Development of Disposable and Flexible Supercapacitor Based on Carbonaceous and Ecofriendly Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
2.3. Production of the Ink and Manufacture of the Disposable Electrodes
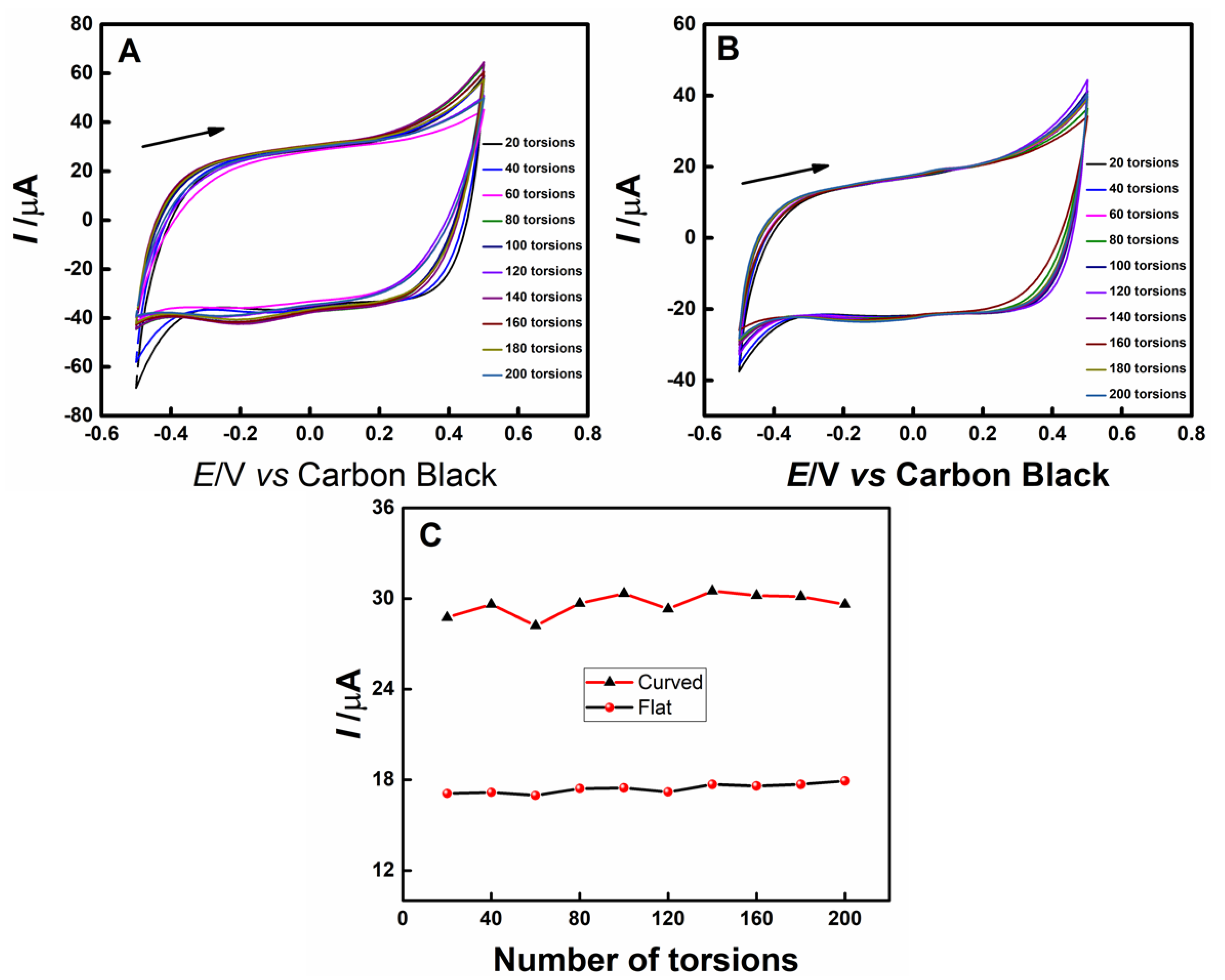
3. Results
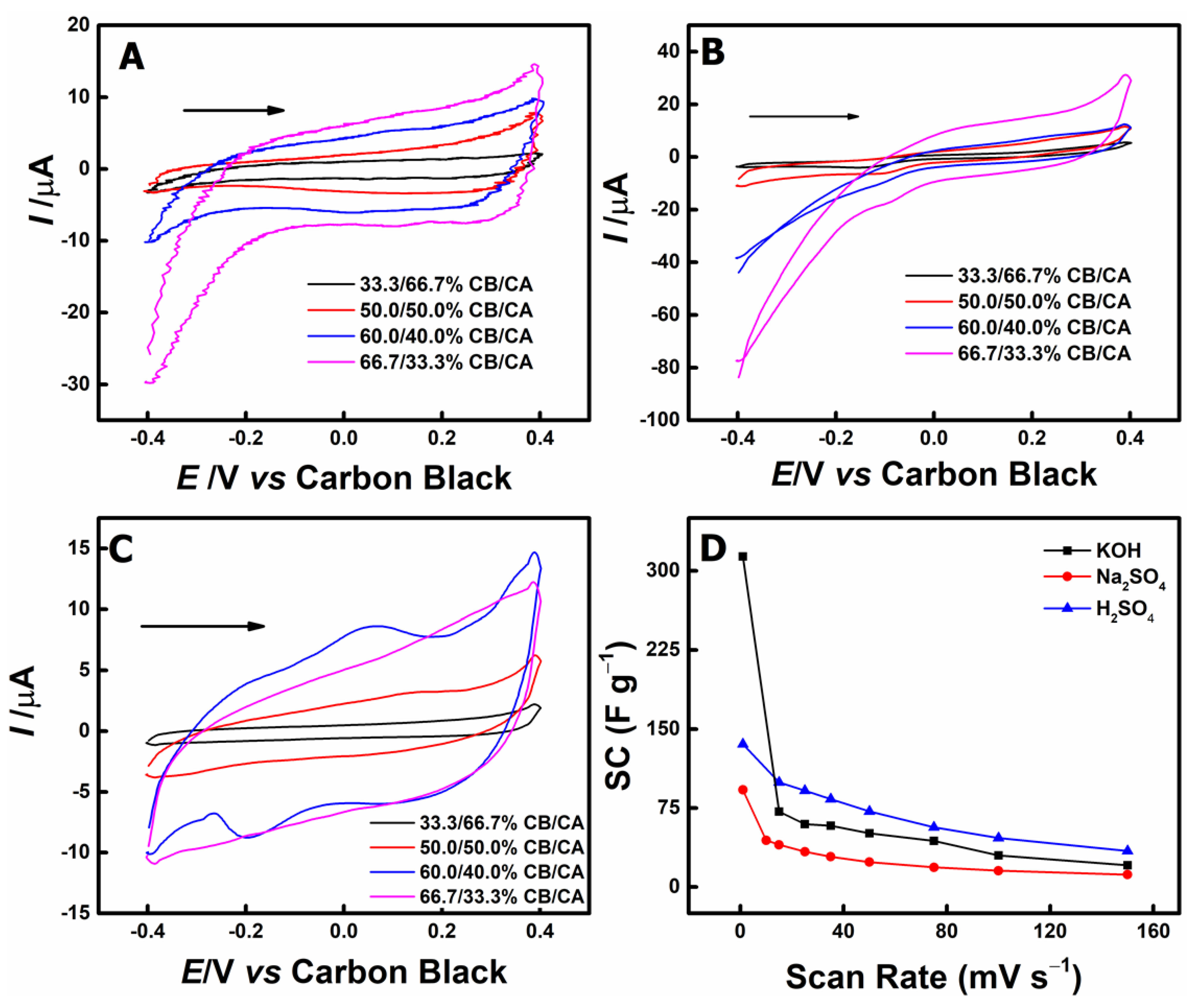
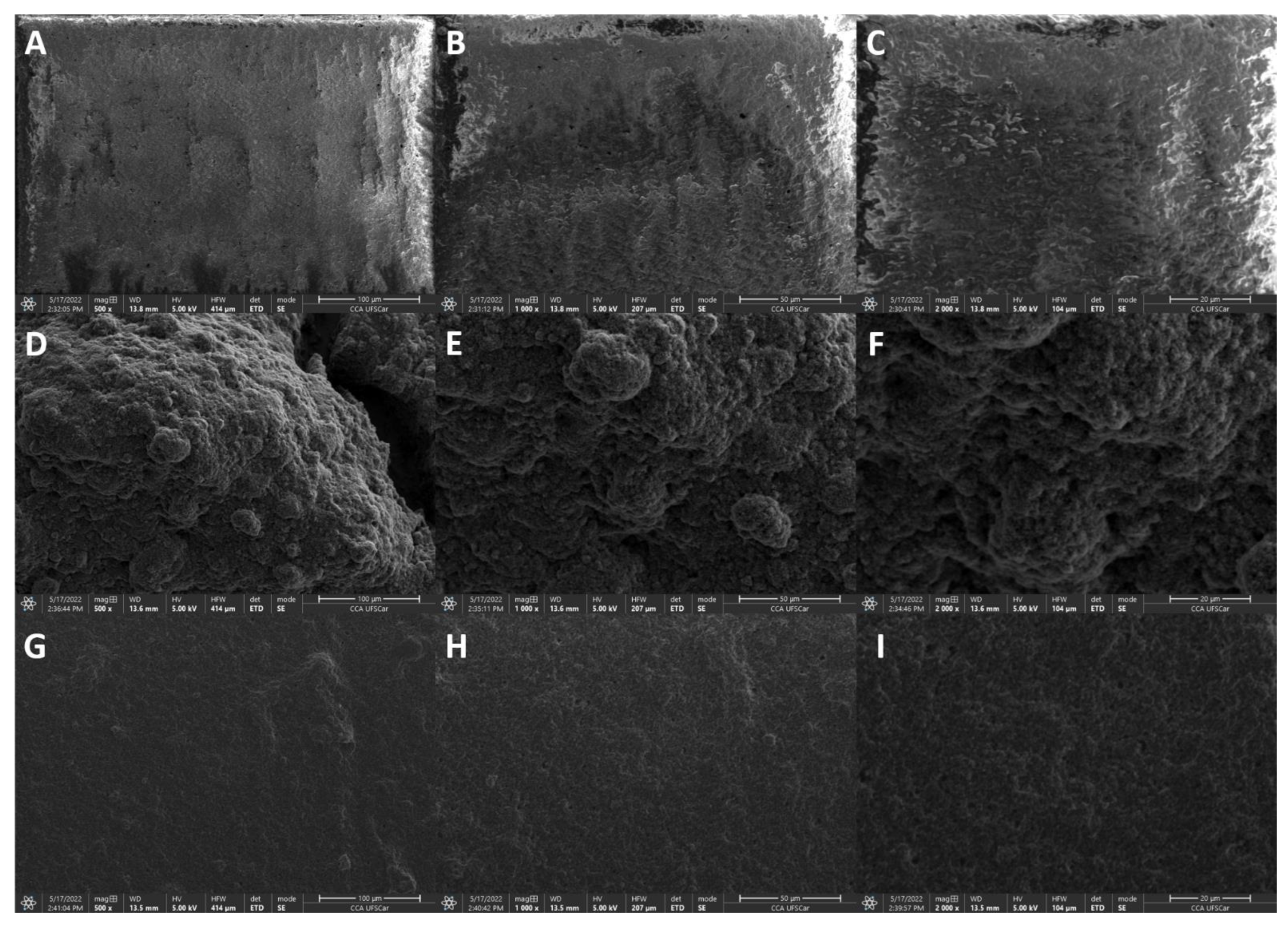
3.1. Electrochemical and Morphological Characterizations
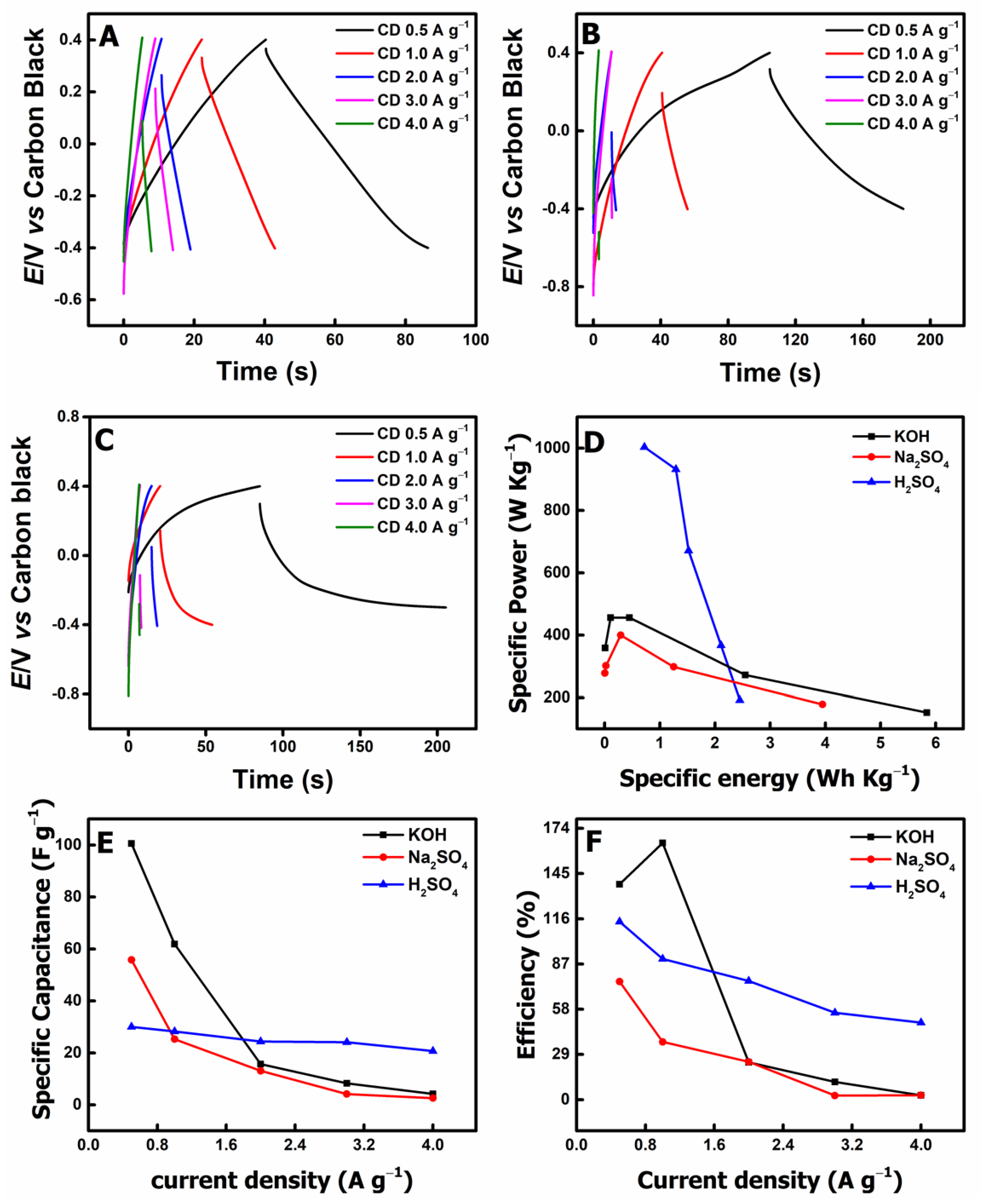
3.2. Potentiostatic Charge/Discharge Evaluation
3.3. Energy and Power Density Calculations
3.4. Flexibility Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapisarda, M.; Marken, F.; Meo, M. Graphene oxide and starch gel as a hybrid binder for environmentally friendly high-performance supercapacitors. Commun. Chem. 2021, 4, 169. [Google Scholar] [CrossRef]
- Faggioli, E.; Rena, P.; Danel, V.; Andrieu, X.; Mallant, R.; Kahlen, H. Supercapacitors for the energy management of electric vehicles. J. Power Sources 1999, 84, 261–269. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Yaici, W.; Entchev, E. Hybrid battery/supercapacitor energy storage system for the electric vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Augusto, G.D.S.; Scarminio, J.; da Silva, P.R.C.; de Siervo, A.; Rout, C.S.; Rouxinol, F.; Gelamo, R.V. Flexible metal-free supercapacitors based on multilayer graphene electrodes. Electrochim. Acta 2018, 285, 241–253. [Google Scholar] [CrossRef]
- Lekakou, C.; Moudam, O.; Markoulidis, F.; Andrews, T.; Watts, J.F.; Reed, G.T. Carbon-based fibrous EDLC capacitors and supercapacitors. J. Nanotechnol. 2011, 2011, 409382. [Google Scholar] [CrossRef] [Green Version]
- Kandalkar, S.; Dhawale, D.; Kim, C.-K.; Lokhande, C. Chemical synthesis of cobalt oxide thin film electrode for supercapacitor application. Synth. Met. 2010, 160, 1299–1302. [Google Scholar] [CrossRef]
