A Review of the Use of Immobilized Ionic Liquids in the Electrochemical Conversion of CO2
Abstract
1. Introduction
2. Immobilizing Ionic Liquids to Create Functionalized Anion Conducting Membranes
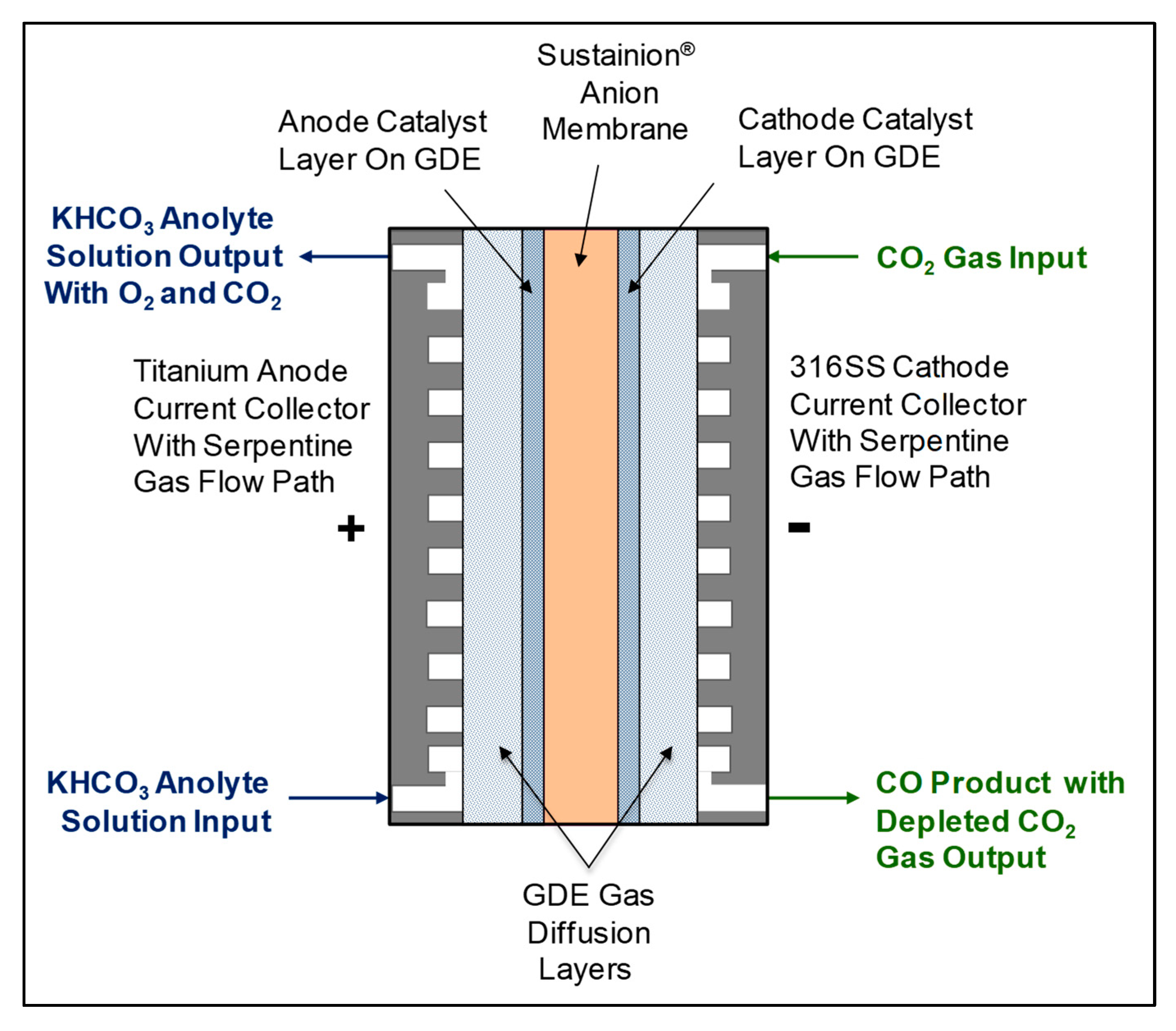
3. Electrochemical Conversion of CO2 to CO
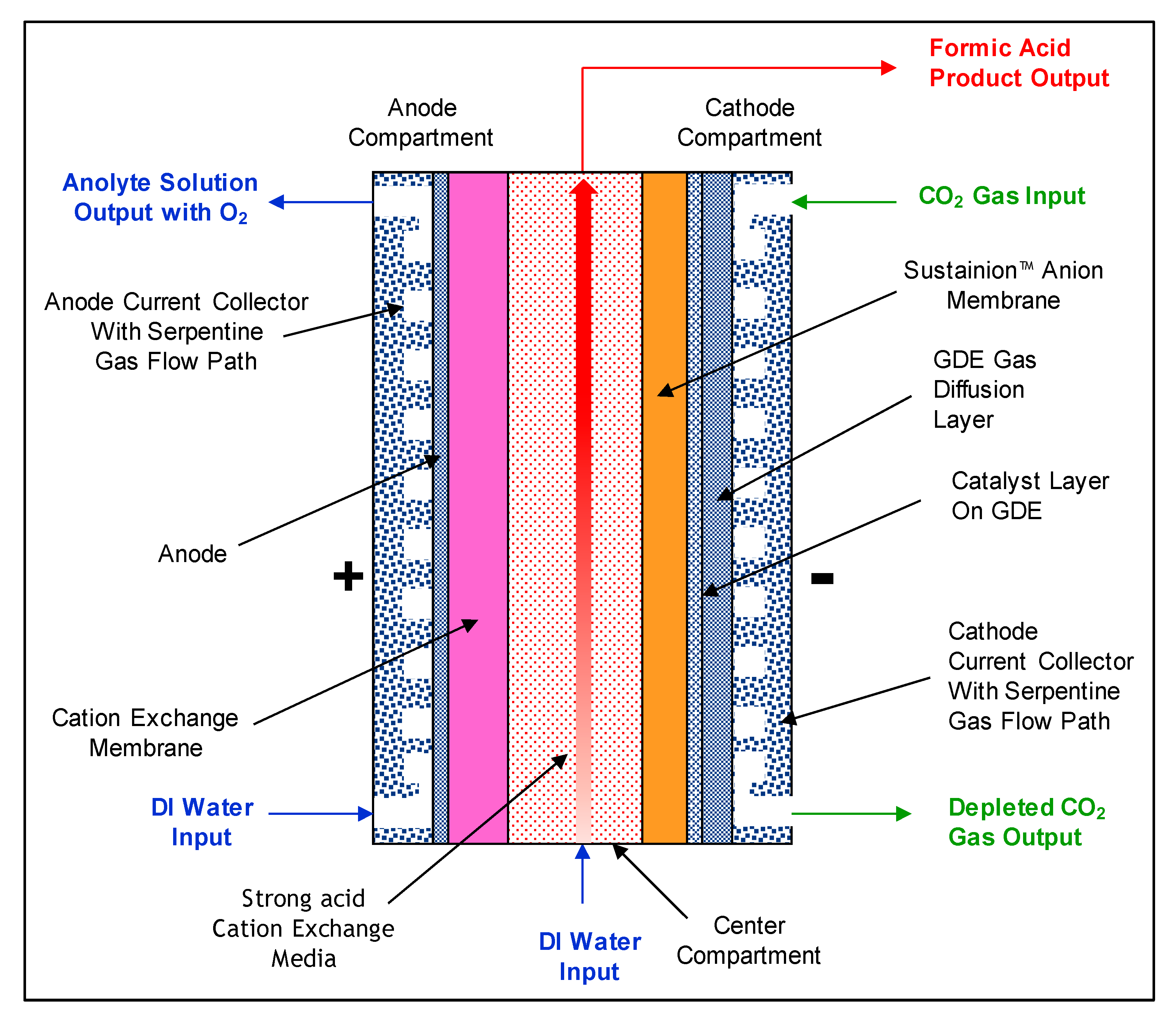
4. Electrochemical Conversion of CO2 to Formic Acid
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic Liquid Mediated Selective Conversion of CO2 to CO At Low Overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.A.; Haan, J.L.; Mukherjee, P.; Braunschweig, B.; Zhu, W.; Salehi-Khojin, A.; Dlott, D.D.; Masel, R.I. In Situ Spectroscopic Examination of a Low Overpotential Pathway for Carbon Dioxide Conversion to Carbon Monoxide. J. Phys. Chem. C 2012, 116, 15307–15312. [Google Scholar] [CrossRef]
- Rosen, B.A.; Zhu, W.; Kaul, G.; Salehi-Khojin, A.; Masel, R.I. Water enhancement of CO2 conversion on silver in 1-ethyl-3-methylimidazolium tetrafluoroborate. J. Electrochem. Soc. 2013, 160, H138–H141. [Google Scholar] [CrossRef]
- Masel, R.I.; Rosen, B.A.; Zhu, W. Devices and Processes for Carbon Dioxide Conversion into Useful Fuels and Chemicals. U.S. Patent 9,181,625, 3 April 2014. [Google Scholar]
- Masel, R.I.; Salahi-Khogin, A.; Rosen, B. Catalysts for Electrochemical Conversion of Carbon Dioxide. U.S. Patent 9,012,345, 21 April 2015. [Google Scholar]
- Zhao, S.-F.; Horne, M.; Bond, A.M.; Zhang, J. Is the Imidazolium Cation a Unique Promoter for Electrocatalytic Reduction of Carbon Dioxide? J. Phys. Chem. C 2016, 120, 23989–24001,. [Google Scholar] [CrossRef]
- Hugar, K.M.; Kostalik, H.A.; Coates, G.W. Imidazolium Cations with Exceptional Alkaline Stability: A Systematic Study of Structure–Stability Relationships. J. Am. Chem. Soc. 2015, 137, 8730–8737. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Elabd, Y.A. Relative Chemical Stability of Imidazolium-Based Alkaline Anion Exchange Polymerized Ionic Liquids. Macromolecules 2011, 44, 8494–8503. [Google Scholar] [CrossRef]
