect of supplementation, temperature and pH on carotenoids and lipids production by Rhodotorula mucilaginosa on sisal bagasse hydrolyzate. Biocatal. Agric. Biotechnol. 2020, 30, 101847. [Google Scholar] [CrossRef]
- Qi, F.; Shen, P.; Hu, R.; Xue, T.; Jiang, X.; Qin, L.; Chen, Y.; Huang, J. Carotenoids and lipid production from Rhodosporidium toruloides cultured in tea waste hydrolysate. Biotechnol. Biofuels 2020, 13, 74. [Google Scholar] [CrossRef]
- Bertacchi, S.; Cantù, C.; Porro, D.; Branduardi, P. Optimization of Carotenoids Production from Camelina sativa Meal Hydrolysate by Rhodosporidium toruloides. Fermentation 2021, 7, 208. [Google Scholar] [CrossRef]
- Martins, L.C.; Palma, M.; Angelov, A.; Nevoigt, E.; Liebl, W.; Sá-Correia, I. Complete Utilization of the Major Carbon Sources Present in Sugar Beet Pulp Hydrolysates by the Oleaginous Red Yeasts Rhodotorula toruloides and R. mucilaginosa. J. Fungi 2021, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.V.D.; Teixeira, E.C.; Macedo, L.P.; dos Santos, G.M.; Burkert CAVBurkert, J.F.M. Agroindustrial byproduct-based media in the production of microbial oil rich in oleic acid and carotenoids. Bioprocess Biosyst. Eng. 2022, 45, 721–732. [Google Scholar] [CrossRef]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and Identification of Carotenoid-Producing Rhodotorula Sp. from Pinaceae Forest Ecosystems and Optimization of in Vitro Carotenoid Production. Biotechnol. Rep. 2021, 32, e00687. [Google Scholar] [CrossRef] [PubMed]
- Machado, W.R.C.; Murari, C.S.; Duarte, A.L.F.; Bianchi, V.L. Optimization of agro-industrial coproducts (molasses and cassava wastewater) for the simultaneous production of lipids and carotenoids by Rhodotorula mucilaginosa. Biocatal. Agric. Biotechnol. 2022, 42, 102342. [Google Scholar] [CrossRef]
- Torres-Alvarez, D.; León-Buitimea, A.; Albalate-Ramírez, A.; Rivas-García, P.; Hernández-Núñez, E.; Morones-Ramírez, J.R. Conversion of banana peel into diverse valuable metabolites using an autochthonous Rhodotorula mucilaginosa strain. Microb. Cell Fact. 2022, 21, 96. [Google Scholar] [CrossRef]
- Maia, F.d.A.; Igreja, W.S.; Xavier, A.A.O.; Mercadante, A.Z.; Lopes, A.S.; Chisté, R.C. Concentrated Manipueira as an Alternative Low-Cost Substrate to Rhodotorula glutinis for Biotechnological Production of High Contents of Carotenoids. Fermentation 2023, 9, 617. [Google Scholar] [CrossRef]
- Szotkowski, M.; Plhalová, Ž.; Sniegoňová, P.; Holub, J.; Chujanov, O.; Špačková, D.; Blažková, J.; Márová, I. Conversion of Mixed Waste Food Substrates by Carotenogenic Yeasts of Rhodotorula sp. Genus. Microorganisms 2023, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Terrones, N.; Quiñones-Cerna, C.E.; Robles, M.; Cruz-Monzon, J.A.; Butrón, F.; Rodríguez, J.C. Optimization of Total Carotenoid Production by Rhodotorula mucilaginosa from Artichoke Agroindustrial Waste Using Response Surface Methodology. Environ. Res. Eng. Manag. 2023, 79, 111–121. [Google Scholar] [CrossRef]
- Fallahi, S.; Habibi, A.; Abbasi, S. Optimized fed-batch cultivation of Rhodotorula toruloides in a bubble column bioreactor progressed the β-carotene production from corn steep liquor. Braz. J. Microbiol. 2023, 54, 2719–2731. [Google Scholar] [CrossRef]
