High-Pressure Phase Equilibrium Studies of Multicomponent (Alcohol-Water-Ionic Liquid-CO2) Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Experimental Procedure
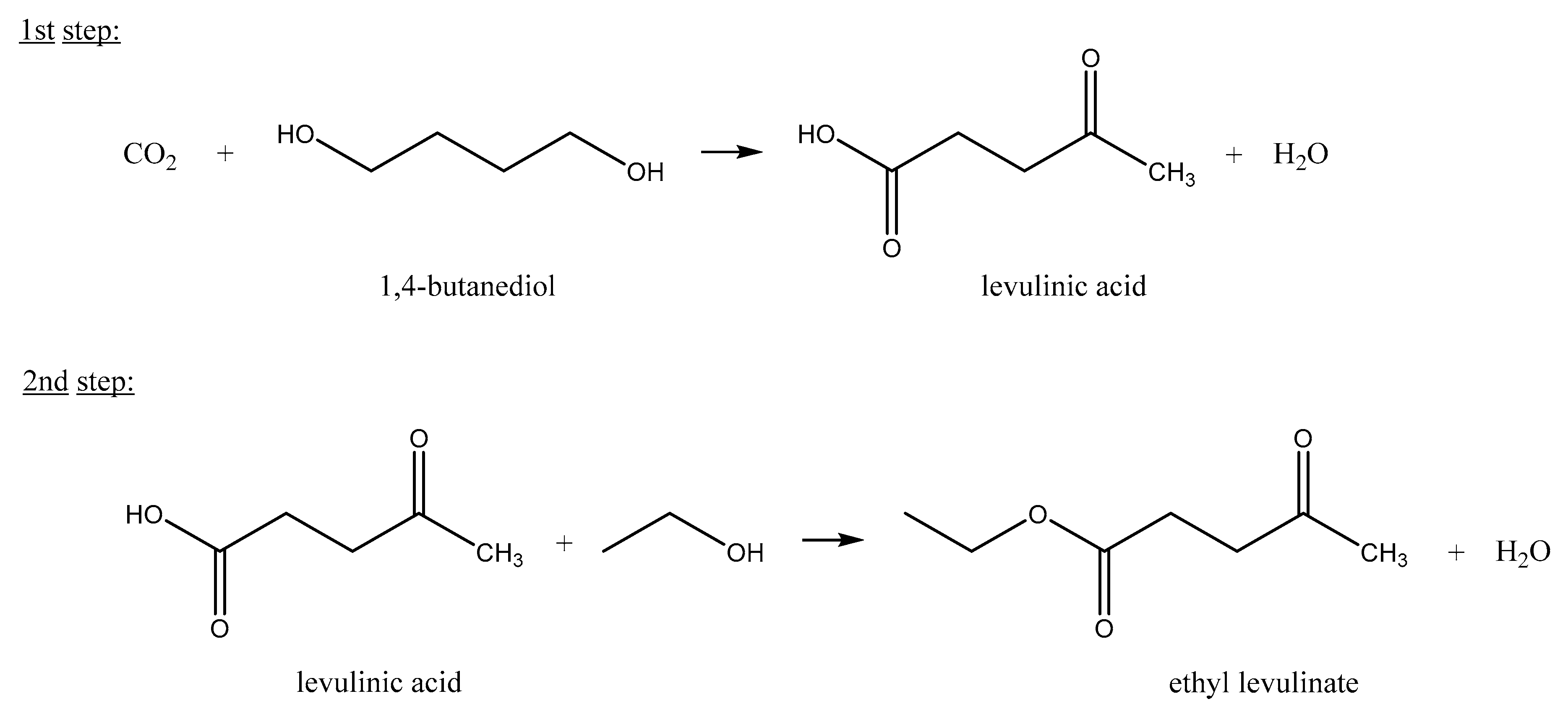
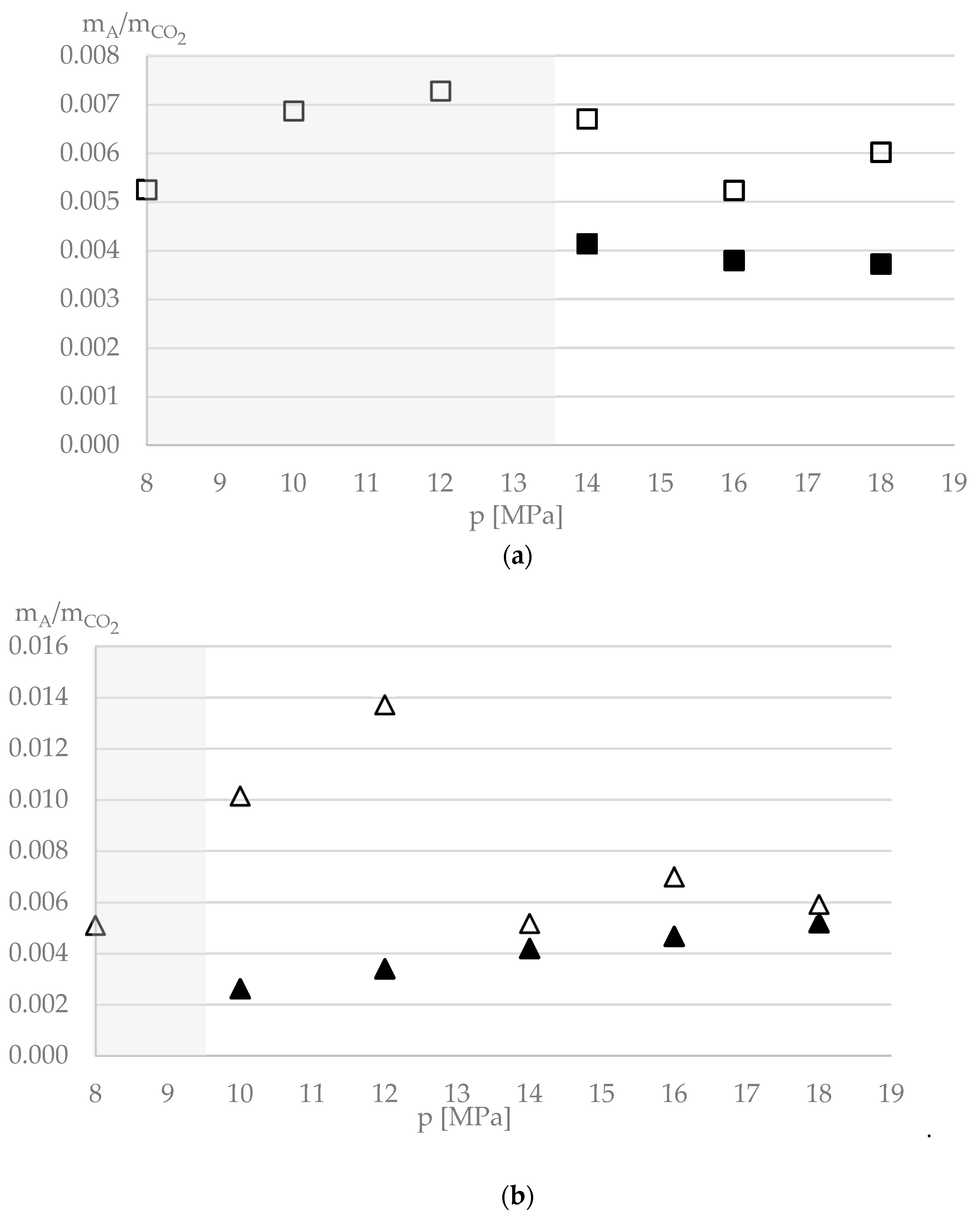
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novamont Press Release. Available online: https://www.novamont.com/eng/read-press-release/mater-biotech/ (accessed on 17 October 2019).
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 2011, 7, 445. [Google Scholar] [CrossRef]
- Burgard, A.; Burk, M.J.; Osterhout, R.; Van Dien, S.; Yim, H. Development of a commercial scale process for production of 1,4-butanediol from sugar. Curr. Opin. Biotechnol. 2016, 42, 118. [Google Scholar] [CrossRef] [PubMed]
- Biddy, M.J.; Scarlata, C.; Kinchin, C. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential, National Renewable Energy Laboratory Technical Report. Available online: www.nrel.gov/publications (accessed on 17 October 2019).
