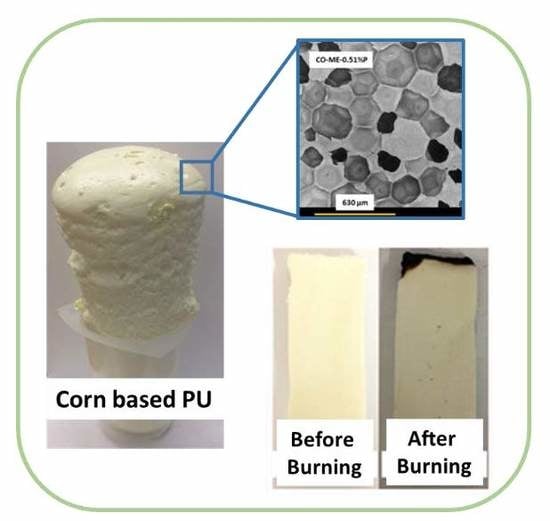
Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams
Abstract
1. Introduction
2. Materials and Methods
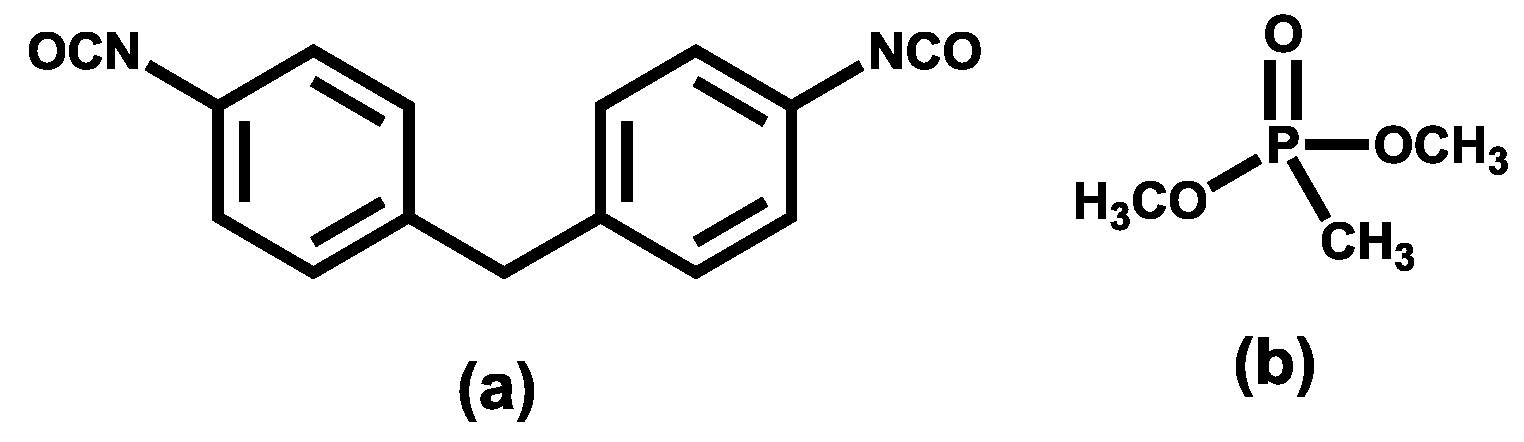
2.1. Materials
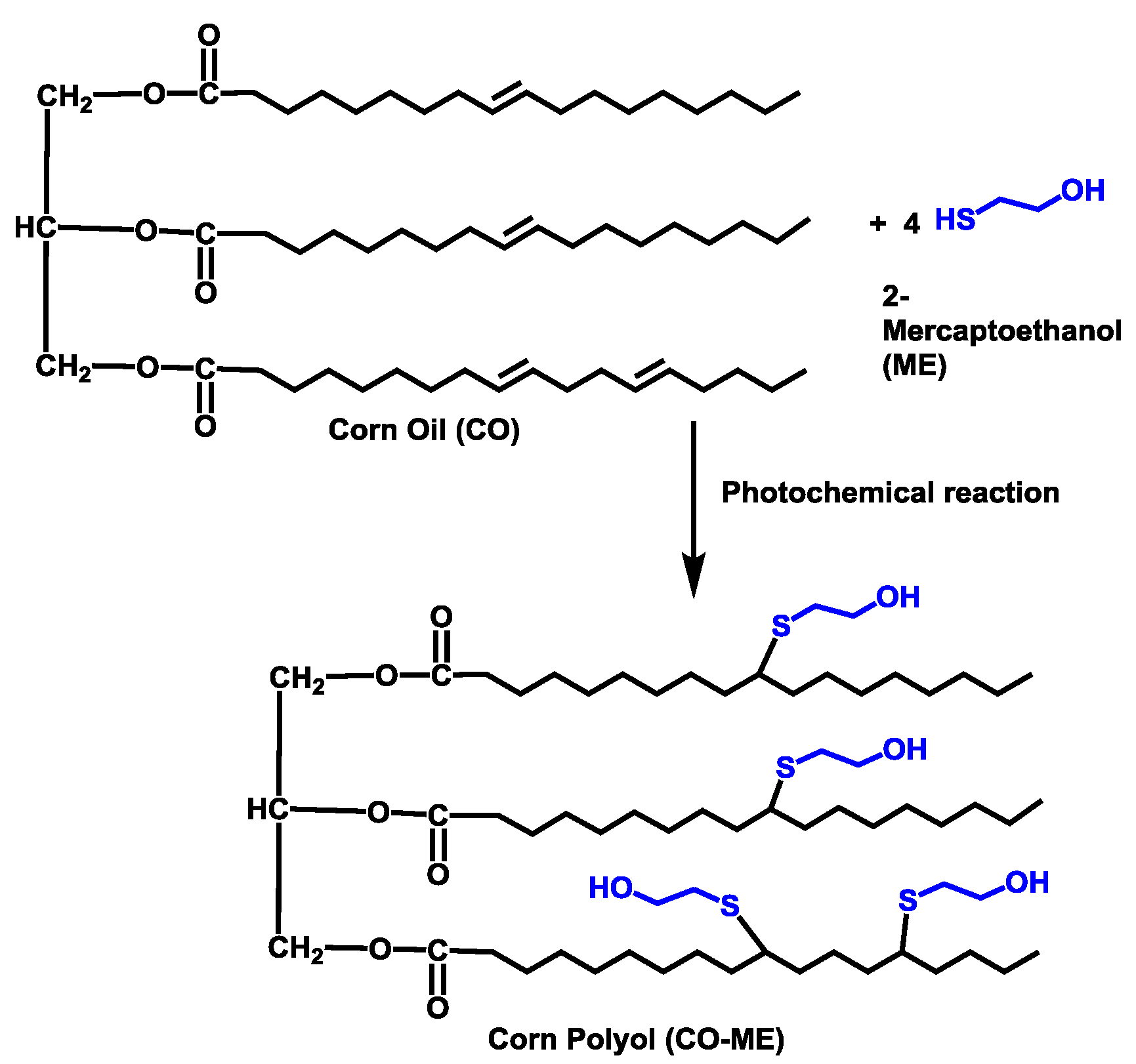
2.2. Synthesis of Corn Oil-Based Polyol
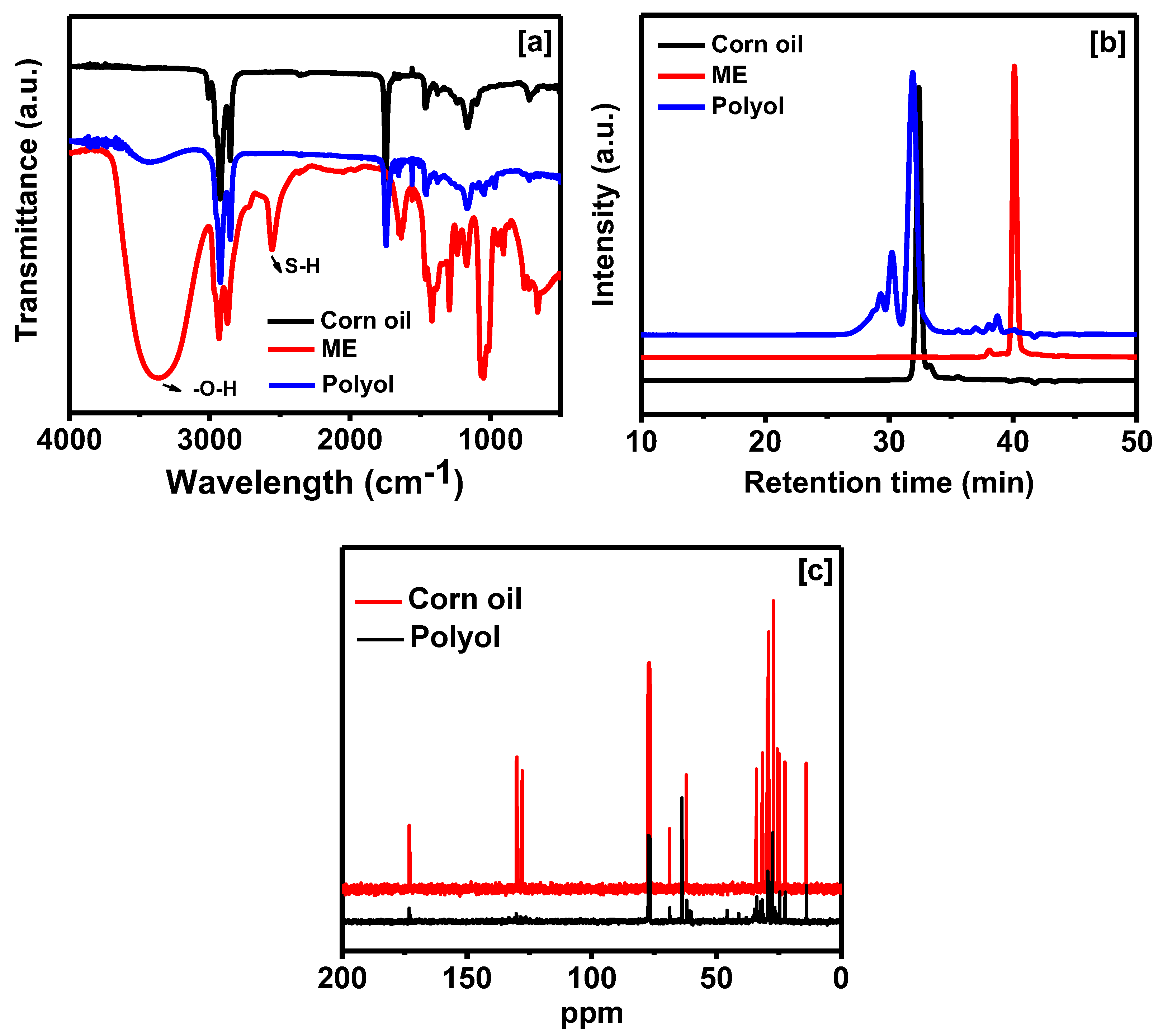
2.3. Characterization of Polyol
2.4. Preparation of Flame Retardant Rigid Polyurethane Foam
2.5. Characterization of Polyurethane Foams
3. Results and Discussion
