Production of Carotenoids by Microorganisms
Abstract
1. Introduction
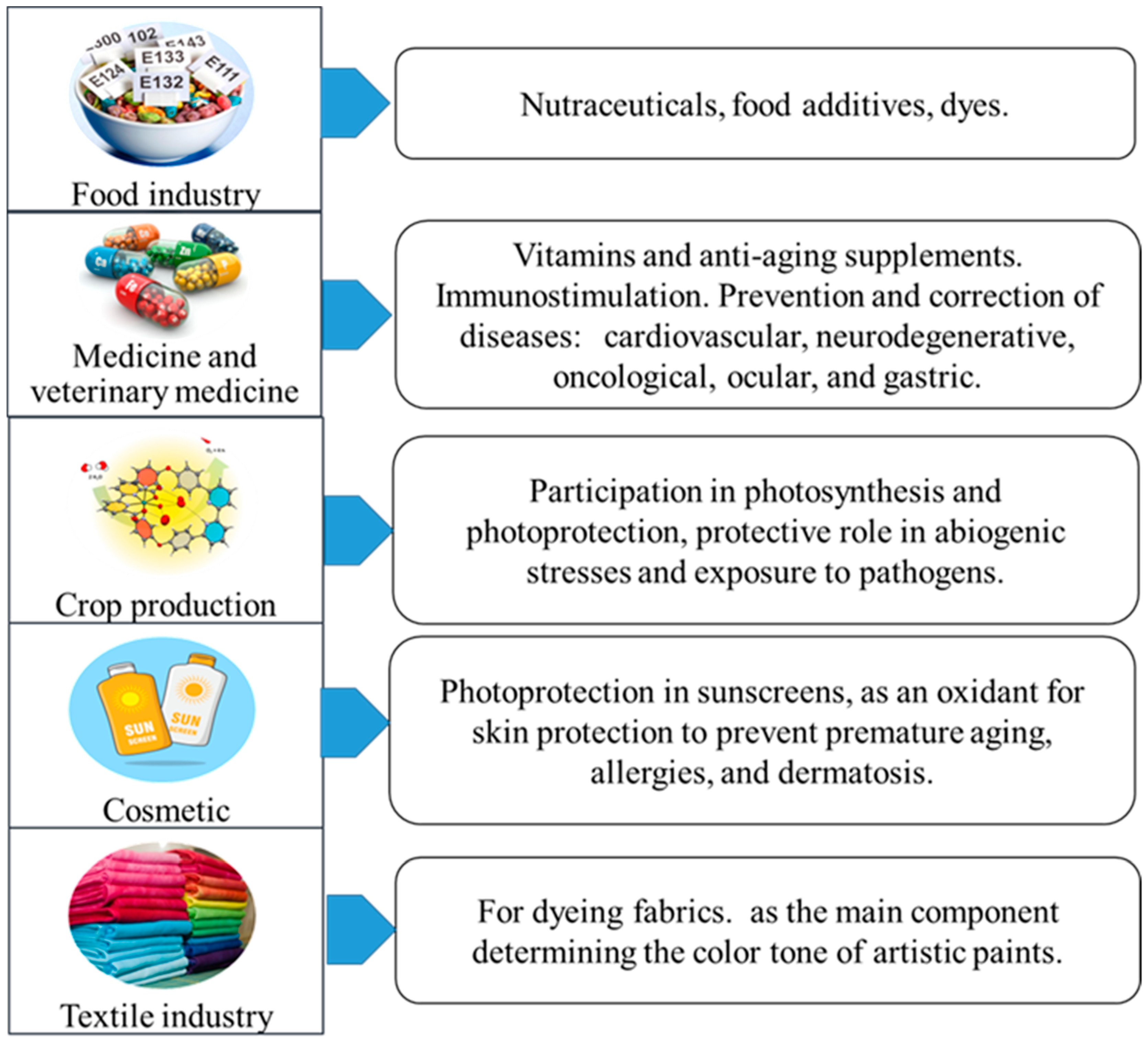
2. Physiological Role of Pigments in Microorganisms
3. Carotenoid-Producing Microorganisms
3.1. Fungi
3.2. Yeast
3.3. Bacteria
3.4. Archaea
4. Effect of Culture Conditions on the Accumulation of Carotenoids by Microorganisms
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Khaneghah, A.M.; Sant’Ana, A.S.; Lorenzoe, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Hucke, H.U.; Ramos, N.F.; Ribeiro, H.F.; Alves, M.B.; Mustafa, A.; Pereira, J.F.B.; Farias, F.O. Tailor-made solvents for microbial carotenoids recovery. Appl. Microbiol. Biotechnol. 2024, 108, 234. [Google Scholar] [CrossRef] [PubMed]
- Kuddus, M.; Singh, P.; Raveendran, S.; Singh, R. Microbial Pigments: Applications in Food and Beverage Industry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; p. 280. [Google Scholar] [CrossRef]
- Raita, S.; Feldmane, L.; Kusnere, Z.; Spalvins, K.; Kuzmika, I.; Berzina, I.; Mika, T. Microbial carotenoids production: Strains, conditions, and yield affecting factors. Environ. Clim. Technol. 2023, 27, 1027–1048. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Brys, J. Torulene and torularhodin: “new” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef]
- Urnau, L.; Colet, R.; Truccolo, R.P.; de Medeiros Burkert, J.F.; Rodrigues, E.; Gomes, R.; Assis Jacques, R.; Valduga, E.; Steffens, C. Use of low-cost agro-industrial substrate to obtain carotenoids from phaffia rhodozyma in a bioreactor. Ind. Biotechnol. 2019, 15, 25–34. [Google Scholar] [CrossRef]
- Grand View Research. Carotenoids Market Analysis by Source (Natural, Synthetic), by Product (Beta-Carotene, Lutein, Lycopene, Astaxanthin, Zeaxanthin, Canthaxanthin), by Application (Food, Supplements, Feed, Pharmaceuticals, Cosmetics), and Segment Forecasts, 2018–2025. 2016. Available online: https://www.grandviewresearch.com/industry-analysis/carotenoids-market (accessed on 1 September 2024).
- Agogué, H.; Joux, F.; Obernosterer, I.; Lebaron, P. Resistance of marine bacterioneuston to solar radiation. Appl. Environ. Microbiol. 2005, 71, 5282–5289. [Google Scholar] [CrossRef] [PubMed]
- Chatragadda, R.; Dufossé, L. Ecological and biotechnological aspects of pigmented microbes: A way forward in development of food and pharmaceutical grade pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Zafar, J.; Aqeel, A.; Shah, F.I.; Ehsan, N.; Gohar, U.F.; Moga, M.A.; Festila, D.; Ciurea, C.; Irimie, M.; Chicea, R. Biochemical and immunological implications of lutein and zeaxanthin. Int. J. Mol. Sci. 2021, 22, 10910. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cui, H.L. In Vitro antioxidant, antihemolytic, and anticancer activity of the carotenoids from halophilic archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Giani, M.; Montero-Lobato, Z.; Garbayo, I.; Vílchez, C.; Vega, J.M.; Martínez-Espinosa, R.M. Haloferax mediterranei cells as C50 carotenoid factories. Mar. Drugs 2021, 19, 100. [Google Scholar] [CrossRef]