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Monolithic carbide-derived carbon films for micro-supercapacitors. Science 2010, 328, 480–483. [Google Scholar] [CrossRef] [Green Version]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. In Nanoscience Technology: A Collection of Reviews from Nature Journals; World Scientific Publishing: Singapore, 2010; pp. 320–329. [Google Scholar]
- Palchoudhury, S.; Ramasamy, K.; Gupta, R.K.; Gupta, A. Flexible supercapacitors: A materials perspective. Front. Mater. 2019, 5, 83. [Google Scholar] [CrossRef] [Green Version]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Khosrozadeh, A.; Darabi, M.A.; Xing, M.; Wang, Q. Flexible electrode design: Fabrication of freestanding polyaniline-based composite films for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 11379–11389. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhao, B.; Zhang, W.; Shi, F.; Zheng, G.P.; Zhang, D.; Yang, J. High-Performance Supercapacitor Applications of NiO-Nanoparticle-Decorated Millimeter-Long Vertically Aligned Carbon Nanotube Arrays via an Effective Supercritical CO2-Assisted Method. Adv. Funct. Mater. 2015, 25, 7381–7391. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, S.; Chen, D.; Dong, L.; Wang, J.; Zhang, T.; Jiao, T.; Liu, B.; Wang, H.; Kai, J.J.; et al. Editable asymmetric all-solid-state supercapacitors based on high-strength, flexible, and programmable 2D-metal–organic framework/reduced graphene oxide self-assembled papers. J. Mater. Chem. A 2018, 6, 20254–20266. [Google Scholar] [CrossRef]
- Yu, L.; Hu, L.; Anasori, B.; Liu, Y.-T.; Zhu, Q.; Zhang, P.; Gogotsi, Y.; Xu, B. MXene-bonded activated carbon as a flexible electrode for high-performance supercapacitors. ACS Energy Lett. 2018, 3, 1597–1603. [Google Scholar] [CrossRef]
