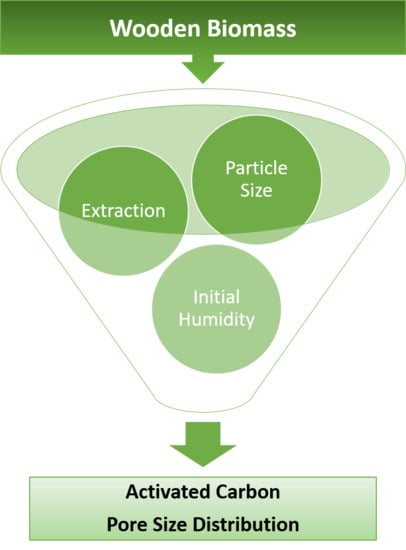
Physical Activation of Wooden Chips and the Effect of Particle Size, Initial Humidity, and Acetic Acid Extraction on the Properties of Activated Carbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomasses Used for Carbonization and Activation
2.2. Determination of Moisture Content
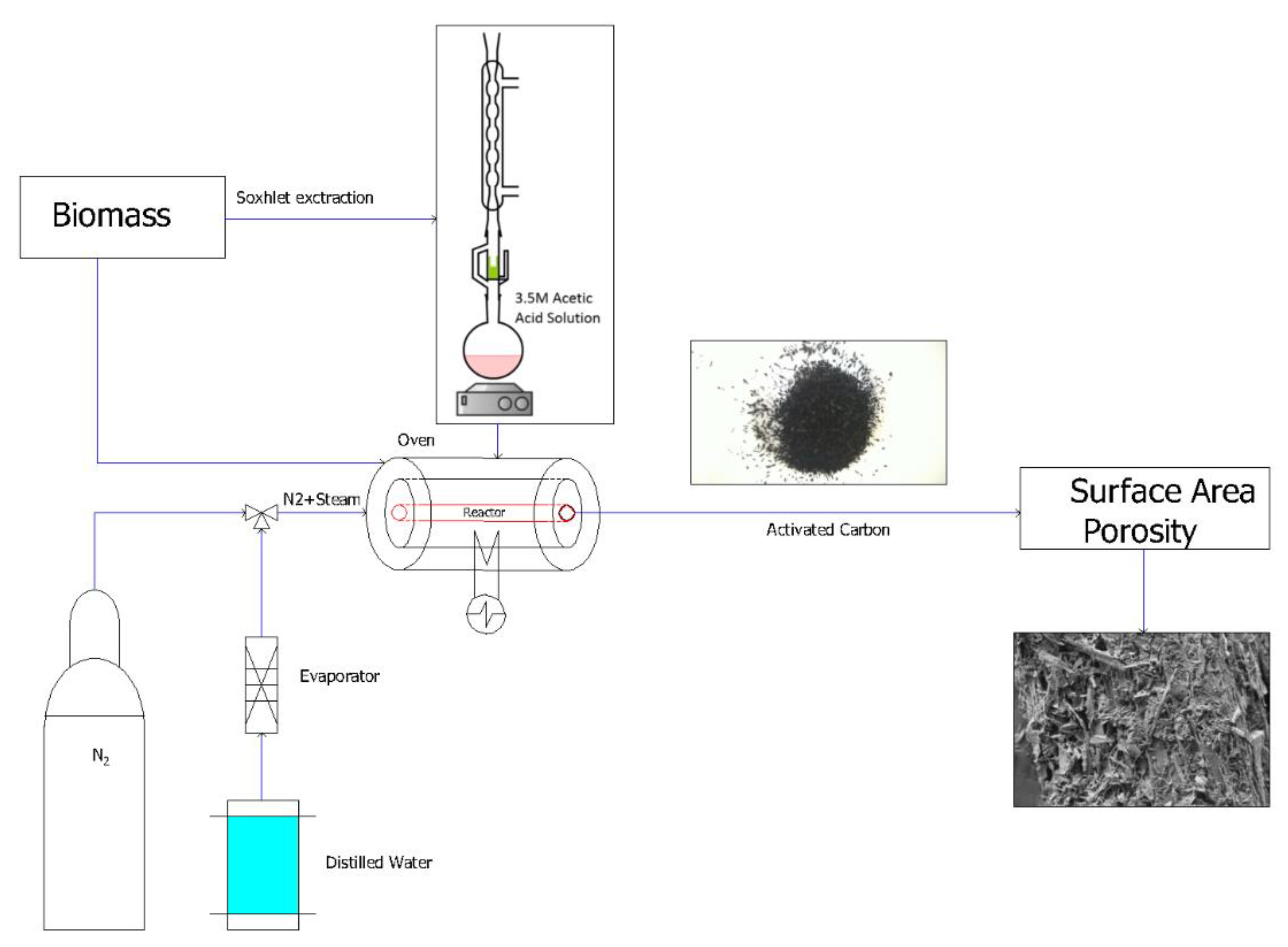
2.3. Carbonization and Activation Procedure
2.4. Determination of Yields and Total Carbon Content
2.5. Specific Surface Area and Pore Size Distribution
2.6. Extraction Process
3. Results and Discussion
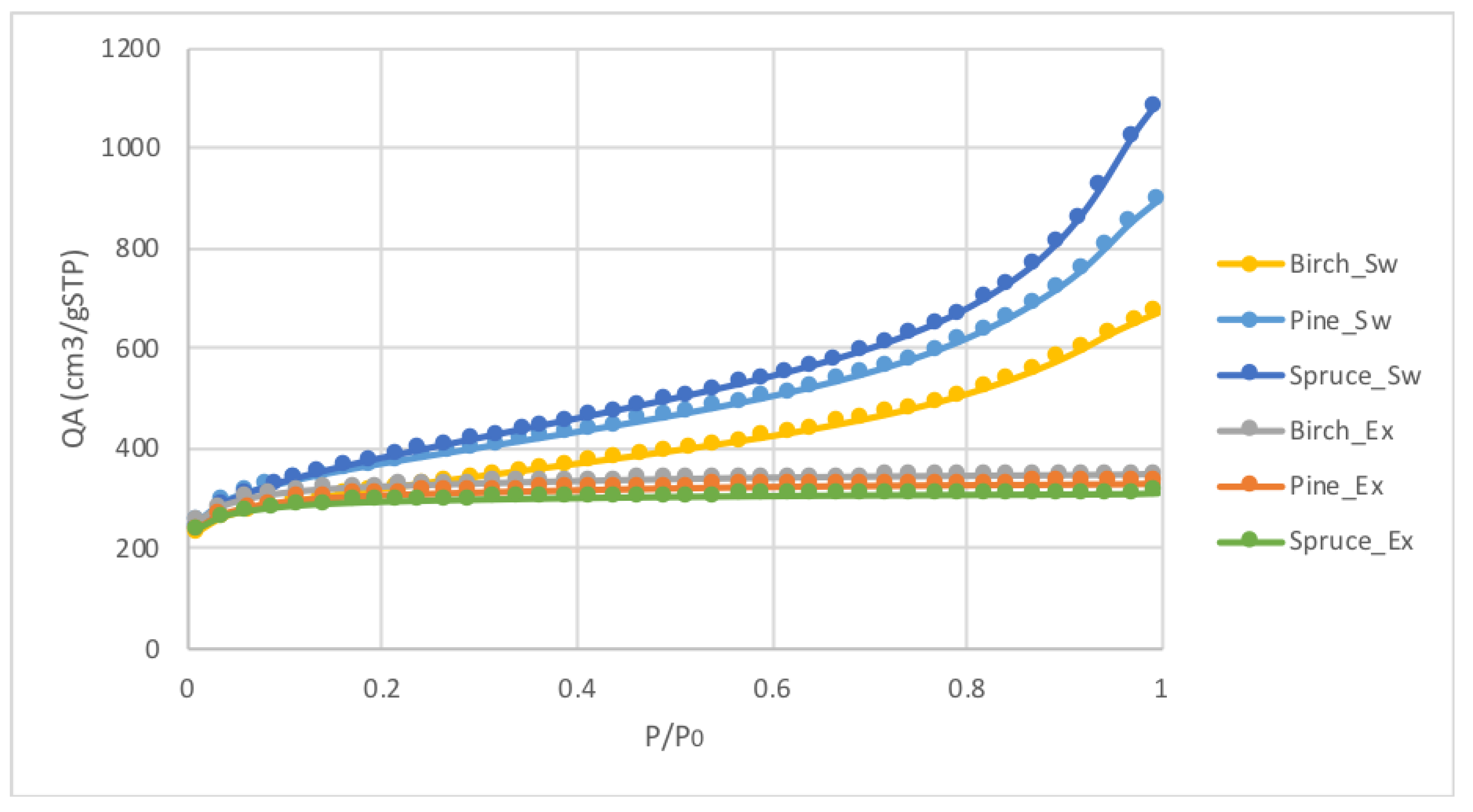
3.1. Effect of Particle Size on AC Porosity
3.2. Effect of the Extraction Process on AC Porosity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daneshvar, N.; Salari, D.; Aber, S. Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solutions by soya cake. J. Hazard. Mater. 2002, 94, 49–61. [Google Scholar] [CrossRef]
- Mohammad-Khah, A.; Ansari, R. Activated Charcoal: Preparation, characterization and Applications: A review article. Int. J. Chem. Tech. Res. 2009, 1, 859–864. [Google