Acidogenic Fermentation of Food Waste for the Production of Short-Chain Fatty Acids: The Impact of Inoculum Type and Inoculum Heat Pretreatment
Abstract
1. Introduction
2. Material and Methods
2.1. Food Waste Characteristics
2.2. Inoculum and Heat Pretreatment
2.3. Batch Fermentation Study
2.4. Analytical Procedure
2.5. Performance Indicator
- (i)
- Hydrolysis yield estimates the conversion efficiency of the particulate organic substrate to soluble organic matter. It can be calculated as follows:
- (ii)
- The SCFA yield was estimated based on total SCFAs produced in milligram COD equivalents to the initial VS (gram) added to the bioreactor.
- (iii)
- The ratio of SCFAs to sCOD (%) indicates the extent to which soluble organic matter is transformed into SCFAs. This ratio is determined by dividing the SCFA yield by the hydrolysis yield.
- (iv)
- Hydrogen yield was estimated based on the total hydrogen gas produced in the reactor compared to the amount of initial VS added to the reactor.
2.6. Microbial Community and Statistical Analysis
3. Results and Discussion
3.1. Hydrolysis of Food Waste
3.1.1. Impact of Inoculum Type
3.1.2. Effect of Heat Pretreatment
3.2. Impact on SCFA Production
3.2.1. Effect of Inoculum Type
3.2.2. Effect of Heat Pretreatment
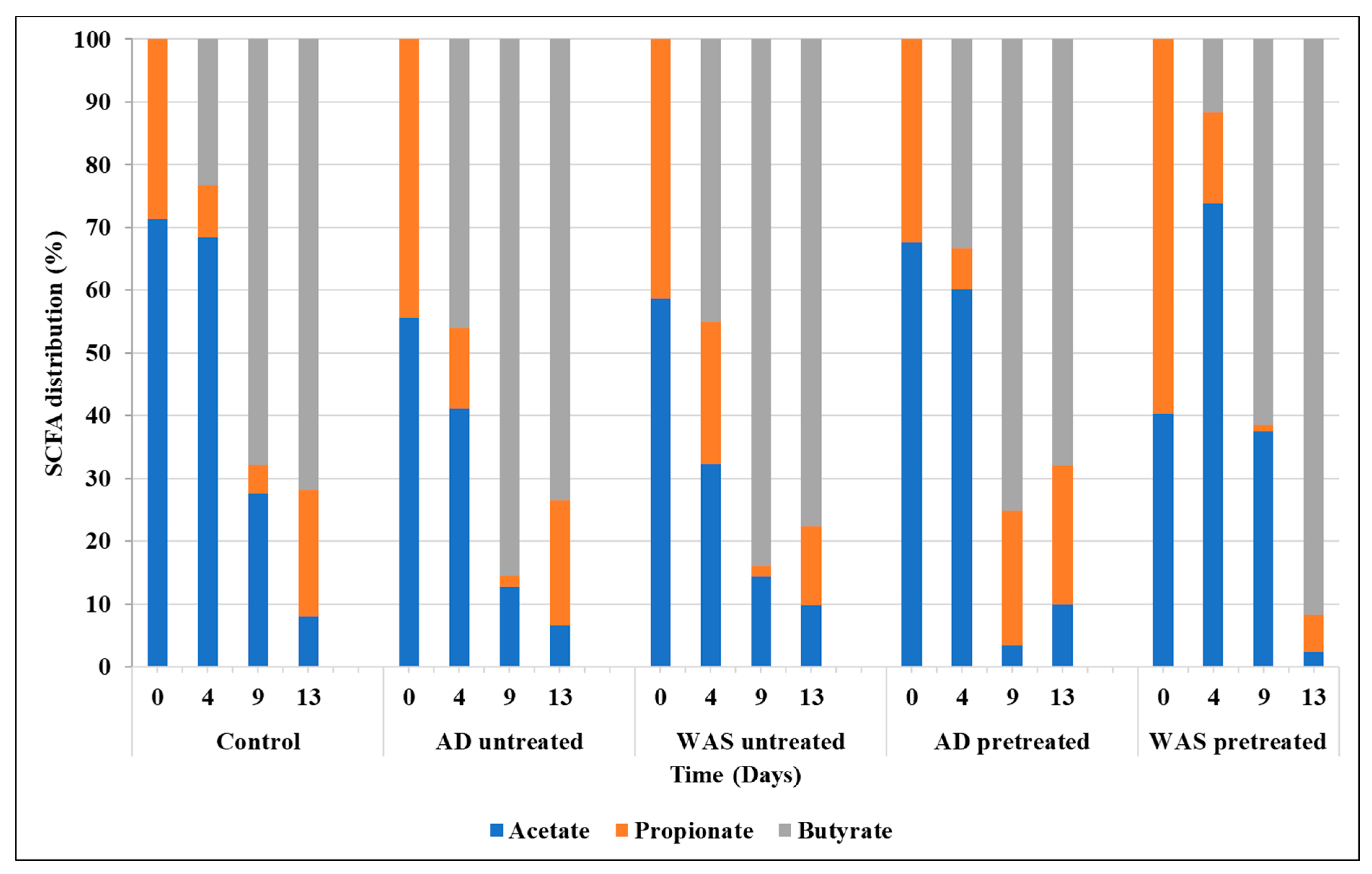
3.3. SCFA Composition
3.4. Hydrogen and Methane Production
3.5. Microbial Community Composition
3.5.1. Microbial Community Composition in Control and Untreated Inoculums
3.5.2. Microbial Community Composition in Treated Inoculums
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blasco, L.; Kahala, M.; Tampio, E.; Vainio, M.; Ervasti, S.; Rasi, S. Effect of Inoculum Pretreatment on the Composition of Microbial Communities in Anaerobic Digesters Producing Volatile Fatty Acids. Microorganisms 2020, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic Fermentation of Food Waste for Volatile Fatty Acid Production with Co-Generation of Biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- van Aarle, I.M.; Perimenis, A.; Lima-Ramos, J.; de Hults, E.; George, I.F.; Gerin, P.A. Mixed Inoculum Origin and Lignocellulosic Substrate Type Both Influence the Production of Volatile Fatty Acids during Acidogenic Fermentation. Biochem. Eng. J. 2015, 103, 242–249. [Google Scholar] [CrossRef]
- Sarkar, O.; Kumar, A.N.; Dahiya, S.; Krishna, K.V.; Yeruva, D.K.; Mohan, S.V. Regulation of Acidogenic Metabolism towards Enhanced Short Chain Fatty Acid Biosynthesis from Waste: Metagenomic Profiling. RSC Adv. 2016, 6, 18641–18653. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Biotechnol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Radadiya, P.; Lee, J.; Venkateshwaran, K.; Benn, N.; Lee, H.S.; Hussain, A. Acidogenic Fermentation of Food Waste in a Leachate Bed Reactor (LBR) at High Volumetric Organic Loading: Effect of Granular Activated Carbon (GAC) and Sequential Enrichment of Inoculum. Bioresour. Technol. 2022, 361, 127705. [Google Scholar] [CrossRef]