- Igreja, W.S.; Maia, F.d.A.; Lopes, A.S.; Chisté, R.C. Biotechnological Production of Carotenoids Using Low Cost-Substrates Is Influenced by Cultivation Parameters: A Review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, Identification of Carotenoid-Producing Rhodotorula sp. from Marine Environment and Optimization for Carotenoid Production. Mar. Drugs 2019, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J.; Reczek, L.; Pobiega, K. Effect of exogenous stress factors on the biosynthesis of carotenoids and lipids by Rhodotorula yeast strains in media containing agro-industrial waste. World J. Microbiol. Biotechnol. 2019, 35, 157. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, M.R.N.; Razavi, S.H. Optimization of β-carotene production by a mutant of the lactose-positive yeast Rhodotorula acheniorum from whey ultrafiltrate. Food Sci. Biotechnol. 2011, 20, 445–454. [Google Scholar] [CrossRef]
- Dias, C.; Silva, C.; Freitas, C.; Reis, A.; da Silva, T.L. Effect of Medium pH on Rhodosporidium toruloides NCYC 921 Carotenoid and Lipid Production Evaluated by Flow Cytometry. Appl. Biochem. Biotechnol. 2016, 179, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, S.M.; Marangoni, A.G. Microbial lipids for foods. Trends Food Sci. Technol. 2022, 119, 593–607. [Google Scholar] [CrossRef]
- Borba, C.M.; Tavares, M.D.; Moraes, C.C.; Burkert, J.F.M. Carotenoid production by Sporidiobolus pararoseus in agroindustrial medium: Optimization of culture conditions in shake flasks and scale-up in a stirred tank fermenter. Braz. J. Chem. Eng. 2018, 35, 509–520. [Google Scholar] [CrossRef]
- Ribeiro, R.M.M.G.P.; Picão, B.W.; Gonçalves, D.O.; Scontri, M.; Mazziero, V.T.; Mussagy, C.U.; Raghavan, V.; Astudillo-Castro, C.; Córdova, A.; Cerri, M.O.; et al. Synergistic Effects of Stirring and Aeration Rate on Carotenoid Production in Yeast Rhodotorula toruloides CCT 7815 Envisioning Their Application as Soap Additives. Fermentation 2023, 9, 828. [Google Scholar] [CrossRef]
- Gong, G.; Zhang, X.; Tan, T. Simultaneously enhanced intracellular lipogenesis and β-carotene biosynthesis of Rhodotorula glutinis by light exposure with sodium acetate as the substrate. Bioresour. Technol. 2020, 295, 122274. [Google Scholar] [CrossRef]
- Pham, K.D.; Shida, Y.; Miyata, A.; Takamizawa, T.; Suzuki, Y.; Ara, S.; Yamazaki, H.; Masaki, K.; Mori, K.; Aburatani, S.; et al. Effect of Light on Carotenoid and Lipid Production in the Oleaginous Yeast Rhodosporidium toruloides. Biosci. Biotechnol. Biochem. 2020, 84, 1501–1512. [Google Scholar] [CrossRef]
- Pinheiro, M.J.; Bonturi, N.; Belouah, I.; Miranda, E.A.; Lahtvee, P.J. Xylose metabolism and the effect of oxidative stress on lipid and carotenoid production in Rhodotorula toruloides: Insights for future biorefinery. Front. Bioeng. Biotechnol. 2020, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cortes, A.; Garcia-Vásquez, J.A.; Aranguren, Y.; Ramirez-Castrillon, M. Pigment Production Improvement in Rhodotorula mucilaginosa AJB01 Using Design of Experiments. Microorganisms 2021, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.V.D.; Contessa, C.R.; Burkert, C.A.V.; Burkert, J.F.M. Evaluation of Light-Emitting Diodes Applied to Rhodotorula mucilaginosa to Produce Carotenoids and Lipids. Food Bioprocess Technol. 2024. [Google Scholar] [CrossRef]