- Serrano, S.; Mendo, S.; Caetano, T. Haloarchaea have a high genomic diversity for the biosynthesis of carotenoids of biotechnological interest. Res. Microbiol. 2022, 173, 103919. [Google Scholar] [CrossRef]
- Nawaz, A.; Chaudhary, R.; Shah, Z.; Dufossé, L.; Fouillaud, M.; Mukhtar, H.; Haq, I.U. An overview on industrial and medical applications of bio-pigments synthesized by marine bacteria. Microorganisms 2021, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, seaweeds and aquatic bacteria, archaea, and yeasts: Sources of carotenoids with potential antioxidant and anti-inflammatory health-promoting actions in the sustainability era. Mar. Drugs 2023, 21, 340. [Google Scholar] [CrossRef] [PubMed]
- Moliné, M.; Libkind, D.; Diéguez, M.C.; van Broock, M. Photoprotective role of carotenoids in yeasts: Response to UV-B of pigmented and naturally-occurring albino strains. J. Photochem. Photobiol. B 2009, 95, 156–161. [Google Scholar] [CrossRef]
- Moliné, M.; Flores, M.R.; Libkind, D.; Dieguéz, M.D.; Farias, M.E.; van Broock, M. Photoprotection by carotenoid pigments in the yeast Rhodotorula mucilaginosa: The role of torularhodin. Photochem. Photobiol. Sci. 2010, 9, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Seel, W.; Baust, D.; Sons, D.; Albers, M.; Etzbach, L.; Fuss, J.; Lipski, A. Carotenoids are used as regulators for membrane fluidity by Staphylococcus xylosus. Sci. Rep. 2020, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid pigmentation in antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arct. Antarct. Alp. Res. 2010, 42, 396–405. [Google Scholar] [CrossRef]
- Buckley, E. Phenotypic and Genomic Insights into Ultraviolet Resistance of Arthrobacter and Pseudarthrobacter Isolated from Desert Soil. Doctoral Dissertation, Auckland University of Technology, Auckland, New Zealand, 2020. [Google Scholar]
- Vasey, J. Characterisation of Pigmentation in a Novel Isolate of Arthrobacter Recovered from Soil of the Namib Desert. Doctoral Dissertation, Auckland University of Technology, Auckland, New Zealand, 2022. [Google Scholar]
- Jones, D.L.; Baxter, B.K. DNA repair and photoprotection: Mechanisms of overcoming environmental ultraviolet radiation exposure in halophilic archaea. Front. Microbiol. 2017, 29, 1882. [Google Scholar] [CrossRef]
- Moliné, M.; Libkind, D.; Garcia, V.; Giraudo, M. Production of pigments and photo-protective compounds by cold-adapted yeasts. In Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzini, P., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 193–224. [Google Scholar] [CrossRef]
- Reis-Mansur, M.C.P.P.; Cardoso-Rurr, J.S.; Silva, J.V.M.A.; de Souza, G.R.; Cardoso, V.D.S.; Mansoldo, F.R.P.; Pinheiro, Y.; Schultz, J.; Lopez Balottin, L.B.; da Silva, A.J.R.; et al. Carotenoids from UV-resistant Antarctic Microbacterium sp. LEMMJ01. Sci. Rep. 2019, 9, 9554. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.N.G.; Seviour, T.; Kjelleberg, S.; Hinks, J. Carotenoids improve bacterial tolerance towards biobutanol through membrane stabilization. Environ. Sci. Nano 2021, 8, 328–341. [Google Scholar] [CrossRef]
