Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature
Abstract
1. Introduction
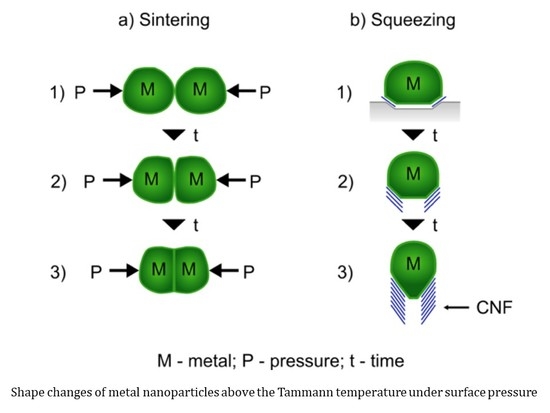
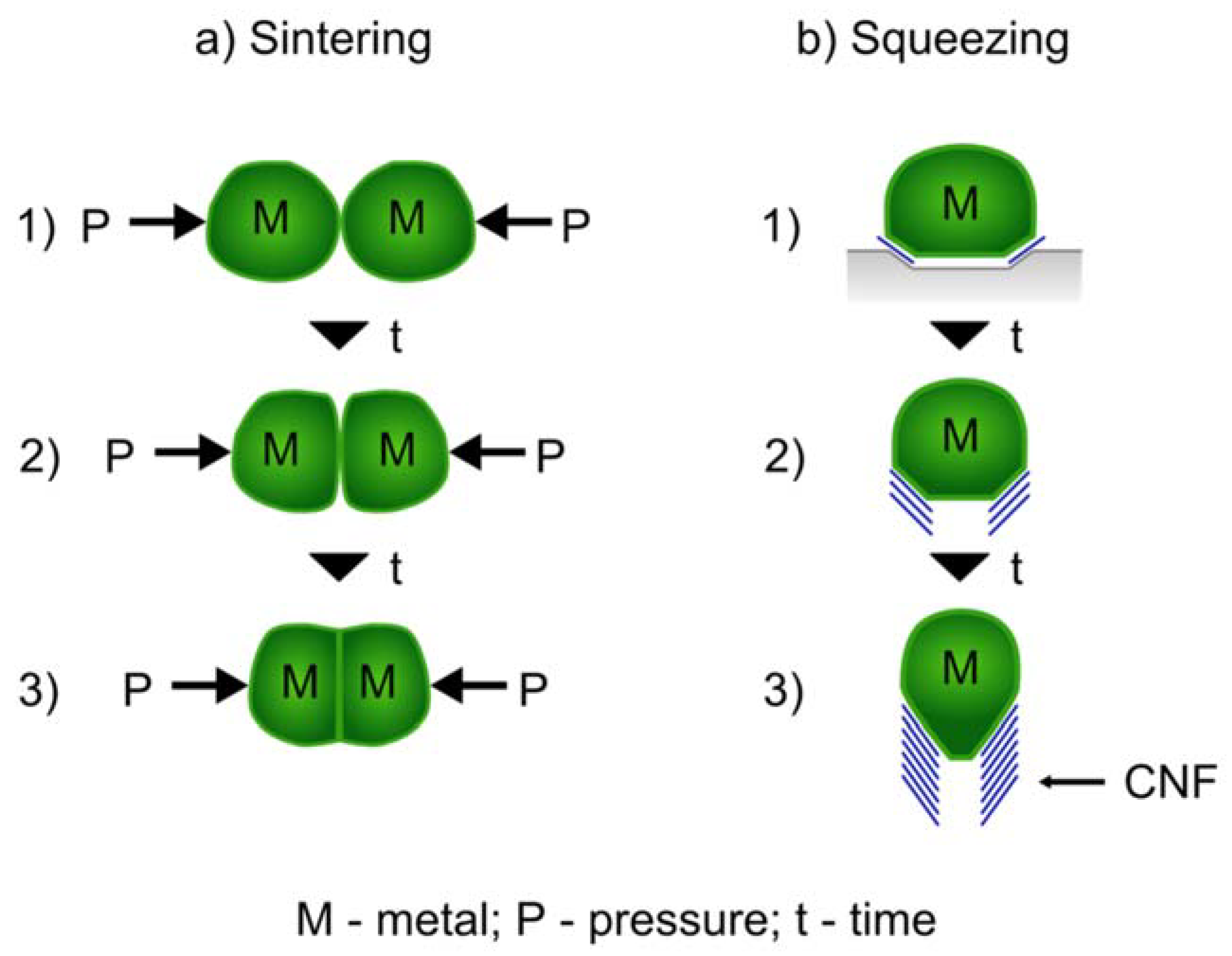
2. Growth of Carbon Nanotubes above Catalysts Tammann Temperature (TTa)
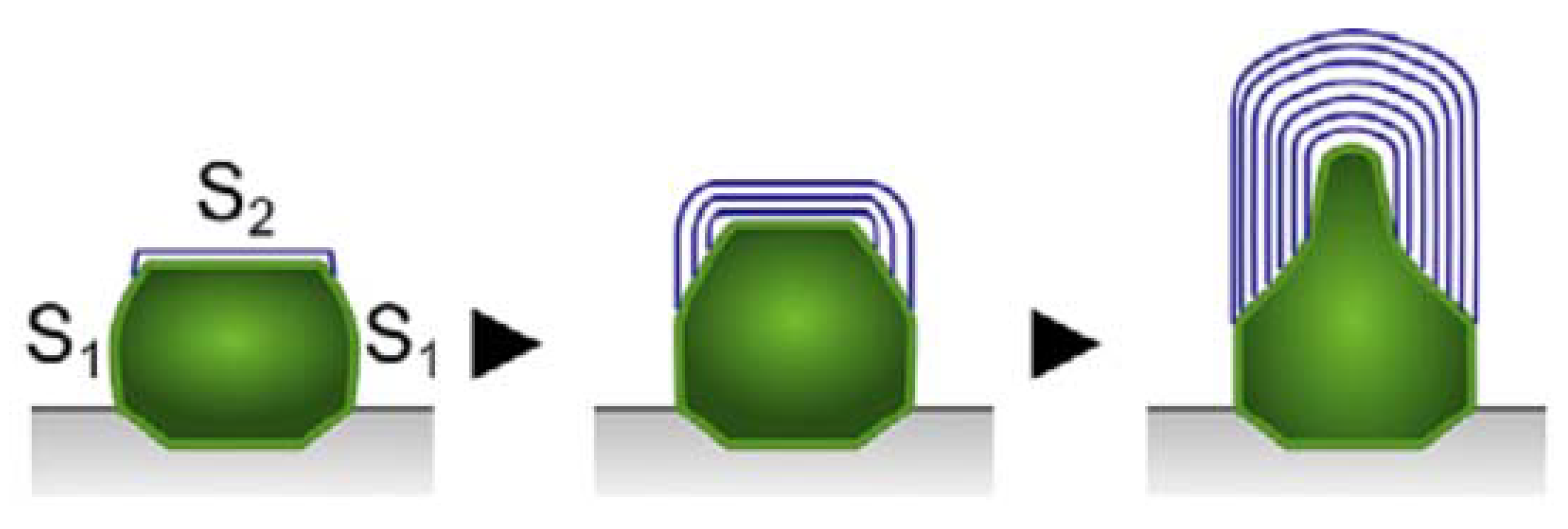
3. Alternative Routes and Shapes
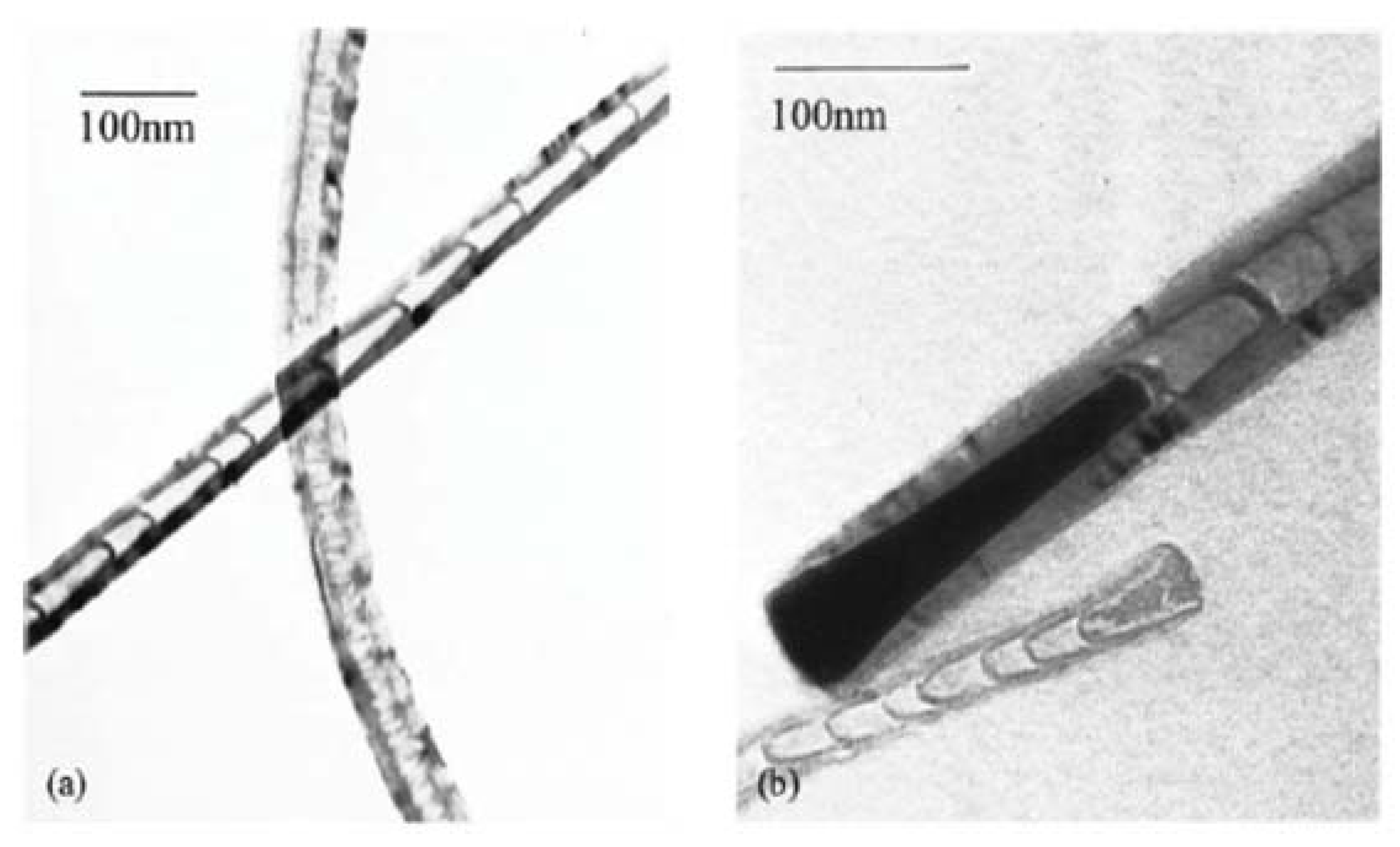
4. Opening and Filling Observed in MWCNTs
5. Bamboo-Like Hexagonal Boron Nitride (h-BN) Nanotubes and Thin Films
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jia, Z.; Kou, K.; Qin, M.; Wu, H.; Puleo, F.; Liotta, F. Controllable and large- scale synthesis of carbon Nanostructures: A Review of Bamboo-Like Nanotubes. Catalysts 2017, 7, 256. [Google Scholar] [CrossRef]
- Lobo, L.S. Catalytic carbon formation: Clarifying the alternative kinetic routes and defining a kinetic linearity for sustained growth concept. React. Kinet. Mech. Cat. 2016, 118, 393–414. [Google Scholar] [CrossRef]
- Barrer, R.M. Aspects of gas-metal equilibrium, interstitial solution and diffusion. Discuss. Faraday Soc. 1948, 4, 68–81. [Google Scholar] [CrossRef]
- Barrer, R.M. Diffusion in and through Solids; The University Press, MacMillan: Cambridge, UK, 1941. [Google Scholar]
- Cordero, B.; Gomez, V.; Platero-Prats, A.E.; Revés, M.; Echeverria, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Lobo, L.S.; Carabineiro, S.A.C. Kinetics and mechanism of catalytic carbon gasification. Fuel 2016, 183, 457–469. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J. Growth model of bamboo-shaped carbon nanotubes by thermal chemical vapor deposition. Appl. Phys. Lett. 2000, 77, 3397–3399. [Google Scholar] [CrossRef]
- Brown, B.; Parker, C.B.; Stoner, B.R.; Glass, J.T. Growth of vertically aligned bamboo-like carbon nanotubes from ammonia/methane precursors using a platinum catalyst. Carbon 2011, 49, 266–274. [Google Scholar] [CrossRef]
- Gonzalez, I.; de Jesus, J.C.; Canizales, E. Bamboo-shaped CNTs generated by methane thermal decomposition using Ni nanoparticles synthesized in water-oil emulsions. Micron 2011, 42, 819–825. [Google Scholar] [CrossRef]
- De Jesus, J.C.; Gonzalez, I.; Garcia, M.; Urbina, C. Preparation of nickel nanoparticles and their catalytic activity in the cracking of methane. J. Vac. Sci. Technol. A 2008, 26, 913–918. [Google Scholar] [CrossRef]
