Removal of Hydrophobic Contaminants from the Soil by Adsorption onto Carbon Materials and Microbial Degradation
Abstract
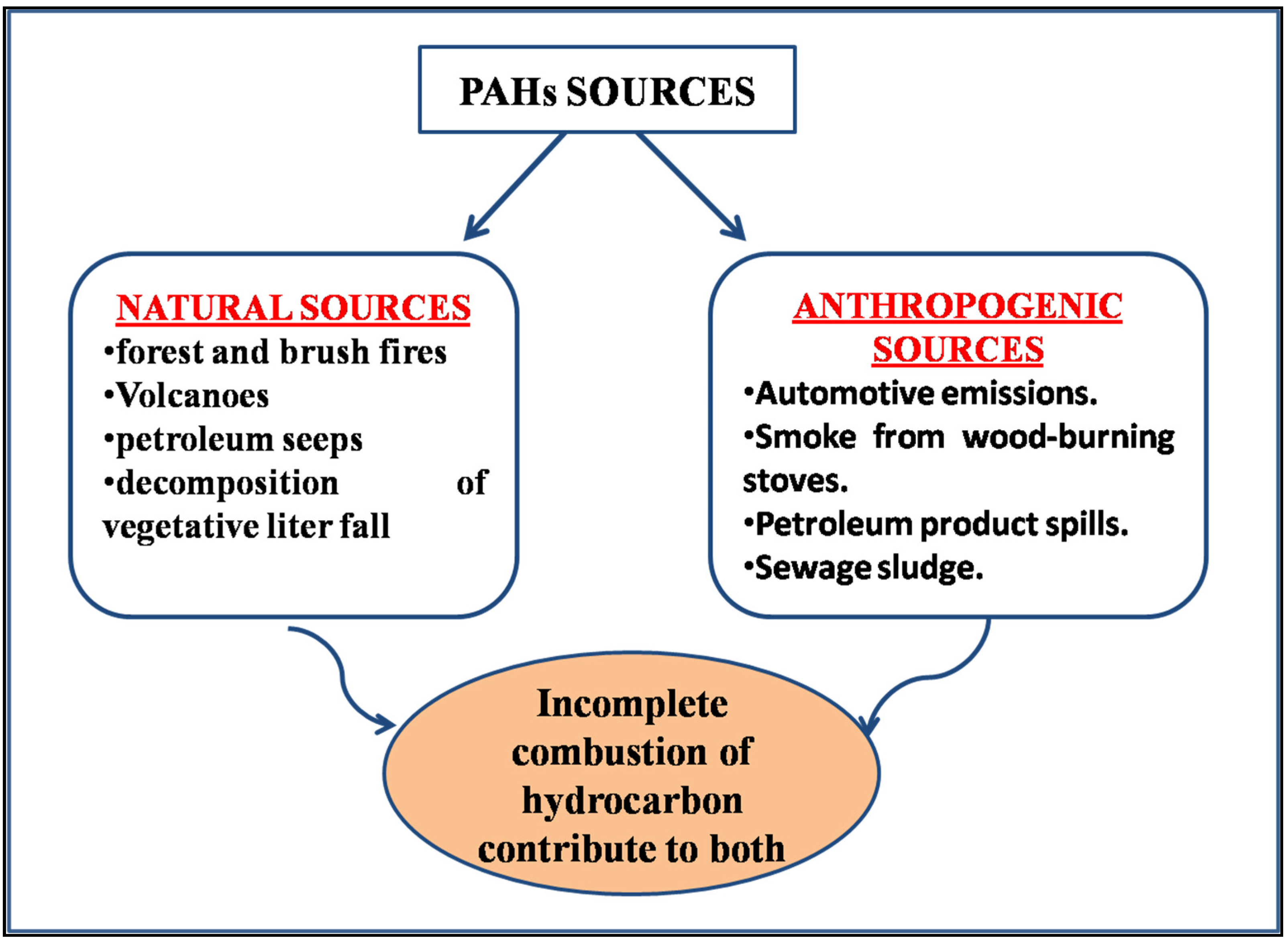
1. Introduction
2. Adsorption
2.1. Types of Adsorption
2.1.1. Physical Adsorption
2.1.2. Chemisorption
2.2. Factors Influencing the Adsorption Process
2.2.1. Temperature
2.2.2. Surface Charge
2.2.3. Surface Area and Porosity
- High efficiency.
- Low operation cost.
- No additional nutrient requirements.
- Minimal chemical and biological sludge.
- Possible valuable metal recovery.
- Possible adsorbent regeneration.
- Successfully operation over a wide range of temperature and pH.
2.3. Adsorption Isotherms
2.3.1. Langmuir Isotherm
2.3.2. Freundlich Isotherm
2.4. Kinetic Adsorption Modeling
3. Removal and Adsorption of Hydrophobic Contaminants
3.1. Adsorption by Biochars
3.1.1. Source, Type, Production and Properties of Biochar
3.1.2. Adsorption Mechanisms for Hoc Removal by Biochar
3.1.3. Removal of Pesticides
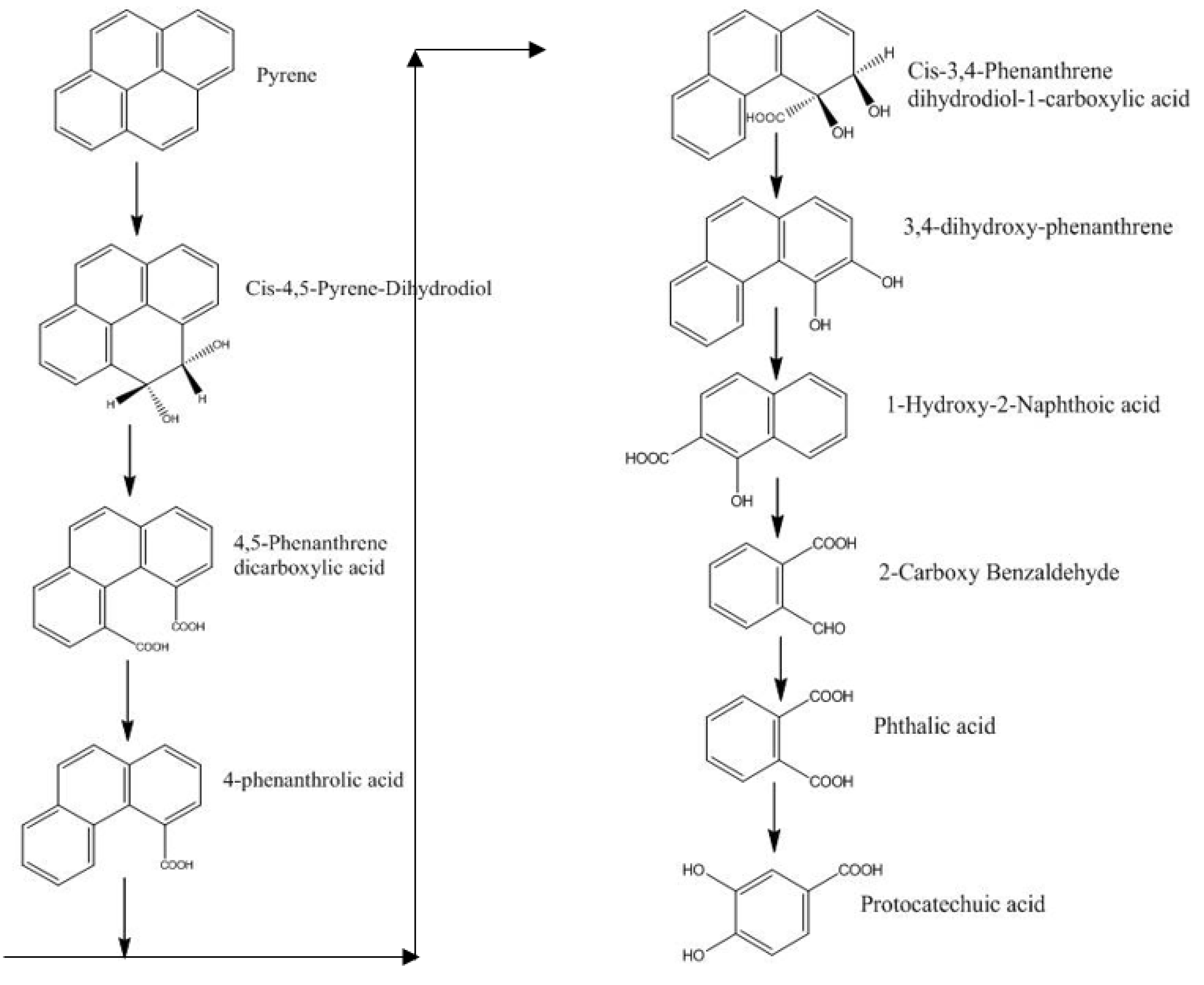
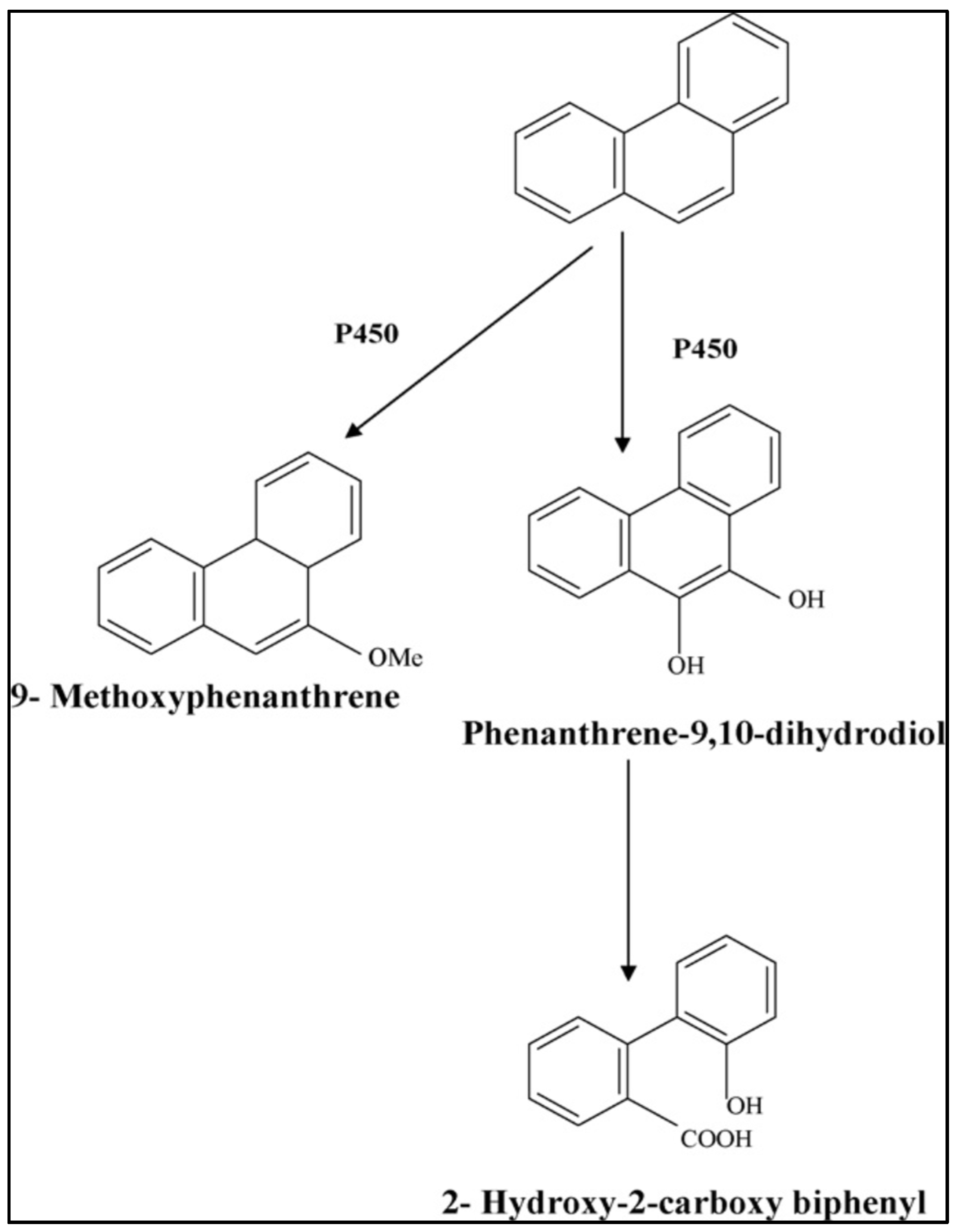
3.1.4. Removal of PAHs
3.2. Adsorption by Activated Charcoal
Removal of PAHs
4. Degradation of Hydrophobic Contaminants
4.1. Microbial Degradation of PAHs
4.1.1. Bacteria
4.1.2. Fungi
4.1.3. Algae
4.2. Microbial Degradation of Pesticides
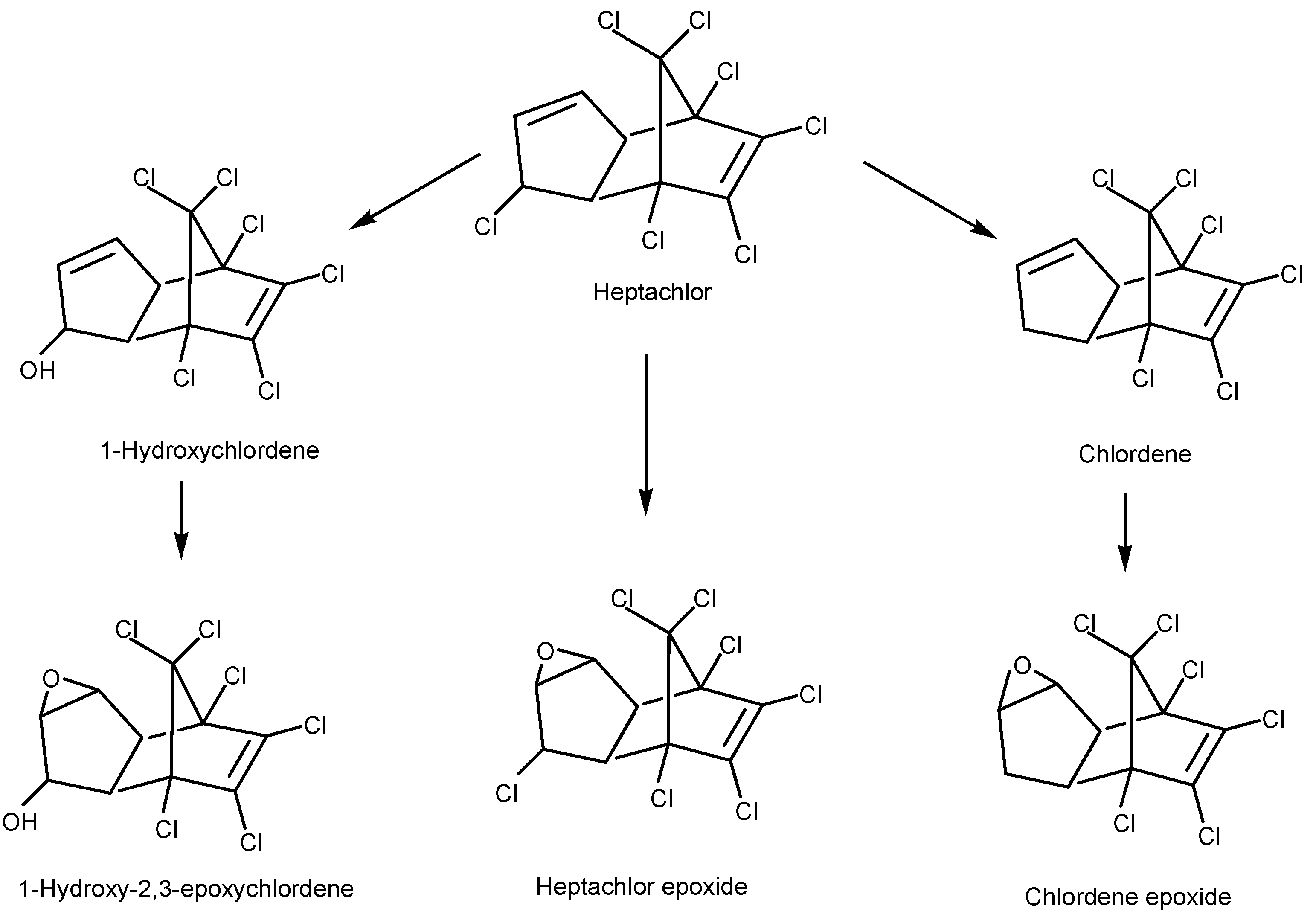
4.2.1. Bacterial Degradation of Organochlorine Pesticides
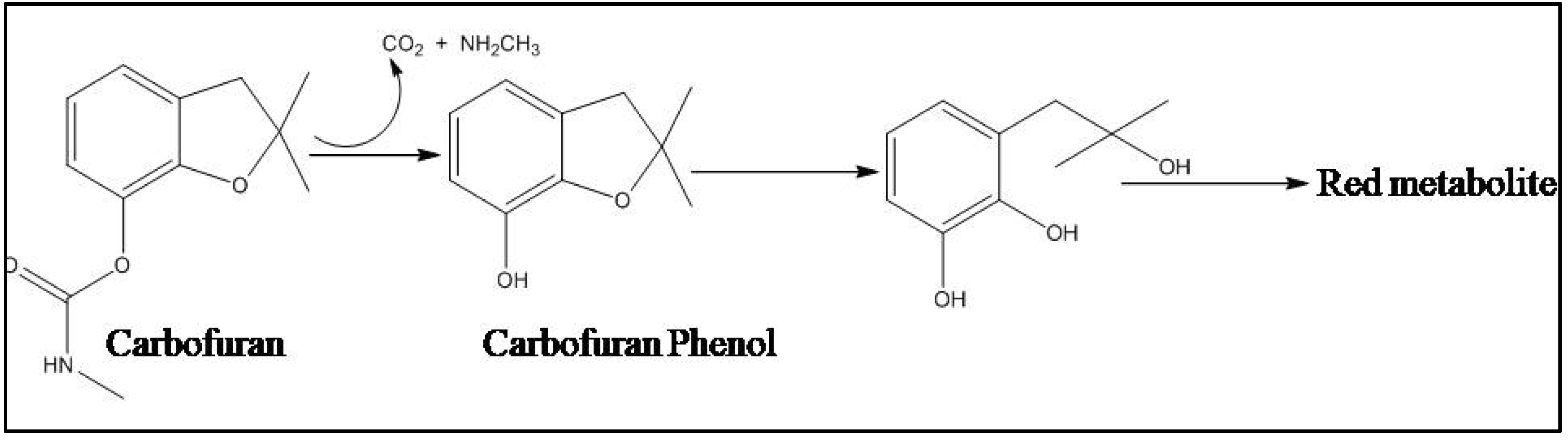
4.2.2. Bacterial Degradation of Carbamate
4.2.3. Fungal Biodegradation of Pesticides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2015, 306, 149–174. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2008/98/CE; European Parliament: Brussels, Belgium, 2008. [Google Scholar]
- European Union. Directive 2008/50/CE; European Parliament: Brussels, Belgium, 2008. [Google Scholar]
- European Union. Directive 2003/87/CE; European Parliament: Brussels, Belgium, 2003. [Google Scholar]
- European Union. Directive 2000/60/CE; European Parliament: Brussels, Belgium, 2000. [Google Scholar]
- FAO. Status of the World’s Soil Resources (SWSR)—Main Report. Natural Resources and Environment Department; Food and Agriculture Organization (FAO) of the United Nations and Intergovernmental Technical Panel on Soils (ITPS): Rome, Italy, 2015; p. 648. [Google Scholar]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Jiang, A.; Cheng, Z.; Shen, Z.; Guo, W. QSAR study on the removal efficiency of organic pollutants in supercritical water based on degradation temperature. Chem. Central J. 2018, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Tian, W.; Qin, H.; Wang, X.; Zhao, W. Optimal design and control of Eastman organic wastewater treatment process. J. Clean. Prod. 2018, 198, 333–350. [Google Scholar] [CrossRef]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2002, 13, 57–149. [Google Scholar] [CrossRef]
- Callier, L.; Clozel, B.; Nowak, C. Methodes de Recherche de Lorigine de Pollution(s) Dans les Sols Oudans les Eauxsouterraines, BRGM RP-51260-FR; Ministère de l’Écologie: Paris, France, 2002. [Google Scholar]
- Nam, K.; Kim, J.Y. Persistence and bioavailability of hydrophobic organic compounds in the environment. Geosci. J. 2002, 6, 13–21. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Samara, C. Persistent organic pollutants (POPs) in the conventional activated sludge treatment process: Fate and mass balance. Environ. Res. 2005, 97, 245–257. [Google Scholar] [CrossRef]
- EnHealth. Australian Exposure Factor Guidance Handbook; EnHealth Council, Environmental Health Committee: Canberra, Australia, 2012; p. 153. [Google Scholar]
- Khan, S.; Cao, Q.; Lin, A.-J.; Zhu, Y.-G. Concentrations and bioaccessibility of polycyclic aromatic hydrocarbons in wastewater-irrigated soil using in vitro gastrointestinal test. Environ. Sci. Pollut. Res. 2008, 15, 344–353. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and Sorptive Properties of Crop Residue-Derived Chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Karlson, U. PAH Degradation Capacity of Soil Microbial Communities—Does It Depend on PAH Exposure? Microb. Ecol. 2005, 50, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Alef, K.; Wilke, B.-M.; Li, P. Activated carbon adsorption of PAHs from vegetable oil used in soil remediation. J. Hazard. Mater. 2007, 143, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Chang, C.Y.; Chen, K.H.; Tsai, W.T.; Shie, J.L.; Chen, Y.H. Adsorption of naphthalene on zeolite from aqueous solution. J. Colloid Interf. Sci. 2004, 277, 29–34. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Wilcke, W. Polycyclic aromatic hydrocarbons (PAHs) in soil e a review. J. Plant Nutr. Soil Sci. 1996, 163, 229–248. [Google Scholar] [CrossRef]
- Nam, J.; Song, B.; Eom, K.; Lee, S.; Smith, A. Distribution of polycyclic aromatic hydrocarbons in agricultural soils in South Korea. Chemosphere 2003, 50, 1281–1289. [Google Scholar] [CrossRef]
- Hao, R.; Wan, H.-F.; Song, Y.-T.; Jiang, H.; Peng, S.-L. Polycyclic Aromatic Hydrocarbons in Agricultural Soils of the Southern Subtropics, China. Pedosphere 2007, 17, 673–680. [Google Scholar] [CrossRef]
- Chung, M.; Hu, R.; Cheung, K.; Wong, M. Pollutants in Hong Kong soils: Polycyclic aromatic hydrocarbons. Chemosphere 2006, 67, 464–473. [Google Scholar] [CrossRef]
- Krauss, M.; Wilcke, W.; Zech, W. Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in forest soils: Depth distribution as indicator of different fate. Environ. Pollut. 2000, 110, 79–88. [Google Scholar] [CrossRef]
- Jensen, H.; Reimann, C.; Finne, T.E.; Ottesen, R.T.; Arnoldussen, A. PAH-concentrations and compositions in the top 2 cm of forest soils along a 120 km long transect through agricultural areas, forest and the city of Oslo, Norway. Environ. Pollut. 2007, 145, 829–838. [Google Scholar]