- Zamudio-Chávez, L.; Suesca, E.; López, G.D.; Carazzone, C.; Manrique-Moreno, M.; Leidy, C. Staphylococcus aureus modulates carotenoid and phospholipid content in response to oxygen-restricted growth conditions, triggering changes in membrane biophysical properties. Int. J. Mol. Sci. 2023, 24, 14906. [Google Scholar] [CrossRef] [PubMed]
- Múnera-Jaramillo, J.; López, G.D.; Suesca, E.; Carazzone, C.; Leidy, C.; Manrique-Moreno, M. The role of staphyloxanthin in the regulation of membrane biophysical properties in Staphylococcus aureus. Biochim. Biophys. Acta Biomembr. 2024, 1866, 184288. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.; Magalon, H.; Dufossé, L.; Fouillaud, M. Production of pigments from the tropical marine-derived fungi Talaromyces albobiverticillius: New resources for natural red-colored metabolites. J. Food Compos. Anal. 2018, 70, 35–48. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef]
- Calegari-Santos, R.; Diogo, R.A.; Fontana, J.D.; Bordin, T.M. Carotenoid production by halophilic archaea under different culture conditions. Curr. Microbiol. 2016, 72, 641–651. [Google Scholar] [CrossRef]
- Sundararajan, P.; Ramasamy, S.P. Current perspectives on industrial application of microbial carotenoid as an alternative to synthetic pigments. Sustain. Chem. Pharm. 2024, 37, 101353. [Google Scholar] [CrossRef]
- Naz, T.; Ullah, S.; Nazir, Y.; Li, S.; Iqbal, B.; Liu, Q.; Mohamed, H.; Song, Y. Industrially important fungal carotenoids: Advancements in biotechnological production and extraction. J. Fungi 2023, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef]
- Coker, J.A. Extremophiles and biotechnology: Current uses and prospects. F1000 Res. 2016, 5, 396. [Google Scholar] [CrossRef] [PubMed]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in chemical and biological methods to identify microorganisms—From past to present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef]
- Afroz Toma, M.; Rahman, M.H.; Rahman, M.S.; Arif, M.; Nazir, K.H.M.N.H.; Dufossé, L. Fungal pigments: Carotenoids, riboflavin, and polyketides with diverse applications. J. Fungi 2023, 9, 454. [Google Scholar] [CrossRef]
- Erasun, C.E.; Johnson, E.A. Fungal carotenoids. Appl. Microbiol. Biotechnol. 2002, 2, 45–85. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant protection from UV- and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Igreja, W.S.; Maia, F.D.A.; Lopes, A.S.; Chisté, R.C. Biotechnological production of carotenoids using low cost-substrates is influenced by cultivation parameters: A review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M. Agro-food wastes utilization by blakeslea trispora for carotenoids production. Acta Biochim. Pol. 2012, 59, 151–153. [Google Scholar] [CrossRef]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Durakli, S.V.; Tirpanci, G.S. Optimizing β-carotene production by Blakeslea trispora using bug damaged wheat. Pigm. Resin. Technol. 2018, 47, 189–195. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Natural β-carotene production by Blakeslea trispora cultivated in Spanish-style green olive processing wastewaters. Foods 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Luthra, U.; Babu, P.; Remya, R.R.; Julius, A.; Patel, Y.; Jajula Veera, R.; Majeed, I. Medium optimization and downstream process design for the augmented yield of β-carotene using fungi Blakeslea trispora. Pigm. Resin. Technol. 2022, 51, 574–580. [Google Scholar] [CrossRef]