- Gao, H.; Tang, Y.; Lv, R.; Jiang, W.; Jiang, Y.; Zhang, W.; Xin, F.; Jiang, M. Transcriptomic Analysis Reveals the Potential Mechanisms for Improving Carotenoid Production in Rhodosporidium toruloides Z11 under Light Stress. J. Agric. Food Chem. 2024, 72, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.D.; Hakozaki, Y.; Takamizawa, T.; Yamazaki, A.; Yamazaki, H.; Mori, K.; Aburatani, S.; Tashiro, K.; Kuhara, S.; Takaku, H.; et al. Analysis of the light regulatory mechanism in carotenoid production in Rhodosporidium toruloides NBRC 10032. Biosci. Biotechnol. Biochem. 2021, 85, 1899–1909. [Google Scholar] [CrossRef]
- Cavelius, P.; Engelhart-Straub, S.; Biewald, A.; Haack, M.; Awad, D.; Brueck, T.; Mehlmer, N. Adaptation of Proteome and Metabolism in Different Haplotypes of Rhodosporidium toruloides during Cu(I) and Cu(II) Stress. Microorganisms 2023, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.B.M.; Mahmoud, G.A. Chemical- vs. Sonochemical-assisted Synthesis of ZnO Nanoparticles from a New Zinc Complex for Improvement of Carotene Biosynthesis from Rhodotorula Toruloides MH023518. Appl. Organomet. Chem. 2020, 35, e6086. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Jiang, L.; Qiu, J.; Gao, Y.; Gu, T.; Li, Z. Responses of Rhodotorula mucilaginosa under Pb(II) stress: Carotenoid production and budding. Environ. Microbiol. 2022, 24, 678–688. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef]
- Illarionov, A.; Lahtvee, P.J.; Kumar, R. Potassium and Sodium Salt Stress Characterization in the Yeasts Saccharomyces cerevisiae, Kluyveromyces marxianus, and Rhodotorula toruloides. Appl. Environ. Microbiol. 2021, 87, e03100-20. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Li, Z.; Cheng, P.; Yu, G. Transcriptomic and Metabolomic Analysis Reveals the Potential Mechanisms Underlying the Improvement of β-Carotene and Torulene Production in Rhodosporidiobolus colostri under Low Temperature Treatment. Food Res. Int. 2022, 156, 111158. [Google Scholar] [CrossRef] [PubMed]
- Watcharawipas, A.; Runguphan, W. Red yeasts and their carotenogenic enzymes for microbial carotenoid production. FEMS Yeast Res. 2023, 23, foac063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, H.; Zhang, X.; Yu, X.; Wang, H.; Xiao, S.; Wang, J.; Zhao, Z.K. Combined mutagenesis of Rhodosporidium toruloides for improved production of carotenoids and lipids. Biotechnol Lett. 2016, 38, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Y.; Zhang, S.; Zhu, Z.; Zhou, Y.J.; Yang, F.; Sun, W.; Wang, X.; Zhao, Z.K. Functional integration of multiple genes into the genome of the oleaginous yeast Rhodosporidium toruloides. FEMS Yeast Res. 2014, 14, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gao, N.; Liu, S.; Zhang, S.; Song, S.; Ji, C.; Dong, X.; Su, Y.; Zhao, Z.K.; Zhu, B. Characterization the carotenoid productions and profiles of three Rhodosporidium toruloides mutants from Agrobacterium tumefaciens-mediated transformation. Yeast 2017, 34, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Watabe, Y.; Takahashi, S. Production of Enhanced Carotenoid-Producing Strains of the Yeast Rhodotorula gracilis Using the Antibiotic Zeocin. Appl. Biochem. Biotechnol. 2023, 12, 7889–7897. [Google Scholar] [CrossRef] [PubMed]
