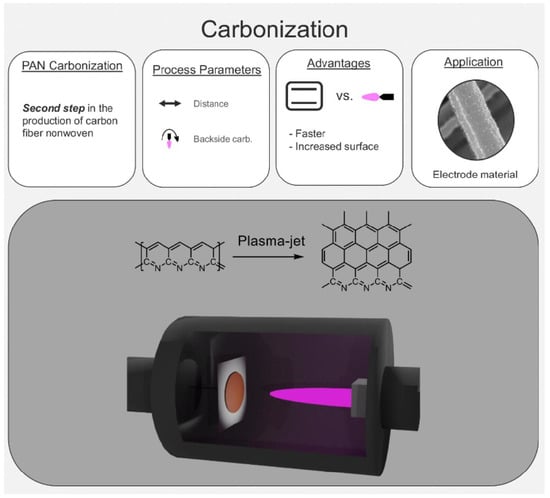
Atmospheric Pressure Plasma-Jet Treatment of PAN-Nonwovens—Carbonization of Nanofiber Electrodes
Abstract
:1. Introduction
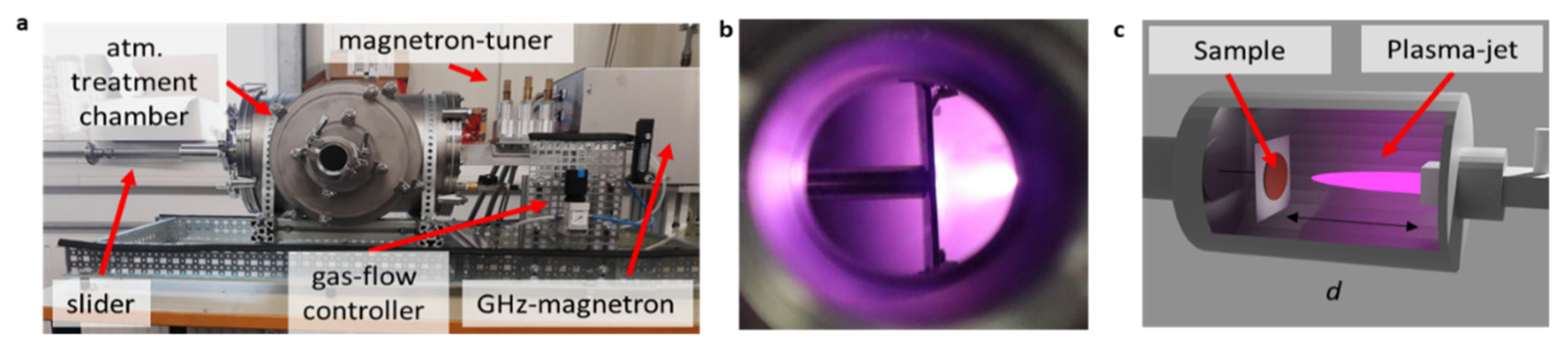
2. Materials and Methods
3. Results and Discussion
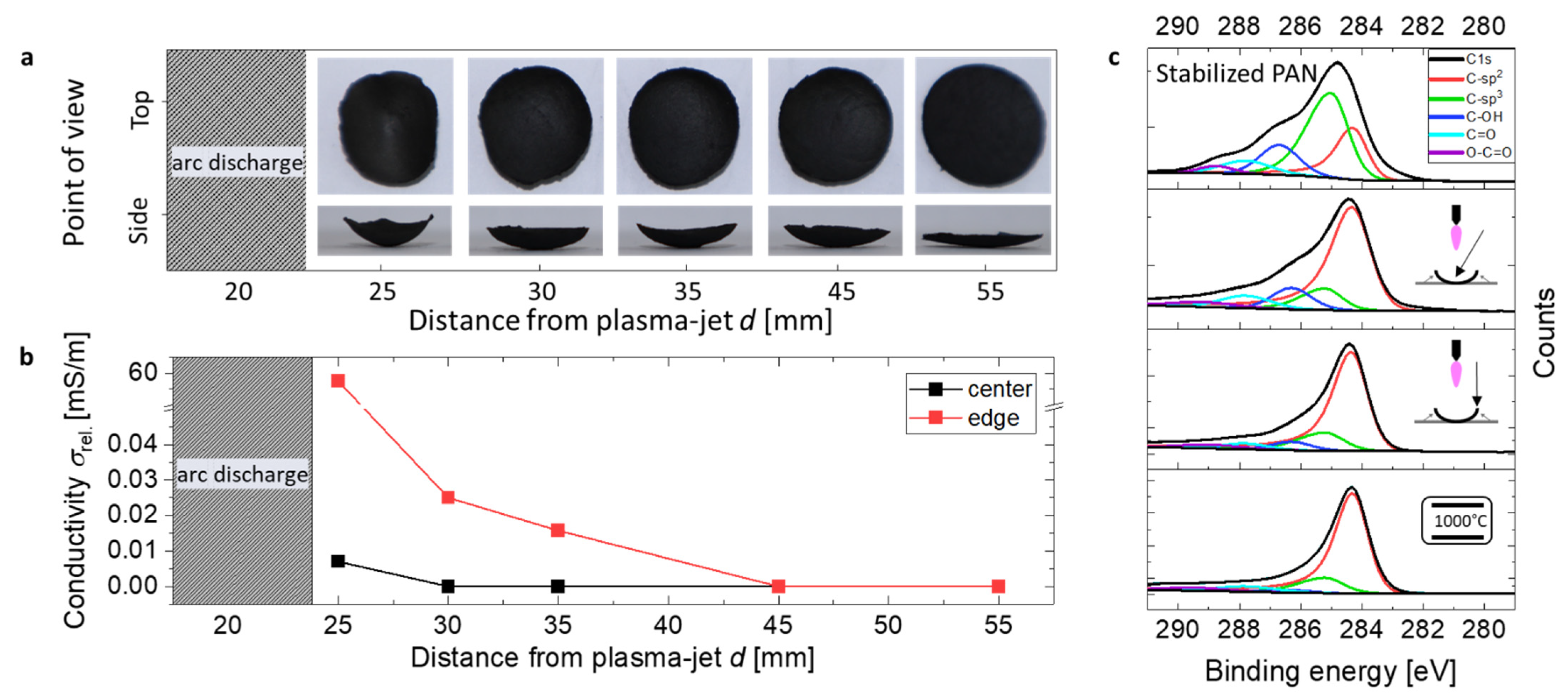
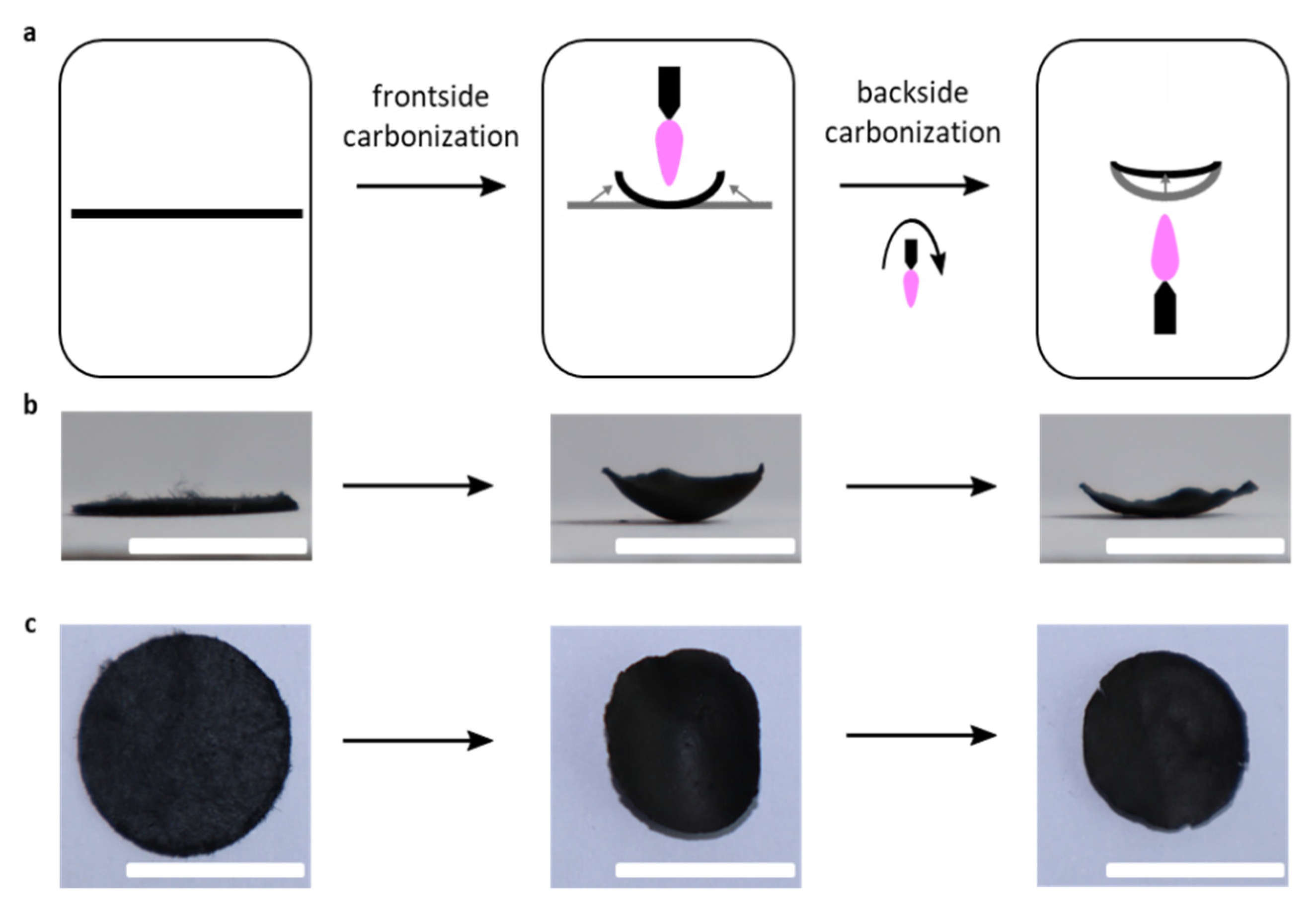
3.1. Carbonization and Shrinkage of the Nonwoven
3.2. Plasma-Jet Carbonized PAN-Nonwoven as Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Hatton, T.A.; Rutledge, G.C. A review of electrospun carbon fibers as electrode materials for energy storage. Curr. Org. Chem. 2013, 17, 1390–1401. [Google Scholar] [CrossRef] [Green Version]
- Mittal, J.; Konno, H.; Inagaki, M.; Bahl, O.P. Denitrogenation behavior and tensile strength increase during carbonization of stabilized PAN fibers. Carbon 1998, 36, 1327–1330. [Google Scholar] [CrossRef]
- Kim, M.A.; Jang, D.; Tejima, S.; Cruz-Silva, R.; Joh, H.I.; Kim, H.C.; Lee, S.; Endo, M. Strengthened PAN-based carbon fibers obtained by slow heating rate carbonization. Sci. Rep. 2016, 6, 22988. [Google Scholar] [CrossRef] [Green Version]
- Laffont, L.; Monthioux, M.; Serin, V.; Mathur, R.B.; Guimon, C.; Guimon, M.F. An EELS study of the structural and chemical transformation of PAN polymer to solid carbon. Carbon 2004, 42, 2485–2494. [Google Scholar] [CrossRef]
- Clingerman, M.L.; King, J.A.; Schulz, K.H.; Meyers, J.D. Evaluation of electrical conductivity models for conductive polymer composites. J. Appl. Polym. Sci. 2002, 83, 1341–1356. [Google Scholar] [CrossRef]
- Opitz, M.; Go, D.; Lott, P.; Müller, S.; Stollenwerk, J.; Kuehne, A.J.C.; Roling, B. On the interplay of morphology and electronic conductivity of rotationally spun carbon fiber mats. J. Appl. Phys. 2017, 122, 105104. [Google Scholar] [CrossRef]