- Choudhari, S.M.; Ananthanarayan, L.; Singhal, R.S. Use of metabolic stimulatorsand inhibitors for enhanced production of -carotene and lycopene by Blakeslea trispora NRRL 2895 and 2896. Bioresour. Technol. 2008, 99, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Nanou, K.; Roukas, T. Stimulation of the biosynthesis of carotenes by oxidative stress in Blakeslea trispora induced by elevated dissolved oxygen levels in the culture medium. Bioresour. Technol. 2011, 102, 8159–8164. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, H.; Tang, P.; Sun, J.; Yuan, Q.; Li, C. GC–MS-based metabolomics study ofthe responses to arachidonic acid in Blakeslea trispora. Fungal Genet. Biol. 2013, 57, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Roukas, T. The role of oxidative stress on carotene production by Blakeslea trispora in submerged fermentation. Crit. Rev. Biotechnol. 2015, 35, 424–433. [Google Scholar]
- Nanou, K.; Roukas, T.; Papadakis, E.; Kotzekidou, P. Carotene production from waste cooking oil by Blakeslea trispora in a bubble column reactor: The role of oxidative stress. Eng. Life Sci. 2017, 17, 775–780. [Google Scholar] [CrossRef]
- He, Z.; Wang, S.; Yang, Y.; Hu, J.; Wang, C.; Li, H.; Ma, B.; Yuan, Q. β-Carotene production promoted by ethylene in Blakeslea trispora and the mechanism involved in metabolic responses. Process Biochem. 2017, 57, 57–63. [Google Scholar] [CrossRef]
- Azizi, M.; Zare, D.; Sepahi, A.A.; Azin, M. Evaluating the effect of microbial stimulation and oxidative stress on increasing β-Carotene production in Blakeslea trispora. Adv. Res. Microb. Metab. Technol. 2021, 4, 1–11. [Google Scholar]
- Ge, X.; Li, R.; Zhang, X.; Zhao, J.; Zhang, Y.; Xin, Q. Transcriptome sequencing and global analysis of blue light-responsive genes provide clues for high carotenoid yields in Blakeslea trispora. Int. Microbiol. 2022, 25, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Watcharawipas, A.; Runguphan, W. Red yeasts and their carotenogenic enzymes for microbial carotenoid production. FEMS Yeast Res. 2023, 23, foac063. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2021, 62, 6932–6946. [Google Scholar] [CrossRef] [PubMed]
- Sartaj, K.; Gupta, P.; Tripathi, S.; Poluri, K.M.; Prasad, R. Insights into the extraction, characterization and antifungal activity of astaxanthin derived from yeast de-oiled biomass. Environ. Technol. Innov. 2022, 27, 102437. [Google Scholar] [CrossRef]
- Keceli, T.M.; Erginkaya, Z.; Turkkan, E.; Kaya, U. Antioxidant and antibacterial effects of carotenoids extracted from Rhodotorula glutinis strains. Asian J. Chem. 2013, 25, 42–46. [Google Scholar] [CrossRef]
- Dumitriu, C.; Ungureanu, C.; Simona, P.; Tofan, V.; Popescu, M.; Pirvu, C. Ti surface modification with a natural antioxidant and antimicrobial agent. Surf. Coat. Int. 2015, 276, 175–185. [Google Scholar] [CrossRef]