- Wang, S.; Liu, N.; Su, J.; Li, L.; Long, F.; Zou, Z.; Jiang, X.; Gao, Y. Highly stretchable and self-healable supercapacitor with reduced graphene oxide based fiber springs. Acs Nano 2017, 11, 2066–2074. [Google Scholar] [CrossRef]
- Ban, S.; Malek, K.; Huang, C.; Liu, Z. A molecular model for carbon black primary particles with internal nanoporosity. Carbon 2011, 49, 3362–3370. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical biosensors based on nanostructured carbon black: A review. J. Nanomater. 2017, 2017, 4571614. [Google Scholar] [CrossRef] [Green Version]
- Delgado, K.P.; Raymundo-Pereira, P.A.; Campos, A.M.; Oliveira, O.N., Jr.; Janegitz, B.C. Ultralow Cost Electrochemical Sensor Made of Potato Starch and Carbon Black Nanoballs to Detect Tetracycline in Waters and Milk. Electroanalysis 2018, 30, 2153–2159. [Google Scholar] [CrossRef]
- Phillips, C.; Al-Ahmadi, A.; Potts, S.-J.; Claypole, T.; Deganello, D. The effect of graphite and carbon black ratios on conductive ink performance. J. Mater. Sci. 2017, 52, 9520–9530. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.M.E.; Pereira, A.S.T.M.; Almeida, A.C.; Roma, U.S.; Soler, A.B.A.; Lacharmoise, P.D.; Ferreira, I.M.D.M.; Simão, C.C.D. Large-Area Paper Batteries with Ag and Zn/Ag Screen-Printed Electrodes. ACS Omega 2019, 4, 16781–16788. [Google Scholar] [CrossRef] [PubMed]
- Pech, D.; Brunet, M.; Taberna, P.-L.; Simon, P.; Fabre, N.; Mesnilgrente, F.; Conédéra, V.; Durou, H. Elaboration of a microstructured inkjet-printed carbon electrochemical capacitor. J. Power Sources 2010, 195, 1266–1269. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, F.J.; Galotto, M.J.; Guarda, A.; Bruna, J.E. Modification of cellulose acetate films using nanofillers based on organoclays. J. Food Eng. 2012, 110, 262–268. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Razak, S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hao, Q.; Mandler, D. Electrochemical detection of dopamine by a calixarene-cellulose acetate mixed Langmuir-Blodgett monolayer. Anal. Chim. Acta 2018, 1042, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, I.A.D.A.; Orzari, L.O.; Camargo, J.R.; Faria, R.C.; Marcolino-Junior, L.H.; Bergamini, M.F.; Gatti, A.; Janegitz, B.C. Disposable and flexible electrochemical sensor made by recyclable material and low cost conductive ink. J. Electroanal. Chem. 2019, 840, 109–116. [Google Scholar] [CrossRef]
- Kondo, T.; Sakamoto, H.; Kato, T.; Horitani, M.; Shitanda, I.; Itagaki, M.; Yuasa, M. Screen-printed diamond electrode: A disposable sensitive electrochemical electrode. Electrochem. Commun. 2011, 13, 1546–1549. [Google Scholar] [CrossRef]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Characterization and fabrication of disposable screen printed microelectrodes. Electrochem. Commun. 2009, 11, 1377–1380. [Google Scholar] [CrossRef]
- Rocha, D.S.; Duarte, L.C.; Silva-Neto, H.A.; Chagas, C.L.; Santana, M.H.; Filho, N.R.A.; Coltro, W.K. Sandpaper-based electrochemical devices assembled on a reusable 3D-printed holder to detect date rape drug in beverages. Talanta 2021, 232, 122408. [Google Scholar] [CrossRef]
- Li, H.; Wang, W.; Lv, Q.; Xi, G.; Bai, H.; Zhang, Q. Disposable paper-based electrochemical sensor based on stacked gold nanoparticles supported carbon nanotubes for the determination of bisphenol A. Electrochem. Commun. 2016, 68, 104–107. [Google Scholar] [CrossRef]
- Camargo, J.R.; Andreotti, I.A.; Kalinke, C.; Henrique, J.M.; Bonacin, J.A.; Janegitz, B.C. Waterproof paper as a new substrate to construct a disposable sensor for the electrochemical determination of paracetamol and melatonin. Talanta 2020, 208, 120458. [Google Scholar] [CrossRef] [PubMed]
- Orzari, L.; Andreotti, I.A.D.A.; Bergamini, M.F.; Marcolino-Junior, L.H.; Janegitz, B.C. Disposable electrode obtained by pencil drawing on corrugated fiberboard substrate. Sens. Actuators B Chem. 2018, 264, 20–26. [Google Scholar] [CrossRef]
