Abstract
β-carotene possesses antioxidant properties and holds significant research value. In our study, we have successfully identified a strain of Pantoea dispersa MSC14 which has the capability to produce β-carotene. By incorporating corn steep liquor powder into culture medium and employing mutagenesis breeding techniques, we have successfully increased the production of β-carotene in the MSC14 strain by 13.97% and 29.22%, respectively. To gain further insights, we conducted genomic and transcriptomics analyses. These analyses revealed a significant mutation in the gndA (6-phosphogluconate dehydrogenase) gene of the mutant strain 14P9, resulting in a 33.74% decrease in 6-phosphogluconate dehydrogenase activity. Using transcriptomics analysis, we investigated the impact of this mutation on β-carotene production and explored the interconnectedness between carbon metabolism, fatty acid metabolism, amino acid metabolism, and β-carotene synthesis. The up-regulation of the trxC (Thioredoxin-2) gene, as observed in both transcriptomics results, prompted us to construct strains that overexpress trxC. This manipulation resulted in a notable 15.89% increase in β-carotene production, highlighting the significant impact of of the trxC gene on the β-carotene content of Pantoea dispersa. In conclusion, our study has successfully identified Pantoea dispersa MSC14 as a proficient producer of β-carotene. Furthermore, we have uncovered two genes implicated in the biosynthesis of β-carotene. These findings enhance our understanding of β-carotene synthesis and provide valuable guidance for carotenoid biosynthesis.
1. Introduction
Carotenoids belong to the isoprenoid family and serve as natural pigment components [1]. They not only provide color but also have diverse biological functions [2], making them highly valuable in commercial and industrial applications [3]. β-carotene (C40H56), one of the carotenoids, is one of the most prevalent and stable natural pigments found in nature. It can serve as a precursor for vitamin A [3,4] and has strong antioxidant properties, which can prevent cellular lipid oxidation, improve body metabolism, and offer protection against cancer, cardiovascular [5] and cerebrovascular diseases, and photosensitive conditions [6].
There exist numerous strains of bacteria in nature that are capable of synthesizing carotenoids, with the Pantoea being recognized as a significant and extensively investigated group. Pantoea is a genus of aerobic or partially anaerobic fermentative Gram-negative bacilli from the Irwiniaceae family, which is within the Enterobacteriaceae family. Pantoea aglumian and Pantoea ananatis [7] are the primary strains used for carotenoid research. These strains have been extensively studied and utilized for carotenoid synthesis research [8]. The nucleotide sequence of the carotenoid biosynthesis genes in Pantoea has been determined [9].
Due to the interest in carotenoid production and its wide range of applications [3], numerous studies have focused on cloning carotenoid synthesis-related genes from Pantoea into engineered bacteria to produce β-carotene [10,11,12,13]. The carotenoid biosynthesis pathway has been a major focus of research, revealing two distinct and independent terpenoid biosynthesis pathways: the mevalonate (MVA) pathway and the methylerythritol phosphate (MEP) pathway. The MEP pathway utilizes glyceraldehyde-3-phosphate and pyruvate as the primary feedstock molecules [14]. However, further investigation is needed to understand the impact of central carbon metabolism on these molecules and, consequently, on carotenoid production. Some studies have involved knocking out the gene that expresses glucose-6-phosphate dehydrogenase (ZWF) [15], effectively blocking the pentose phosphate pathway and increasing the lycopene content. It has also been demonstrated that a reduction in pentose phosphate pathway flux leads to an increase in carotenoid content. Genetic breeding studies which aimed to increase the β-carotene production in bacteria have also been conducted [16,17]. Furthermore, the optimization of the medium plays a crucial role in improving the carotenoid yield. Corn steep liquor powder, a valuable by-product of the corn starch industry, offers not only cost-effectiveness but also a wealth of nutrients [18]. Numerous studies have investigated its inclusion in culture media for fermentation production [19,20]. Additionally, corn steep liquor powder meal serves as a vital and affordable ingredient in carotenoid production research [21,22]. Nevertheless, the effects of corn steep liquor powder meal on the carotenoid synthesis process remain unclear.
Carotenoids act as antioxidants that scavenge harmful substances from cells [23]. During carotenoid biosynthesis, the overexpression of specific enzymes in the redox system can enhance the carotenoid content. For instance, the overexpression of glutathione S-transferase has been shown to increase the biomass of, and carotenoid and astaxanthin content in, Phaffia rhodozyma strains [24]. Therefore, increasing the redox level in organisms may provide a novel approach to boost the production of biosynthesized carotenoids.
In this study, we identify a bacterium (Pantoea dispersa) that is capable of synthesizing β-carotenoids and increased its β-carotenoid production capacity through mutagenesis. Furthermore, we enhanced the β-carotenoid yield by adding corn steep liquor powder to the culture medium. Additionally, we investigated the effects of mutant strains and corn steep liquor powder on β-carotenoid synthesis in P. dispersa through whole-genome sequencing and transcriptomics. We also explored relevant regulators of carotenoid synthesis, aiming to gain a deeper understanding of the mechanism behind carotenoid synthesis in P. dispersa and provide insights for carotenoid production and applications.
2. Materials and Methods
2.1. Strain and Culture Conditions
The wild-type strain MSC14 was isolated from soil. Strain 14P9 was selected for high β-carotene production through the UV mutagenesis of strain MSC14. The above strains are kept in the laboratory of Prof. Tangbing Cui (B6-441-2, South China University of Technology, Guangzhou, China).
The cultivation base medium used was LB medium, which consisted of 10 g tryptone, 5 g yeast extract, and 10 g NaCl per liter. The fermentation medium was either LB-glucose medium (LB medium) (10 g tryptone, 5 g yeast extract, 10 g NaCl, and 1 g glucose per liter) or LB-glucose corn steep liquor syrup medium (LB-C medium) (10 g tryptone, 5 g yeast extract, 10 g NaCl, 1 g glucose, and 1 g corn steep liquor powder per liter). Tryptone, yeast extract, NaCl, glucose, and agar were bought from HuanTai Biotechnology Co. (Guangzhou, China). Corn steep liquor powder was bought from Beijing Hongrun Baoshun Technology Co. (Beijing, China). All other chemical reagents used in this experiment were of analytical grade.