- Mielke, H.W.; Wang, G.; Gonzales, C.R.; Powell, E.T.; Le, B.; Quach, V.N. PAHs and metals in the soils of inner-city and suburban New Orleans, Louisiana, USA. Environ. Toxicol. Phar. 2004, 18, 243–247. [Google Scholar] [CrossRef]
- Morillo, E.; Romero, A.S.; Maqueda, C.; Madrid, L.; Ajmone-Marsan, F.; Grcman, H.; Davidson, C.M.; Hursthouse, A.S.; Villaverde, J. Soil pollution by PAHs in urban soils: A comparison of three European cities. J. Environ. Monit. 2007, 9, 1001–1008. [Google Scholar] [CrossRef]
- Jiang, Y.-F.; Wang, X.-T.; Wang, F.; Jia, Y.; Wu, M.-H.; Sheng, G.-Y.; Fu, J.-M. Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in Urban soil of Shanghai, China. Chemosphere 2009, 75, 1112–1118. [Google Scholar] [CrossRef]
- Wild, S.R.; Jones, K.C. Polynuclear aromatic hydrocarbons in the United Kingdom environment: A preliminary source inventory and budget. Environ. Pollut. 1995, 88, 91–108. [Google Scholar] [CrossRef]
- Lee, P.-H.; Ong, S.K.; Golchin, J.; Nelson, G.L. Use of solvents to enhance PAH biodegradation of coal tar-contaminated soils. Wat. Res. 2001, 35, 3941–3949. [Google Scholar] [CrossRef]
- Bogan, B.W.; Trbovic, V.; Paterek, J. Inclusion of vegetable oils in Fenton’s chemistry for remediation of PAH-contaminated soils. Chemosphere 2002, 50, 15–21. [Google Scholar] [CrossRef]
- Gong, Z.; Wilke, B.-M.; Alef, K.; Li, P.; Zhou, Q. Removal of polycyclic aromatic hydrocarbons from manufactured gas plant-contaminated soils using sunflower oil: Laboratory column experiments. Chemosphere 2006, 62, 780–787. [Google Scholar] [CrossRef]
- Eom, I.; Rast, C.; Veber, A.; Vasseur, P. Ecotoxicity of a polycyclic aromatic hydrocarbon (PAH)-contaminated soil. Ecotoxicol. Environ. Saf. 2007, 67, 190–205. [Google Scholar] [CrossRef]
- White, P.A.; Claxton, L. Mutagens in contaminated soil: A review. Mutat. Res. 2004, 567, 227–345. [Google Scholar] [CrossRef]
- Mumtaz, M.; George, J. Toxicological Profile for Polycyclic Aromatic Hydrocarbons; U.S. Department of Health and Human Services, Public Health Service. Agency for Toxic Substances and Disease Registry: Washington, DC, USA, 1995. [Google Scholar]
- Trellu, C.; Pechaud, Y.; Oturan, N.; Mousset, E.; van Hullebusch, E.D.; Huguenot, D.; Oturan, M.A. Remediation of soils contaminated by hydrophobic organic compounds: How to recover extracting agents from soil washing solutions? J. Haz. Mater. 2021, 404, 124–137. [Google Scholar] [CrossRef]
- Karimi-Lotfabad, S.; Pickard, M.A.; Gray, M.R. Reactions of Polynuclear Aromatic Hydrocarbons on Soil. Environ. Sci. Technol. 1996, 30, 1145–1151. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Chen, C.; Zhu, L. Removal of polycyclic aromatic hydrocarbons from surfactant solutions by selective sorption with organo-bentonite. Chem. Eng. J. 2013, 233, 251–257. [Google Scholar] [CrossRef]
- Steiner, W. Soil Washing Process for the Removal of Hydrophobic Organic Compounds from Soils and Sediments. Ph.D. Thesis, New York University, New York, NY, USA, 1988. [Google Scholar]
- Peng, R.-H.; Fu, X.-Y.; Zhao, W.; Tian, Y.-S.; Zhu, B.; Han, H.-J.; Xu, J.; Yao, Q.-H. Phytoremediation of Phenanthrene by Transgenic Plants Transformed with a Naphthalene Dioxygenase System from Pseudomonas. Environ. Sci. Technol. 2014, 48, 12824–12832. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Gao, Y.; Jin, L.; Gu, Y.; Wang, W. Isolation, plant colonization potential, and phenanthrene degradation performance of the endophytic bacterium Pseudomonas sp. Ph6 gfp. Sci. Rep. 2014, 4, 5462. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of Polycyclic Aromatic Hydrocarbons by Graphene and Graphene Oxide Nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef]
- Li, C.-H.; Wong, Y.-S.; Wang, H.-Y.; Tam, N.F.-Y. Anaerobic biodegradation of PAHs in mangrove sediment with amendment of NaHCO3. J. Environ. Sci. 2015, 30, 148–156. [Google Scholar] [CrossRef]
- Shih, K.L.; Lederberg, J. Chloramine Mutagenesis in Bacillus subtilis. Science 1976, 192, 1141–1143. [Google Scholar] [CrossRef]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Oller, I.; Malato, S.; Pérez, J.A.S. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total. Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Xi, Z.; Chen, B. Removal of polycyclic aromatic hydrocarbons from aqueous solution by raw and modified plant residue materials as biosorbents. J. Environ. Sci. 2014, 26, 737–748. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Wang, X.; Wei, C. Efficient adsorption of phenanthrene by simply synthesized hydrophobic MCM-41 molecular sieves. Appl. Surf. Sci. 2014, 311, 825–830. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Jiang, L.; Yin, X. Adsorption of mixed polycyclic aromatic hydrocarbons in surfactant solutions by activated carbon. J. Ind. Eng. Chem. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- Lian, F.; Chang, C.; Du, Y.; Zhu, L.; Xing, B.; Liu, C. Adsorptive removal of hydrophobic organic compounds by carbonaceous adsorbents: A comparative study of waste-polymer-based, coal-based activated carbon, and carbon nanotubes. J. Environ Sci. 2012, 24, 1549–1558. [Google Scholar] [CrossRef]
- Staninska, J.; Szczepaniak, Z.; Cyplik, P.; Piotrowska-Cyplik, A. The effect of organic and clay fraction on polycyclic aromatic hydrocarbons mobility in soil model systems. J. Res. Appl. Agric. Eng. 2015, 60, 98–101. [Google Scholar]