- Zaikovski, V.I.; Chesnokov, V.V.; Buyanov, R.A. The Relationship between the State of Active Species in a Ni/Al2O3 Catalyst and the Mechanism of Growth of Filamentous Carbon. Kinet. Catal. 2001, 42, 890–931. [Google Scholar] [CrossRef]
- McKee, D.W. Metal oxides as catalysts for oxidation of graphite. Carbon 1970, 8, 623–626. [Google Scholar] [CrossRef]
- Sharma, R.; Rez, P.; Brown, M.; Du, G.; Treacy, M.M.J. Dynamic observations of the effect of pressure and temperature conditions on the selective synthesis of carbon nanotubes. Nanotechnology 2007, 18, 125602–125609. [Google Scholar] [CrossRef]
- Hofmann, S.; Sharma, R.; Ducati, C.; Du, G.; Mattevi, C.; Cepek, C.; Cantoro, M.; Pisana, S.; Parvez, A.; Cervantes-Sodi, F.; et al. In situ observations of Catalyst Dynamics during Surface-Bound Carbon Nanotube Nucleation. Nano Lett. 2007, 7, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, D.; Totdal, B.; Holmen, A.H. Effect of support and Reactant on the Yield and Structure of Carbon Growth by Chemical vapor deposition. J. Phys. Chem. B 2005, 109, 6096–6102. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Ascensio, J.A.; Perez-Alvarez, M.; Yacaman, M.J. Melting behavior of nanometer sized gold isomers. Surf. Sci. 2001, 491, 88–98. [Google Scholar] [CrossRef]
- Saito, Y. Nanoparticles and filled nanocapsules. Carbon 1995, 33, 979–988. [Google Scholar] [CrossRef]
- Li, Y.D.; Chen, J.; Ma, Y.; Zhao, J.; Qin, Y.; Chang, L. Formation of bamboo-like nanocarbon and evidence for the quasi-liquid state of nanosized metal particles at moderate temperatures. Chem. Comm. 1999, 1141–1142. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, O.; Stoner, B.R. Deposition of aligned bamboo-like carbon nanotubes via microwave plasma enhanced chemical vapor deposition. J. Appl. Phys. 2000, 88, 6072–6074. [Google Scholar] [CrossRef]
- He, C.; Zhao, N.; Shi, C.; Du, X.; Li, J. TEM studies of the initial stage growth and morphologies of bamboo-shaped carbon nanotubes synthesized by CVD. J. Alloys Comp. 2007, 433, 79–83. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Ma, Y.; Qin, Y.; Chang, L. Formation of bamboo-shaped carbon filaments and dependence of their morphology on catalyst composition and reaction conditions. Carbon 2001, 39, 1467–1475. [Google Scholar] [CrossRef]
- Jung, M.; Eun, K.Y.; Lee, J.-K.; Baik, Y.-J.; Lee, K.-R.; Park, J.W. Growth of CNTs by CVD. Diamond Rel. Mater. 2001, 10, 1235–1240. [Google Scholar] [CrossRef]