- Naisi, S.; Bayat, M.; Salehi, T.Z.; Zarif, B.R.; Yahyaraeyat, R. Antimicrobial and antibiofilm effects of carotenoid pigment extracted from Rhodotorula glutinis strain on food-borne bacteria. Iran. J. Microbiol. 2023, 15, 79. [Google Scholar] [CrossRef]
- Ungureanu, C.; Popescu, S.; Purcel, G.; Tofan, V.; Popescu, M.; Sălăgeanu, A.; Pîrvu, C. Improved antibacterial behavior of titanium surface with torularhodin-polypyrrole film. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Kot, A.M.; Sęk, W.; Kieliszek, M.; Błażejak, S.; Pobiega, K.; Brzezińska, R. Diversity of red yeasts in various regions and environments of poland and biotechnological potential of the isolated strains. Appl. Biochem. Biotechnol. 2023, 196, 3274–3316. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Ribeiro, H.F.; Santos-Ebinuma, V.C.; Schuur, B.; Pereira, J.F.B. Rhodotorula sp.-based biorefinery: A source of valuable biomolecules. Appl. Microbiol. Biotechnol. 2022, 106, 7431–7447. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Characterization and cytotoxic activity of pigment extracted from Rhodotorula mucilaginosa to assess its potential as bio-functional additive in confectionary products. J. Food Sci. Technol. 2021, 58, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.Y.; Alnaeeli, M.; Park, J.K. Production control and characterization of antibacterial carotenoids from the yeast Rhodotorula mucilaginosa AY-01. Process Biochem. 2016, 51, 463–473. [Google Scholar] [CrossRef]
- Lee, J.; Chen, L.; Cao, B.; Chen, W.N. Engineering Rhodosporidium toruloides with a membrane transporter facilitates production and separation of carotenoids and lipids in a bi-phasic culture. Appl. Microbiol. Biotechnol. 2016, 100, 869–877. [Google Scholar] [CrossRef]
- Xue, S.J.; Li, X.C.; Huang, X.; Liu, J.; Li, Y.; Zhang, X.T.; Zhang, J.Y. Diversity investigation of cultivable yeasts associated with honeycombs and identification of a novel Rhodotorula toruloides strain with the robust concomitant production of lipid and carotenoid. Bioresour. Technol. 2023, 370, 128573. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, Z.; Zhao, D.; Li, B.; Wang, D.; Chang, L.; Feng, F.; Zheng, L.; Wang, X.; Shao, M.; et al. Multi-omics analysis provides insights into the enhancement of β-carotene and torularhodin production in oleaginous red yeast Sporobolomyces pararoseus under H2O2-induced oxidative stress. LWT 2024, 197, 115947. [Google Scholar] [CrossRef]
- Xie, Z.T.; Mi, B.Q.; Lu, Y.J.; Chen, M.T.; Ye, Z.W. Research progress on carotenoid production by Rhodosporidium Toruloides. Appl. Microbiol. Biotechnol. 2024, 108, 7. Available online: https://link.springer.com/article/10.1007/s00253-023-12943-0 (accessed on 28 September 2024). [CrossRef] [PubMed]