- Kobayashi, T.; Sumida, H. Effects of humic acids on the sorption and bioavailability of pyrene and 1,2-dihydroxynaphthalene. Soil Sci. Plant Nutr. 2015, 61, 113–122. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Yu, N.; Zhang, C.; Wang, S.; Ma, L.; Zhao, J.; Lohmann, R. Particulate matter, gaseous and particulate polycyclic aromatic hydrocarbons (PAHs) in an urban traffic tunnel of China: Emission from on-road vehicles and gas-particle partitioning. Chemosphere 2015, 134, 52–59. [Google Scholar]
- Wang, J.; Wang, C.; Huang, Q.; Ding, F.; He, X. Adsorption of PAHs on the Sediments from the Yellow River Delta as a Function of Particle Size and Salinity. Soil Sediment Contam. Int. J. 2015, 24, 103–115. [Google Scholar] [CrossRef]
- Tong, D.S.; Zhou, C.H.; Lu, Y.; Yu, H.Y.; Zhang, G.F.; Yu, W.H. Adsorption of acid red G dye on octadecyltrimethylammonium montmorillonite. Appl. Clay Sci. 2010, 50, 427–431. [Google Scholar] [CrossRef]
- Alcantara, M.T.; Gomez, J.; Pazos, M.; Sanroman, M.A. PAHs soil decontamination in two steps: Desorption and electrochemical treatment. J. Hazard. Mater. 2009, 166, 462–468. [Google Scholar] [CrossRef]
- Toul, J.; Bezděk, J.; Kovářová, M.; Boháček, Z.; Hanák, J.; Milička, J.; Müller, P. Sorption of hydrophobic organic pollutants on soils and sediments. Bull. Geosci. 2003, 78, 205–223. [Google Scholar]
- Lamichhane, S.; Krishna, K.C.B.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Removal of Emerging Contaminants from Water and Wastewater by Adsorption Process. In Emerging Compounds Removal from Wastewater; Springer: Dordrecht, The Netherlands, 2012; pp. 15–37. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Zhang, L.; Zeng, Z.; Dong, W.; Guo, Q. The temperature dependence of adsorption coefficients of 222 Rn on activated charcoal: An experimental study. Appl. Radiat. Isot. 2017, 125, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Sahmoune, M.N. Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environ. Chem. Lett. 2018, 17, 697–704. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Meijboom, R. Determination of the surface area and sizes of supported copper nanoparticles through organothiol adsorption—Chemisorption. Appl. Surf. Sci. 2016, 390, 224–235. [Google Scholar] [CrossRef]
- Kratochvil, D.; Volesky, B. Advances in the Biosorption of heavy metals. Tib Tech. 1998, 16, 291–300. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef]
- Saswati, G.; Ghosh, U.C. Studies on adsorption behaviour of Cr (VI) onto synthetic hydroxyl stannic oxide. Water SA. 2005, 31, 597–602. [Google Scholar]
- Mamdouth, N.N.; Kamar, T.E.; Ebrahiem, E.E.; Yehia, H.M.; Mansour, H.M. Adsorption of iron and manganese ions using low cost materials as adsorbents. Adsorption Sci. Technol. 2004, 22, 25–37. [Google Scholar]
- Tebbutt, T.H.Y. Principles of Water Quality Control, 3rd ed.; Pergamon: Oxford, UK, 1991. [Google Scholar]
- Salam, M.A. Coating carbon nanotubes with crystalline manganese dioxide nanoparticles and their application for lead ions removal from model and real water. Colloids Surf. A Physicochem. Eng. Asp. 2013, 419, 69–79. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Baltazar, S.E.; García, A.; Romero, A.H.; Rubio, M.A.; Altbir, D. Lead removal by nano-scale zero valent iron: Surface analysis and pH effect. Mater. Res. Bull. 2014, 59, 341–348. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Erhan, D.; Kobya, M.; Elif, S.; Ozkan, T. Adsorption kinetics for the removal of chromium III from aqueous solutions on the activated carbonaceous prepared from agricultural wastes. Water S.A. 2004, 30, 533–540. [Google Scholar]
- Oke, I.A.; Olarinoye, N.O.; Adewusi, S.R.A. Adsorption kinetics for arsenic removal from aqueous solutions by untreated powdered eggshell. Adsorption 2007, 14, 73–83. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J.; Majdanska, M.; Fila, D. Sorption of lanthanide ions on biochar composites. J. Rare Earths 2018, 36, 1212–1220. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, B.; Song, Z.; Wang, H.; He, F.; Han, X. Wheat straw biochar amendments on the removal of polycyclic aromatic hydrocarbons (PAHs) in contaminated soil. Ecotoxicol. Environ. Saf. 2016, 130, 248–255. [Google Scholar] [CrossRef]
- Li, Z.B.; Wang, C.Y.; Jiang, X.; Wang, F. Progress of the research on potential environmental risk of polycyclic aromatic hydrocarbons (PAHs) in biochar. Acta Pedol. Sin. 2016, 53, 1357–1370. [Google Scholar]
- Zhelezova, A.; Cederlund, H.; Stenström, J. Effect of biochar amendment and ageing on adsorption and degradation of two herbicides. Water Air Soil Pollut. 2017, 228, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Chen, M.; Xi, P.; Li, J. Study on the effect of different carbon sorbents on the bioavailability of sediment-associated pyrethroids based on tenax desorption technology. Asian J. Ecotoxicol. 2017, 12, 382–390. [Google Scholar]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Zhong, J.K.; Liu, L.; Zhong, Z.W.; Yang, Q.Z.; Zhang, J.Y.; Wang, L.G. Advances on the research of the effect of biochar on the environmental behavior of antibiotics. J. Saf. Environ. 2018, 18, 657–663. [Google Scholar]
- Liu, H.L.; Xu, F.; Xie, Y.L.; Wang, C.; Zhang, A.; Li, L.L.; Xu, H. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S.M. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, H.; Min, L.; Ren, C. Biochars change the sorption and degradation of thiacloprid in soil: Insights into chemical and biological mechanisms. Environ. Pollut. 2018, 236, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lv, J.; Li, Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline Bioresour. Technol. 2018, 263, 475–482. [Google Scholar]