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, X.; Liu, Z. Synthesis of chemicals using CO2 as a building block under mild Conditions. Curr. Opin. Green Sustain. Chem. 2016, 1, 13. [Google Scholar] [CrossRef]
- Roh, K.; Frauzem, R.; Gani, R.; Lee, J.H. Process systems engineering issues and applications towards reducing carbon dioxide emissions through conversion technologies. Chem. Eng. Res. Des. 2016, 116, 27. [Google Scholar] [CrossRef]
- Putra, Z.A.; Kurnia, J.C.; Sasmito, A.P.; Muraza, O. Process design and techno-economic analysis of ethyl levulinate production from carbon dioxide and 1,4-butanediol as an alternative biofuel and fuel additive. Int. J. Energy Res. 2019, 43, 5932. [Google Scholar] [CrossRef]
- Leal Silva, J.F.; Grekin, R.; Mariano, A.P.; Maciel Filho, R. Making Levulinic Acid and Ethyl Levulinate Economically Viable: A Worldwide Technoeconomic and Environmental Assessment of Possible Routes. Energy Technol. 2018, 6, 613. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28. [Google Scholar] [CrossRef]
- Ahosseini, A.; Ortega, E.; Sensenich, B.; Scurto, A.M. Viscosity of n-Alkyl-3-methyl-imidazolium Bis(trifluoromethylsulfonyl)amide Ionic Liquids Saturated with Compressed CO2. Fluid Phase Equilibr. 2009, 286, 72. [Google Scholar] [CrossRef]
- Tomida, D.; Kenmochi, S.; Qiao, K.; Bao, Q.; Yokoyama, C. Viscosity of Ionic Liquid Mixtures of 1-Alkyl-3-methylimidazolium Hexafluorophosphate + CO2. Fluid Phase Equilibr. 2011, 307, 185. [Google Scholar] [CrossRef]
- Moosavi, M.; Rostami, A.A. Densities, viscosities, refractive indices, and excess properties of aqueous 1,2-etanediol, 1,3-propanediol, 1,4-butanediol, and 1,5-pentanediol binary mixtures. J. Chem. Eng. Data 2017, 62, 156. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowski, M. Extraction of butan-1-ol from water with ionic liquids at T = 308.15 K. J. Chem. Thermodyn. 2012, 53, 108. [Google Scholar] [CrossRef]