- Singh, V.; Örmeci, B.; Singh, A.; Saha, S.; Hussain, A. A Novel Solid-State Submerged Fermenter (3SF) for Acidogenic Fermentation of Food Waste at High Volumetric Loading: Effect of Inoculum to Substrate Ratio, Design Optimization, and Inoculum Enrichment. Chem. Eng. J. 2023, 475, 146173. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food Waste Biorefinery: Sustainable Strategy for Circular Bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Shewa, W.A.; Hussain, A.; Chandra, R.; Lee, J.; Saha, S.; Lee, H.S. Valorization of Food Waste and Economical Treatment: Effect of Inoculation Methods. J. Clean. Prod. 2020, 261, 121170. [Google Scholar] [CrossRef]
- Slezak, R.; Grzelak, J.; Krzystek, L.; Ledakowicz, S. The Effect of Initial Organic Load of the Kitchen Waste on the Production of VFA and H2 in Dark Fermentation. Waste Manag. 2017, 68, 610–617. [Google Scholar] [CrossRef]
- Cappai, G.; De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. An Experimental Study on Fermentative H2 Production from Food Waste as Affected by PH. Waste Manag. 2014, 34, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Khan, A.R.; Kwon, M.K.; Jung, Y.J.; Yun, Z.; Kiso, Y. Acidogenic Fermentation of Blended Food-Waste in Combination with Primary Sludge for the Production of Volatile Fatty Acids. J. Chem. Technol. Biotechnol. 2005, 80, 909–915. [Google Scholar] [CrossRef]
- Tampio, E.A.; Blasco, L.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile Fatty Acids (VFAs) and Methane from Food Waste and Cow Slurry: Comparison of Biogas and VFA Fermentation Processes. GCB Bioenergy 2019, 11, 72–84. [Google Scholar] [CrossRef]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of Operational Parameters on Dark Fermentative Hydrogen Production from Biodegradable Complex Waste Biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef]
- Shin, H.S.; Youn, J.H.; Kim, S.H. Hydrogen Production from Food Waste in Anaerobic Mesophilic and Thermophilic Acidogenesis. Int. J. Hydrogen Energy 2004, 29, 1355–1363. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic Digestion of Food Waste for Volatile Fatty Acids (VFAs) Production with Different Types of Inoculum: Effect of PH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Zou, D.; Yuan, H.; Zhu, B.; Li, X.; Pang, Y. Influence of Temperature on Hydrolysis Acidification of Food Waste. Procedia Environ. Sci. 2012, 16, 85–94. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Short-Chain Fatty Acids and Hydrogen Production in One Single Anaerobic Fermentation Stage Using Carbohydrate-Rich Food Waste. J. Clean. Prod. 2021, 284. [Google Scholar] [CrossRef]