- Carvalho, J.H.; Gogola, J.L.; Bergamini, M.F.; Marcolino-Junior, L.H.; Janegitz, B.C. Disposable and low-cost lab-made screen-printed electrodes for voltammetric determination of L-dopa. Sens. Actuators Rep. 2021, 3, 100056. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Jiang, H.-L.; Zhang, X.; Xu, Q. Metal-organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor. Carbon 2010, 48, 456–463. [Google Scholar] [CrossRef]
- Danaee, I.; Jafarian, M.; Forouzandeh, F.; Gobal, F.; Mahjani, M. Mahjani, Electrochemical impedance studies of methanol oxidation on GC/Ni and GC/NiCu electrode. Int. J. Hydrogen Energy 2009, 34, 859–869. [Google Scholar] [CrossRef]
- Krause, A.; Kossyrev, P.; Oljaca, M.; Passerini, S.; Winter, M.; Balducci, A. Electrochemical double layer capacitor and lithium-ion capacitor based on carbon black. J. Power Sources 2011, 196, 8836–8842. [Google Scholar] [CrossRef]
- Ma, X.; Song, X.; Yu, Z.; Li, S.; Wang, X.; Zhao, L.; Zhao, L.; Xiao, Z.; Qi, C.; Ning, G.; et al. S-doping coupled with pore-structure modulation to conducting carbon black: Toward high mass loading electrical double-layer capacitor. Carbon 2019, 149, 646–654. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Asavamongkolkul, T.; Perngyai, T.; Jongsomjit, B.; Phisalaphong, M. Interconnected Micro, Meso, and Macro Porous Activated Carbon from Bacterial Nanocellulose for Superior Adsorption Properties and Effective Catalytic Performance. Molecules 2020, 25, 4063. [Google Scholar] [CrossRef]
- Toupin, M.; Bélanger, D.; Hill, I.R.; Quinn, D. Performance of experimental carbon blacks in aqueous supercapacitors. J. Power Sources 2005, 140, 203–210. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Whily: Hoboken, NJ, USA, 2008; pp. 383–389. [Google Scholar]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications, Modern Aspects of Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2002; pp. 143–248. [Google Scholar]