- Xu, C.; Xiang, Q.; Zhu, H.H.; Wang, S.; Qi, H.Z.; Huang, D.; Zhang, Y. Effect of biochar from peanut shell on speciation and availability of lead and zinc in an acidic paddy soil. Ecotoxicol. Environ. Saf. 2018, 164, 554–561. [Google Scholar]
- Zazycki, M.A.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. New biochar from pecan nutshells as an alternative adsorbent for removing Reactive Red 141 from aqueous solutions. J. Clean. Prod. 2018, 171, 57–65. [Google Scholar] [CrossRef]
- Aggelopoulosa, C.A.; Moschopoulou, E.; Klepetsanis, P.G.; Tsakiroglou, C.D. Valorization of fruit wastes (pistachio shells) as adsorbent for the removal of Zn from aqueous solutions under adverse acidic conditions. Desalin. Water Treat. 2017, 74, 174–183. [Google Scholar] [CrossRef]
- Nie, C.; Yang, X.; Niazi, N.K.; Xu, X.; Wen, Y.H.; Rinklebe, J.; Ok, Y.S.; Xu, S.; Wang, H. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: A field study. Chemosphere 2018, 200, 274–282. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; Poon, C.S. Phosphoric acid-activated wood biochar for catalytic conversion of starchrich food waste into glucose and 5-hydroxymethylfurfural. Bioresour. Technol. 2018, 267, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kua, H.W.; Koh, H.J. Application of biochar from food and wood waste as green admixture for cement mortar. Sci. Total Environ. 2018, 619, 418–435. [Google Scholar] [CrossRef]
- Al-wabel, M.; Hussain, Q.; Usman, A.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land Degrad. Dev. 2017, 29, 2124–2161. [Google Scholar] [CrossRef]
- Vause, D.; Heaney, N.; Lin, C. Differential release of sewage sludge biochar-borne elements by common low-molecular-weight organic acids. Ecotoxicol. Environ. Saf. 2018, 165, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrol. 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Huang, R.; Tian, D.; Liu, J.; Lv, S.; He, X.; Gao, X. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Kalsoom, T.; Yasmeen, S.; Kalsoom, A.; Raina, S.; Zhuang, Q.; Kong, J. Animal manure-derived biochars produced via fast pyrolysis for the removal of divalent copper from aqueous media. J. Environ. Manag. 2018, 213, 109–118. [Google Scholar] [CrossRef]
- Dong, Q.; Li, H.; Niu, M.; Luo, C.; Zhang, J.; Qi, B.; Li, X.; Zhong, W. Microwave pyrolysis of moso bamboo for syngas production and bio-oil upgrading over bamboo-based biochar catalyst. Bioresour. Technol. 2018, 266, 284–290. [Google Scholar] [CrossRef]
- Lu, S.; Zong, Y. Pore structure and environmental serves of biochars derived from different feedstocks and pyrolysis conditions. Environ. Sci. Pollut. Res. Int. 2018, 25, 30401–30409. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Luo, L.; Cheng, G.; Wei, Y.; Mei, R.; Xun, B.; Xu, X.; Hu, B.; Chen, Y. The sorption of pentachlorophenol by aged sediment supplemented with black carbon produced from rice straw and fly ash. Bioresour. Technol. 2012, 112, 61–66. [Google Scholar] [CrossRef]
- Zhu, X.M.; Chen, B.L.; Zhu, L.Z.; Xing, B.S. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Longterm effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61196–61205. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Sun, F.F.; Lu, S.G. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Shinogi, Y.; Kanri, Y. Pyrolysis of plant, animal and human waste: Physical and chemical characterization of the pyrolytic products. Bioresour. Technol. 2003, 90, 241–247. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.M.; Dallmeyer, I.; Garcia Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, E.L.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Hyvaluoma, J.; Kulju, S.; Hannula, M.; Wikberg, H.; Kalli, A.; Rasa, K. Quantitative characterization of pore structure of several biochars with 3D imaging, Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanroman, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxidebiochar nano-composites derived from pulp and paper sludge. Appl. Water Sci. 2017, 7, 2175–2186. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.Q.; Zhang, Q.P.; Liu, Q.C.; Li, Y.D.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar]
- Li, H.; Mahyoub, S.A.A.; Liao, W.; Xia, S.; Zhao, H.; Guo, M.; Ma, P. Effect of pyrolysis temperature on characteristics and aromatic contaminants adsorption behavior of magnetic biochar derived from pyrolysis oil distillation residue. Bioresour. Technol. 2017, 223, 20–26. [Google Scholar] [CrossRef]
- Zhao, H.X.; Lang, Y.H. Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar. J. Taiwan Inst. Chem. Eng. 2018, 88, 152–160. [Google Scholar] [CrossRef]
- Chen, T.W.; Luo, L.; Deng, S.H.; Shi, G.Z.; Zhang, S.R.; Zhang, Y.Z.; Deng, O.P.; Wang, L.L.; Zhang, J.; Wei, L.Y. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Khorram, M.S.; Lin, D.; Zhang, Q.; Zheng, Y.; Fang, H.; Yu, Y.L. Effects of aging process on adsorption-desorption and bioavailability of fomesafen in an agricultural soil amended with rice hull biochar. J. Environ. Sci. 2017, 56, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qi, S.; Zheng, F.; Huang, L.; Pan, J.; Jiang, Y.; Hou, W.; Xiao, L. Organics removal, nitrogen removal and N2O emission in subsurface wastewater infiltration systems amended with/without biochar and sludge. Bioresour. Technol. 2018, 249, 57–61. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhoua, W.; Lin, D. Sorption characteristics of Nitrosodimethylamine onto biochar from aqueous solution. Bioresour. Technol. 2015, 179, 359–366. [Google Scholar] [CrossRef]