- Stoffers, M.; Górak, A. Continuous multi-stage extraction of n-butanol from aqueous solutions with 1-hexyl-3-methylimidazolium tetracyanoborate. Sep. Purif. Technol. 2013, 120, 415. [Google Scholar] [CrossRef]
- Rebocho, S.; Nunes, A.V.M.; Najdanovic-Visak, V.; Barreiros, S.; Simões, P.; Paiva, A. High pressure vapor–liquid equilibrium for the ternary system ethanol (±) menthol/carbon dioxide. J. Supercrit. Fluids 2014, 92, 282. [Google Scholar] [CrossRef]
- Bogel-Łukasik, R.; Najdanovic-Visak, V.; Barreiros, S.; Nunes da Ponte, M. Distribution Ratios of Lipase-Catalyzed Reaction Products in Ionic Liquid Supercritical CO2 Systems: Resolution of 2-Octanol Enantiomers. Ind. Eng. Chem. Res. 2008, 47, 4473. [Google Scholar] [CrossRef]
- Nunes, A.V.M.; Matias, A.A.; Nunes da Ponte, M.; Duarte, C.M.M. Quaternary Phase Equilibria for scCO2 plus Biophenolic Compound plus Water plus Ethanol. J. Chem. Eng. Data 2007, 52, 244. [Google Scholar] [CrossRef]
- Nunes, A.V.M.; Nunes da Ponte, M. Phase Equilibrium and Kinetics of O2-Oxidation of Limonene in High Pressure Carbon Dioxide. J. Supercrit. Fluids 2012, 66, 23. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Nunes da Ponte, M. Volumetric and phase behaviour of mixtures of tetracyanoborate-based ionic liquids with high pressure carbon dioxide. J. Supercrit. Fluids 2016, 113, 31. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Nunes da Ponte, M. Influence of Water on the Carbon Dioxide Solubility in [OTf]- and [eFAP]-Based Ionic Liquids. J. Chem. Eng. Data 2018, 63, 907. [Google Scholar] [CrossRef]