- Nasibi, M.; Golozar, M.A.; Rashed, G. Nano zirconium oxide/carbon black as a new electrode material for electrochemical double layer capacitors. J. Power Sources 2012, 206, 108–110. [Google Scholar] [CrossRef]
- Bobacka, J.; Lewenstam, A.; Ivaska, A. Electrochemical impedance spectroscopy of oxidized poly (3, 4-ethylenedioxythiophene) film electrodes in aqueous solutions. J. Electroanal. Chem. 2000, 489, 17–27. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Wang, J.; Pentecost, A.; Long, D.; Ling, L.; Qiao, W. Effect of graphitic structure on electrochemical ion intercalation into positive and negative electrodes. J. Solid State Electrochem. 2014, 18, 2673–2682. [Google Scholar] [CrossRef]
- Mitchell, J.B. Understanding the Role of Structural Water for High Power Electrochemical Energy Storage in Tungsten Oxide Hydrates; North Carolina State University: Raleigh, NC, USA, 2021. [Google Scholar]
- Orellana, K.P.D. Low Cost, Carbon-Based Micro-and Nano-Structured Electrodes for High Performance Supercapacitors. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2016. [Google Scholar]
- Thissandier, F.; Le Comte, A.; Crosnier, O.; Gentile, P.; Bidan, G.; Hadji, E.; Brousse, T.; Sadki, S. Highly doped silicon nanowires based electrodes for micro-electrochemical capacitor applications. Electrochem. Commun. 2012, 25, 109–111. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.; Zhong, W.; Yang, W. Hydrothermal direct synthesis of polyaniline, graphene/polyaniline and N-doped graphene/polyaniline hydrogels for high performance flexible supercapacitors. J. Mater. Chem. A 2018, 6, 9245–9256. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, T.; Xing, W.; Li, Z.; Shen, H.; Zhuo, S. Activated polyaniline-based carbon nanoparticles for high performance supercapacitors. Electrochim. Acta 2015, 160, 152–159. [Google Scholar] [CrossRef]