- Cornelissen, G.; Gustafsson, O. Sorption of phenanthrene to environmental black carbon in sediment with and without organic matter and native sorbates. Environ. Sci. Technol. 2004, 38, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, A.G. Sorption of PAHs in the Soil Environment with Emphasis on the Role of Soil Organic Matter: A Review. World Appl. Sci. J. 2010, 11, 759–765. [Google Scholar]
- Gregory, S.T.; Shea, D.; Guthrie-Nichols, E. Impact of vegetation on sedimentary organic matter composition and polycyclic aromatic hydrocarbon attenuation. Environ. Sci. Technol. 2005, 39, 5285–5292. [Google Scholar] [CrossRef]
- Cao, J.; Guo, H.; Zhu, H.M.; Jiang, L.; Yang, H. Effects of SOM, surfactant and pH on the sorption-desorption and mobility of prometryne in soils. Chemosphere 2008, 70, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Abelmann, K.; Kleineidam, S.; Knicker, H.; Grathwohl, P.; Kogel-Knabner, I. Sorption of HOC in soils with carbonaceous contamination: Influence of organic-matter composition. J. Plant Nutr. Soil Sci. 2005, 168, 293–306. [Google Scholar] [CrossRef]
- Ni, N.; Wang, F.; Song, Y.; Bian, Y.; Shi, R.; Yang, X.; Gu, C.; Jiang, X. Mechanisms of biochar reducing the bioaccumulation of PAHs in rice from soil: Degradation stimulation vs immobilization. Chemosphere 2018, 196, 288–296. [Google Scholar] [CrossRef]
- Yargicoglu, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef]
- Anderson, N.; Jones, J.; Page-Dumroese, D.; McCollum, D.; Baker, S.; Loeffler, D.; Chung, W. A comparison of producer gas, biochar, and activated carbon from two distributed scale thermochemical conversion systems used to process forest biomass. Energies 2013, 6, 164–183. [Google Scholar] [CrossRef]
- Ogbonnaya, U.; Semple, K.T. Impact of biochar on organic contaminants in soil: A tool for mitigating risk? Agronomy 2013, 3, 349–375. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Hale, S.E.; Lehmann, J.; Cornelissen, G. Activated carbon and biochar amendments decrease pore-water concentrations of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge. Bioresour. Technol. 2012, 111, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Sato, T.; Xing, B.S. Competitive sorption of pyrene on wood chars. Environ. Sci. Technol. 2006, 40, 3267–3272. [Google Scholar] [CrossRef]
- Hale, S.E.; Hanley, K.; Lehmann, J.; Zimmerman, A.; Cornelissen, G. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ. Sci. Technol. 2011, 45, 10445–10453. [Google Scholar] [CrossRef]
- Zheng, W.; Guo, M.; Chow, T.; Bennett, D.N.; Rajagopalan, N. Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater. 2010, 181, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, R.; Li, C.; Guo, W.; Han, X.; He, F.; Ma, Y.; Xing, B. Selective removal of polycyclic aromatic hydrocarbons (PAHs) from soil washing effluents using biochars produced at different pyrolytic temperatures. Bioresour. Technol. 2014, 163, 193–198. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition on nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Bornemann, L.C.; Kookana, R.S.; Welp, G. Differential sorption behaviour of aromatic hydrocarbons on charcoals prepared at different temperatures from grass and wood. Chemosphere 2007, 67, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Yuan, M.X. Enhanced sorption of polycyclic aromatic hydrocarbons by soil amended with biochar. J. Soils Sediments 2011, 11, 62–71. [Google Scholar] [CrossRef]
- Werner, D.; Karapanagioti, H.K. Comment on “modeling maximum adsorption capacities of soot and soot-like materials for PAHs and PCBs”. Environ. Sci. Technol. 2005, 39, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Awoyemi, A. Understanding the Adsorption of PAHs from Aqueous Phase onto Activated Carbon. Master’s Thesis, University of Toronto, Toronto, Canada, 2011. [Google Scholar]
- Kong, H.; He, J.; Gao, Y.; Han, J.; Zhu, X. Removal of polycyclic aromatic hydrocarbons from aqueous solution on soybean stalk-based carbon. J. Environ. Qual. 2011, 40, 1737–1744. [Google Scholar] [CrossRef]
- Gupta, H. Removal of phenanthrene from water using activated carbon developed from orange rind. Int. J. Sci. Res. Environ. Sci. 2015, 3, 248–255. [Google Scholar] [CrossRef]
- Valderrama, C.; Gamisans, X.; de las Heras, X.; Farran, A.; Cortina, J.L. Sorption kinetics of polycyclic aromatic hydrocarbons removal using granular activated carbon: Intraparticle diffusion coefficients. J. Hazard. Mater. 2008, 157, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Amstaetter, K.; Eek, E.; Cornelissen, G. Sorption of PAHs and PCBs to activated carbon: Coal versus biomass-based quality. Chemosphere 2012, 87, 573–578. [Google Scholar]
- Brandli, R.C.; Hartnik, T.; Henriksen, T.; Cornelissen, G. Sorption of native polyaromatic hydrocarbons (PAH) to black carbon and amended activated carbon in soi. Chemosphere 2008, 73, 1805–1810. [Google Scholar] [CrossRef]
- Yakout, S.M.; Daifullah, A.A.M.; El-Reefy, S.A. Adsorption of naphthalene, phenanthrene and pyrene from aqueous solution using low-cost activated carbon derived from agricultural wastes. Adsorpt. Sci. Technol. 2013, 31, 293–302. [Google Scholar] [CrossRef]
- Bansal, R.C.; Donnet, J.B.; Stoeckli, F.; Dekker, M. A review of: “Active Carbon.” R.C. Bansal, J.B. Donnet and F. Stoeckli; Marcel Dekker, New York, 1988. pp. 482, $135.00. J. Dispers. Sci. Technol. 1990, 11, 323. [Google Scholar]
- Burchell, T.D. Carbon news goes online. Carbon 1999, 37, 1672–1672. [Google Scholar]
- Kalderis, D.; Kayan, B.; Akay, S.; Kulaksız, E.; Gözmen, B. Adsorption of 2,4-dichlorophenol on paper sludge/wheat husk biochar: Process optimization and comparison with biochars prepared from wood chips, sewage sludge and hog fuel/demolition waste. J. Environ. Chem. Eng. I. 2017, 5, 2222–2231. [Google Scholar] [CrossRef]