- Si, Z.; Qiu, L.; Dong, H.; Gu, F.; Li, Y.; Yan, F. Effects of substituents and substitution positions on alkaline stability of imidazolium cations and their corresponding anion-exchange membranes. ACS Appl. Mater. Interfaces 2014, 6, 4346–4355. [Google Scholar] [CrossRef]
- Qiu, B.; Lin, B.; Qiu, L.; Yan, F. Alkaline imidazolium- and quaternary ammonium-functionalized anion exchange membranes for alkaline fuel cell applications. J. Mater. Chem. 2012, 22, 1040–1045. [Google Scholar] [CrossRef]
- Qiu, B.; Lin, B.; Si, Z.; Qiu, L.; Chu, F.; Zhao, J.; Yan, F. Bis-imidazolium-based anion-exchange membranes for alkaline fuel cells. J. Power Sources 2012, 217, 329–335. [Google Scholar] [CrossRef]
- Guo, M.; Fang, J.; Xu, H.; Li, W.; Lu, X.; Lan, C.; Li, K. Synthesis and characterization of novel anion exchange membranes based on imidazolium-type ionic liquid for alkaline fuel cells. J. Membr. Sci. 2010, 362, 97–104. [Google Scholar] [CrossRef]
- Marinkas, A.; Struzynska-Piron, I.; Lee, Y.; Lim, A.; Park, H.S.; Jang, J.H.; Kim, H.J.; Kim, J.H.; Maljusch, A.; Conradi, O.; et al. Anion-conductive membranes based on 2-mesityl-benzimidazolium functionalised poly(2,6-dimethyl-1,4-phenylene oxide) and their use in alkaline water electrolysis. Polymer 2018, 145, 242–251. [Google Scholar] [CrossRef]
- Tham, D.D. C2 and N3 substituted imidazolium functionalized poly(arylene ether ketone) anion exchange membrane for water electrolysis with improved chemical stability. J. Membr. Sci. 2019, 581, 139–149. [Google Scholar] [CrossRef]
- Masel, R.I.; Rosen, B. Catalyst Mixtures. U.S. Patent 8,956,990, 17 February 2015. [Google Scholar]
- Masel, R.I.; Chen, Q.; Liu, Z.; Kutz, R. Ion-Conducting Membranes. U.S. Patent 9,370,773, 21 June 2016. [Google Scholar]
- Masel, R.I.; Chen, Q.; Liu, Z.; Kutz, R. Electrochemical Device for Converting Carbon Dioxide to a Reaction Product. U.S. Patent 9,481,939, 1 November 2016. [Google Scholar]