- Katzman, H.A.; Adams, P.M.; Le, T.D.; Hemminger, C.S. Characterization of low thermal conductivity pan-based carbon fibers. Carbon 1994, 32, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Ko, T. The influence of pyrolysis on physical properties and microstructure of modified PAN fibers during carbonization. J. Appl. Polym. Sci. 1991, 43, 589–600. [Google Scholar] [CrossRef]
- Kurban, Z.; Lovell, A.; Jenkins, D.; Bennington, S.; Loader, I.; Schober, A.; Skipper, N.; Nanotechnology, M. Turbostratic graphite nanofibres from electrospun solutions of PAN in dimethylsulphoxide. Eur. Polym. J. 2010, 46, 1194–1202. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, Z.H.; Ma, W.J.; Ma, Q.S. Influence of heat treatment on physical–chemical properties of PAN-based carbon fiber. Ceram. Int. 2006, 32, 291–295. [Google Scholar] [CrossRef]
- Tsai, E.W.; Pajkossy, T.; Rajeshwar, K.; Reynolds, J.R. Anion-exchange behavior of polypyrrole membranes. J. Phys. Chem. 1988, 92, 3560–3565. [Google Scholar] [CrossRef]
- Go, D.; Lott, P.; Stollenwerk, J.; Thomas, H.; Möller, M.; Kuehne, A.J.C. Laser carbonization of PAN-nanofiber mats with enhanced surface area and porosity. ACS Appl. Mater. Interfaces 2016, 8, 28412–28417. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Jiménez-Calvo, P.; Bansmann, J.; Strauss, V.; Kuehne, A.J.C. On-Chip Direct Laser Writing of PAN-Based Carbon Supercapacitor Electrodes. Macromol. Rapid Commun. 2022, 43, 2100731. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guo, C.; Lu, Y.; Zhang, R.; Wang, Z.; Jin, M. Comparison of microwave and conventional heating methods in carbonization of polyacrylonitrile-based stabilized fibers at different temperature measured by an in-situ process temperature control ring. Polym. Degrad. Stab. 2017, 140, 32–41. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.Y.; Lee, S.; Jo, S.; Im, Y.H.; Lee, H.S. Microwave plasma carbonization for the fabrication of polyacrylonitrile-based carbon fiber. Polymer 2015, 56, 590–595. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.Y.; Choi, J.; Lee, S.; Jo, S.M.; Joo, J.; Lee, H.S. Two step microwave plasma carbonization including low plasma power pre-carbonization for polyacrylonitrile based carbon fiber. Polymer 2015, 69, 123–128. [Google Scholar] [CrossRef]