- Najdanovic-Visak, V.; Serbanovic, A.; Esperança, J.M.S.S.; Guedes, H.J.R.; Rebelo, L.P.N.; Nunes da Ponte, M. Supercritical Carbon Dioxide-Induced Phase Changes in (Ionic Liquid, Water and Ethanol Mixture) Solutions: Application to Biphasic Catalysis. ChemPhysChem 2003, 4, 520. [Google Scholar] [CrossRef] [PubMed]
- Inomata, H.; Kondo, A.; Kakehashi, H. Vapor–liquid equilibria for CO2–fermentation alcohol mixtures Application of a new group contribution equation of state to isomeric compounds. Fluid Phase Equilibr. 2005, 228–229, 335. [Google Scholar] [CrossRef]
- Chyliński, K.; Gregorowicz, J. Solubilities of 1-propanol and 1,2-propanediol in supercritical carbon dioxide. New analytical procedure and measurements. Fluid Phase Equilibr. 1998, 143, 163. [Google Scholar] [CrossRef]
- Škerget, M.; Čuček, D.; Knez, Ž. Phase equilibria of the propylene glycol/CO2 and propylene glycol/ethanol/CO2 systems. J. Supercrit. Fluids 2014, 95, 129. [Google Scholar] [CrossRef]
- King, M.B.; Mubarak, A.; Kim, J.D.; Bott, T.R. The Mutual Solubilities of Water with Supercritical and Liquid Carbon Dioxide. J. Supercrit. Fluids 1992, 5, 296. [Google Scholar] [CrossRef]
- Han, X.; Poliakoff, M. Continuous reactions in supercritical carbon dioxide: Problems, solutions and possible ways forward. Chem. Soc. Rev. 2012, 41, 1428. [Google Scholar] [CrossRef]
- Stouten, S.C.; Noël, T.; Wang, Q.; Hessel, V. Catalyst retention in continuous flow with supercritical carbon dioxide. Chem. Eng. Process. 2014, 83, 26. [Google Scholar] [CrossRef]

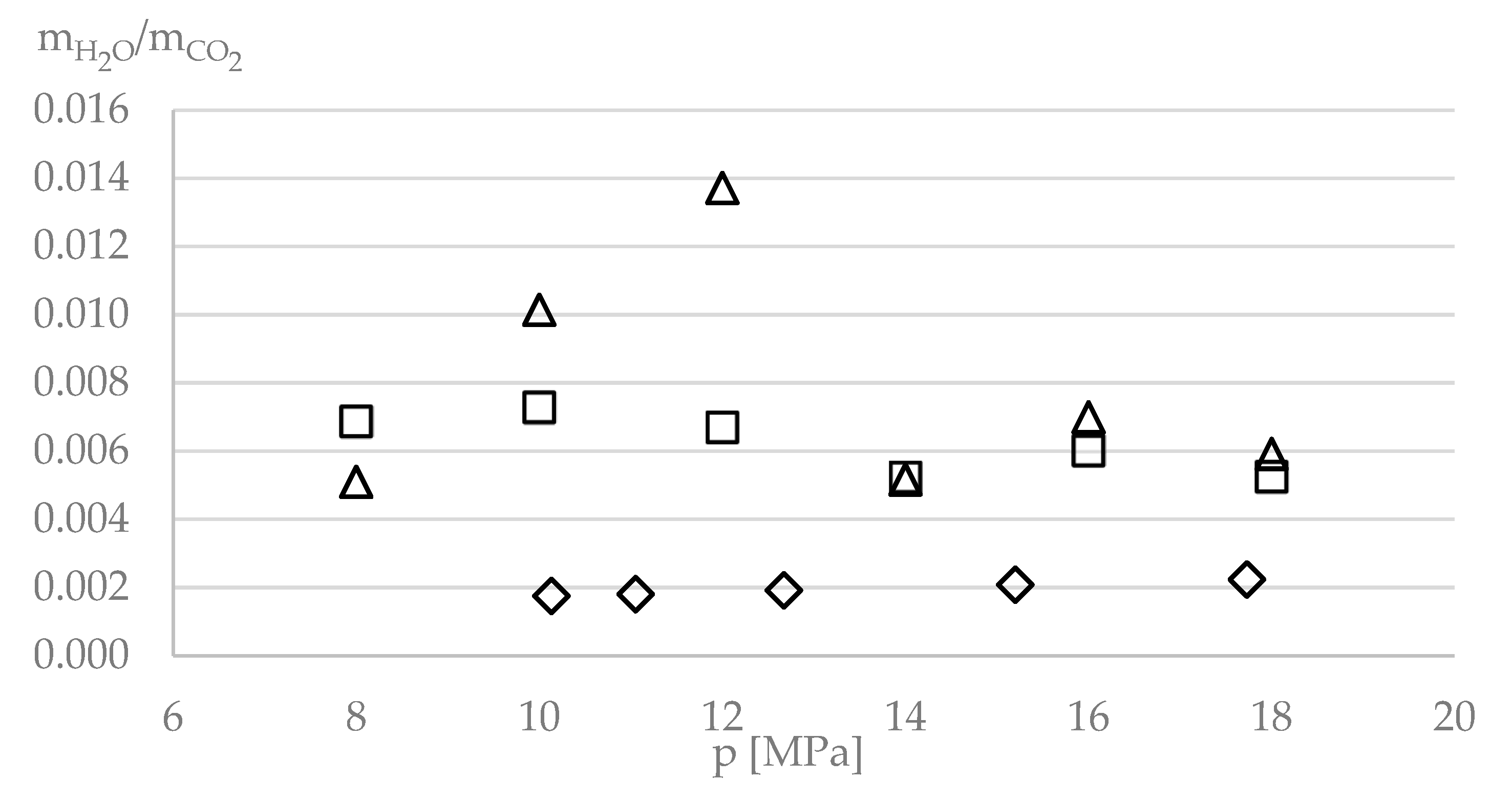
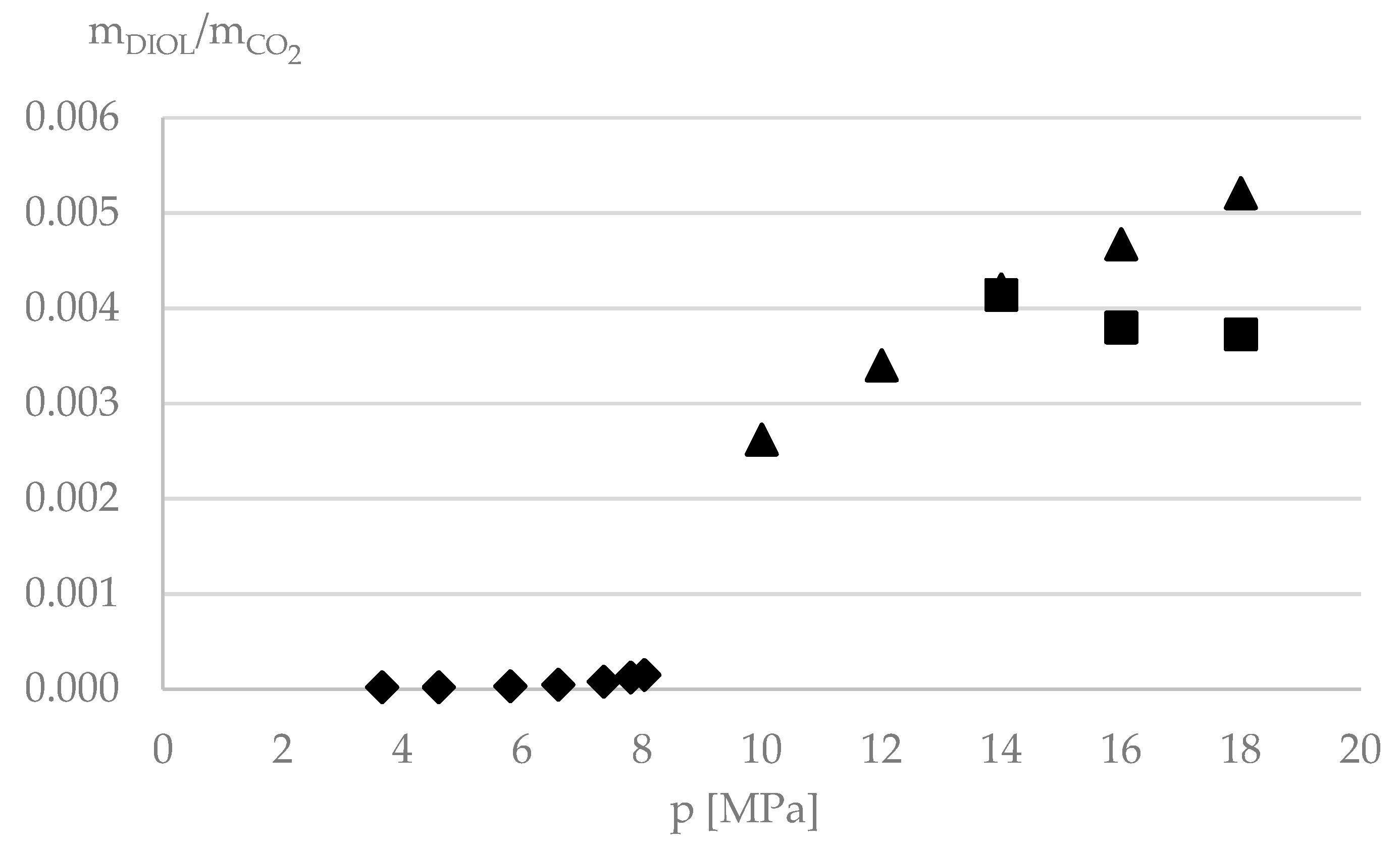
| P/MPa | xCO2 | x1,4-BDO | xIL | xH2O | yCO2 | y1,4-BDO | yIL | yH2O |
|---|---|---|---|---|---|---|---|---|
| 8 | 0.324 | 0.072 | 0.475 | 0.129 | 0.9870 | nd | nd | 0.0130 |