- Masel, R.I.; Chen, Q.; Liu, Z.; Kutz, R. Ion-Conducting Membranes. U.S. Patent 9,580,824, 28 February 2017. [Google Scholar]
- Masel, R.I.; Chen, Q.; Liu, Z. Ion-Conducting Membranes. U.S. Patent 9,849,450, 26 December 2017. [Google Scholar]
- Kutz, R.B.; Chen, Q.; Yang, H.; Sajjad, S.D.; Liu, Z.; Masel, I.R. Sustainion Imidazolium-Functionalized Polymers for Carbon Dioxide Electrolysis. Energy Technol. 2017, 5, 929–936. [Google Scholar] [CrossRef]
- Endrődi, B.; Kecsenovity, E.; Samu, A.; Darvas, F.; Jones, R.V.; Török, V.; Danyi, A.; Janáky, C. Multilayer Electrolyzer Stack Converts Carbon Dioxide to Gas Products at High Pressure with High Efficiency. ACS Energy Lett. 2019, 4, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-Y.; Balamurugan, M.; Choutipalli, V.S.K.; Jeong, E.-S.; Subramanian, V.; Sim, U.; Nam, K.T. Achieving highly efficient CO2 to CO electroreduction exceeding 300 mA cm−2 with single-atom nickel electrocatalysts. J. Mater. Chem. A 2019, 7, 10651–10661. [Google Scholar] [CrossRef]
- Lee, J.; Lim, J.; Roh, C.-W.; Whang, H.S.; Lee, H. Electrochemical CO2 reduction using alkaline membrane electrode assembly on various metal electrodes. J. CO2 Util. 2019, 31, 244–250. [Google Scholar] [CrossRef]
- Ren, S.; Joulie, D.; Berlinguette, C.P.; Joulie, D.; Torbensen, K.; Wang, M.; Robert, M.; Salvatore, D. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365, 367–369. [Google Scholar] [CrossRef]
- Wang, M.; Torbensen, K.; Joulie, D.; Mendoza, D.; Robert, M.; Salvatore, D.; Berlinguette, C.P.; Ren, S.; Joulie, D.; Berlinguette, C.P.; et al. CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine. Nat. Commun. 2019, 10, 3602. [Google Scholar] [CrossRef]
- Gabardo, C.M.; O’Brien, C.P.; Edwards, J.P.; McCallum, C.; Xu, Y.; Dinh, C.-T.; Li, J.; Sargent, E.H.; Sinton, D. Continuous Carbon Dioxide Electroreduction to Concentrated Multi-carbon Products Using a Membrane Electrode Assembly. Joule 2019, 3, 2777–2791. [Google Scholar] [CrossRef]