- Pi, H.W.; Anandharaj, M.; Kao, Y.Y.; Lin, Y.J.; Chang, J.J.; Li, W.H. Engineering the oleaginous red yeast Rhodotorula glutinis for simultaneous β-carotene and cellulase production. Sci. Rep. 2018, 8, 10850. [Google Scholar] [CrossRef]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Siziya, I.N.; Hwang, C.Y.; Seo, M.J. Antioxidant potential and capacity of microorganism-sourced c30 carotenoids—A Review. Antioxidants 2022, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, P.; Ramasamy, S.P. Bacteria as promising biofactory for pigment production: A prospective insights into production strategies and industrial applications. AsPac J. Mol. Biol. Biotechnol. 2023, 31, 53–61. [Google Scholar] [CrossRef]
- Sun, X.Y.; Dong, H.; Zhang, Y.; Gao, J.W.; Zhou, P.; Sun, C.; Xu, L. Isolation and Cultivation of Carotenoid Producing Strains from Tidal Flat Sediment and Proposal of Croceibacterium aestuarii sp. nov., a Novel Carotenoid-Producing Species in the Family Erythrobacteraceae. J. Mar. Sci. Eng. 2024, 12, 99. [Google Scholar] [CrossRef]
- Kandasamy, G.D.; Kathirvel, P. Production, characterization and in vitro biological activities of crude pigment from endophytic Micrococcus luteus associated with Avicennia marina. Arch. Microbiol. 2023, 206, 26. [Google Scholar] [CrossRef] [PubMed]
- Fariq, A.; Yasmin, A.; Jamil, M. Production, characterization and antimicrobial activities of bio-pigments by Aquisalibacillus elongatus MB592, Salinicoccus sesuvii MB597, and Halomonas aquamarina MB598 isolated from Khewra Salt Range, Pakistan. Extremophiles 2019, 23, 435–449. [Google Scholar] [CrossRef]
- Hagaggi, N.S.A.; Abdul-Raouf, U.M. Production of bioactive β-carotene by the endophytic bacterium Citricoccus parietis AUCs with multiple in vitro biological potentials. Microb. Cell Fact. 2023, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Akulava, V.; Byrtusova, D.; Zimmermann, B.; Smirnova, M.; Kohler, A.; Miamin, U.; Valentovich, L.; Shapaval, V. Screening for pigment production and characterization of pigment profile and photostability in cold-adapted Antarctic bacteria using FT-Raman spectroscopy. J. Photochem. Photobiol. A Chem. 2024, 450, 115461. [Google Scholar] [CrossRef]
- Grivard, A.; Goubet, I.; Duarte Filho, L.M.S.; Thiéry, V.; Chevalier, S.; de Oliveira-Junior, R.G.; El Aouad, N.; Guedes da Silva Almeida, J.R.; Sitarek, P.; Quintans-Junior, L.J.; et al. Archaea carotenoids: Natural pigments with unexplored innovative potential. Mar. Drugs 2022, 20, 524. [Google Scholar] [CrossRef] [PubMed]
- Yabuzaki, J. Carotenoids database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [PubMed]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Bonete, M.J.; León, R.; Kharroub, K. Characterization and biological activities of carotenoids produced by three haloarchaeal strains isolated from Algerian salt lakes. Arch. Microbiol. 2022, 204, 6. [Google Scholar] [CrossRef] [PubMed]
- Marova, I.; Carnecka, M.; Halienova, A.; Koci, R.; Breierova, E. Production of carotenoid/ergosterol supplemeted biomass by red yeast Rhodotorula glutinis grown under external stress. Food Technol. Biotech. 2010, 48, 56–61. [Google Scholar]