- Eustache, E.; Frappier, R.; Porto, R.L.; Bouhtiyya, S.; Pierson, J.-F.; Brousse, T. Asymmetric electrochemical capacitor microdevice designed with vanadium nitride and nickel oxide thin film electrodes. Electrochem. Commun. 2013, 28, 104–106. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.A.; Yoo, C.-Y.; Yoo, J.J.; Gwak, R.; Cho, W.K.; Kim, B.; Yoon, H. Self-templated synthesis of interconnected porous carbon nanosheets with controllable pore size: Mechanism and electrochemical capacitor application. Microporous Mesoporous Mater. 2018, 261, 119–125. [Google Scholar] [CrossRef]
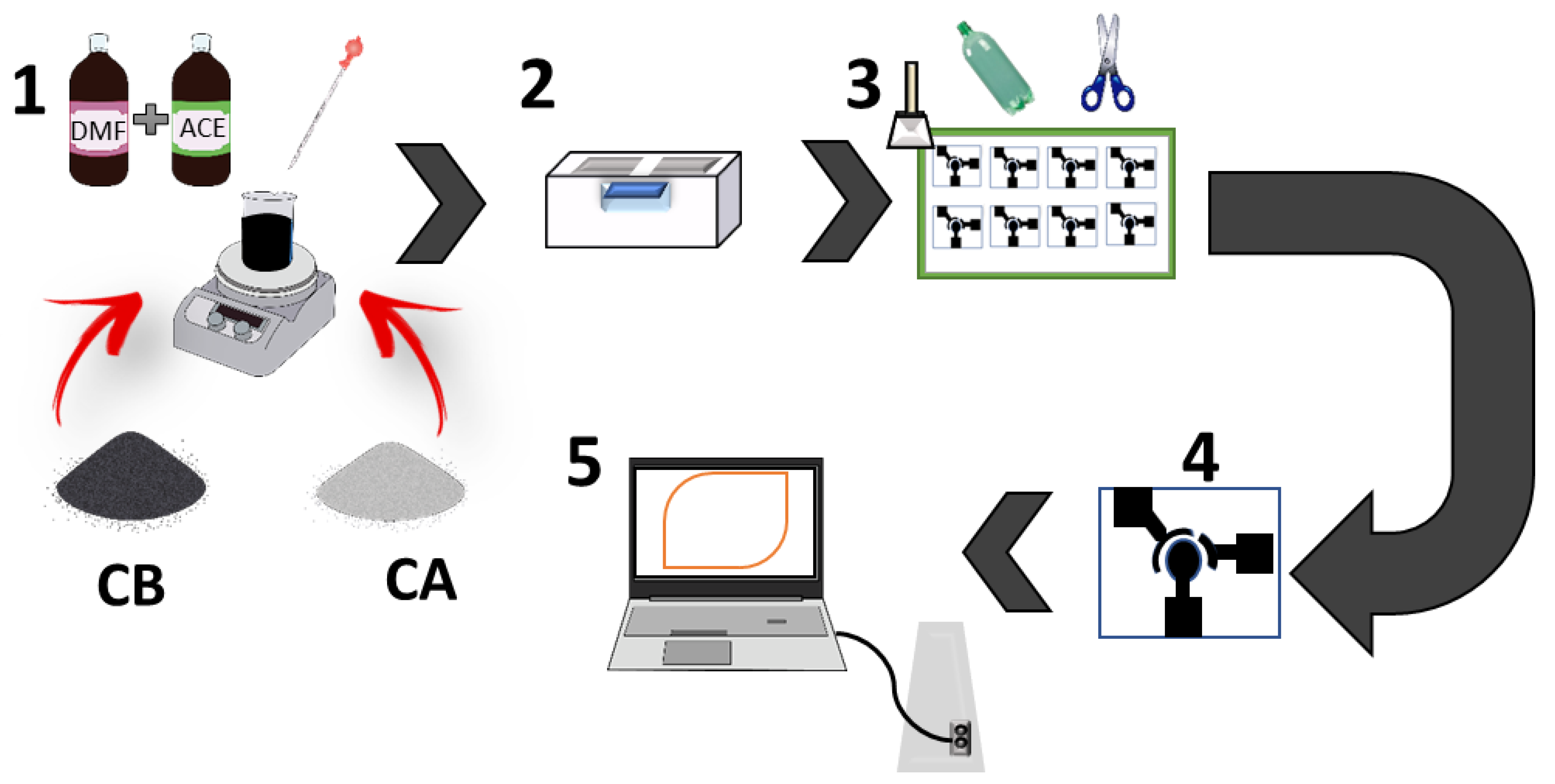

| Formulation Nº | CB (mg) | CA (mg) | CB/CA (wt%/wt%) |
|---|---|---|---|
| 1 | 125 | 250 | 33.3/66.7 |
| 2 | 250 | 250 | 50.0/50.0 |
| 3 | 375 | 250 | 60.0/40.0 |
| 4 | 500 | 250 | 66.7/33.3 |
| Electrolyte | Rate Capacitance (F g−1) | ||||
|---|---|---|---|---|---|
| 0.5 A g−1 | 1.0 A g−1 | 2.0 A g−1 | 3.0 A g−1 | 4.0 A g−1 | |
| H2SO4 | 30.01 | 28.23 | 24.38 | 24.13 | 20.7 |
| KOH | 100.6 | 64.85 | 15.70 | 8.280 | 4.242 |
| Na2SO4 | 55.79 | 25.29 | 13.10 | 4.172 | 2.590 |
| Types of Cell | Composition | Specific Capacitance | Specific Power | Specific Energy | Works |
|---|---|---|---|---|---|
| Three electrodes | Highly doped silicon nanowires | 46 µF cm−2 | 1.6 mF cm−2 | Not specified | [50] |
| Two electrodes | N-doped Graphene/PANI hydrogel | 584.7 mF cm−2 | − 0.5 mW cm−2 | ~0.1 mWh cm−2 | [51] |
| Two electrodes | PANI-N/CNT | 341.0 F g−1 | 40.0 W kg−1 | 11.5 Wh kg−1 | [52] |
| Two electrodes | ACTIVATED CARBON-PTFE | 2.1 mF cm−2 | Non-specified | Non-specified | [23] |
| Two electrodes | PANI-MLG | 110 mF cm−2 | 0.15 mW cm−2 | ~0.01 mWh cm−2 | [6] |
| Two electrodes | PANI/cellulose/Ag | 310.9 F g−1 | 238.2 W kg−1 | 0.7 Wh kg−1 | [13] |