- Koshikawa, H.; Murase, H.; Hayashi, T.; Nakajima, K.; Mashiko, H.; Shiraishi, S.; Tsuji, Y. Single Nanometer-Sized NiFe-Layered Double Hydroxides as Anode Catalyst in Anion Exchange Membrane Water Electrolysis Cell with Energy Conversion Efficiency of 74.7% at 1.0 A cm−2. ACS Catal. 2020, 10, 1886–1893. [Google Scholar] [CrossRef]
- Liu, Z.; Sajjad, S.D.; Gao, Y.; Yang, H.; Kaczur, J.J.; Masel, R.I. The effect of membrane on an alkaline water electrolyzer. Int. J. Hydrog. Energy 2017, 42, 29661–29665. [Google Scholar] [CrossRef]
- Pushkareva, I.V.; Pushkarev, A.S.; Grigoriev, S.A.; Modisha, P.; Bessarabov, D.G. Comparative study of anion exchange membranes for low-cost water electrolysis. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Nwabara, U.O.; Cofell, E.R.; Verma, S.; Negro, E.; Kenis, P.J.A. Durable Cathodes and Electrolyzers for the Efficient Aqueous Electrochemical Reduction of CO2. ChemSusChem 2020, 13, 855–875. [Google Scholar] [CrossRef] [PubMed]
- Masel, R.I.; Liu, Z.; Kutz, R.; Sajjad, S.D. Catalyst Layers and Electrolyzers. U.S. Patent 9,945,040, 17 April 2018. [Google Scholar]
- Kaczur, J.J.; Yang, H.; Liu, Z.; Sajjad, S.D.; Masel, R.I. Carbon dioxide and water electrolysis using new alkaline stable anion membranes. Front. Chem. 2018, 6, 263. [Google Scholar] [CrossRef]
- Haas, T.; Krause, R.; Weber, R.; Demler, M.; Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 2018, 1, 32–39. [Google Scholar] [CrossRef]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. Electrochemical conversion of CO2 to formic acid utilizing Sustainion™ membranes. J. CO2 Util. 2017, 20, 208–217. [Google Scholar] [CrossRef]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. CO2 conversion to formic acid in a three compartment cell with sustainion membranes. ECS Trans. 2017, 77, 1425–1431. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, P.; Jiang, Q.; Pan, Y.; Liang, W.; Stavitski, E.; Alshareef, H.N.; Wang, H. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 2019, 4, 776–785. [Google Scholar] [CrossRef]