- Dimesso, L.; Spanheimer, C.; Jaegermann, W.; Zhang, Y.; Yarin, A.L. LiCoPO4—3D carbon nanofiber composites as possible cathode materials for high voltage applications. Electrochim. Acta 2013, 95, 38–42. [Google Scholar] [CrossRef]
- Li, X.; Sun, N.; Tian, X.; Yang, T.; Song, Y.; Xu, B.; Liu, Z. Electrospun coal liquefaction residues/polyacrylonitrile composite carbon nanofiber nonwoven fabrics as high-performance electrodes for lithium/potassium batteries. Energy Fuels 2020, 34, 2445–2451. [Google Scholar] [CrossRef]
- Manickam, S.S.; Karra, U.; Huang, L.; Bui, N.N.; Li, B.; McCutcheon, J.R. Activated carbon nanofiber anodes for microbial fuel cells. Carbon 2013, 53, 19–28. [Google Scholar] [CrossRef]
- Su, C.I.; Huang, Y.X.; Wong, J.W.; Lu, C.H.; Wang, C.M. PAN-based carbon nanofiber absorbents prepared using electrospinning. Fibers Polym. 2012, 13, 436–442. [Google Scholar] [CrossRef]
- Waisi, B.I.; Arena, J.T.; Benes, N.E.; Nijmeijer, A.; McCutcheon, J.R. Activated carbon nanofiber nonwoven for removal of emulsified oil from water. Microporous Mesoporous Mater. 2020, 296, 109966. [Google Scholar] [CrossRef]
- Go, D.; Opitz, M.; Lott, P.; Rahimi, K.; Stollenwerk, J.; Thomas, H.; Möller, M.; Roling, B.; Kuehne, A.J.C. Electrochemical characterization of laser-carbonized polyacrylonitrile nanofiber nonwovens. J. Appl. Polym. Sci. 2018, 135, 46398. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Chhetri, K.; Lee, M.; Dahal, B.; Lohani, P.C.; Kim, H.-Y. A review of electrospun carbon nanofiber-based negative electrode materials for supercapacitors. Electrochem 2021, 2, 236–250. [Google Scholar] [CrossRef]