- Mannazzu, I.; Landolfo, S.; Lopes da Silva, T.; Buzzini, P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, T.; Guo, C.; Chen, G.; Fan, J.; Zhang, Q. Carotenoid biosynthesis is associated with low-temperature adaptation in Rhodosporidium kratochvilovae. BMC Microbiol. 2022, 22, 319. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J.; Reczek, L.; Pobiega, K. Effect of exogenous stress factors on the biosynthesis of carotenoids and lipids by Rhodotorula yeast strains in media containing agro-industrial waste. World J. Microbiol. Biotechnol. 2019, 35, 157. [Google Scholar] [CrossRef]
- Singh, G.; Jawed, A.; Paul, D.; Bandyopadhyay, K.K.; Kumari, A.; Haque, S. Concomitant production of lipids and carotenoids in Rhodosporidium toruloides under osmotic stress using response surface methodology. Front. Microbiol. 2016, 7, 1686. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.D.; Shida, Y.; Miyata, A.; Takamizawa, T.; Suzuki, Y.; Ara, S.; Yamazaki, H.; Masaki, K.; Mori, K.; Aburatani, S.; et al. Effect of light on carotenoid and lipid production in the oleaginous yeast Rhodosporidium toruloides. Biosci. Biotechnol. Biochem. 2020, 84, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, C.J. Multi-omics profiling reveals potential mechanisms of culture temperature modulating biosynthesis of carotenoids, lipids, and exopolysaccharides in oleaginous red yeast Rhodotorula glutinis ZHK. LWT 2022, 171, 114103. [Google Scholar] [CrossRef]
- Roukas, T.; Varzakakou, M.; Kotzekidou, P. From cheese whey to carotenes by Blakeslea trispora in a bubble column reactor. Appl. Biochem. Biotechnol. 2015, 175, 182–193. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Tsimidou, M.Z.; Roukas, T. Performance of crude olive pomace oil and soybean oil during carotenoid production by Blakeslea trispora in submerged fermentation. J. Agric. Food Chem. 2006, 54, 2575–2581. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Naziri, E.; Tsimidou, M.Z. Industrial glycerol as a supplementary carbon source in the production of beta-carotene by Blakeslea trispora. J. Agric. Food Chem. 2008, 56, 2668–2675. [Google Scholar] [CrossRef] [PubMed]
- Varzakakou, M.; Roukas, T. Identification of carotenoids produced from cheese whey by Blakeslea trispora in submerged fermentation. Prep. Biochem. Biotechnol. 2009, 40, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Nguyen, T.T.L.; Nguyen, T.T.; Vo, N.N.N.; Vo, N.T.; Nguyen, Y.T.N. Optimization of In Vitro Carotenoid Production by Rhodotorula Toruloides. Chem. Eng. Trans. 2024, 108, 55–60. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.; Lee, S.; Oh, J.Y.; Jeon, E.J.; Ryu, H.S.; Lee, C.H. Primary and secondary metabolite profiling of doenjang, a fermented soybean paste during industrial processing. Food Chem. 2014, 165, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, Y.N.; Burkina, V.; Okmane, L.; Blomqvist, J.; Rapoport, A.; Sandgren, M.; Pickova, J.; Sampels, S.; Passoth, V. Identification, quantification and kinetic study of carotenoids and lipids in Rhodotorula toruloides CBS 14 cultivated on wheat straw hydrolysate. Fermentation 2022, 8, 300. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, I.; Akermi, S.; Louati, M.; Gargouri, A.; Mellouli, L.; Guerfali, M. Microbial bioactive compounds from oleaginous yeast culture: Insights into molecular docking interactions and toxicity prediction. Biomass Convers. Bior. 2024. [Google Scholar] [CrossRef]