All experiments were carried out in 250 mL conical flasks containing 100 mL of medium at 180 rpm. Strain MSC14 and strain 14P9 cells were transferred from 4 °C LB slants to fresh LB medium and incubated at 30 °C for 48 h or more, respectively. Then, 10% of the pre-incubated stock was inoculated into the seed medium and incubated for another 10 h. Following that, 10% of the stock from the previous step was further inoculated into the fermentation medium and fermented at 30 °C for 48 h on a shaker at 150 rpm. All experiments were performed in triplicate. The dry weight of the cells was determined by centrifuging 10 mL of the bacterial solution, washing with distilled water, and drying at 65 °C until a constant weight was reached.
2.2. Measurement of β-Carotene
We centrifuged 50 mL of the bacterial solution at 16,000× g for 10 min and rinsed twice with distilled water. After centrifuging the bacterial solution and discarding the supernatant, 10 mL of saturated sodium bicarbonate solution was added, and the solution was saponified for 10 min. The cells were then dissolved in ethyl acetate in the dark and subjected to ultrasonic extraction, with 5 s of ultrasonic breakage and a 5 s pause, repeated for a total of 20 min. The supernatant was centrifuged and extracted.
β-carotene analysis was performed using HPLC. The HPLC used was Shimadzu LC-20AT, Agilent C18 column (4.6 mm × 250 mm, 5 μm) with mobile phases A (methanol) and B (ethyl acetate) at a volume ratio of 40:60. The flow rate was set at 1 mL/min, the detection wavelength was 453 nm, the injection volume was 10 μL, and the column temperature was maintained at 30 °C.
2.3. UV Mutagenesis
The UV (253.7 nm) irradiation was carried out under dark conditions. First preheated for about 30 min, 5 mL of prepared bacterial solution (OD600 = 1.00) was placed in a petri dish with a diameter of 9 cm, which was placed on a magnetic stirrer at a distance of 30 cm (vertical distance) from the UV lamp and irradiated for 0, 30, 60, 90, and 120 s, respectively. The irradiated bacterial solution was diluted in the dark with a certain gradient (after the pre-test results, it was more appropriate to choose to dilute it 10–4 times), coated on the LB agar plate, and then incubated at 30 °C to grow single colonies to be counted to calculate the UV lethality rate.
Diphenylamine is an inhibitor of carotenoid synthesis. After mutagenesis, the discolored strains were rescreened on LB agar plate medium containing 40 μmol/L diphenylamine. We determined through preliminary experimentation that the utilization of diphenylamine at a concentration of 40 μmol/L on LB agar plates was more appropriate and efficient.
2.4. RT-qPCR
RNA extraction was performed using TRIzol Reagent/RNeasy Mini Kit (Qiagen, Hilden, Germany). Total RNA from each sample was quantified and characterized using an Agilent 2100/2200 Bioanalyzer (Agilent, Santa Clara, CA, USA), NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA).
RT-PCR was performed in part using PrimeScriptTM RT reagent kit (Cat. # RR036A, TaKaRa, Tokyo, Japan)). qPCR was performed in part using FastStart Universal SYBR Green Master (ROX) (Cat. #04913850001, Roche, Basel, Switzerland). Real-time polymerase chain reaction (RT-qPCR) analysis was performed using LightCycler96 (Roche, Basel, Switzerland) and lightcycler software v1.1 (Roche, Basel, Switzerland)). Dissociation curves were constructed to test the validity of amplification. The validated gene sequences, amplicon sizes, and primers are shown in Tables S1 and S2, and 16sRNA was the control gene. The relative expression of the genes was determined using the 2−ΔΔCt method of the Sequence Detection software program v1.2.2. The RT-qPCR validation of the transcriptomics results was analyzed as shown in Figure S1.
RNA extraction and RT-qPCR methods for attaining transcript level of trxC gene in different strains were performed as above, and primers taxCF (for RT-qPCR) and taxCR (for RT-qPCR) are shown in Table S6.
2.5. Whole-Genome Resequencing
The genomic DNA of strain MSC14 and strain 14P9 was extracted by magnetic bead method and then sequenced by Illumina HiSeq platform. The above work was performed by Jin Wei Zhi Biotechnology Co., Ltd. (Suzhou, China). The SAMTOOLS bioinformatics tool was used to compare the samples with the reference genome P. dispersa (GeneBank accession number: GCA_019890955.1). The variant sites mentioned in the text can be found in Table S3.
2.6. Transcriptomics Analysis
Transcriptomics analysis was conducted on strain MSC14 and strain 14P9, which were grown in LB-glucose or LB-glucose corn steep liquor powder medium at 30 °C for 48 h. The RNA sequencing procedures were performed by Jinwei Zhi Biotechnology Co. (Suzhou, China). Clean data were assembled using the Trinity software program (v2.6.6), and genes were functionally classified using FunCat from the Munich Protein Sequence Information Center, with a log2 > 1.5-fold cutoff. Three parallel setups were performed for each sample. Genes mentioned in the text can be found in Tables S4 and S5.
2.7. Structure and Activity Analysis of Phosphogluconate Dehydrogenase
The three-dimensional structures of gndA-b (from strain 14P9) and gndA-y (from strain MSC14) were modeled using SWISS-MODEL, with gndA (PDB 3fwn.1, 88.03% sequence identity) serving as a template. UCSF Chimera software(chimera-1.17.3-win64) was used for 3D model analysis. The 6-Phosphogluconate dehydrogenase (6PGDH) Activity Assay Kit from (Solarbio, Beijing, China), was used to test the phosphogluconate dehydrogenase enzyme activity. The enzyme activity unit was defined as the amount of enzyme that catalyzes the production of 1 nmol of NADPH per minute per gram of bacterium.