- Katayama, T.; Araki, H.; Yoshino, K. Multiwalled nanotubes with bamboo-like structure and effects of heat treatment. J. Appl. Phys. 2002, 91, 6675–6678. [Google Scholar] [CrossRef]
- Chadderton, L.T.; Chen, Y. A model for the growth of bamboo and skeletal nanotubes: Catalytic capillarity. J. Crystal Growth 2002, 240, 164–169. [Google Scholar] [CrossRef]
- Bartsch, K.; Biedermann, J.; Gemming, T.; Leonhardt, A. On the diffusion-controlled growth of multi-walled CNTs. J. Appl. Phys. 2005, 97, 114301. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, C.E.; Lee, T.J.; Lyu, S.C.; Lee, C.J. Lateral force microscopy of bamboo-shaped multiwalled CNTs. Curr. Appl. Phys. 2006, 6, 141–144. [Google Scholar] [CrossRef]
- Ting, J.-M.; Lin, S.-H. Growth and characteristics of CNTs obtained under different C2H2/H2/NH3 concentrations. Carbon 2007, 45, 1934–1940. [Google Scholar] [CrossRef]
- Lin, M.T.; Tan, J.P.I.; Boothroyd, C.; Loh, K.P.; Tok, E.S.; Foo, Y.-L. Dynamical Observation of Bamboo-like Carbon Nanotube Growth. Nano Lett. 2007, 7, 2234–2238. [Google Scholar] [CrossRef]
- Katar, S.L.; Gonzalez-Berrios, A.; de Jesus, J.; Weiner, B.; Morell, G. Direct deposition of Bamboo-Like Carbon Nanotubes on Copper Substrates by Sulfur-Assisted HFCVD. J. Nanomat. 2008. article 515890 (7 pages). [Google Scholar] [CrossRef]
- Xue, B.; Liu, R.; Huang, W.-Z.; Zheng, Y.-F.; Xu, Z.-D. Growth and characterization of bamboo-like multiwalled carbon nanotubes over Cu/Al2O3 catalyst. J. Mater. Sci. 2009, 44, 4040–4046. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, L.; Wang, Y.-L.; Qiau, W.-M.; Liang, X.; Ling, L.-C. Effect of Sulfur on the growth of carbon nanotubes by detonation-assisted CVD. Appl. Surf. Sci. 2010, 257, 932–936. [Google Scholar] [CrossRef]
- Lin, J.-H.; Chen, C.-S.; Zheng, Z.-Y.; Chang, C.-W.; Chen, H.-W. Sulphate-activated growth of bamboo-like carbon nanotubes over copper catalysts. Nanoscale 2012, 4, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jia, J.; Kwong, F.; Ng, D.H.L. Synthesis of bamboo-like CNTs on a Cu foil by catalytic CVD from ethanol. Carbon 2012, 50, 2504–2512. [Google Scholar] [CrossRef]