- Tkáčová, J.; Zara, G.; Ianiri, G.; Castoria, R.; Čertík, M.; Mannazzu, I. Impairment of carotenoid biosynthesis through CAR1 gene mutation results in CoQ10, sterols, and phytoene accumulation in Rhodotorula mucilaginosa. Appl. Microbiol. Biotechnol. 2022, 106, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lv, J.; Ji, C.; Liang, H.; Li, S.; Yang, Z.; Xu, W.; Zhang, S.; Lin, X. Analysis of carotenoid profile changes and carotenogenic genes transcript levels in Rhodosporidium toruloides mutants from an optimized Agrobacterium tumefaciens-mediated transformation method. Biotechnol. Appl. Biochem. 2021, 68, 71–81. [Google Scholar] [CrossRef]
- Tran, T.N.; Ngo, D.-H.; Tran, Q.T.; Nguyen, H.C.; Su, C.-H.; Ngo, D.-N. Enhancing Astaxanthin Biosynthesis by Rhodosporidium toruloides Mutants and Optimization of Medium Compositions Using Response Surface Methodology. Processes 2020, 8, 497. [Google Scholar] [CrossRef]
- Pi, H.W.; Anandharaj, M.; Kao, Y.Y.; Lin, Y.-J.; Chang, J.-J.; Li, W.-H. Engineering the oleaginous red yeast Rhodotorula glutinis for simultaneous beta-carotene and cellulase production. Sci. Rep. 2018, 8, 10850. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Yang, Q.; Wang, P. Enhancing carotenoid production in Rhodotorula mucilaginosa KC8 by combining mutation and metabolic engineering. Ann. Microbiol. 2017, 67, 425–431. [Google Scholar] [CrossRef]
- Yolmeh, M.; Khomeiri, M. Using physical and chemical mutagens for enhanced carotenoid production from Rhodotorula glutinis (PTCC 5256). Biocatal. Agric. Biotechnol. 2016, 8, 158–166. [Google Scholar] [CrossRef]
- Issa, S.; Alhajali, A.; Alamir, L. Improving carotenoid pigments production in Rhodotorula mucilaginosa using UV irradiation. Int. Food Res. J. 2016, 23, 873–878. [Google Scholar]
- Huang, J.; Yang, S.; Jian, H. Comparative Genomic Analysis of the Mutant Rhodotorula mucilaginosa JH-R23 Provides Insight into the High-Yield Carotenoid Mechanism. Fermentation 2024, 10, 176. [Google Scholar] [CrossRef]
- Bhosale, P.; Gadre, R. Production of β-carotene by a mutant of Rhodotorula glutinis. Appl Microbiol Biotechnol. 2001, 55, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Bonturi, N.; Pinheiro, M.J.; de Oliveira, P.M.; Rusadze, E.; Eichinger, T.; Liudžiūtė, G.; De Biaggi, J.S.; Brauer, A.; Remm, M.; Miranda, E.A.; et al. Development of a Dedicated Golden Gate Assembly Platform (RtGGA) for Rhodotorula toruloides. Metab. Eng. Commun. 2022, 15, e00200. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.N.; da Silveira, J.M.; Burkert, J.F.; Ores, J.C.; Burkert, C.A.V. Simultaneous lipid and carotenoid production by stepwise fed-batch cultivation of Rhodotorula mucilaginosa with crude glycerol. Braz. J. Chem. Eng. 2019, 36, 1099–1108. [Google Scholar] [CrossRef]
- Sriphuttha, C.; Boontawan, P.; Boonyanan, P.; Ketudat-Cairns, M.; Boontawan, A. Simultaneous Lipid and Carotenoid Production via Rhodotorula paludigena CM33 Using Crude Glycerol as the Main Substrate: Pilot-Scale Experiments. Int. J. Mol. Sci. 2023, 24, 17192. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.-M.; Ebrahiminezhad, A.; Ghoshoon, M.B.; Dehshahri, A.; Berenjian, A.; Ghasemi, Y. Magnetic Immobilization of Pichia pastoris Cells for the Production of Recombinant Human Serum Albumin. Nanomaterials 2020, 10, 111. [Google Scholar] [CrossRef]