2.8. Overexpression Strains
Primers were synthesized by TsingkeBiotechnology Co., Ltd. (Guangzhou, China), and the enzyme was purchased from TransGen Biotech Co., Ltd. (Beijing, China). The sensory states of P. dispersa MSC14 and P. dispersa 14P9 were obtained by preparation with 0.1 M/L CaCl2 solution.
Using P. dispersa MSC14 as a template, the trxC target fragment was amplified using primer trxCF and primer trxCR; the trxC target fragment was amplified using Hind III and the target fragment of trxC was amplified by HindIII and BamHI; and pUC19 and the fragment trxC were double digested by Hind III and BamH I. The gel was recovered and ligated into a new plasmid, pUC19-trxC, with T4 DNA ligase in the appropriate enzyme conjugation system. It was then transformed into the pre-prepared susceptible P. dispersa MSC14 and P. dispersa 14P9, and was verified by the screening of ampicillin resistance (AmpR). The sequences of the gene and primer are shown in Table S6.
2.9. Statistical Analysis
All statistical analyses were subjected to t-tests, * for p < 0.05, ** for p < 0.01, and error bars for standard deviation.
3. Results
3.1. Analysis of the Pathway and Ability of Strain MSC14 to Produce β-Carotene and the Positive Effect of Corn Steep Liquor Powder on β-Carotene Synthesis
P. dispersa MSC14, an organism isolated from soil and maintained in our laboratory, exhibits a yellowish hue. Through whole-genome sequencing, it has been determined that MSC14 produces β-carotene using the MEP pathway, with DMAPP (Dimethylallyl diphosphate) serving as a precursor in a four-step dehydrogenation reaction and a one-step cyclization reaction to yield β-carotene (Figure 1c). HPLC experiments have verified that the predominant pigment produced is indeed β-carotene (Figure 1a,b). Figure 1d illustrates the accumulation curves of β-carotene in different media over time. Following four days of incubation in LB fermentation medium (LB), MSC14 produced 255.98 μg/g of β-carotene. However, when 1 g/L of corn steep liquor powder was added to the LB fermentation medium (LB-C), the β-carotene content in the MSC14 increased to 291.75 μg/g, representing a 13.97% increase.
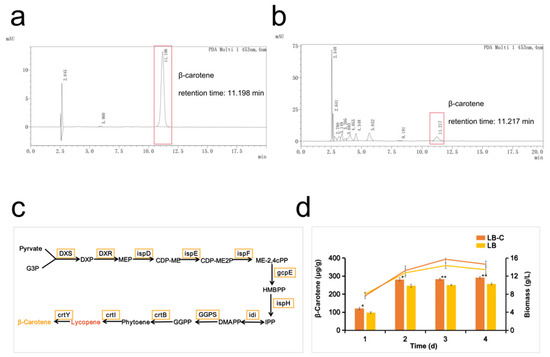
Figure 1.
Identification of the ability of MSC14 to synthesize carotenoids and differences in yield in different media. (a) Liquid chromatograms of β-carotene standards; (b) liquid chromatograms of carotenoids extracted from MSC14; (c) genes associated with carotenoid synthesis by strain MSC14 in whole-genome sequencing; (d) comparison of the biomass and β-carotene yield of strain MSC14 in LB medium supplemented with corn steep liquor powder with that of strain MSC14 in regular LB medium. Biomass and β-carotene production of MSC14 in LB medium supplemented with corn steep liquor powder were compared with those of MSC14 in normal LB medium. LB in the legend is an abbreviation for LB medium, which represents the data measured when strain MSC14 was grown in LB medium, and LB-C in the legend is an abbreviation for LB medium supplemented with corn steep liquor powder, which represents the data measured when strain MSC14 was grown in LB medium supplemented with corn steep liquor powder. After t-test, the β-carotene production of MSC14 strain grown in LB medium supplemented with corn steep liquor powder was significantly different from that in normal LB medium. * represents p < 0.05, ** represents p < 0.01.
3.2. The Ability of the Mutagenized Strain 14P9 to Produce β-Carotene Was Significantly Increased
The mutagenized strain 14P9 displayed a significantly enhanced capacity for β-carotene production. The bacterial fluids were incubated at 30 °C and 150 rpm for 48 h, then diluted and plated after 80 s of UV irradiation (resulting in approximately 85% lethality) (Figure 2a,b). Colonies exhibiting an orange-yellow hue were selected from the mutant population following overnight culturing for diphenylamine resistance screening. A mutant strain called 14P9, showing a darker color than the MSC14 strain, was obtained through the preliminary comparison of the strains’ pigment synthesis capabilities (Figure 2d). The β-carotene synthesis ability of strain 14P9 remained stable after 15 passages (Figure 2c). Compared to strain MSC14, strain 14P9 demonstrated a 29.22% higher β-carotene synthesis ability.
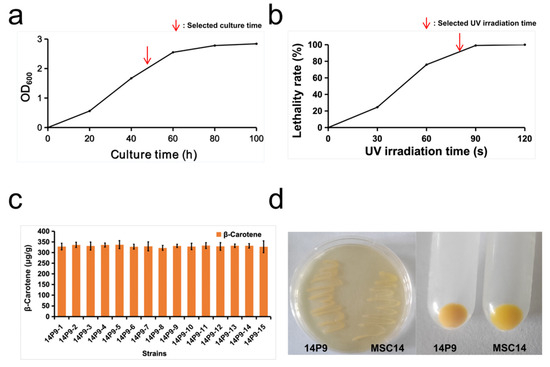
Figure 2.
Analysis of UV mutagenesis to obtain strain 14P9 and its ability to synthesize β-carotene. (a) Growth curves of strain 14P9; (b) lethality curves of UV against strain 14P9; (c) evolution of β-carotene synthesis capacity during 14P9 passaging, t-test, no significant difference; (d) comparison of color differences between strain 14P9 and strain MSC14.