- Arampatzidou, A.C.; Deliyanni, E.A. Comparison of activation media and pyrolysis temperature for activated carbons development by pyrolysis of potato peels for effective adsorption of endocrine disruptor bisphenol. J. Colloid. Interface Sci. 2016, 466, 101–112. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Zhang, H.; Ghosh, S.; Pan, B. Fast and slow adsorption of carbamazepine on biochar as affected by carbon structure and mineral composition. Sci. Total Environ. 2017, 579, 598–605. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response ofmicrobial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jung, C.; Han, J.; Her, N.; Park, C.M.; Jang, M.; Son, A.; Yoon, Y. Sorptive removal of selected emerging contaminants using biochar in aqueous solution. J. Ind. Eng. Chem. 2016, 36, 364–371. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Gao, F.; Li, M.; Luo, X. Effects of biochar-derived sewage sludge on heavy metal adsorption and immobilization in soils. Int. J. Environ. Res. Public Health 2017, 14, 681–690. [Google Scholar] [CrossRef]
- Rao, M.A.; Simeone, G.D.R.; Scelza, R.; Conte, P. Biochar based remediation of water and soil contaminated by phenanthrene and pentachlorophenol. Chemosphere 2017, 186, 193–201. [Google Scholar] [CrossRef]
- Fu, H.; Wei, C.; Qu, X.; Li, H.; Zhu, D. Strong binding of apolar hydrophobic organic contaminants by dissolved black carbon released from biochar: A mechanism of pseudomicelle partition and environmental implications. Environ. Pollut. 2018, 232, 402–410. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Y.; Gao, B.; Chen, R.; Wu, F. Removal of sulfamethoxazole (SMX) and sulfapyridine (SPY) from aqueous solutions by biochars derived from anaerobically digested bagasse. Environ. Sci. Pollut. Res. 2017, 25, 25659–25667. [Google Scholar] [CrossRef]
- Vyavahare, G.D.; Gurav, R.G.; Jadhav, P.P.; Patil, R.R.; Aware, C.B.; Jadhav, J.P. Response surface methodology optimization for sorption of malachite green dye on sugarcane bagasse biochar and evaluating the residual dye for phyto and cytogenotoxicity. Chemosphere 2018, 94, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, D.A. Aerobic and anaerobic biodegradation of PCB: A review. Crit. Rev. Biotechnol. 1990, 10, 241–251. [Google Scholar] [CrossRef]
- Cajthaml, T.; Pacakova, V.; Sasek, V. Microbial degradation of polycyclic aromatic hydrocarbons. Chem. Listy. 2001, 95, 404. [Google Scholar]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mat. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Thakur, I.S. Colour removal of anaerobically treated pulp and paper mill effluent by microorganisms in two steps bioreactor. Biores. Technol. 2006, 97, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Parte, S.G.; Mohekar, A.D.; Kharat, A.S. Microbial degradation of pesticide: A review. Afr. J. Microbiol.Res. 2017, 11, 992–1012. [Google Scholar]
- Ye, B.; Siddiqi, M.A.; Maccubbin, A.E.; Kumar, S.; Sikka, H.C. Degradation of polynuclear aromatic hydrocarbons by Sphingomonas paucimobilis. Environ. Sci. Technol. 1996, 30, 136–142. [Google Scholar] [CrossRef]
- Romero, M.C.; Cazau, M.C.; Giorgieri, S.; Arambarri, A.M. Phenanthrene degradation by microorganisms isolated from a contaminated stream. Environ. Pollut. 1998, 101, 355–359. [Google Scholar] [CrossRef]
- Rehmann, K.; Noll, H.P.; Steiberg, C.E.W.; Kettrup, A.A. Pyrene degradation by Mycobacterium sp. Strain KR2. Chemosphere 1998, 36, 2977–2992. [Google Scholar] [CrossRef]
- Aitken, M.D.; Stringfellow, W.T.; Nagel, R.D.; Kazunga, C.; Chen, S.H. Characteristics of phenanthrene-degrading bacteria isolated from soils contaminated with polycyclic aromatic hydrocarbons. Can. J. Microbiol. 1998, 44, 743–752. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Shiung, L.C.; Chang, B.V. Biodegradation of polycyclic aromatichydrocarbons by inoculated microorganisms in soil. Bull. Environ. Conatm. Toxicol. 2002, 69, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Dean-Ross, D.; Moody, J.; Cerniglia, C.E. Utilisation of mixtures of Polycyclic aromatic hydrocarbons by bacteria isolated from contaminated sediment. FEMS Microbiol. Ecol. 2002, 41, 1–7. [Google Scholar] [CrossRef]
- Cutright, T.J. Polycyclic aromatic hydrocarbon biodegradation and kinetics using Cunninghamella echinulata var. elegans. Int. Biodet. Biodeg. 1995, 35, 397–408. [Google Scholar] [CrossRef]
- Hofrichter, M.; Schneibner, K.; Schneegab, I.; Fritzche, W. Enzymatic combustion of aromatic and aliphatic compounds by manganese peroxidase from Nematoloma frowardii. Appl. Environ. Microbiol. 1998, 64, 399–404. [Google Scholar] [CrossRef]
- Vyas, B.R.M.; Bakowski, S.; Sasek, V.; Matucha, M. Degradation of anthracene by selected white rot fungi. FEMS Microbiol. Ecol. 1994, 14, 65–70. [Google Scholar] [CrossRef]
- Clemente, A.R.; Anazawa, T.A.; Durrant, L.R. Biodegradation of polycyclic aromatic hydrocarbons by soil fungi. Braz. J. Microbiol. 2001, 32, 255–261. [Google Scholar] [CrossRef]
- Silva, I.S.; Grossman, M.; Durrant, L.R. Degradation of polycyclic aromatic hydrocarbons (2–7 rings) under microaerobic and very-low-oxygen conditions by soil fungi. Int. Biodet. Biodeg. 2009, 63, 224–229. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Gibson, D.T.; Baalen, C.V. Algal oxidation of aromatic hydrocarbons: Formation of 1-Naphthol from naphthalene by Agmenellum quadruplicatum strain PR-6. Biochem. Biophys. Res. Commun. 1979, 88, 50–58. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Baalen, C.V.; Gibson, D.T. Metabolism of naphthalene by cyanobacterium Oscillatoria sp. Strain JCM. J. Gen. Microbiol. 1980, 116, 485–494. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Gibson, D.T.; Baalen, C.V. Oxidation of naphthalene by cyanobacteria and microalgae. J. Gen. Microbiol. 1980, 116, 495–500. [Google Scholar] [CrossRef][Green Version]