- Alonso-Estrada, D.; Ochoa-Viñals, N.; Ramos-González, R.; Michelena-Álvarez, G.; Hurtado-López, G.F.; Núñez-Caraballo, A.; Aguilar-González, M.A.; Ilyina, A. Invertase production by Rhodotorula toruloides in submerged and surface adhesion on magnetic nanoparticles fermentations. Biocatal. Agric. Biotechnol. 2024, 56, 103035. [Google Scholar] [CrossRef]
- Taghizadeh, S.-M.; Ghoshoon, M.B.; Ghasemi, Y.; Dehshahr, A.; Berenjian, A.; Ebrahiminezhad, A. Efficiency of magnetic immobilization for recombinant Pichia pastoris cells harvesting over consecutive production cycles. Sep. Sci. Technol. 2022, 58, 420–434. [Google Scholar] [CrossRef]
- Konopacka, A.; Rakoczy, R.; Konopacki, M. The effect of rotating magnetic field on bioethanol production by yeast strain modified by ferrimagnetic nanoparticles. J. Magn. Magn. Mater. 2019, 473, 176–183. [Google Scholar] [CrossRef]
- Núñez Caraballo, A.; Iliná, A.; Ramos González, R.; Aguilar, C.N.; Michelena Álvarez, G.; Flores Gallegos, A.C.; Sandoval-Cortés, J.; Aguilar-Gonzalez, M.A.; Soto-Cruz, N.O.; García García, J.D.; et al. Sustainable Ethanol Production From Sugarcane Molasses by Saccharomyces cerevisiae Immobilized on Chitosan-Coated Manganese Ferrite. Front. Sustain. Food Syst. 2021, 5, 683170. [Google Scholar] [CrossRef]
- Firoozi, F.R.; Raee, M.J.; Lal, N.; Ebrahiminezhad, A.; Teshnizi, S.H.; Berenjian, A.; Ghasemi, Y. Application of magnetic immboilization for ethanol biosynthesis using Saccharomyces cerevisiae. Sep. Sci. Technol. 2022, 57, 777–787. [Google Scholar] [CrossRef]
| Yeast Strain | Sustrate | Carotenoid | Fermentation | Reference |
|---|---|---|---|---|
| R. glutinis LOCKR13 | Potato wastewater/glycerol | Total carotenoids (3.4–3.7 mg/L) | Batch 28 °C, 200 rpm, pH 4.0–7.072 h | [36] |
| R. toruloides ATCC 204091 | Vegetable market waste (Mandi)-derived medium containing glucose, xylose, and glycerol. | β-carotene (62 mg/L) | Batch 100 h | [33] |
| R. toruloides NCYC 921 | Carob pulp syrup | Total carotenoids (0.42 mg/g) | Fed-batch 30 °C | [37] |
| R. glutinis | Cassava wastewater | Total carotenoids (167 μg/g) | Batch 30 °C, 200 rpm 120 h | [38] |
| R. mucilaginosa | Sisal bagasse hydrolyzate | Total carotenoids (1.13 g/L) | Batch 22 °C, 200 rpm 96 h, pH 7 | [39] |
| R. mucilaginosa CCT3892 | Hydrolyzed sugarcane molasses at 40 g/L | Total carotenoids (53.0 μg/g) | Batch 30 °C, pH 6.49 200 rpm, 120 h | [10] |
| R. mucilaginosa | Olive mill waste (Alperujo) | Total carotenoids (7.3 mg/L) | Batch 30 °C, pH 5.15–5.39 150 rpm, 144 h | [34] |
| R. gracilis ATCC 10788 | Potato wastewater/glycerol | Total carotenoid (6.24 mg/L) 47% β-carotene 51% torulene | Batch 20 °C/28 °C 120 h, 300 rpm | [11] |
| R. toruloides M18 | Tea waste hydrolysate | β-carotene (10.08 mg/g)torularhodin (481.9 μg/g) torulene (501 μg/g) | Batch 30 °C pH 6.0, 200 rpm,120 h | [40] |
| R. mucilaginosa MTCC-1403 | Onion peel powder and mung bean husks | Total carotenoids (819.2 μg/g) | Batch 25.8 °C, pH 6 119.6 rpm, 84 h | [9] |
| R. mucilaginosa | Sisal bagasse hydrolyzate | Total carotenoids (1.13 g/L) | Bach 22.0 °C pH 7.0 | [39] |
| R. toruloides DSM 4444 | Camelina sativa meal hydrolysates Flower of Calendula officinalis, Zea mays seed flour, potato seed flour | β-Carotene (5.5 mg/L, 12.6 mg/L, 16.0 mg/L) | Batch 30 °C, 160 rpm | [41] |