3.3. Genomic Analysis and Transcriptomics Analysis between Strain MSC14 and Strain 14P9
P. dispersa (GeneBank accession number: GCA_019890955.1) was used as the reference genome for the analysis. We conducted bioinformatic analysis using Sentieon (V202112.02), and SNV/InDel analysis using Annovar (V21 April 2018) with Annotation. Mutations were considered concordant if they shared the same chromosome number, location, and mutation type. After excluding mutations that were identical to the reference genome in strain MSC14 and strain 14P9, the analysis revealed that strain 14P9 had 294 genes with single nucleotide variants (SNV) and 114 genes with insertion and deletion mutations (InDel). Most of the genes located on or near the mutation sites in strain 14P9 were associated with the ABC transport system, central carbon metabolism, and amino acid metabolism.
A decrease in the flux of the pentose phosphate pathway [11] can lead to increased β-carotene production. In strain 14P9, the gndA gene, which encodes the NADP-dependent phosphogluconate dehydrogenase, exhibited six single nucleotide site variants. Phosphogluconate dehydrogenase is a crucial enzyme in the pentose phosphate pathway. Figure 3a presents a sequence comparison highlighting the two amino acids (from 460 D to N, and 462 I to V) affected by the variants. Using SWISS-MODEL, we employed gndA (PDB 3fwn.1, 88.03% sequence identity) as a template to model the three-dimensional structure of gndA-b (from strain 14P9) and gndA-y (from strain MSC14). The three-dimensional structure of phosphogluconate dehydrogenase was modeled as a dimer. Figure 3c demonstrates that the amino acid at the mutated position in gndA-b (gold) is closer to the substrate (orange) compared to the amino acid in gndA-y (blue). This suggests that the change in phosphogluconate dehydrogenase activity in strain 14P9 may be caused by this proximity. We then tested the activity of phosphogluconate dehydrogenase and found that it decreased by 33.74% (t-test, p < 0.01) in strain 14P9 compared to strain MSC14 (Figure 3b). The decreased phosphogluconate dehydrogenase activity in strain 14P9 leads to a decrease in the flow of carbon through the pentose phosphate pathway. As a result, there is an increased flow of carbon into the glycolytic pathway. This increase in carbon flow enhances the availability of glyceraldehyde-3-phosphate and pyruvate, which could be a crucial factor in the higher production of β-carotene observed in strain 14P9.
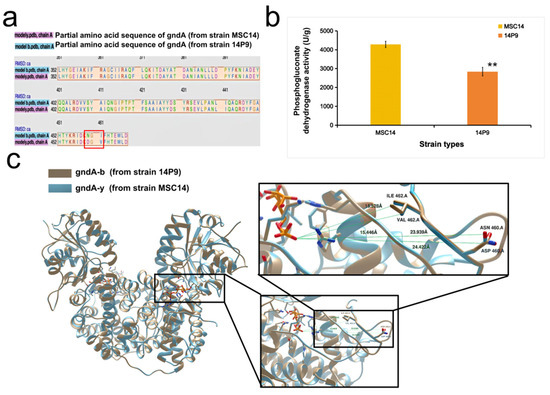
Figure 3.
Identification of the ability of MSC14 to synthesize carotenoids and differences in yield in different media. (a) Amino acid sequences of proteins corresponding to sequence variation in the gndA gene in strain 14P9 compared to strain MSC14, with the selected differential proteins in the red boxes; (b) comparison of phosphogluconate dehydrogenase activity, with error bars representing the standard deviation in the bar graphs, and ** representing the t-test, p < 0.01; (c) phosphogluconate dehydrogenase of strain MSC14 and strain 14P9 three-dimensional structure modeling.
We used Cgview software to generate a genomic circle map (Figure 4a), which displayed gene, GC content, and variant site information. Transcriptomic analysis revealed significant differences in gene expression between the strains 14P9 and MSC14. According to the KEGG annotation, the differential expression genes were primarily related to carbon metabolism pathways, transporter systems, and redox systems (Figure 4b).
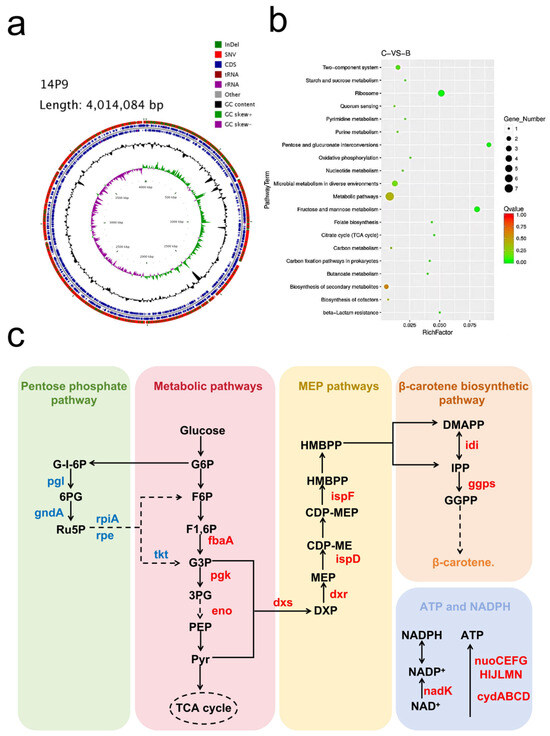
Figure 4.
Mutation information of strain 14P9 and transcriptomics analysis with strain MSC14. (a) Gene completion diagram for strain 14P9; (b) differential gene KEGG enrichment bubble diagram for strain 14P9 vs. strain MSC14; (c) changes in the expression of genes related to β-carotene synthesis in strain 14P9 compared to strain MSC14, genes marked in red indicates up-regulated expression and genes marked in blue indicates down-regulated expression.