- Narro, M.L.; Cerniglia, C.E.; Baalen, C.V.; Gibson, D.T. Evidence of NIH shift in naphthalene oxidation by the marine cyanobacterium, Oscillatoria species strain JCM. Appl. Environ. Microbiol. 1992, 58, 1360–1363. [Google Scholar] [CrossRef]
- Narro, M.L.; Cerniglia, C.E.; Baalen, C.V.; Gibson, D.T. Metabolism of phenanthrene by the marine cyanobacterium Agmenellum quadruplicatum strain PR-6. Appl. Environ. Microbiol. 1992, 58, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Warshawsky, D.; Cody, T.; Radike, M.; Reilman, R.; Schumann, B.; LaDow, K.; Schneider, J. Biotransformation of Benzo[a]pyrene and other polycyclic aromatic hydrocarbons and heterocyclic analogs by several green algae and other algal species under gold and white light. Chemico-Biol. Interact. 1995, 97, 131–148. [Google Scholar] [CrossRef]
- Warshawsky, D.; Radike, M.; Jayasimhulu, K.; Cody, T. Metabolism of Benzo[a]pyrene by a dioxygenase systemof freshwater green alga Selenastrum capricornutum. Biochem. Biophys. Res. Commun. 1988, 152, 540–544. [Google Scholar] [CrossRef]
- Borde, X.; Guieysse, B.; Delgado, O.; Munoz, R.; Hatti-Kaul, R.; Nugier-Chauvin, C.; Patin, H.; Mattiasson, B. Synergistic relationships in algal–bacterial microcosms for the treatment of aromatic pollutants. Bioresour. Technol. 2003, 86, 293–300. [Google Scholar] [CrossRef]
- Lei, A.P.; Hu, Z.L.; Wong, Y.S.; Tam, N.F.Y. Removal of fluoranthene and pyrene by different microalgal species. Bioresour. Technol. 2007, 98, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Jeyaratnam, J. Health problems of pesticide usage in the third world. Br. J. Ind. Med. 1985, 42, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Igbedioh, S.O. Effects of agricultural pesticides on humans, animals and higher plants in developing countries. Archiv. Environ. Health 1991, 46, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Forget, G. Balancing the need for pesticides with the risk to human health. In Impact of Pesticide Use on Health in Developing Countries; Forget, G., Goodman, G., de Villiers, A.T., Eds.; International Development Research Centre: Ottawa, ON, Canada, 1993; pp. 2–16. [Google Scholar]
- Galli, C. Degradaciónpormediosbacterianos de Compuestosquímicostóxicos; ComisiónTécnicaAsesora en Ambiente y desarrollosostenible: Buenos Aires, Argentina, 2002. [Google Scholar]
- Qiu, X.; Zhong, Q.; Li, M.; Bai, W.; Li, B. Biodegradation of pnitrophenol by methyl parathion-degrading Ochrobactrum sp. B2. Int. Biodeterior. Biodegradation 2007, 59, 297–301. [Google Scholar] [CrossRef]
- Aislabie, J.; Lloyd-Jones, G. A review of bacterial-degradation of pesticides. Aust. J. Soil Res. 1995, 33, 925–942. [Google Scholar] [CrossRef]
- Iranzo, M.; Sain-Pardo, I.; Boluda, R.; Sanchez, J.; Mormeneo, S. The use of microorganisms in environmental remediation. Ann. Microbiol. 2001, 51, 135–143. [Google Scholar]
- Vischetti, C.; Casucci, C.; Perucci, P. Relationship between changes of soil microbial biomass content and imazamox and benfluralin degradation. Biol. Fertil. Soils 2002, 35, 13–17. [Google Scholar] [CrossRef]
- Chaudhry, G.R.; Chapalamadugu, S. Biodegradation of halogenated organic compounds microbiological reviews. Microbiol. Mol. Biol. 1991, 55, 59–79. [Google Scholar]
- Diaz, E. Bacterial degradation of aromatic pollutants: A paradigm of metabolic versatility. Int. Microbiol. 2004, 7, 173–180. [Google Scholar]
- Dua, M.; Singh, A.; Sethunathan, N.; Johri, A.K. Biotechnology and Bioremediation: Successes and Limitations. Appl. Microbiol. Biotechnol. 2002, 59, 143–152. [Google Scholar]
- Menone, M.L.; Bortolus, A.; de Moreno, J.E.A.; Moreno, V.J.; Lanfranchi, A.L.; Metcalfe, T.L.; Metcalfe, C.D. Organochlorine pesticides and PCBs in a Southern Atlantic Coastal Lagoon Watershed, Argentina. Archiv. Environ. Contam. Toxicol. 2001, 40, 355–362. [Google Scholar]
- Patnaik, P. A Comprehensive Guide to the Hazardous Properties of Chemical Substances; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Langlois, B.E.; Collins, J.A.; Sides, K.G. Some factors affecting degradation of organochlorine pesticide by bacteria. J. Dairy Sci. 1970, 53, 1671–1675. [Google Scholar] [CrossRef]
- Matsumura, F.; Boush, G.M.; Tai, A. Breakdown of dieldrin in the soil by a microorganism. Nature 1968, 219, 965–967. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, C.; Qiao, C.L. Biodegradation of nitrophenol and 4-chlorophenol by Stenotrophomonas sp. FEMS Microbiol. Lett. 2007, 277, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, R.; Vasudevan, N. Effect of tween 80 added to the soil on the degradation of endosulfan by Pseudomonas aeruginosa. Int. J. Environ. Sci. Tech. 2007, 4, 203–210. [Google Scholar] [CrossRef]
- Kumar, M.; Lakshmi, C.V.; Khanna, S. Biodegradation and bioremediation of endosulfan contaminated soil. Biores. Technol. 2008, 99, 3116–3122. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.M.; Jiménez, B.; Font, G.; Moltó, J.C. Analysis of Carbamate Pesticides and Their Metabolites in Water by Solid Phase Extraction and Liquid Chromatography: A Review. Crit. Rev. Anal. Chem. 2001, 31, 19–52. [Google Scholar] [CrossRef]
- Tomasek, P.H.; Karns, J.S. Cloning of a carbofuran hydrolase gene from Achromobacter sp. strain WM111 and its expression in Gramnegative bacteria. J. Bacteriol. 1989, 171, 4038–4044. [Google Scholar] [CrossRef]
- Desaint, S.; Hartmann, A.; Parekh, N.R.; Fournier, J.C. Genetic diversity of carbofuran-degrading soil bacteria. FEMS Microbiol. Ecol. 2000, 34, 173–180. [Google Scholar] [CrossRef]