- Hussain, A.; Lee, J.; Xiong, Z.; Wang, Y.; Lee, H.S. Butyrate Production and Purification by Combining Dry Fermentation of Food Waste with a Microbial Fuel Cell. J. Environ. Manag. 2021, 300. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile Fatty Acids Production from Food Wastes for Biorefinery Platforms: A Review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Kraemer, J.T.; Bagley, D.M. Improving the Yield from Fermentative Hydrogen Production. Biotechnol. Lett. 2007, 29, 685–695. [Google Scholar] [CrossRef]
- Baghchehsaraee, B.; Nakhla, G.; Karamanev, D.; Margaritis, A.; Reid, G. The Effect of Heat Pretreatment Temperature on Fermentative Hydrogen Production Using Mixed Cultures. Int. J. Hydrogen Energy 2008, 33, 4064–4073. [Google Scholar] [CrossRef]
- Xiong, Z.; Hussain, A.; Lee, H.S. Food Waste Treatment with a Leachate Bed Reactor: Effects of Inoculum to Substrate Ratio and Reactor Design. Bioresour. Technol. 2019, 285, 121350. [Google Scholar] [CrossRef]
- Cao, J.; Xu, C.; Zhou, R.; Duan, G.; Lin, A.; Yang, X.; You, S.; Zhou, Y.; Yang, G. Potato Peel Waste for Fermentative Biohydrogen Production Using Different Pretreated Culture. Bioresour. Technol. 2022, 362, 127866. [Google Scholar] [CrossRef]
- Cappai, G.; De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Effect of Inoculum to Substrate Ratio (ISR) on Hydrogen Production through Dark Fermentation of Food Waste; CISA Publisher: Atlanta, GA, USA, 2015; Volume 5. [Google Scholar]
- Yin, J.; Yu, X.; Zhang, Y.; Shen, D.; Wang, M.; Long, Y.; Chen, T. Enhancement of Acidogenic Fermentation for Volatile Fatty Acid Production from Food Waste: Effect of Redox Potential and Inoculum. Bioresour. Technol. 2016, 216, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced Volatile Fatty Acids Production from Anaerobic Fermentation of Food Waste: A Mini-Review Focusing on Acidogenic Metabolic Pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Dahiya, S.; Mohan, S.V. Selective Control of Volatile Fatty Acids Production from Food Waste by Regulating Biosystem Buffering: A Comprehensive Study. Chem. Eng. J. 2019, 357, 787–801. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, B.; Gong, X.; Liu, Y.; Li, G. Effects of PH Buffering Agents on the Anaerobic Hydrolysis Acidification Stage of Kitchen Waste. Waste Manag. 2017, 68, 603–609. [Google Scholar] [CrossRef]