Two crucial cofactors, ATP and NADPH, are also necessary for increased β-carotene production. Zhao et al. achieved an increase in β-carotene production by regulating genes related to the ATP synthesis module and TCA module. Compared to strain MSC14, the expression levels of ubiquinone oxidoreductase I (encoded by nuoCEFGHIJLMN) and cytochrome bo oxidase (encoded by CydABCD) were up-regulated in strain 14P9, leading to an increased ATP supply. Furthermore, the expression level of the nadK gene [25] in strain 14P9 was increased by 1.15-fold, enhancing the supply of NADPH. The increased availability of these two important cofactors, ATP and NADPH, may contribute to the higher β-carotene production in strain 14P9.
The MEP synthesis module is crucial for increased β-carotene production [26]. Further analysis of the transcriptomic results showed that the expression levels of key enzymes [27], including dxs (1-deoxy-D-xylulose-5-phosphate synthase), dxr (1-deoxy-D-xylulose-5-phosphate reductoisomerase, ispD (2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase), and ispF (2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase), were increased in strain 14P9 compared to strain MSC14. Specifically, the expression levels of dxs, dxr, ispD, and ispF were increased by 1.03-fold, 1.19-fold, 1.09-fold, and 1.17-fold, respectively. Additionally, the expression levels of the idi (isopentenyl-diphosphate Delta-isomerase) and ispA (farnesyl diphosphate synthase) genes, involved in the β-carotene synthesis pathway, were also increased. These genes with enhanced expression are important steps in the MEP synthesis process [28]. Moreover, the redox system was found to influence carotenoid synthesis, as evidenced by the 4.29-fold up-regulation of the trxC gene, encoding a thioredoxin enzyme in strain 14P9.
In conclusion, the combination of our genomic and transcriptomic findings provides strong evidence which supports the higher β-carotene content observed in strain 14P9 compared to strain MSC14 (Figure 4c).
3.4. Transcriptomic Analysis of Strain MSC14 in Different Media
To further explore the factors associated with β-carotene synthesis and assess the impact of corn steep liquor dry powder on this process, we conducted a transcriptome analysis. This was aimed to scrutinize the changes in the gene expression of strain MSC14 when it was exposed to a medium either with or without corn steep liquor powder. By doing so, we could gain insights into the relationship between β-carotene synthesis and the presence of corn steep liquor powder. The analysis revealed that, in the corn steep liquor powder-containing medium, 677 genes were up-regulated while 759 genes were down-regulated in strain MSC14. This result is compared to the medium without corn steep liquor powder in Figure 5a. KEGG enrichment analysis of the differentially expressed genes indicated significant differences in the carbon metabolism, citric acid cycle, glyoxylate and dicarboxylic acid metabolism, fatty acid degradation, amino acid degradation, and ABC transporter pathways (Figure 5b). Corn steep liquor powder is composed primarily of polysaccharides and various amino acids. Figure 5c illustrates the impact of corn steep liquor powder on carotenoid production.
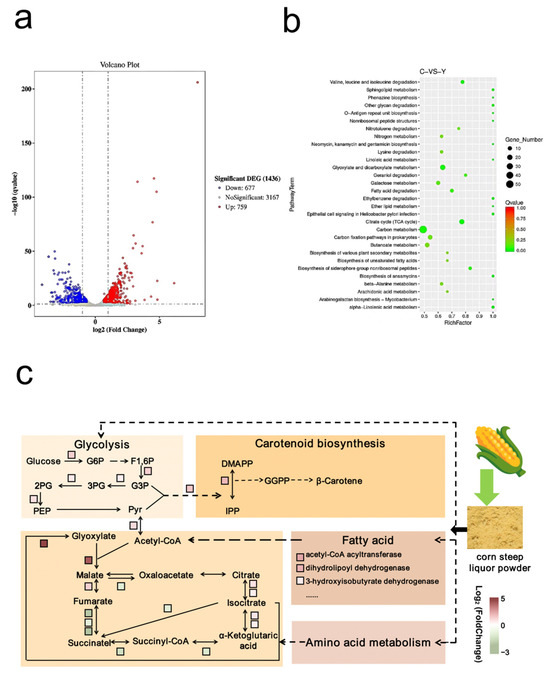
Figure 5.
Transcriptomics analysis of strain MSC14 in LB medium containing corn steep liquor powder and regular LB medium. (a) Differential gene volcano map; (b) differential gene KEGG enrichment bubble diagram; (c) schematic diagram of up- and down-regulated genes in the pathway associated with corn steep liquor powder affecting β-carotene yield, the colors of different squares represent the folds of differential expression of the genes.
Firstly, there is an increase in the expression of genes responsible for enzymes that are involved in glycolytic metabolism, leading to elevated pyruvate levels. Secondly, the down-regulation of genes which encode enzymes associated with the tricarboxylic acid (TCA) cycle, such as succinate-CoA ligase and succinate dehydrogenase, hinders the conversion of pyruvate into acetyl-coenzyme A through the TCA cycle [29]. Lastly, the up-regulation of genes encoding isocitrate-cleaving enzymes of the glyoxylate cycle promotes the accumulation of acetyl-coenzyme A, thereby increasing the supply of pyruvate. This surplus of pyruvate contributes to enhanced β-carotene production through the MEP pathway. Additionally, in order to compensate for the reduced flow in the pentose phosphate pathway, the expression level of isocitrate dehydrogenase, which generates more reducing equivalents in the TCA cycle, is up-regulated to ensure an adequate supply of the cofactor NADPH [30].
Fatty acid metabolism was found to be closely related to carotenoid synthesis [31]. The enhanced expression of genes encoding enzymes involved in the fatty acid degradation pathway, such as long-chain acyl-CoA synthetase, 3-hydroxyacyl-CoA dehydrogenase, acyl-CoA dehydrogenase, acyl-CoA acyltransferase, dihydrolipoyl dehydrogenase, and alcohol dehydrogenase, enhances the fatty acid degradation pathway. The further increase in pyruvate flux [32] to the MEP pathway ultimately leads to an increase in carotenoid production. Furthermore, a higher up-regulation of the gene encoding the amino acid ABC transporter protein was observed, facilitating the utilization of amino acids from corn steep liquor powder by strain MSC14 for carotenoid synthesis.