- Lee, S.; Ju, H.; Machunda, R.; Uhm, S.; Lee, J.K.; Lee, H.J.; Lee, J. Sustainable production of formic acid by electrolytic reduction of gaseous carbon dioxide. J. Mater. Chem. A 2015, 3, 3029–3034. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, X.; Jung, J.; Cai, Y.; Hou, X.; Zhang, Q.; Qiao, J. Continuous electroreduction of carbon dioxide to formate on Tin nanoelectrode using alkaline membrane cell configuration in aqueous medium. Catal. Today 2018, 318, 32–38. [Google Scholar] [CrossRef]
- Ramdin, M.; Morrison, A.R.T.; de Groen, M.; van Haperen, R.; de Kler, R.; Irtem, E.; Laitinen, A.T.; van den Broeke, L.J.P.; Breugelmans, T.; Trusler, J.P.M.; et al. High-Pressure Electrochemical Reduction of CO2 to Formic Acid/Formate: Effect of pH on the Downstream Separation Process and Economics. Ind. Eng. Chem. Res. 2019, 58, 22718–22740. [Google Scholar] [CrossRef]




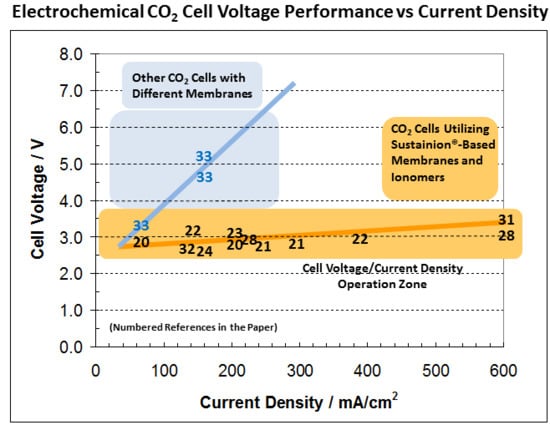
| References | Cell Configuration | Cell Area | Cell Voltage | Current Density | CO Selectivity | Run Length | ||
|---|---|---|---|---|---|---|---|---|
| Anode/Catalyst | Cathode/Catalysts | Membrane | cm² | V | mA/cm2 | % | h | |
| Kutz et al. [20] | Nano IrO2 on GDE | Nano Ag on GDE/PSMIM ionomer | Sustainion® PSMIM | 5 | 2.8–3.0 | 50 | 85–95% | 4500 |
| Nano IrO2 on GDE | Nano Ag on GDE/XA-9 ionomer | Sustainion® PSTMIM | 5 | 2.8–3.0 | 200 | 85–95% | 1000 | |
| Liu et al. [28] | Nano IrO2 on GDE | Nano Ag on GDE/XA-9 ionomer | Sustainion® X37-50 | 5 | 2.8–3.0 | 200 | 90–95% | 3800 |
| Nano IrO2 on GDE | Nano Ag on GDE/XA-9 ionomer | Sustainion® X37-50 | 5 | 2.8–3.0 | 600 | 93–96% | 4 | |
| Kaczur et al. [32] | Nano IrO2 on GDE | Nano Ag on GDE/XA-9 ionomer | Sustainion® X37-50 | 250 | 2.8–3.0 | 120 | 98% | 760 |
| Endrődi et al. [21] | IrO2 catalyst on Ti Frit | Nano Ag on GDE/XA-9 ionomer | Sustainion® X37T | 61 cm2 each cell, 3 cell stack | 2.75–3.0 | 200–300 | 70–85% | 8–100 |