- Keczenovity, E.; Fejes, D.; Reti, B.; Hernadi, K. Growth and characterization of bamboo-like carbon nanotubes synthesized on Fe-Co-Cu catalysts prepared by high-energy ball milling. Phys. Status Solidi B 2013, 250, 2544–2548. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.H. Purity-controllable growth of bamboo-like multi-walled CNTs over copper-based catalysts. Catal. Commun. 2013, 34, 41–44. [Google Scholar] [CrossRef]
- Krishna, V.M.; Abilarassu, A.; Somanathan, T.; Gokulakrishnan, N. Effective synthesis of well graphitized high yield bamboo-like MWCNTs on copper loaded α-alumina nanoparticles. Diamond Rel. Mater. 2014, 50, 20–25. [Google Scholar] [CrossRef]
- Velasquez, M.; Batiot-Dupeyrat, C.; Gallego, J.; Santamaria, A. Chemical and morphological characterization of MWCNTs synthesized by C deposition from ethanol-glycerol blend. Diamond Rel. Mater. 2014, 50, 30–48. [Google Scholar] [CrossRef]
- Boi, F.S.; Wang, S.; He, Y. Mapping the transition from catalyst-pool to bamboo-like growth-mechanism in VA free-standing films of CNTs filled with Fe3C: The key role of water. AIP Adv. 2016, 6, 0851012016. [Google Scholar] [CrossRef]
- Sarawast, S.K.; Sinha, B.; Pant, K.K.; Gupta, R.B. Kinetic Study and Modeling of Homogeneous Thermocatalytic Decomposition of Methane over a Ni-Cu-Zn/Al2O3 Catalyst for the Production of Hydrogen and Bamboo-Shaped CNTs. Ind. Eng. Chem. Res. 2016, 55, 11672–11680. [Google Scholar] [CrossRef]
- Wang, Y.; McIntyre, P.C.; Cai, W. Phase Field Model for Morphological Transition in Nanowire Vapor-Liquid-Solid Growth. Crystal Growth Des. 2017, 17, 2211–2227. [Google Scholar] [CrossRef]
- Arnaiz, N.; Martin-Gullon, I.; Font, R.; Gomez-Rico, M.F. Production of bamboo-type carbon nanotubes doped with nitrogen from polyamide pyrolysis gas. J. Anal. Appl. Pyrol. 2018, 130, 52–61. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, W. Synthesis and Characterization of Bamboo-like MWCNTs By Alcohothermal Process. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012058. [Google Scholar] [CrossRef]
- Kumi, D.O.; Phaahlamohlaka, T.N.; Dlamini, M.W.; Mangezvo, I.T.; Mhlanga, S.D.; Scurrell, M.S.; Coville, N.J. Effect of titania covering on CNTS as support for the Ru catalyzed selective Co metanation. Appl. Cat. B Environ. 2018, 232, 492–500. [Google Scholar] [CrossRef]
- Xu, F.-F.; Bando, Y. Structures of a hollow filamentary conical helix. Acta Cryst. A 2003, 59, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Merkulov, V.I.; Guillom, M.A.; Lowndes, D.H.; Simpson, M.L.; Voelkl, E. Shaping carbon nanostructures by controlling the synthesis process. Appl. Phys. Lett. 2001, 79, 1178–1180. [Google Scholar] [CrossRef]