- Chhetri, K.; Subedi, S.; Muthurasu, A.; Ko, T.H.; Dahal, B.; Kim, H.Y. A review on nanofiber reinforced aerogels for energy storage and conversion applications. J. Energy Storage 2022, 46, 103927. [Google Scholar] [CrossRef]
- Waisi, B.I.; Manickam, S.S.; Benes, N.E.; Nijmeijer, A.; McCutcheon, J.R. Activated Carbon Nanofiber Nonwovens: Improving Strength and Surface Area by Tuning Fabrication Procedure. Ind. Eng. Chem. Res. 2019, 58, 4084–4089. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kuehne, A.J.C. High throughput centrifugal electrospinning of polyacrylonitrile nanofibers for carbon fiber nonwovens. Polymers 2021, 13, 1313. [Google Scholar] [CrossRef]
- Schütze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, F.; Fracassi, F. Atmospheric pressure non-equilibrium plasma jet technology: General features, specificities and applications in surface processing of materials. Surf. Coat. Technol. 2017, 322, 174–201. [Google Scholar] [CrossRef]
- Joshi, N.K.; Sahasrabudhe, S.N.; Sreekumar, K.P.; Venkatramani, N. Variation of axial temperature in thermal plasma jets. Meas. Sci. Technol. 1997, 8, 1146–1150. [Google Scholar] [CrossRef]
- Selvan, B.; Ramachandran, K.; Pillai, B.C.; Subhakar, D. Numerical modelling of Ar-N2 plasma jet impinging on a flat substrate. J. Therm. Spray Technol. 2011, 20, 534–548. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Mei, B.A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical interpretations of Nyquist plots for EDLC electrodes and devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Lei, C.; Markoulidis, F.; Ashitaka, Z.; Lekakou, C. Reduction of porous carbon/Al contact resistance for an electric double-layer capacitor (EDLC). Electrochim. Acta 2013, 92, 183–187. [Google Scholar] [CrossRef]
- Masarapu, C.; Zeng, H.F.; Hung, K.H.; Wei, B. Effect of temperature on the capacitance of carbon nanotube supercapacitors. ACS Nano 2009, 3, 2199–2206. [Google Scholar] [CrossRef]