The expression of the idi gene [33], which catalyzes the isomerization of isopentenyl pyrophosphate (IPP) to dimethylpropenyl pyrophosphate (DMADP), increased 4.05-fold during β-carotene synthesis. The idi gene is an important regulator and rate-limiting enzyme in carotenoid synthesis, and the increase in its expression is favorable for β-carotene biosynthesis. Additionally, the expression of the ispF gene increased 2.35-fold. The ispF gene is another important enzyme in the MEP pathway and is beneficial for carotenoid synthesis.
The maintenance of cellular redox homeostasis is crucial, and redox systems, including the sulfur redox system and glutathione redox system, play an important role in this process [24]. Transcriptome analysis revealed the enhanced expression of genes encoding enzymes involved in the sulfur-oxidoreduction system, such as S-(hydroxymethyl)glutathione dehydrogenase, glutathione S-transferase, and sulfur-oxidoreductase. This suggests a significance of redox homeostasis in carotenoid synthesis.
In conclusion, corn steep liquor powder provides strain MSC14 with more nutrient factors, alters the central carbon metabolic pathway and MEP synthesis pathway, and affects the redox system, ultimately leading to an increased synthesis of β-carotene by strain MSC14.
3.5. Effect of trxC Gene on Growth Performance and Ability to Synthesize β-Carotene in P. dispersa
Cross-clustering analysis of the two transcriptomics datasets revealed significant up-regulation of the trxC gene, which encodes thioredoxin 2, in strain MSC14 (2.77-fold) and strain 14P9 (4.29-fold) when cultivated in medium containing corn steep liquor powder. To investigate the impact of the trxC gene on β-carotene synthesis, we cloned the trxC gene using the DNA of strain MSC14 as a template and generated the overexpression strains MSC14 (trxC) and 14P9 (trxC).
The RT-qPCR results showed that the trxC transcript level of strain MSC14 (trxC) was 4.08 times higher than that of the empty vector control strain MSC14, and that the trxC transcript level of strain 14P9 (trxC) was 1.20 times higher than that of the empty vector control strain 14P9 (Figure 6a). The fermentation results showed that, after 48 h of fermentation in LB medium, the β-carotene content of strain MSC14 (trxC) increased by 15.47% compared to the control strain MSC14, and the β-carotene content of strain 14P9 (trxC) increased by 5.79% compared to that of strain 14P9 (Figure 6b).
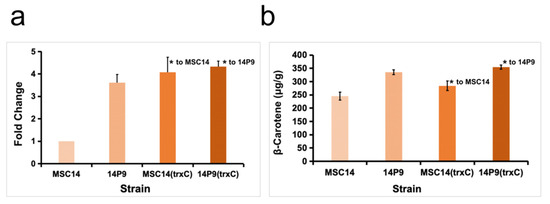
Figure 6.
Construction of strain MSC14 and strain 14P9 overexpressing trxC gene and analysis of their ability to synthesize β-carotene. (a) RT-qPCR verified the differences in the transcript levels of trxC in different strains; (b) comparison of the ability of different strains to synthesize β-carotene, * represents p < 0.05 by t-test, and error bars represent the standard deviation in the bar graphs.
The results showed that overexpression of the trxC gene significantly increased the ability of strain MSC14 to synthesize β-carotene, and its transcriptional level (4.08-fold) was also similar to that of the trxC gene in the transcriptomics of strain 14P9 (4.29-fold), which could explain why the β-carotene contents of strains MSC14 (trxC) and 14P9 were similar. However, the β-carotene content of strain MSC14 (trxC) still did not reach the level of strain 14P9, which may be related to factors such as the variation in the gndA gene in strain 14P9. In addition, the β-carotene content of strain 14P9 (trxC) did not increase much compared with that of strain 14P9. We hypothesized that this might be due to the fact that there might be an upper limit to the ability of the trxC gene to increase β-carotene, and that it might need to be regulated in multiple ways together with other pathways to further increase the β-carotene content.
The thioredoxin system, which comprises thioredoxin [34] (Trx), thioredoxin reductase TrxR (TR), and the reducing substrate nicotinamide adenine dinucleotide phosphate (NADPH), plays a critical role in maintaining a stable redox state in the body. trxC is involved in regulating cell growth, inhibiting apoptosis, and controlling gene transcription. Previous studies have demonstrated that trxC regulates pigment accumulation in certain cyanobacteria, and both carotenoids and trxC have been found to mitigate oxidative damage [35]. However, the relationship between trxC and carotenoids in bacteria has not been extensively explored. Interestingly, the overexpression of genes associated with the redox system [36] in yeast has been shown to enhance the carotenoid content. We suggest that thioredoxin not only reduces intracellular damage caused by reactive oxygen species but also stabilizes the intracellular environment and enhances cell viability by reducing ribonucleotide reductase, a critical enzyme for DNA synthesis [37]. More importantly, β-carotene acts as an antioxidant, and the overexpression of thioredoxin may primarily rely on the thioredoxin system to eliminate reactive oxygen species, resulting in reduced β-carotene consumption and increased β-carotene accumulation within the cells.
4. Discussion
In the current study, our objective was to investigate the ability of MSC14, a P. dispersa strain isolated from soil, to produce β-carotene. This discovery aligns with previous research that has identified carotenoid production in Pantoea, expanding the range of carotenoid-producing strains within the genus. By analyzing the transcriptomic analysis conducted herein, we have gained a deeper understanding of how corn steep liquor powder, a cost-effective medium, contributes to the process of β-carotene synthesis. This has significant implications for optimizing media for β-carotene synthesis by bacteria. As found by most studies [21,23,38], corn steep liquor powder can also serve as a nitrogen source in industrial production. We also speculate on the potential success of using corn steep liquor powder as the sole carbon and nitrogen source for bacterial fermentation in β-carotene production. We have planned to explore this intriguing experiment in our upcoming study, aiming to further reduce production costs. This investigation aligns with our purpose of utilizing transcriptomics to analyze the connection between corn steep liquor powder and the β-carotene synthesis process.