| Jeong et al. [22] | Nano IrO2 on GDE | Ni-SA-NCs on GDE | Sustainion® X37-50 | 5 | 2.60 3.00 | 140 380 | 99% 99% | 9 9 |
| Lee et al. [23] | Catalyst on Ti Felt | Nano Pd, Ag, Zn on GDE/XA-9 ionomer | Sustainion® X37-50 | 5 | 3.0 | 200 | 96.7% | 10 |
| Wang et al. [25] | Pt/Ti alloy | Cobalt phthalocyanine catalysts CoPc1 and CoPc2 | Sustainion® X37-50 | 10 | - | 22–165 | 92–94% | 2 |
| Ren et al. [24] | Nickel Foam | Cobalt phthalocyanine | Sustainion® X37-50 | 4 | 2.5 | 150 | 95% | 3–5 |
| Masel et al. [31] | Nano RuO2 or IrO2 on GDE | Nano Ag on GDE /PSMIM ionomer | Sustainion® PSMIM | 5 | 3.0 | 600 | 95% | 8 |
| Haas et al. [33] | IrO2 on titanium | Ag-Based GDE w/o Carbon | ZrO2-based Diaphragm | 10 | 4.90 | 150 | 82–88% | - |
| IrO2 on titanium | Ag-Based GDE w/o Carbon | ZrO2-based Diaphragm | 10 | 7.0–7.5 | 300 | 60% | 1250 | |
| IrO2 on titanium | Ag-Based GDE w/o Carbon | Nafion | 10 | 3.20 (30 °C) | 50 | 45–60% | 1000 | |
| IrO2 on titanium | Ag-Based GDE w/o Carbon | Nafion | 10 | 4.80 (30 °C) | 150 | 60% | 250 | |
| References | Cell Configuration | Cell Area | Cell Voltage V | Current Density mA/cm2 | Formic Acid Wt% | FE % | Run Length h | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Anode/Catalyst | Cathode/Catalysts | Membrane | ||||||||
| Anode Side | Cathode Side | |||||||||
| Yang et al. [34] | Graphite/Nano IrO2 on GDE | Nano Sn on GDE with PSMIM ionomer | Nafion 212 | Sustainion® X37-50 | 5 | 3.3–3.4 | 140 | 15%–18% | 30% | 550 |
| Yang et al. [34,35] Kaczur et al. [32] | Titanium/Ti Fiber Paper with IrO2 | Nano Sn on GDE with PSMIM ionomer | Nafion 324 | Sustainion® X37-50 | 5 | 3.5 | 140 | 10% | 80% -> 94% | 142 |
| This Paper | Titanium/Ti Fiber Paper coated with IrO2 | Nano XX catalyst on GDE with PSMIM ionomer | Nafion 324 | Sustainion® X37-50 | 5 | 3.5 | 200 | 10% | 80% -> 65% | 500 |
| Xia et al. [36] | Graphite/IrO2 on Carbon | 2D-Bismuth | Nafion (1110) | Sustainion® X37-50 | 4 | 3.0 | 30 | 0.5% | 80% | 100 |