- Merkulov, V.I.; Hensley, D.K.; Melechko, A.V.; Guillorn, M.A.; Lowndes, D.H.; Simpson, M.L. Control Mechanisms for the Growth of Isolated Vertically Aligned Carbon Nanofibers. J. Phys. Chem. B 2002, 106, 10570–10577. [Google Scholar] [CrossRef]
- Melechko, A.V. Vertically aligned CNF and related structures: Controlled synthesis and directed assembly. J. Appl. Phys. 2005, 97, 041301. [Google Scholar] [CrossRef]
- Merkulov, I.A.; Yoon, M.; Geohegan, D.B. How the shape of catalyst nanoparticles determines their crystallographic orientation during carbon nanofiber growth. Carbon 2013, 60, 41–45. [Google Scholar] [CrossRef]
- Becker, M.J.; Xia, W.; Tessonier, J.-P.; Blume, R.; Yao, L.; Schlögl, R.; Muhler, M. Optimizing the synthesis of cobalt-based catalysts for the selective growth of multiwalled CNTs under industrially relevant conditions. Carbon 2011, 49, 5253–5264. [Google Scholar] [CrossRef]
- Maurice, J.-L.; Pribat, D.; He, Z.; Patriarche, G.; Cojocaru, C.S. Catalyst faceting during graphene layer crystallization in the course of carbon nanofiber growth. Carbon 2014, 79, 93–102. [Google Scholar] [CrossRef]
- Kovalevski, V.V.; Safronov, A.N. Pyrolysis of Hollow Carbons Melted Catalyst. Carbon 1998, 36, 963–968. [Google Scholar] [CrossRef]
- Bartsch, K.; Leonhardt, A. An approach to the structural diversity of aligned grown multi-walled CNTs on catalyst layers. Carbon 2004, 42, 1731–1736. [Google Scholar] [CrossRef]
- Mohammad, S.N. Systematic investigation of the growth mechanism for conventional, doped and bamboo-shaped nanotubes. Carbon 2014, 75, 133–148. [Google Scholar] [CrossRef]
- Harris, P.J.F. Carbon Nanotube Science: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2011. [Google Scholar]
- Wu, C.; Sahajwalla, V. Influence of Melt Carbon and Sulfur on the Wetting of Solid Graphite by Fe-C-S Melts. Metallurg. Mater. Trans. B 1998, 29, 471–477. [Google Scholar] [CrossRef]
- Luo, N.; Jiu, H. Gaseous detonation fabrication of CNTs and CNTs doping with Fe based composites. Fuller. Nanotub. Carb. Nanostruct. 2016, 24, 494–499. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, Z.; Wu, W.; Liu, Z. Detonation chemistry of a CHNO explosive: Catalytic assembly of carbon nanotubes at low pressure and temperature state. Chem. Commun. 2002, 2740–2741. [Google Scholar] [CrossRef]