Furthermore, we observed that mutations in the gndA gene, which is responsible for the production of phosphogluconate dehydrogenase in strain 14P9, resulted in a decrease in flux through the pentose phosphate pathway. This decrease, in turn, led to an increase in the β-carotene content. These findings suggest a correlation between changes in central carbon metabolism and β-carotene production, with the effect of the gndA gene being similar to that observed in previous studies involving the zwf [15] gene. Thus, our study provides a new gene locus for blocking the pentose phosphate pathway and thereby increasing the β-carotene yield. It is crucial to note that an enhanced supply of ATP and NADPH is essential for β-carotene synthesis. Consequently, our future studies will focus on how to further enhance these two cofactors. Furthermore, we have discovered that the trxC gene, which is responsible for encoding thioredoxin, plays a pivotal role in the synthesis of β-carotene. To the best of our knowledge, this finding may be the first of its kind to be reported. However, further investigation is needed to fully understand the mechanism behind the interaction between the redox system and β-carotene synthesis. It is worth noting that this study should not solely concentrate on thioredoxin but should also explore other redox systems.
Despite implementing mutagenesis and optimizing the medium, the β-carotene content of P. dispersa MSC14 was not the highest among the experimental strains [39], and there was no significant improvement in the expression of carotenoid synthesis genes, such as crtI. Given these challenges, our next step will involve reconstructing the β-carotene synthesis pathway of strain MSC14 and strain 14P9 in the engineered strains. This will allow us to further investigate the relationship between the gndA gene, the trxC gene, and β-carotene synthesis. Additionally, we will continue to explore the regulatory mechanisms of P. dispersa MSC14 and P. dispersa 14P9 in order to develop P. dispersa strains with higher β-carotene contents. This research will provide valuable insights into carotenoid production and the diverse applications of carotenoids in various fields.
In conclusion, the present study showcases the capability of P. dispersa cerevisiae in producing β-carotene. Moreover, we established a direct correlation between corn steep liquor powder as a medium component and the synthesis of β-carotene. Additionally, the role of central carbon metabolism and the thioredoxin reduction system in regulating carotenoid synthesis was thoroughly examined. Notably, we were the pioneers in reporting the impact of two specific genes, gndA and trxC, on β-carotene synthesis. These exciting findings have the potential to guide the engineering of bacteria for enhanced carotenoid production and even facilitate the production of natural products such as isoprenoids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation10020083/s1. Figure S1: Correlation analysis of RT-qPCR with differential gene log2 Fold Change; Table S1: RT-qPCR validation genes and their primers between MSC14 and 14P9; Table S2: RT-qPCR validation genes and their primers for culture medium with and without dried corn syrup powder. Table S3: Carbohydrate metabolism-related gene variant locus; Table S4: Genes Containing Significantly Increased MSC14 Expression in Corn Slurry Medium in Transcriptome Analysis; Table S5: Genes with significantly increased expression in strain 14P9 compared to strain MSC14; Table S6: The sequences of gene or primer.
Author Contributions
Conceptualization, T.C.; methodology, T.C. and N.L.; validation, T.C. and N.L.; formal analysis, N.L.; investigation, N.L.; data curation, T.C. and N.L.; writing—original draft preparation, N.L.; writing—review and editing, T.C.; supervision, T.C.; project administration, T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The high throughput sequencing data about this study are stored at NCBI (https://www.ncbi.nlm.nih.gov/) (accessed on 6 December 2023) under the accession number: PRJNA1049143.
Acknowledgments
We would like to thank Shuqi Li for the gift of P. dispersa MSC14 strains.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albermann, C. High versus low level expression of the lycopene biosynthesis genes from Pantoea ananatis in Escherichia coli. Biotechnol. Lett. 2011, 33, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ananda, N.; Vadlani, P.V. Production and optimization of carotenoid-enriched dried distiller’s grains with solubles by Phaffia rhodozyma and Sporobolomyces roseus fermentation of whole stillage. J. Ind. Microbiol. Biotechnol. 2010, 37, 1183–1192. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Schürmann, P.; Jacquot, J.-P. Thioredoxin and metabolic regulation. Semin. Cell Biol. 1994, 5, 285–293. [Google Scholar] [CrossRef]
- Burton, G.W.; Mogg, T.J.; Riley, W.W.; Nickerson, J.G. β-Carotene oxidation products—Function and safety. Food Chem. Toxicol. 2021, 152, 112207. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Kang, B.; Lee, Y.; Lee, Y.; Kim, J. Pantoea ananatis carotenoid production confers toxoflavin tolerance and is regulated by Hfq-controlled quorum sensing. Microbiologyopen 2021, 10, e1143. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Fulco, A.J. Fatty acid metabolism in bacteria. Prog. Lipid Res. 1983, 22, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Gassel, S.; Breitenbach, J.; Sandmann, G. Genetic engineering of the complete carotenoid pathway towards enhanced astaxanthin formation in Xanthophyllomyces dendrorhous starting from a high-yield mutant. Appl. Microbiol. Biotechnol. 2014, 98, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.I.; Inostroza, I.; Pizarro, M.; Pérez, J. From genetic improvement to commercial-scale mass culture of a Chilean strain of the green microalga Haematococcus pluvialis with enhanced productivity of the red ketocarotenoid astaxanthin. AoB Plants 2013, 5, plt026. [Google Scholar] [CrossRef] [PubMed]