| 10 | 0.438 | 0.047 | 0.396 | 0.119 | 0.9842 | nd | nd | 0.0158 |
| 12 | 0.467 | 0.042 | 0.391 | 0.100 | 0.9817 | nd | nd | 0.0183 |
| 14 | 0.551 | 0.028 | 0.353 | 0.067 | 0.9819 | 0.00198 | nd | 0.0161 |
| 16 | 0.616 | 0.017 | 0.310 | 0.057 | 0.9856 | 0.00183 | nd | 0.0126 |
| 18 | 0.646 | 0.014 | 0.299 | 0.041 | 0.9837 | 0.00179 | nd | 0.0145 |
| P/MPa | yCO2 | y1,4-BDO | yIL |
|---|---|---|---|
| 8 | 1.0000 | nd | nd |
| 10 | 1.0000 | nd | nd |
| 12 | 1.0000 | nd | nd |
| 14 | 1.0000 | nd | nd |
| 16 | 0.9883 | 0.01168 | nd |
| 18 | 0.9921 | 0.00795 | nd |
| P/MPa | yCO2 | y1,4-BDO | yH2O |
|---|---|---|---|
| 8 | 0.9877 | nd | 0.0123 |
| 10 | 0.9744 | 0.00148 | 0.0242 |
| 12 | 0.9657 | 0.00201 | 0.0324 |
| 14 | 0.9852 | 0.00239 | 0.0125 |
| 16 | 0.9806 | 0.00265 | 0.0168 |
| 18 | 0.9828 | 0.00296 | 0.0142 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewska, M.E.; Paninho, A.B.; Guedes da Silva, M.F.C.; Nunes, A.V.M. High-Pressure Phase Equilibrium Studies of Multicomponent (Alcohol-Water-Ionic Liquid-CO2) Systems. C 2020, 6, 9. https://doi.org/10.3390/c6010009
Zakrzewska ME, Paninho AB, Guedes da Silva MFC, Nunes AVM. High-Pressure Phase Equilibrium Studies of Multicomponent (Alcohol-Water-Ionic Liquid-CO2) Systems. C. 2020; 6(1):9. https://doi.org/10.3390/c6010009
Chicago/Turabian StyleZakrzewska, Małgorzata E., Ana B. Paninho, M. Fátima C. Guedes da Silva, and Ana V. M. Nunes. 2020. "High-Pressure Phase Equilibrium Studies of Multicomponent (Alcohol-Water-Ionic Liquid-CO2) Systems" C 6, no. 1: 9. https://doi.org/10.3390/c6010009
APA StyleZakrzewska, M. E., Paninho, A. B., Guedes da Silva, M. F. C., & Nunes, A. V. M. (2020). High-Pressure Phase Equilibrium Studies of Multicomponent (Alcohol-Water-Ionic Liquid-CO2) Systems. C, 6(1), 9. https://doi.org/10.3390/c6010009