- Shaikjee, A.; Coville, N.J. The role of the hydrocarbon source on the growth of carbon materials. Carbon 2012, 50, 33376–33398. [Google Scholar] [CrossRef]
- Sun, L.; Banhart, F.; Krasheninnikov, A.V.; Rodrigues-Manzo, J.A.; Terrones, H.; Ajyan, P.M. Carbon nanotubes as high-pressure cylinders and nanoextruders. Science 2006, 312, 1199–1202. [Google Scholar] [CrossRef]
- Amelinckx, S.; Zhang, X.B.; Bernaerts, D.; Zhang, X.F.; Ivanov, V.; Nagy, B.A. Formation Mechanism for Catalytically Grown Helix-Shaped Graphite Nanotubes. Science 1994, 265, 635–639. [Google Scholar] [CrossRef]
- Usman, I.B.; Matsoso, M.; Ranganathan, K.; Naidoo, D.; Coville, N.J.; Wamwangi, D. Magnetic properties of iron containing N-doped MWCNTs. Mater. Chem. Phys. 2018, 209, 280–290. [Google Scholar] [CrossRef]
- Coville, N.J.; Mhlanga, S.D.; Nxumalo, E.N.; Saikjee, A. A review of shaped carbon nanomaterials. S. Afr. J. Sci. 2011, 107, 1–15. [Google Scholar] [CrossRef]
- Han, W.; Bando, Y.; Kurashima, K.; Sato, T. Synthesis of boron nitride nanotubes from carbon nanotubes by a substitution reaction. Appl. Phys. Lett. 1998, 73, 3085–3087. [Google Scholar] [CrossRef]
- Ma, Y.; Bando, Y.; Sato, T. Controlled Synthesis of BN Nanotubes, Nanobamboos and Nanocables. Adv. Mater. 2002, 14, 366–368. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Gu, Y.; Zhao, G.; Qian, Q.; Li, J.; Pan, X.; Zhang, Z. Catalytic growth of bamboo-like boron nitride nanotubes using self-propagation HT synthesized porous precursor. Mater. Lett. 2012, 67, 17–20. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Zhao, G.; Gu, Y.; Zhang, Z. Selective synthesis of boron nitride nanotubes by self-propagation high temperature synthesis and annealing process. J. Solid State Chem. 2011, 184, 2478–2484. [Google Scholar] [CrossRef]
- Sharma, B.B.; Parashar, A. A review on thermo-mechanical properties of bi-crystalline and polycrystalline 2D nanomaterials. Crit. Rev. Solid State Mater. Sci. 2019, 45, 134–170. [Google Scholar] [CrossRef]