| Lee et al. [37] | Nano Pt on Carbon | Nano Sn on GDE with Nafion ionomer | Nafion 115 | - | 9 | Cathode Potential Constant: −0.7 V | 5–20 | Formic Acid Vapor | 12.5% -> 7.0% | 3–10 |
| Lei et al. [38] | Not specified | Nano Sn on GDE with Nafion ionomer | Toray A901 (anion) | - | 25 | 3 --> 5 | 5 --> 100 | Formate | 80% --> 15% | 2 |
| Not specified | Nano Sn on GDE with Nafion ionomer | Yichen (anion) | - | 25 | 3 --> 5 | 5 --> 100 | Formate | 80% --> 12% | 2 | |
| Not specified | Nano Sn on GDE with Nafion ionomer | FAD (anion) | - | 25 | 3 --> 5 | 5 --> 100 | Formate | 85% --> 12% | 2 | |
| Not specified | Nano Sn on GDE with Nafion ionomer | Nafion 117 Cation | - | 25 | 3 --> 5 | 5 --> 100 | Formate | 85% --> 16% | 2 | |
| Ramdin et al. [39] | Not specified | Sn electrode | Fumatech Bipolar FBM-PK | - | 80 | 4.0 | 60 @50 bar CO2 | Formate 12500 ppm | 80% | 0.33 |
| Not specified | Sn electrode | Fumatech FKB-PK (cation) | - | 80 | 3.5 | 40 @50 bar CO2 | Formate 9000 ppm | 80% | 0.33 | |
| Not specified | Sn electrode | Fumatech Bipolar FBM-PK | Fumatech Bipolar FBM-PK | 80 | 3.5 | 30 @50 bar CO2 | 1.0% | 90% | 0.33 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczur, J.J.; Yang, H.; Liu, Z.; Sajjad, S.D.; Masel, R.I. A Review of the Use of Immobilized Ionic Liquids in the Electrochemical Conversion of CO2. C 2020, 6, 33. https://doi.org/10.3390/c6020033
Kaczur JJ, Yang H, Liu Z, Sajjad SD, Masel RI. A Review of the Use of Immobilized Ionic Liquids in the Electrochemical Conversion of CO2. C. 2020; 6(2):33. https://doi.org/10.3390/c6020033
Chicago/Turabian StyleKaczur, Jerry J., Hongzhou Yang, Zengcai Liu, Syed D. Sajjad, and Richard I. Masel. 2020. "A Review of the Use of Immobilized Ionic Liquids in the Electrochemical Conversion of CO2" C 6, no. 2: 33. https://doi.org/10.3390/c6020033
APA StyleKaczur, J. J., Yang, H., Liu, Z., Sajjad, S. D., & Masel, R. I. (2020). A Review of the Use of Immobilized Ionic Liquids in the Electrochemical Conversion of CO2. C, 6(2), 33. https://doi.org/10.3390/c6020033