- Chen, C.C.; Chuang, Y.S.; Lin, C.Y.; Lay, C.H.; Sen, B. Thermophilic Dark Fermentation of Untreated Rice Straw Using Mixed Cultures for Hydrogen Production. Int. J. Hydrogen Energy 2012, 37, 15540–15546. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile Fatty Acids Production via Mixed Culture Fermentation: Revealing the Link between PH, Inoculum Type and Bacterial Composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Wang, X.; Wu, C.; Li, T.; Wang, M.; Liu, S.; Wang, Q.; Shimaoka, T. Dechlorination of Municipal Solidwaste Incineration Fly Ash by Leaching with Fermentation Liquid of Food Waste. Sustainability 2020, 12, 4389. [Google Scholar] [CrossRef]
- Kawagoshi, Y.; Hino, N.; Fujimoto, A.; Nakao, M.; Fujita, Y.; Sugimura, S.; Furukawa, K. Effect of Inoculum Conditioning on Hydrogen Fermentation and PH Effect on Bacterial Community Relevant to Hydrogen Production. J. Biosci. Bioeng. 2005, 100, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hu, J.; Wang, J. Enriching Hydrogen-Producing Bacteria from Digested Sludge by Different Pretreatment Methods. Int. J. Hydrogen Energy 2014, 39, 13550–13556. [Google Scholar] [CrossRef]
- Hernández, C.; Alamilla-Ortiz, Z.L.; Escalante, A.E.; Navarro-Díaz, M.; Carrillo-Reyes, J.; Moreno-Andrade, I.; Valdez-Vazquez, I. Heat-Shock Treatment Applied to Inocula for H2 Production Decreases Microbial Diversities, Interspecific Interactions and Performance Using Cellulose as Substrate. Int. J. Hydrogen Energy 2019, 44, 13126–13134. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Sung, S.; Lay, J.J. Biohydrogen Production as a Function of PH and Substrate Concentration. Environ. Sci. Technol. 2001, 35, 4726–4730. [Google Scholar] [CrossRef]
- Zhu, H.; Béland, M. Evaluation of Alternative Methods of Preparing Hydrogen Producing Seeds from Digested Wastewater Sludge. Int. J. Hydrogen Energy 2006, 31, 1980–1988. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile Fatty Acids Production during Mixed Culture Fermentation—The Impact of Substrate Complexity and PH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Qi, B.C.; Aldrich, C.; Lorenzen, L.; Wolfaardt, G.W. Acidogenic Fermentation of Lignocellulosic Substrate with Activated Sludge. Chem. Eng. Commun. 2005, 192, 1221–1242. [Google Scholar] [CrossRef]
- Karadag, D.; Puhakka, J.A. Effect of Changing Temperature on Anaerobic Hydrogen Production and Microbial Community Composition in an Open-Mixed Culture Bioreactor. Int. J. Hydrogen Energy 2010, 35, 10954–10959. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Ballesteros, M.; González-Fernández, C. Acidogenesis and Chain Elongation for Bioproduct Development. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 391–414. [Google Scholar]
- Holtzapple, M.T.; Wu, H.; Weimer, P.J.; Dalke, R.; Granda, C.B.; Mai, J.; Urgun-Demirtas, M. Microbial Communities for Valorizing Biomass Using the Carboxylate Platform to Produce Volatile Fatty Acids: A Review. Bioresour. Technol. 2022, 344, 126253. [Google Scholar] [CrossRef] [PubMed]