| R. mucilaginosa IST 390 R. toruloides PYCC 5615 | Sugar beet pulp hydrolysates | Total carotenoids (1.4 mg/L) (5.4 mg/L) | Batch 30 °C, pH 5 250 rpm, 150 h | [42] |
| R. mucilaginosa CCT7688 | Sugarcane molasses/corn steep liquor | Total carotenoids (121.4 μg/g) | Batch/fed-batch 25 °C, pH 6.0 180 rpm, 144 h | [43] |
| R. toruloides NRRL Y-1091 | Wheat straw hydrolysate | Total carotenoids (14.09 mg/L) | Batch 30 °C, 250 rpm, 72 h | [15] |
| R. toruloides ATCC 204091 | Lignocellulosic waste hydrolysate | Total carotenoids (19 mg/L) | Batch 25 °C 120 rpm | [33] |
| R. mucilaginosa CCT 3892 | Sisal bagasse hydrolyzate | Total carotenoids (223.5 μg/g) | Batch 28 °C, pH 6.0 150 rpm, 120 h | [44] |
| R. mucilaginosa URM 7409 | Sugarcane molasses/cassava wastewater | Total carotenoids (168.08 μg/g) | Batch 25 °C, pH 6.0 130 rpm | [45] |
| R. mucilaginosa ANL-001L | Banana peel extract | Total carotenoids (317 μg/g) | Batch 28 °C 300 rpm, 144 h | [46] |
| R. glutinis CCT-2186 | Sugarcane Bagasse Hydrolysate | β-carotene (118.6 mg/L) | Batch 72 h | [13] |
| R. glutinis | Manipueira | Total carotenoids (1410 μg/g) | Batch 35 °C, pH 5.0 150 rpm | [47] |
| R. toruloides R. kratochvilovae | Coffee oil/waste glycerol. | Total carotenoids (10.51 mg/g) (10.75 mg/g) | Fed-batch 25 °C, pH 6.5 60 rpm,168 h | [48] |
| R. mucilaginosa | Artichoke Agro-industrial Waste | Total carotenoids (1228.53 µg/g) | Batch pH 5, 120 rpm and 30 °C for 72 h | [49] |
| R. glutinis | Olive mill wastewater | Total carotenoid (192.09 μg/g) torulene 85% torularhodin 80% β-carotene 39.45% | Batch | [12] |
| R. toruloides KP324973 | Corn steep liquor | Total carotenoid (12.31 mg/g/h) | Batch/fed-batch 11.7 °C, pH 6.1 | [50] |
| R. glutinis P4M422 | Hydrolyzed goat milk whey | Total carotenoid (4075 µg/L) | Batch 30 °C, pH 4.5 200 rpm, 72 h | [32] |
| R. kratochvilovae Y-42 | Cheese whey/fermented wheat bran | Total carotenoids (36.4 mg/L) 97% lycopene | Batch | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa-Viñals, N.; Alonso-Estrada, D.; Pacios-Michelena, S.; García-Cruz, A.; Ramos-González, R.; Faife-Pérez, E.; Michelena-Álvarez, L.G.; Martínez-Hernández, J.L.; Iliná, A. Current Advances in Carotenoid Production by Rhodotorula sp. Fermentation 2024, 10, 190. https://doi.org/10.3390/fermentation10040190
Ochoa-Viñals N, Alonso-Estrada D, Pacios-Michelena S, García-Cruz A, Ramos-González R, Faife-Pérez E, Michelena-Álvarez LG, Martínez-Hernández JL, Iliná A. Current Advances in Carotenoid Production by Rhodotorula sp. Fermentation. 2024; 10(4):190. https://doi.org/10.3390/fermentation10040190
Chicago/Turabian StyleOchoa-Viñals, Nayra, Dania Alonso-Estrada, Sandra Pacios-Michelena, Ariel García-Cruz, Rodolfo Ramos-González, Evelyn Faife-Pérez, Lourdes Georgina Michelena-Álvarez, José Luis Martínez-Hernández, and Anna Iliná. 2024. "Current Advances in Carotenoid Production by Rhodotorula sp." Fermentation 10, no. 4: 190. https://doi.org/10.3390/fermentation10040190
APA StyleOchoa-Viñals, N., Alonso-Estrada, D., Pacios-Michelena, S., García-Cruz, A., Ramos-González, R., Faife-Pérez, E., Michelena-Álvarez, L. G., Martínez-Hernández, J. L., & Iliná, A. (2024). Current Advances in Carotenoid Production by Rhodotorula sp. Fermentation, 10(4), 190. https://doi.org/10.3390/fermentation10040190