- Yu, B.-S.; Ha, T.-J. High-performance Solution-Processed IGZO Thin-Film Transistors with Al2O3/BN Composite dielectrics Fabricated at Low Temperature. Phys. Status Solidi A 2018, 215, 1700802. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, B.; Mattevi, C.; Li, K. Dynamic microstructure of graphene oxide membranes and permeation flux. J. Membr. Sci. 2018, 549, 385–392. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide Semiconductor Thin-Film Transistors: A Review of Recent Advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef]
- Atkinson, J.D.; Fortunato, M.A.; Dastgheib, S.A.; Rostam-Abadia, M.; Rooda, M.J.; Suslick, K.S. Synthesis and characterization of iron-impregnated porous carbon spheres by ultrasonic spray pyrolysis. Carbon 2011, 49, 587–598. [Google Scholar] [CrossRef]
- Kim, H.; Fortunato, M.E.; Xu, H.; Bang, J.H.; Suslick, K.S. Carbon microspheres as Supercapacitors. J. Phys. Chem. C 2011, 115, 20481–20486. [Google Scholar] [CrossRef]
- Motuma, B.K.; Matoso, B.J.; Momodu, D.; Oyedotun, K.O.; Coville, N.J.; Manyala, N. Deciphering the Structural, Textural, and Electrochemical Properties of Activated BN-Doped Spherical Carbons. Nanomaterials 2019, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Yang, G.; Zhao, B.; Yang, J. Catalyst-free synthesis of multi-walled carbon nanotubes from carbon spheres and its implications for the formation mechanism. Carbon 2013, 53, 137–144. [Google Scholar] [CrossRef]
- Lobo, L.S. Mechanism of catalytic CNTs growth in 400–650 °C range: Explaining volcano shape Arrhenius plot and catalytic synergism using both Pt (or Pd) and Ni, Co or Fe. C-J. Carb. Res. 2019, 5, 42. [Google Scholar] [CrossRef]


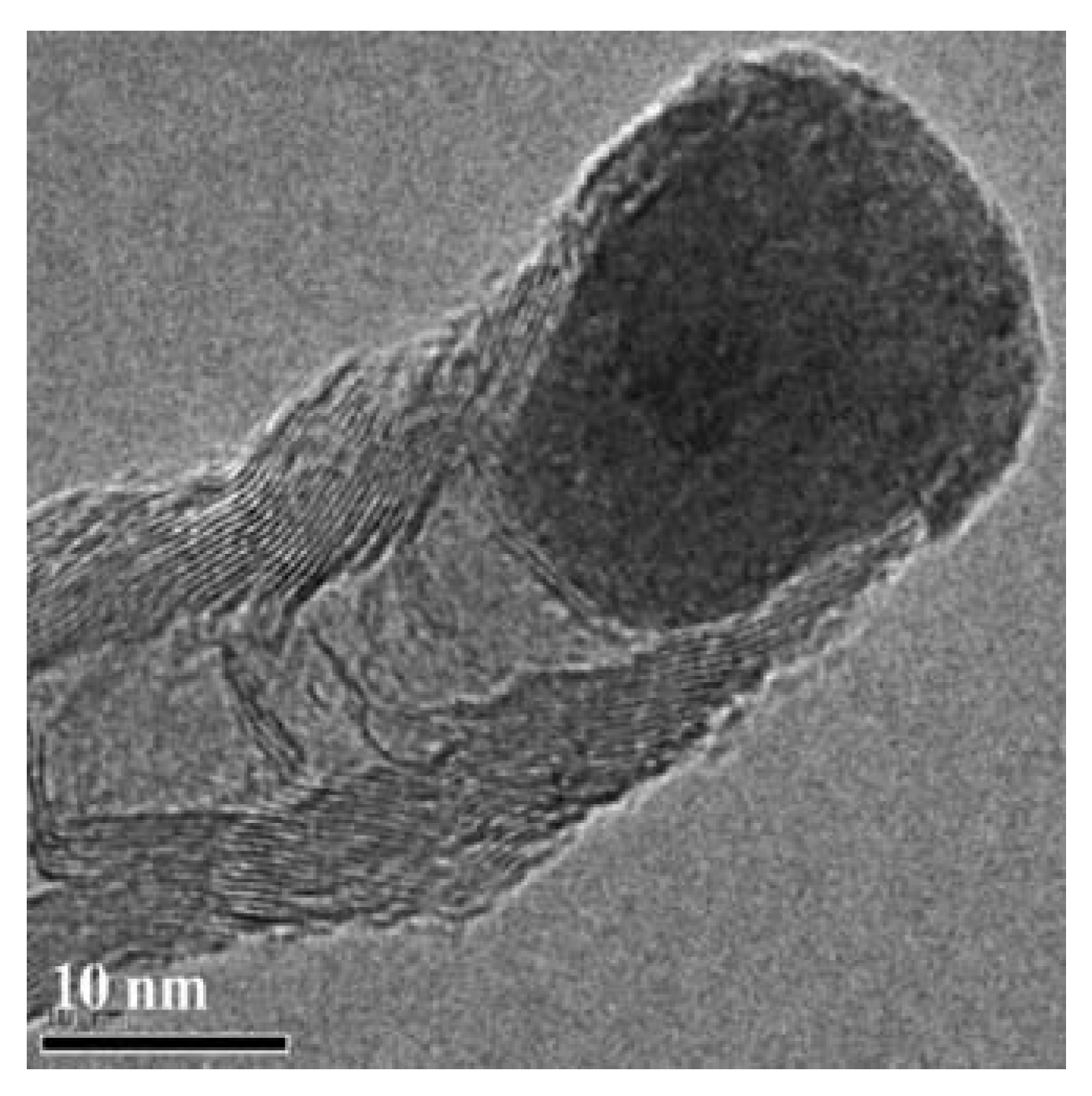
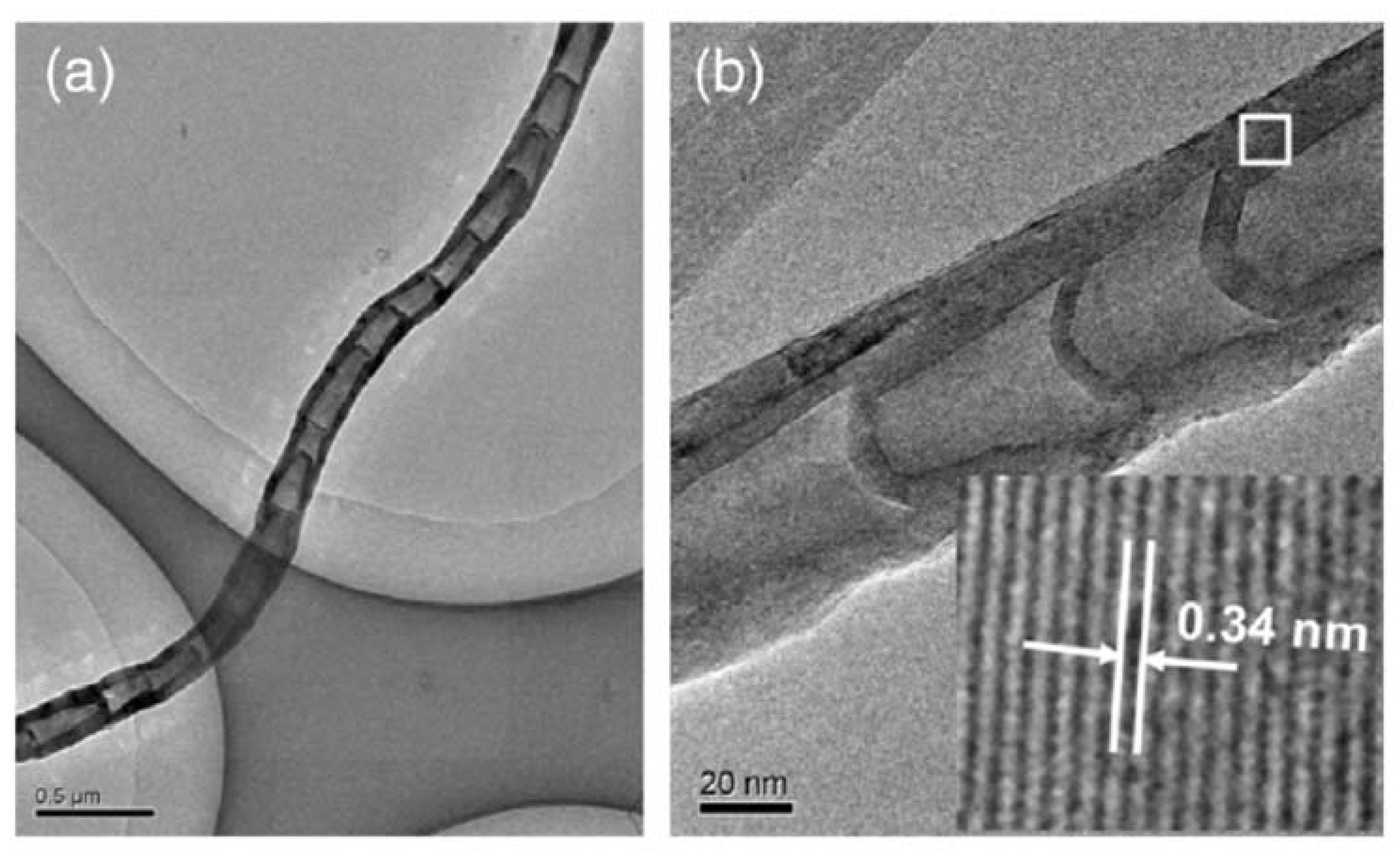

| Kinetic Routes | Temperature Range (°C) | C Growth Type | Active Catalysts |
|---|---|---|---|
| I Catalytic | 300–550 | Surface catalysis | Fe, Co, Ni |
| II Hybrid | 550–(700) | C black atoms dissolve/grow | Pt, Ru, Mo, Ni, Cu |
| III Pyrolytic | 600–(1200) | C black (C2/C3) forms layers | No catalysis, shape adjusts |
| Metal Solvent | Melting (°C) | TTa (°C) | ARsolv (pm) | ARsoluC/ARsolv | ARsoluN/ARsolv | ARsoluB/ARsolv |
|---|---|---|---|---|---|---|
| Fe | 1538 | 632 | 140 | 0.50 | 0.36 | 0.56 |
| Co | 1495 | 611 | 135 | 0.52 | 0.37 | 0.57 |
| Ni | 1455 | 590 | 135 | 0.52 | 0.38 | 0.58 |
| Cu | 1083 | 405 | 135 | 0.52 | 0.39 | 0.60 |
| Ru | 2334 | 1030 | 130 | 0.54 | 0.31 | 0.49 |
| Rh | 1964 | 845 | 135 | 0.52 | 0.32 | 0.50 |
| Pd | 1555 | 641 | 140 | 0.50 | 0.33 | 0.51 |
| Catalyst | Gas | Temperature (°C) | First Author | Year | Reference |