3.1. Properties of Polyol
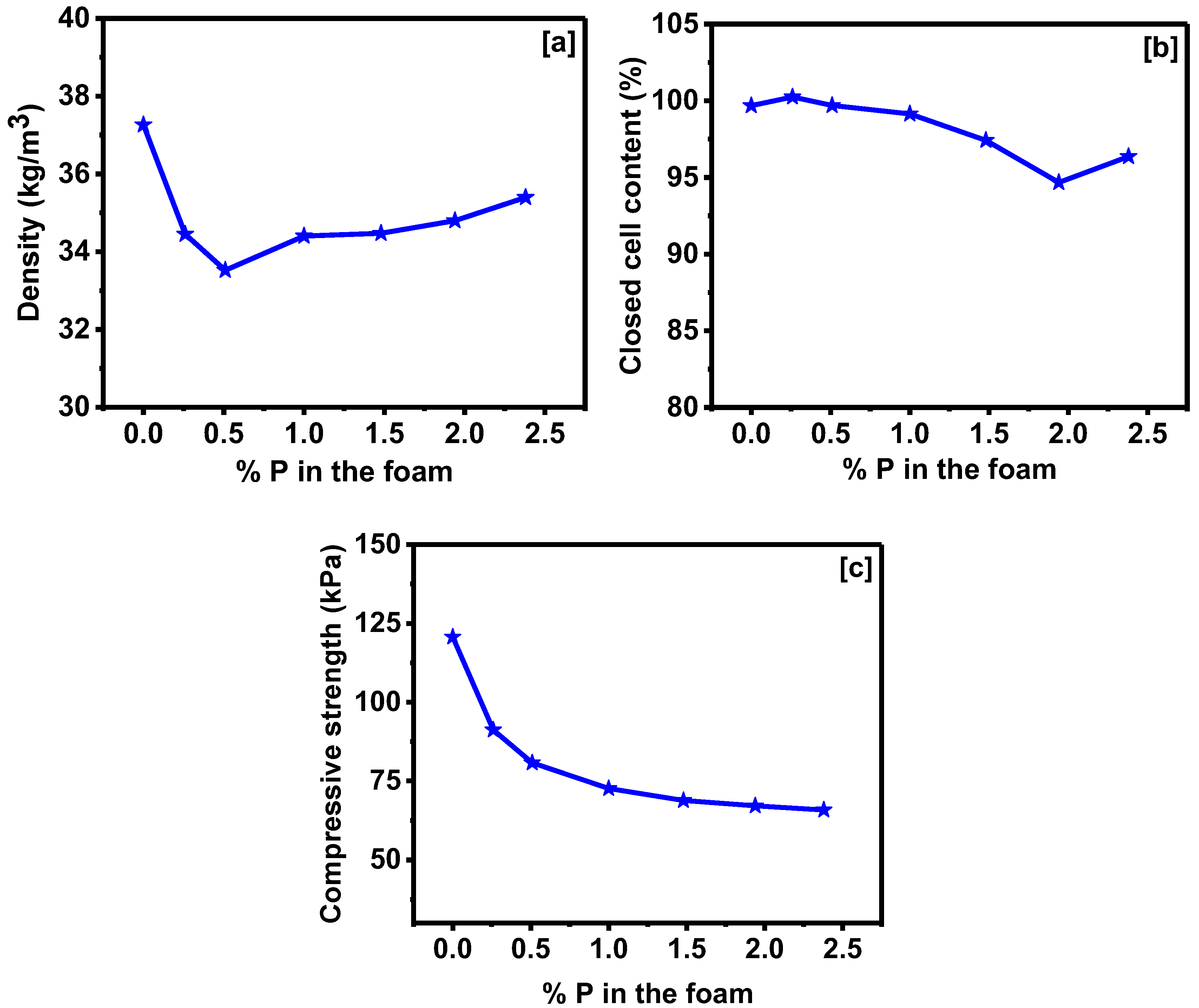
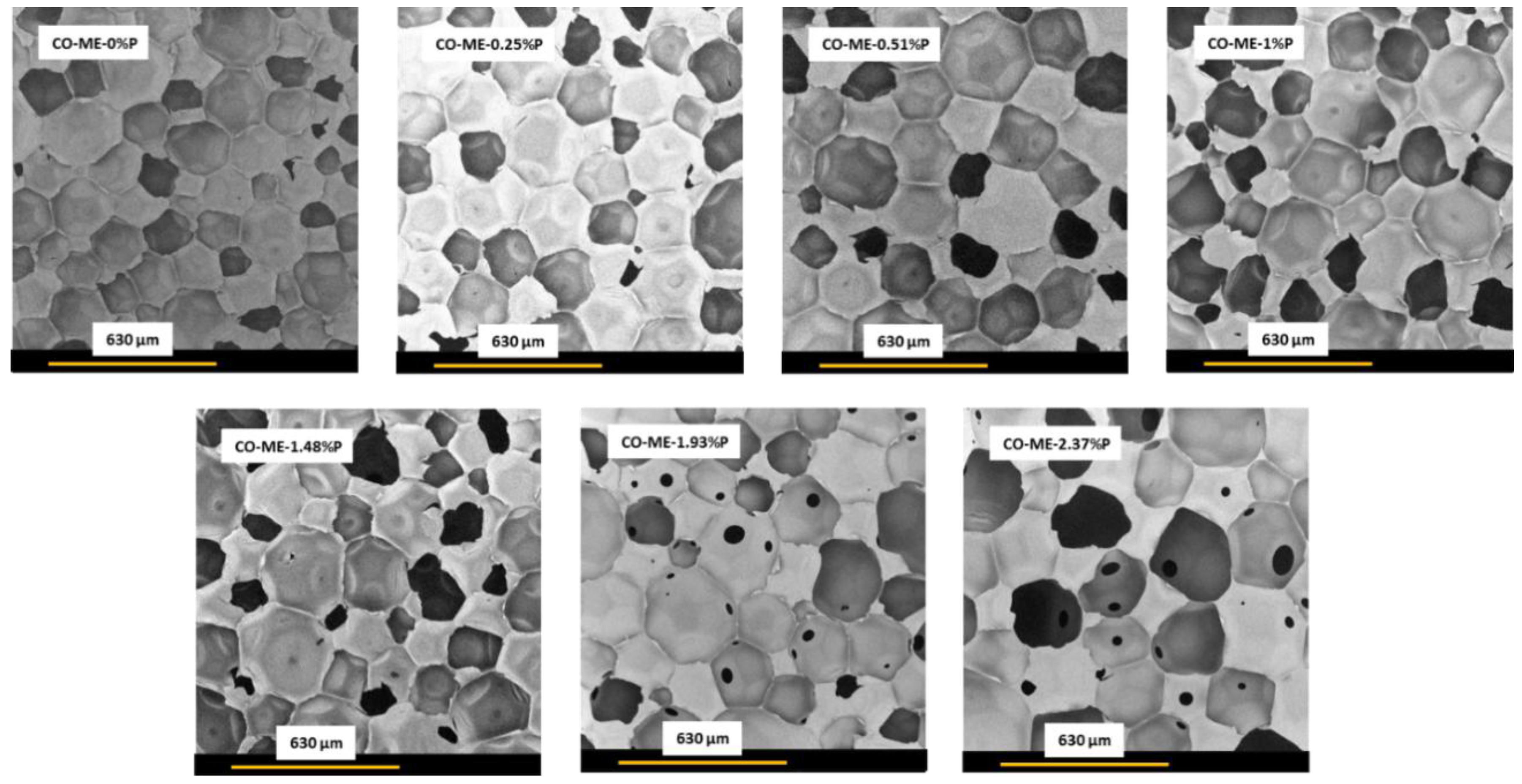
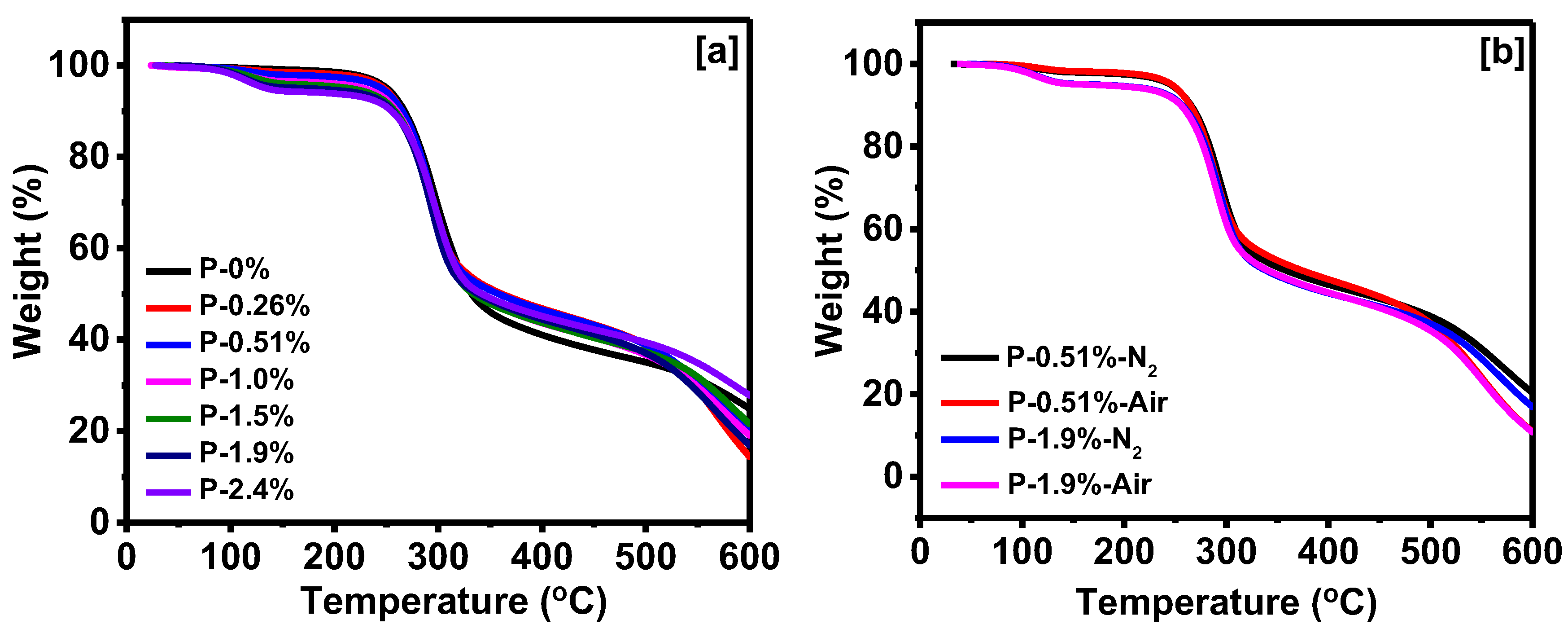
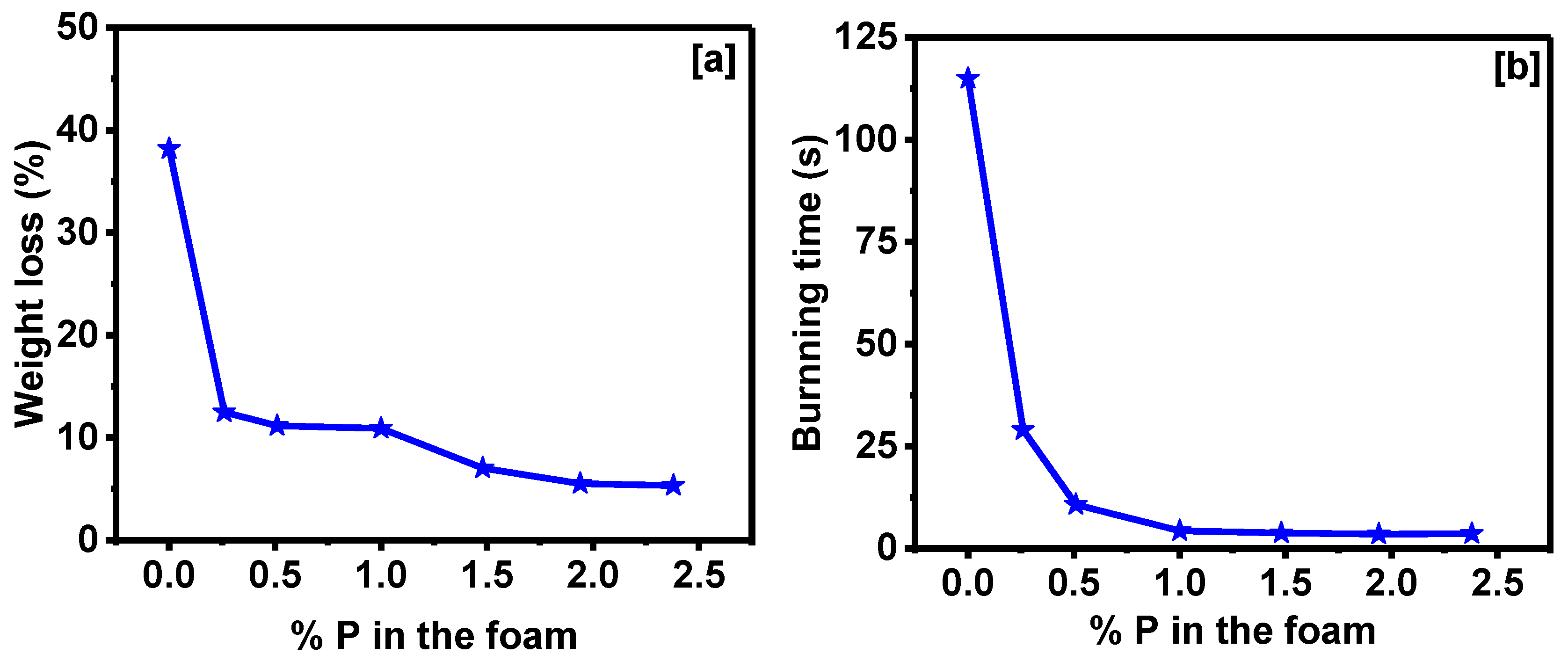
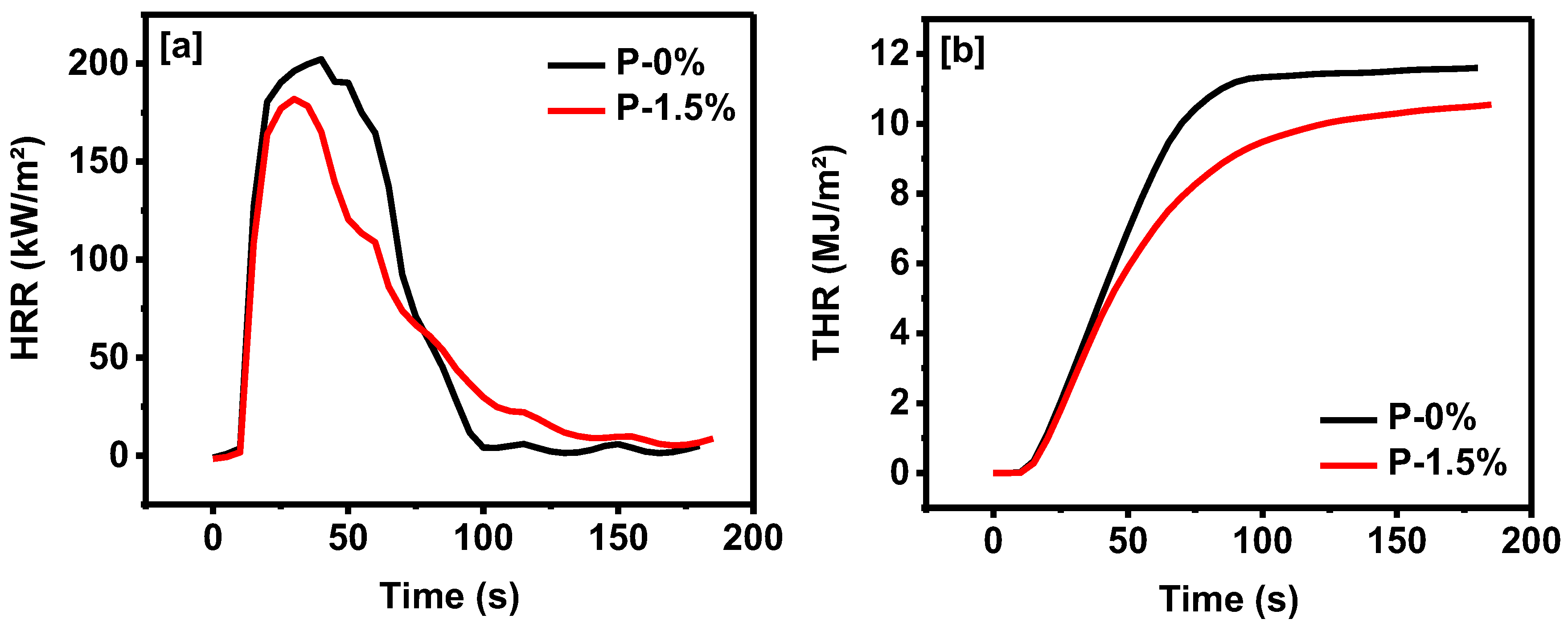
3.2. Properties of Corn Oil-Based Polyurethane Foams
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Li, Y.; Chen, R.; Kessler, M.R. Polyurethanes from Solvent-Free Vegetable Oil-Based Polyols. ACS Sustain. Chem. Eng. 2014, 2, 2465–2476. [Google Scholar] [CrossRef]