- Cabrol, L.; Marone, A.; Venegas, E.T.; Steyer, J.-P.; Ruiz-Filippi, G.; Trably, E. Microbial Ecology of Fermentative Hydrogen Producing Bioprocesses: Useful Insights for Driving the Ecosystem Function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.H.; Wu, J.H.; Hsiao, C.L.; Chang, J.J.; Chen, C.C.; Lin, C.Y. Biohydrogen Production from Soluble Condensed Molasses Fermentation Using Anaerobic Fermentation. Int. J. Hydrogen Energy 2010, 35, 13445–13451. [Google Scholar] [CrossRef]
- Feng, K.; Li, H.; Zheng, C. Shifting Product Spectrum by PH Adjustment during Long-Term Continuous Anaerobic Fermentation of Food Waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef]
- Ma, J.; Xie, S.; Yu, L.; Zhen, Y.; Zhao, Q.; Frear, C.; Chen, S.; Wang, Z.W.; Shi, Z. PH Shaped Kinetic Characteristics and Microbial Community of Food Waste Hydrolysis and Acidification. Biochem. Eng. J. 2019, 146, 52–59. [Google Scholar] [CrossRef]



| Substrate | Inoculum Type/Pretreatment | Reactor Type | Temperature | pH | Hydrolysis Yield (g sCOD/ kg VSfed) | Acidification Yield (g COD SCFA/ kg VS added) | SCFA Concentration (g/L) | Hydrogen Yield (mL H2/ gm VS added) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Urban biowaste | AD/thermal | Batch | 37 °C | NC | 228 | [1] | |||
| Food waste | AD/thermal | Batch | 37 °C | Initial 9.3 | 12 | 284 | [15] | ||
| Food waste | AD/thermal | Batch | 52–56 °C | Initial pH 7 | 700 | [16] | |||
| Food waste | AD/thermal | Batch | 37 °C | NC | 205 | 105 | [12,14] | ||
| Synthetic food waste | WAS/thermal | Batch | 39 °C | 6 6.5 7 | 62 * 73 * 42 * | [13] | |||
| Food waste | Primary sludge | Plug flow reactor | 35 °C 18 °C | pH 6.5/NC | 365 490 | [14] | |||
| Food waste | Mesophilic AD | Batch semi-continuously fed | 35 °C/55 °C | 4.5 5.5 6.5 | 576 668 643 | 5 2.5 1.3 | [17] | ||
| Food waste | AD | Batch | 30 °C | 4 5 6 NC | 4.71 ** 1.14 ** 1.87 ** 2.4 ** | 124 § 651 § 918 § 337 § | [18] | ||
| WAS | Batch | 30 °C | 4 5 6 NC | 2.03 ** 1.07 ** 1.52 ** 1.25 ** | 206 § 445 § 481 § 229 § | ||||
| Food waste | AD | Batch | 35 °C | 7 | 24 g/L | 17 | [19] | ||
| 55 °C | 7 | 28 g/L | 10 | ||||||
| 70 °C | 7 | 36 g/L | 12 | ||||||
| Food waste | WAS | LBR | 22 °C | 6.5 | 491 | 375 | [8] | ||
| Food waste | AD/thermal | LBR | 22 °C | 7 | 693 | 649 | [11] | ||
| Food waste | AD sludge | Batch | 21 °C | 5.5–6 | 27 | 395 | [20] | ||
| Food waste | Enriched inoculum | LBR | 21 °C | 6 | 774 | 697 | [21] | ||
| Food waste | AD sludge | Batch | 22 °C | 6.5 | 567 | 462 | [9] |
| Parameter | Food Waste Slurry | Centrifuged AD Inoculum | Centrifuged WAS Inoculum |
|---|---|---|---|
| TS (g/kg) | 234.4 ± 0.42 | 102.3 ± 1.83 | 88.6 ± 1.88 |
| VS (g/kg) | 224.2 ± 0.39 | 60.4 ± 1.05 | 64.5 ± 0.96 |
| VS/TS (%) | 95.64 ± 0.01 | 59.1 ± 0.06 | 72.8± 0.50 |