|---|---|---|---|---|---|
| Ni-Cu/Al | CH4/N2 | 500–730 | Li YD | 1999 | [18] |
| Fe/SiO2 | C2H2 | 750–950 | Lee CJ | 2000 | [7] |
| Fe/SiO2 | CH4/NH3 | 650–950 | Cui H | 2000 | [19] |
| Ni/Al | CH4/N2 | 500–600 | He C | 2007 | [20] |
| Ni-Cu/Al2O3 | CH4/H2 | 720–830 | Chen J | 2001 | [21] |
| Ni | C2H2/N2/H2 | 750–950 | Jung M | 2001 | [22] |
| Ni | Phthalocyanine | 600–850 | Katayama T | 2002 | [23] |
| Fe | Phthalocyanine | 1000 | Chadderton LT | 2002 | [24] |
| Fe,Co,Ni | CH4/H2 | 850–1100 | Bartsch K | 2005 | [25] |
| Co/Al2O3-Ti | C2H2/NH3 | 750–950 | Jang JY | 2006 | [26] |
| Fe | C2H2/NH3/H2 | 700 | Ting JM | 2007 | [27] |
| Ni | C2H2 | 650 | Lin MT | 2007 | [28] |
| Cu | CH4/H2/H2S | 500–900 | Katar SL | 2008 | [29] |
| Cu/Al2O3 | C2H5OH | 700–850 | Xue B | 2009 | [30] |
| Ni (AC)2 | C4H4S/H2-S | Detonation | Wang C | 2010 | [31] |
| Ni, Ni-Cu | CH4/N2 | 550–830 | Gonzalez I | 2011 | [9] |
| Pt/SiO2 | CH4/NH3 | 1000/Plasma | Brown B | 2011 | [8] |
| Cu/Al2O3 | C2H4/He | 700–900 | Lin JH | 2012 | [32] |
| Cu | Ethanol | 700–1000 | Zhu J | 2012 | [33] |
| Fe,Co,Ni,Al2O3 | C2H2 | 720 | Keczenovity E | 2013 | [34] |
| Cu/SiO2 | C2H4/He | 500–900 | Lin YC | 2013 | [35] |
| Cu/Al2O3 | C2H2/N2 | 550–800 | Krishna VM | 2014 | [36] |
| La/NiO3 | Glicerol/Ethanol | 700–900 | Velasquez M | 2014 | [37] |
| Ferrocene/SiO2 | Dichlorobenzene | 800–900 | Boi FS | 2016 | [38] |
| Ni,Cu,Zn | CH4 | 600–800 | Saraswat SK | 2016 | [39] |
| Fe-Mo/Al2O3 | C3H4N2 | 800–900 | Wang Q | 2017 | [40] |
| Fe/Al2O3 | Polyamide | 750 | Arnaiz N | 2018 | [41] |
| Cobaltocene | Ethanol | 500 | Tang Y | 2018 | [42] |
| Co-Fe/Ru | CO/H2 | 750 | Kumi DO | 2018 | [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo, L.S.; Carabineiro, S.A.C. Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature. C 2020, 6, 18. https://doi.org/10.3390/c6020018
Lobo LS, Carabineiro SAC. Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature. C. 2020; 6(2):18. https://doi.org/10.3390/c6020018
Chicago/Turabian StyleLobo, Luís Sousa, and Sónia A.C. Carabineiro. 2020. "Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature" C 6, no. 2: 18. https://doi.org/10.3390/c6020018
APA StyleLobo, L. S., & Carabineiro, S. A. C. (2020). Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature. C, 6(2), 18. https://doi.org/10.3390/c6020018