- Gaidukova, G.; Ivdre, A.; Fridrihsone, A.; Verovkins, A.; Cabulis, U.; Gaidukovs, S. Polyurethane rigid foams obtained from polyols containing bio-based and recycled components and functional additives. Ind. Crops Prod. 2017, 102, 133–143. [Google Scholar] [CrossRef]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased polyurethanes prepared from different vegetable oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Elbers, N.; Ranaweera, C.K.; Ionescu, M.; Wan, X.; Kahol, P.K.; Gupta, R.K. Synthesis of Novel Biobased Polyol via Thiol-Ene Chemistry for Rigid Polyurethane Foams. J. Renew. Mater. 2017, 5, 74–83. [Google Scholar] [CrossRef]
- Ding, H.; Huang, K.; Li, S.; Xu, L.; Xia, J.; Li, M. Synthesis of a novel phosphorus and nitrogen-containing bio-based polyol and its application in flame retardant polyurethane foam. J. Anal. Appl. Pyrolysis. 2017, 128, 102–113. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Radojcic, D.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly flame-retardant bio-based polyurethanes using novel reactive polyols. J. Appl. Polym. Sci. 2018, 135, 46027. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Highly flame retardant and bio-based rigid polyurethane foams derived from orange peel oil. Polym. Eng. Sci. 2018, 58, 2078–2087. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly flame-retardant polyurethane foam based on reactive phosphorus polyol and limonene-based polyol. J. Appl. Polym. Sci. 2018, 135, 46224. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Sustainable flame-retardant polyurethanes using renewable resources. Ind. Crops Prod. 2018, 123, 480–488. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Ionescu, M.; Bilic, N.; Wan, X.; Kahol, P.K.; Gupta, R.K. Biobased Polyols Using Thiol-Ene Chemistry for Rigid Polyurethane Foams with Enhanced Flame-Retardant Properties. J. Renew. Mater. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Soybean-oil-based waterborne polyurethane dispersions: Effects of polyol functionality and hard segment content on properties. Biomacromolecules 2008, 9, 3332–3340. [Google Scholar] [CrossRef]
- de Espinosa, L.M.; Meier, M.A. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Economic Research Service (ERS). Workbook: World Agricultural Supply and Demand Estimates at a Glance; United States Department of Agriculture (USDA): Washington, DC, USA, 2019.