- Belaustegui, Y.; Pantò, F.; Urbina, L.; Corcuera, M.A.; Eceiza, A.; Palella, A.; Triolo, C.; Santangelo, S. Bacterial-cellulose-derived carbonaceous electrode materials for water desalination via capacitive method: The crucial role of defect sites. Desalination 2020, 492, 114596. [Google Scholar] [CrossRef]
- Xu, R.; Guo, F.; Cui, X.; Zhang, L.; Wang, K.; Wei, J. High performance carbon nanotube based fiber-shaped supercapacitors using redox additives of polypyrrole and hydroquinone. J. Mater. Chem. A 2015, 3, 22353–22360. [Google Scholar] [CrossRef]
- Yang, I.; Kim, S.-G.; Kwon, S.H.; Kim, M.-S.; Jung, J.C. Relationships between pore size and charge transfer resistance of carbon aerogels for organic electric double-layer capacitor electrodes. Electrochim. Acta 2017, 223, 21–30. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, A.; Uhl, M.; Ceblin, M.; Rohrbach, F.; Bansmann, J.; Mallah, M.; Heuermann, H.; Jacob, T.; Kuehne, A.J.C. Atmospheric Pressure Plasma-Jet Treatment of PAN-Nonwovens—Carbonization of Nanofiber Electrodes. C 2022, 8, 33. https://doi.org/10.3390/c8030033
Hoffmann A, Uhl M, Ceblin M, Rohrbach F, Bansmann J, Mallah M, Heuermann H, Jacob T, Kuehne AJC. Atmospheric Pressure Plasma-Jet Treatment of PAN-Nonwovens—Carbonization of Nanofiber Electrodes. C. 2022; 8(3):33. https://doi.org/10.3390/c8030033
Chicago/Turabian StyleHoffmann, Andreas, Matthias Uhl, Maximilian Ceblin, Felix Rohrbach, Joachim Bansmann, Marcel Mallah, Holger Heuermann, Timo Jacob, and Alexander J. C. Kuehne. 2022. "Atmospheric Pressure Plasma-Jet Treatment of PAN-Nonwovens—Carbonization of Nanofiber Electrodes" C 8, no. 3: 33. https://doi.org/10.3390/c8030033
APA StyleHoffmann, A., Uhl, M., Ceblin, M., Rohrbach, F., Bansmann, J., Mallah, M., Heuermann, H., Jacob, T., & Kuehne, A. J. C. (2022). Atmospheric Pressure Plasma-Jet Treatment of PAN-Nonwovens—Carbonization of Nanofiber Electrodes. C, 8(3), 33. https://doi.org/10.3390/c8030033