| TS (%) | 23.4 ± 0.04 | 10.2 ± 0.18 | 8.9 ± 0.19 |
| VS (%) | 22.4 ± 0.04 | 6 ± 0.11 | 6.5 ± 0.10 |
| Experiment | Designation | Inoculum | Substrate | Heat Pretreatment Applied to Inoculum | pH |
|---|---|---|---|---|---|
| 1 | Control | No inoculum | Food waste | - | 7 |
| 2 | AD pretreated (heated) | AD sludge | Food waste | Yes | 7 |
| 3 | AD untreated | AD sludge | Food waste | No | 7 |
| 4 | WAS pretreated (heated) | Waste-activated sludge | Food waste | Yes | 7 |
| 5 | WAS untreated | Waste-activated sludge | Food waste | No | 7 |
| Control | AD Untreated | WAS Untreated | AD Pretreated | WAS Pretreated | |
|---|---|---|---|---|---|
| Cumulative sCOD production, mg sCOD | 1475 ± 317 | 2542 ± 208 | 2166 ± 132 | 2171± 150 | 1260 ± 161 |
| Hydrolysis yield, g sCOD/kg VS added | 243 ± 62 | 399 ± 14 | 366 ± 17 | 333 ± 38 | 194 ± 23 |
| Acetate, mg COD | 51 ± 5 | 78 ± 30 | 85 ± 20 | 153 ± 16 | 18 ± 2 |
| Propionate, mg COD | 80 ± 11 | 229 ± 50 | 108 ± 40 | 335 ± 89 | 45 ± 13 |
| Butyrate, mg COD | 505 ± 30 | 851 ± 40 | 671 ± 80 | 1036 ± 59 | 698 ± 60 |
| Total SCFA production, mg CODSCFA | 636 ± 55 | 1158 ± 150 | 864 ± 30 | 1525 ± 65 | 761 ± 25 |
| SCFA yield, g CODSCFA/kg VS added | 104 ± 9 | 182 ± 3 | 131 ± 5 | 238 ± 6 | 117 ± 2 |
| SCFA/sCOD, (%) | 43 | 46 | 36 | 71 | 60 |
| Hydrogen yield, LH2/kg VS added | 20 ± 4 | 34 ± 5 | 32 ± 2 | 18 ± 2 | 20 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jodhani, S.; Sebastian, J.; Lee, J.; Venkiteshwaran, K.; Lee, H.-S.; Singh, V.; Ormeci, B.; Hussain, A. Acidogenic Fermentation of Food Waste for the Production of Short-Chain Fatty Acids: The Impact of Inoculum Type and Inoculum Heat Pretreatment. Fermentation 2024, 10, 162. https://doi.org/10.3390/fermentation10030162
Jodhani S, Sebastian J, Lee J, Venkiteshwaran K, Lee H-S, Singh V, Ormeci B, Hussain A. Acidogenic Fermentation of Food Waste for the Production of Short-Chain Fatty Acids: The Impact of Inoculum Type and Inoculum Heat Pretreatment. Fermentation. 2024; 10(3):162. https://doi.org/10.3390/fermentation10030162
Chicago/Turabian StyleJodhani, Sharli, Joseph Sebastian, Jangho Lee, Kaushik Venkiteshwaran, Hyung-Sool Lee, Virender Singh, Banu Ormeci, and Abid Hussain. 2024. "Acidogenic Fermentation of Food Waste for the Production of Short-Chain Fatty Acids: The Impact of Inoculum Type and Inoculum Heat Pretreatment" Fermentation 10, no. 3: 162. https://doi.org/10.3390/fermentation10030162
APA StyleJodhani, S., Sebastian, J., Lee, J., Venkiteshwaran, K., Lee, H.-S., Singh, V., Ormeci, B., & Hussain, A. (2024). Acidogenic Fermentation of Food Waste for the Production of Short-Chain Fatty Acids: The Impact of Inoculum Type and Inoculum Heat Pretreatment. Fermentation, 10(3), 162. https://doi.org/10.3390/fermentation10030162