- Ash, M.; Knisley, S. Oil Crops Outlook; OCS-16-H; United States Department of Agriculture (USDA): Washington, DC, USA, 2016. [Google Scholar]
- Kirpluks, M.; Cabulis, U.; Avots, A. Flammability of Bio-Based Rigid Polyurethane Foam as Sustainable Thermal Insulation Material. In Insulation Materials in Context of Sustainability; IntechOpen: London, UK, 2016. [Google Scholar]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.; Boutevin, B. Synthesis of Biobased Polyols by Thiol−Ene Coupling from Vegetable Oils. Macromolecules 2011, 44, 2489–2500. [Google Scholar] [CrossRef]
- Konig, A.; Fehrenbacher, U.; Hirth, T.; Kroke, E. Flexible Polyurethane Foam with the Flame-retardant Melamine. J. Cell. Plast. 2008, 44, 469–480. [Google Scholar] [CrossRef]
- Feng, F.; Qian, L. The flame retardant behaviors and synergistic effect of expandable graphite and dimethyl methylphosphonate in rigid polyurethane foams. Polym. Compos. 2014, 35, 301–309. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, C.; Fan, R.; Yuan, Y.; Xing, Y.; Ma, X. Halogen-free flame-retardant rigid polyurethane foam with a nitrogen–phosphorus flame retardant. J. Fire Sci. 2017, 35, 99–117. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Castor-oil derived nonhalogenated reactive flame-retardant-based polyurethane foams with significant reduced heat release rate. J. Appl. Polym. Sci. 2019, 136, 47276. [Google Scholar] [CrossRef]
- Tohka, A.; Zevenhoven, R. Processing Wastes and Waste-Derived Fuels Containing Brominated Flame Retardants; Helsinki University of Technology: Espoo, Finland, 2002. [Google Scholar]
- Chen, M.J.; Shao, Z.B.; Wang, X.L.; Chen, L.; Wang, Y.Z. Halogen-free flame-retardant flexible polyurethane foam with a novel nitrogen-phosphorus flame retardant. Ind. Eng. Chem. Res. 2012, 51, 9769–9776. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology: Shrewsbury, UK, 2007. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Shea, J.J. Polymeric foams: Mechanisms and materials. IEEE Electr. Insul. Mag. 2005, 21, 56. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The study of mechanical behavior and flame retardancy of castor oil phosphate-based rigid polyurethane foam composites containing expanded graphite and triethyl phosphate. Polym. Degrad. Stab. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- Liu, W.; Tang, Y.; Li, F.; Ge, X.G.; Zhang, Z.J. TG-FTIR characterization of flame retardant polyurethane foams materials. IOP Conf. Ser. Mater. Sci. Eng. 2016, 137, 012033. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Huang, Z.; Cao, Y.; Li, L. Continuous flame-retardant actions of two phosphate esters with expandable graphite in rigid polyurethane foams. Polym. Degrad. Stab. 2016, 130, 97–102. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of phosphorus valency on thermal behaviour of flame retarded polyurethane foams. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Pettigrew, A. Halogenated Flame Retardants. In Kirk-Othmer encyclopedia of chemical technology, 4th ed.; John Wiley & Sons: New York, NY, USA, 1993; Volume 10, pp. 954–976. [Google Scholar]
- Pan, H.; Wang, W.; Pan, Y.; Song, L.; Hu, Y.; Liew, K.M. Formation of layer-by-layer assembled titanate nanotubes filled coating on flexible polyurethane foam with improved flame retardant and smoke suppression properties. ACS Appl. Mater. Interfaces 2015, 7, 101–111. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, Z.; Luo, Y.; Jia, D.; Li, B. Environmentally Friendly Flame-Retardant and Its Application in Rigid Polyurethane Foam. Int. J. Polym. Sci. 2014, 2014, 7. [Google Scholar] [CrossRef]


| Compounds | P-0 wt.% | P-0.26 wt.% | P-0.51 wt.% | P-1 wt.% | P-1.5 wt.% | P-1.9 wt.% | P-2.4 wt.% |
|---|---|---|---|---|---|---|---|
| Jeffol-360 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| CO-ME | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| DMMP | 0 | 0.50 | 1.00 | 2.00 | 3.00 | 4.00 | 3.00 |
| Tegostab B-8404 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Niax-A1 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| T-12 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Water | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| MDI | 26.20 | 26.20 | 26.20 | 26.20 | 26.20 | 26.20 | 26.20 |
| Characteristics | P-0 wt.% | P-0.26 wt.% | P-0.51 wt.% | P-1 wt.% | P-1.5 wt.% | P-1.9 wt.% | P-2.4 wt.% |
|---|---|---|---|---|---|---|---|
| Mix time | 7 | 6 | 7 | 6 | 6 | 7 | 7 |
| Cream time | 6 | 6 | 6 | 7 | 6 | 6 | 7 |
| Rise time | 12 | 11 | 12 | 12 | 11 | 12 | 11 |
| Tack free time | 16 | 16 | 15 | 16 | 15 | 16 | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramanujam, S.; Zequine, C.; Bhoyate, S.; Neria, B.; Kahol, P.K.; Gupta, R.K. Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams. C 2019, 5, 13. https://doi.org/10.3390/c5010013
Ramanujam S, Zequine C, Bhoyate S, Neria B, Kahol PK, Gupta RK. Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams. C. 2019; 5(1):13. https://doi.org/10.3390/c5010013
Chicago/Turabian StyleRamanujam, Sneha, Camila Zequine, Sanket Bhoyate, Brooks Neria, Pawan K. Kahol, and Ram K. Gupta. 2019. "Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams" C 5, no. 1: 13. https://doi.org/10.3390/c5010013
APA StyleRamanujam, S., Zequine, C., Bhoyate, S., Neria, B., Kahol, P. K., & Gupta, R. K. (2019). Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams. C, 5(1), 13. https://doi.org/10.3390/c5010013