- Gromer, S.; Urig, S.; Becker, K. The thioredoxin system—From science to clinic. Med. Res. Rev. 2004, 24, 40–89. [Google Scholar] [CrossRef] [PubMed]
- Harker, M.; Bramley, P. Expression of prokaryotic 1-deoxy-d-xylulose-5-phosphatases in Escherichia coli increases carotenoid and ubiquinone biosynthesis. FEBS Lett. 1999, 448, 115–119. [Google Scholar] [CrossRef]
- Kar, A.; Paramasivam, B.; Jayakumar, D.; Swaroop, A.K.; Jubie, S. Thioredoxin Interacting Protein Inhibitors in Diabetes Mellitus: A Critical Review. Curr. Drug Res. Rev. 2023, 15, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Keasling, J.D. Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol. Bioeng. 2001, 72, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Momen, A.Z.R.; Mijts, B.N.; Schmidt-Dannert, C. Biosynthesis of Structurally Novel Carotenoids in Escherichia coli. Chem. Biol. 2003, 10, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, Y.; Li, L.; Ma, Y.; Huang, J.; Ye, J. Engineering Sphingobium sp. to Accumulate Various Carotenoids Using Agro-Industrial Byproducts. Front. Bioeng. Biotechnol. 2021, 9, 784559. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yi, H.; Zhan, H.; Wang, L.; Wang, J.; Li, Y.; Liu, B. Gibberellic acid-induced fatty acid metabolism and ABC transporters promote astaxanthin production in Phaffia rhodozyma. J. Appl. Microbiol. 2022, 132, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Mantzouridou, F.; Roukasa, T.; Kotzekidoua, P.; Liakopoulou, M. Optimization of β-Carotene Production from Synthetic Medium by Blakeslea trispora: A Mathematical Modeling. Appl. Biochem. Biotechnol. 2002, 101, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef]
- Mehta, B.J.; Obraztsova, I.N.; Cerdá-Olmedo, E. Mutants and Intersexual Heterokaryons of Blakeslea trispora for Production of β-Carotene and Lycopene. Appl. Environ. Microbiol. 2003, 69, 4043–4048. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M. Agro-food wastes utilization by Blakeslea trispora for carotenoids production. Acta Biochim. Pol. 2012, 59, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Nanou, K.; Roukas, T.; Papadakis, E.; Kotzekidou, P. Carotene production from waste cooking oil by Blakeslea trispora in a bubble column reactor: The role of oxidative stress. Eng. Life Sci. 2017, 17, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; He, X.; Zhang, H.; Guo, X.; Cheng, Y.; Liu, X.; Wang, Z.; He, X. Whole Genome Sequencing and RNA-seq-Driven Discovery of New Targets That Affect Carotenoid Synthesis in Phaffia rhodozyma. Front. Microbiol. 2022, 13, 837894. [Google Scholar] [CrossRef]
- Oka, S.-I.; Titus, A.S.; Zablocki, D.; Sadoshima, J. Molecular properties and regulation of NAD+ kinase (NADK). Redox Biol. 2023, 59, 102561. [Google Scholar] [CrossRef]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Rohdich, F.; Hecht, S.; Gärtner, K.; Adam, P.; Krieger, C.; Amslinger, S.; Arigoni, D.; Bacher, A.; Eisenreich, W. Studies on the nonmevalonate terpene biosynthetic pathway: Metabolic role of IspH (LytB) protein. Proc. Natl. Acad. Sci. USA 2002, 99, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Rohdich, F.; Zepeck, F.; Adam, P.; Hecht, S.; Kaiser, J.; Laupitz, R.; Gräwert, T.; Amslinger, S.; Eisenreich, W.; Bacher, A.; et al. The deoxyxylulose phosphate pathway of isoprenoid biosynthesis: Studies on the mechanisms of the reactions catalyzed by IspG and IspH protein. Proc. Natl. Acad. Sci. USA 2003, 100, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Carotenoids of biotechnological importance. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 148, pp. 449–467. [Google Scholar]
- Wu, Y.; Yan, P.; Li, Y.; Liu, X.; Wang, Z.; Chen, T.; Zhao, X. Enhancing β-Carotene Production in Escherichia coli by Perturbing Central Carbon Metabolism and Improving the NADPH Supply. Front. Bioeng. Biotechnol. 2020, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, J.; Chen, H. Progress in metabolic engineering of β-carotene synthesis. Sheng Wu Gong Cheng Xue Bao 2017, 33, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Song, G.H.; Kim, S.H.; Choi, B.H.; Han, S.J.; Lee, P.C. Heterologous Carotenoid-Biosynthetic Enzymes: Functional Complementation and Effects on Carotenoid Profiles in Escherichia coli. Appl. Environ. Microbiol. 2013, 79, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.-L.; Chen, K.-Q.; Dai, W.-Y.; Ma, J.-F.; Zhang, M.; Jiang, M.; Wei, P.; Ouyang, P.-K. Succinic acid production by Actinobacillus succinogenes NJ113 using corn steep liquor powder as nitrogen source. Bioresour. Technol. 2013, 136, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Lv, X.; Yu, H. Engineering microbes for isoprene production. Metab. Eng. 2016, 38, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Kim, J.-E.; Lee, S.-H.; Park, H.-M.; Choi, M.-S.; Kim, J.-Y.; Lee, S.-H.; Shin, Y.-C.; Keasling, J.D.; Kim, S.-W. Engineering the lycopene synthetic pathway in E. coli by comparison of the carotenoid genes of Pantoea agglomerans and Pantoea ananatis. Appl. Microbiol. Biotechnol. 2007, 74, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Li, K.; Klug, G. Expression of the trxC Gene of Rhodobacter capsulatus: Response to Cellular Redox Status Is Mediated by the Transcriptional Regulator OxyR. J. Bacteriol. 2006, 188, 7689–7695. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.-G.; Han, M.; Zhang, W.-G.; Qian, H. Carotene production from agro-industrial wastes by Arthrobacter globiformis in shake-flask culture. Prep. Biochem. Biotechnol. 2014, 44, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Sun, T.; Zhu, X.; Xu, H.; Tang, J.; Zhang, X.; Ma, Y. Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab. Eng. 2013, 17, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nambou, K.; Wei, L.; Cao, J.; Imanaka, T.; Hua, Q. Lycopene production in recombinant strains of Escherichia coli is improved by knockout of the central carbon metabolism gene coding for glucose-6-phosphate dehydrogenase. Biotechnol. Lett. 2013, 35, 2137–2145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).