- Parekh, N.R.; Hartmann, A.; Charnay, M.P.; Fournier, J.C. Diversity of carbofuran-degrading soil bacteria and detection of plasmid-encoded sequences homologous to the mcd gene. FEMS Microbiol. Ecol. 1995, 17, 149–160. [Google Scholar] [CrossRef]
- De Schrijver, A.; De Mot, R. Degradation of pesticides by Actinomycetes. Crit. Rev. Microbiol. 1999, 5, 85–119. [Google Scholar] [CrossRef]
- Xiao, P.; Mori, T.; Kamei, I.; Kondo, R. Metabolism of Organochlorine Pesticide Heptachlor and its Metabolite Heptachlor Epoxide by White-rot Fungi, Belonging to Genus Phlebia. FEMS Microbiol. Lett. 2010, 314, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Katayama, A.; Matsumura, F. Degradation of organochlorine pesticides, particularly endosulfan, by Trichoderma harzianum. Environ. Toxicol. Chem. 2009, 12, 1059–1065. [Google Scholar] [CrossRef]
- Bhalerao, T.S.; Puranik, P.R. Biodegradation of organochlorine pesticide, endosulfan, by a fungal soil isolate, Aspergillus niger. Int. Biodeterior. Biodegradation 2007, 59, 315–321. [Google Scholar] [CrossRef]
- Zhongli, C.; Shunpeng, L.; Guoping, F. Isolation of methyl parathion-degrading strain m6 and cloning of the methyl parathion hydrolase gene. Appl. Environ. Microbiol. 2001, 67, 4922–4925. [Google Scholar] [CrossRef]
- Pieper, D.H.; Reineke, W. Engineering bacteria for bioremediation. Curr. Opin. Biotechnol. 2000, 11, 262–70. [Google Scholar] [CrossRef]
- Qing, Z.; Yang, L.; Huan, L.Y. Purification and characterization of a novel carbaryl hydrolase from Aspergillus niger PY168. FEMS Microbiol. Lett. 2006, 228, 39–44. [Google Scholar] [CrossRef]
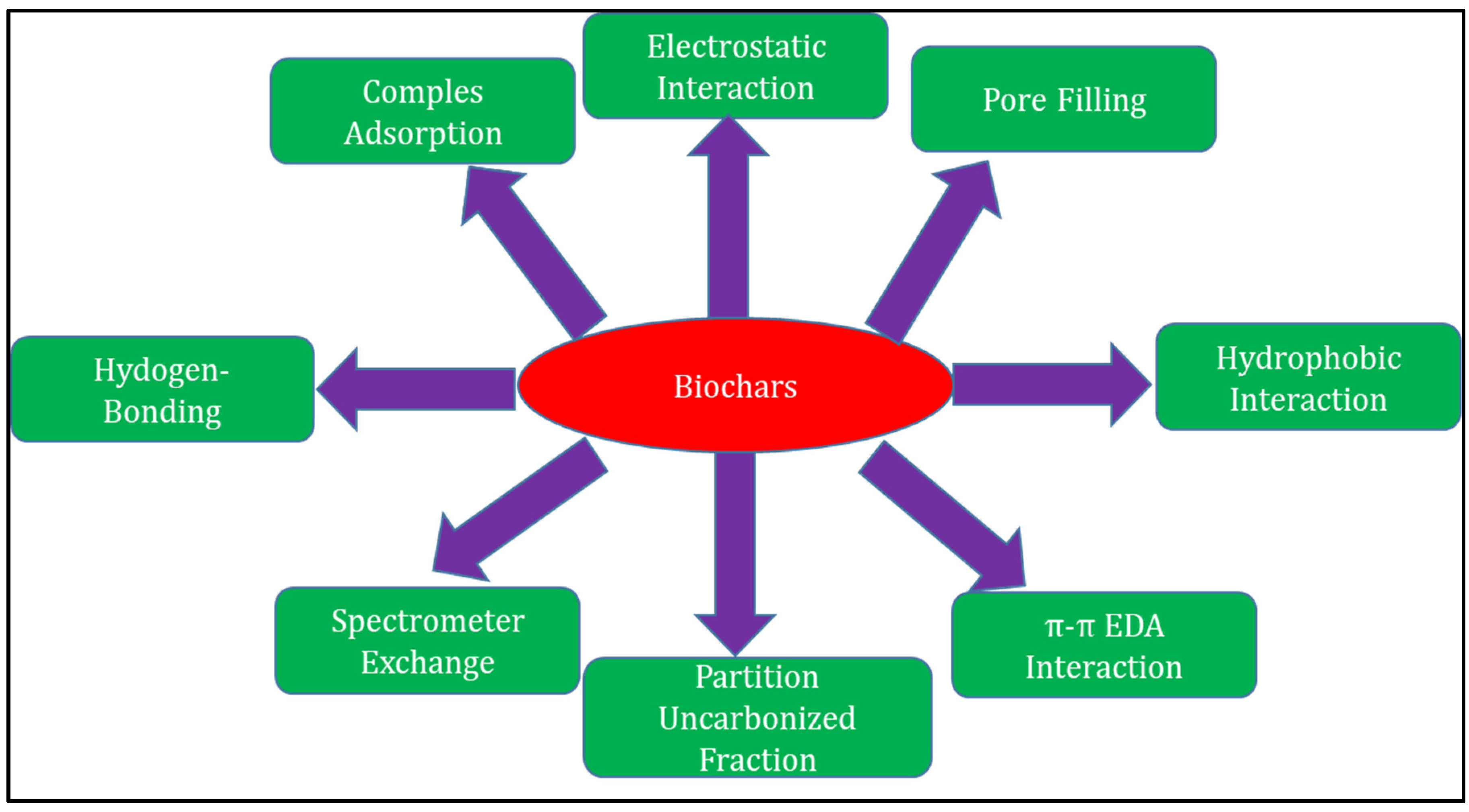

| Hydrophobic Organic Contaminants | Types of Adsorbents | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Pore Size (nm) | Surface Groups | Removal Efficiency and/or Adsorption Efficiency | Adsorption Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| Dimethoate | Sugarcane bagasse Biochar | - | - | - | - | - | Physical sorption and intraparticle diffusion | Sun et al. [122] |
| Thiacloprid | Maize straw Biochar | - | - | - | - | - | Hydrophobic interaction, pore-filling, and π–π interaction | Zhang et al. [87] |
| Imidacloprid | Swine manure Biochar | 0.68–8.45 | - | Rougher and more porous | (−COOH and −COH) | - | Pore-filling | Jin et al. [123] |
| N-nitrosodimethyl-amine | Bamboo biochar | 33.50–39.25 | - | - | O-containing moieties (−C−O, C=O, −COOH) | 61.68% | Hydrophobic interaction | Chen et al. [124] |
| 2,4-Dichlorophenol | Sewage sludge/wood Chip Biochar | 25.6–360 | - | - | Hyrophobic surface | 99.95% | Electrostatic interactions and π-electron donor-acceptor interaction | Kalderis et al. [152] |
| Bisphenol A | Potato peels Biochar | 0.907–1041.43 | 0.004–1.216 | C−OH, C=O moieties in aromatic carboxyl groups, C=C of the aromatic ring structures and conjugated systems such as diketone, ketoester, quinone | 454.62 mg g−1 | π–π interaction | Arampatzidou and Deliyanni [153] | |
| Carbamazepine | Eucalyptus wood and bamboo Biochar | 64.728–85.07 | 0.057–0.069 | - | - | 104.85 to 861.70 mg L−1 | π–π interaction | Chen et al. [154,155] |
| Benzophene (BZP) Benzotriazole (BZT) Bisphenol A (BPA) 17 β-estradiol (E2) | Pine chips | 1360 | 0.307–0.643 | - | Alkyl (0–45 ppm), methoxyl (45–63 ppm), carbohydrate (63–108 ppm), and carboxyl carbons (165–187 ppm) | 6.79 mg L−1 9.22 mg L−1 28.4 mg L−1 30.2 mg L−1 | Hydrophobic interaction | Kim et al. [156] |
| Tetraethyltin | Sawdust | 17.35 | 0.038 | 8.85 | - | 91.6% | π–π interaction Complexation | Zhou et al. [157,158] |
| Tetracycline | Saw dust 600 | - | - | - | - | 10 to 25 mg L−1 | Hydrogen bonding and π–π EDA | Zhou et al. [157,158] |
| Phenanthrene Pentachlorophenol | Poplar and coniferwood chips | 76.88 114.67 | 0.046 0.067 | 24.08 23.23 | C−H (3050 cm−1), C=C and C=O stretching (1707 cm−1), aromatic C=C and C−H alkenes (1591−1455 cm−1), C=O streching (1080 cm−1) | 11.9 mg kg−1 132 mg kg−1 | π–π interactions | Rao et al. [159] |
| PAHs | Bacterial Species | References |
|---|---|---|
| Benzo(a)pyrene | Sphingomonas paucimobilis, EPA 505 strain | Ye et al. [168] |
| Phenanthrene | Pseudomonas aeruginosa | Romero et al. [169] |
| Pyrene | Mycobacterium spp., KR2 strain | Rehmann et al. [170] |
| BaP. | Pseudomonas, Agrobacterium, Bacillus, Burkholderia, and Sphingomonas species | Aitken et al. [171] |
| Acenaphthene, fluorene, phenanthrene, anthracene, and pyrene | Pseudomonas fluoresens and Haemophilus spp. | Yuan et al. [172] |
| Pyrene Anthracene | Mycobacterium flavescens and Rhodococcus spp. | Dean-Ross et al. [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewangan, S.; Bhatia, A.K.; Singh, A.K.; Carabineiro, S.A.C. Removal of Hydrophobic Contaminants from the Soil by Adsorption onto Carbon Materials and Microbial Degradation. C 2021, 7, 83. https://doi.org/10.3390/c7040083
Dewangan S, Bhatia AK, Singh AK, Carabineiro SAC. Removal of Hydrophobic Contaminants from the Soil by Adsorption onto Carbon Materials and Microbial Degradation. C. 2021; 7(4):83. https://doi.org/10.3390/c7040083
Chicago/Turabian StyleDewangan, Shippi, Amarpreet K. Bhatia, Ajaya Kumar Singh, and Sónia A. C. Carabineiro. 2021. "Removal of Hydrophobic Contaminants from the Soil by Adsorption onto Carbon Materials and Microbial Degradation" C 7, no. 4: 83. https://doi.org/10.3390/c7040083
APA StyleDewangan, S., Bhatia, A. K., Singh, A. K., & Carabineiro, S. A. C. (2021). Removal of Hydrophobic Contaminants from the Soil by Adsorption onto Carbon Materials and Microbial Degradation. C, 7(4), 83. https://doi.org/10.3390/c7040083