| Two electrodes | PANI-MLG | 451.0 F g−1 | 3610.0 W kg−1 | 17.0 Wh kg−1 | [6] |
| Three electrodes | Vanadium nitride and nickel oxide thin film | 1.85 mF cm−2 | 28 mW cm−2 | 0.7 μWh cm−2 | [53] |
| Two electrodes | IPCN-alkaline carbon metal | 200 F g−1 | Non-specified | Non-specified | [54] |
| Three electrodes | Carbon black-cellulose acetate | 313.53 F g−1 | 455.691W kg−1 | 5.84 Wh kg−1 | This work |
| 168.05 mF cm−2 | 0.24437 mW cm−2 | 0.00313 mWh cm−2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniele, G.G.; de Souza, D.C.; de Oliveira, P.R.; Orzari, L.O.; Blasques, R.V.; Germscheidt, R.L.; da Silva, E.C.; Pocrifka, L.A.; Bonacin, J.A.; Janegitz, B.C. Development of Disposable and Flexible Supercapacitor Based on Carbonaceous and Ecofriendly Materials. C 2022, 8, 32. https://doi.org/10.3390/c8020032
Daniele GG, de Souza DC, de Oliveira PR, Orzari LO, Blasques RV, Germscheidt RL, da Silva EC, Pocrifka LA, Bonacin JA, Janegitz BC. Development of Disposable and Flexible Supercapacitor Based on Carbonaceous and Ecofriendly Materials. C. 2022; 8(2):32. https://doi.org/10.3390/c8020032
Chicago/Turabian StyleDaniele, Giovanni G., Daniel C. de Souza, Paulo Roberto de Oliveira, Luiz O. Orzari, Rodrigo V. Blasques, Rafael L. Germscheidt, Emilly C. da Silva, Leandro A. Pocrifka, Juliano A. Bonacin, and Bruno C. Janegitz. 2022. "Development of Disposable and Flexible Supercapacitor Based on Carbonaceous and Ecofriendly Materials" C 8, no. 2: 32. https://doi.org/10.3390/c8020032
APA StyleDaniele, G. G., de Souza, D. C., de Oliveira, P. R., Orzari, L. O., Blasques, R. V., Germscheidt, R. L., da Silva, E. C., Pocrifka, L. A., Bonacin, J. A., & Janegitz, B. C. (2022). Development of Disposable and Flexible Supercapacitor Based on Carbonaceous and Ecofriendly Materials. C, 8(2), 32. https://doi.org/10.3390/c8020032