- Keskin, A.; Ünlü, A.E.; Takat, S. Utilization of olive mill wastewater for selective production of lipids and carotenoids by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2023, 107, 4973–4985. [Google Scholar] [CrossRef] [PubMed]
- Mata-Gómez, L.C.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Microbial carotenoid synthesis optimization in goat cheese whey using the robust taguchi method: A sustainable approach to help tackle vitamin A deficiency. Foods 2023, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Xin, X.l.; Yuan, Q.P. Improved lycopene production by Blakeslea trispora with isopentenyl compounds and metabolic precursors. Biotechnol. Lett. 2012, 34, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; He, S.; Chen, T.; Lu, Y.; Ng, I.S. Enhancing beta-carotene biosynthesis and gene transcriptional regulation in Blakeslea trispora with sodium acetate. Biochem. Eng. J. 2016, 114, 10–17. [Google Scholar] [CrossRef]
- Vargas-Sinisterra, A.F.; Ramírez-Castrillón, M. Yeast carotenoids: Production and activity as antimicrobial biomolecule. Arch. Microbiol. 2021, 203, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Koldaev, V.M.; Kropotov, A.V. Carotenoids in practical medicine. Pac. Med. J. 2022, 1, 65–71. (In Russian) [Google Scholar] [CrossRef]
- Long, Y.; Paengkoum, S.; Lu, S.; Niu, X.; Thongpea, S.; Taethaisong, N.; Han, Y.; Paengkoum, P. Physicochemical properties, mechanism of action of lycopene and its application in poultry and ruminant production. Front. Vet. Sci. 2024, 11, 1364589. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Yi, Y.; Zhu, L.; Liu, M.; Zhang, Z.; Xie, Q.; Jiang, L. Insights into the synthesis, engineering, and functions of microbial pigments in Deinococcus bacteria. Front. Microbiol. 2024, 15, 1447785. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant defense of Deinococcus radiodurans: How does it contribute to extreme radiation resistance? Int. J. Radiat. Biol. 2023, 99, 1803–1829. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saubenova, M.; Rapoport, A.; Venkatachalam, M.; Dufossé, L.; Yermekbay, Z.; Oleinikova, Y. Production of Carotenoids by Microorganisms. Fermentation 2024, 10, 502. https://doi.org/10.3390/fermentation10100502
Saubenova M, Rapoport A, Venkatachalam M, Dufossé L, Yermekbay Z, Oleinikova Y. Production of Carotenoids by Microorganisms. Fermentation. 2024; 10(10):502. https://doi.org/10.3390/fermentation10100502
Chicago/Turabian StyleSaubenova, Margarita, Alexander Rapoport, Mekala Venkatachalam, Laurent Dufossé, Zhanerke Yermekbay, and Yelena Oleinikova. 2024. "Production of Carotenoids by Microorganisms" Fermentation 10, no. 10: 502. https://doi.org/10.3390/fermentation10100502
APA StyleSaubenova, M., Rapoport, A., Venkatachalam, M., Dufossé, L., Yermekbay, Z., & Oleinikova, Y. (2024). Production of Carotenoids by Microorganisms. Fermentation, 10(10), 502. https://doi.org/10.3390/fermentation10100502