Scholar]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies, Bioresour. Technology 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Prati, L.; Bergna, D.; Villa, A.; Spontoni, P.; Bianchi, C.L.; Romar, H.; Lassi, U. Carbons from second generation biomass as sustainable supports for catalytic systems. Catal. Today 2018, 301, 239–243. [Google Scholar] [CrossRef]
- Lahti, R.; Bergna, D.; Romar, H.; Hu, T.; Comazzi, A.; Pirola, C.; Bianchi, C.L.; Lassi, U. Characterization of Cobalt Catalysts on Biomass-Derived Carbon Supports. Top. Catal. 2017, 16, 1415–1428. [Google Scholar] [CrossRef]
- Mäkelä, E.; Lahti, R.; Jaatinen, S.; Romar, H.; Hu, T.; Puurunen, R.L.; Lassi, U.; Karinen, R. Study of Ni, Pt, and Ru Catalysts on Wood-based Activated Carbon Supports and their Activity in Furfural Conversion to 2-Methylfuran. Chem. Cat Chem. 2018, 10, 1–16. [Google Scholar] [CrossRef]
- Yabushita, M.; Kobayashi, H.; Haraa, K.; Fukuoka, A. Quantitative evaluation of ball-milling effects on the hydrolysis of cellulose catalysed by activated carbon. Catal. Sci. Technol. 2014, 4, 2312. [Google Scholar] [CrossRef]
- Klinke, H.B.; Ahring, B.K.; Schmidt, A.S.; Thomsen, A.B. Characterization of degradation products from alkaline wet oxidation of wheat straw, Bioresour. Technology 2002, 82, 15–26. [Google Scholar]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage, Bioresour. Technology 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.E.; Rao, R.M. Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties1, Bioresour. Technology 2000, 71, 113–123. [Google Scholar]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Molina, A.; Mondragon, F. Reactivity of coal gasification with steam and CO2. Fuel 1998, 77, 1831–1839. [Google Scholar] [CrossRef]
- Varila, T.; Bergna, D.; Lahti, R.; Romar, H.; Hu, T.; Lassi, U. Activated Carbon Production from Peat Using ZnCl2: Characterization and Applications. Bioresources 2017, 12, 8078–8092. [Google Scholar]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Lladó, J.; Gil, R.R.; Lao-Luque, C.; Solé-Sardans, M.; Fuente, E.; Ruiz, B. Highly microporous activated carbons derived from biocollagenic wastes of the leather industry as adsorbents of aromatic organic pollutants in water. J. Environ. Chem. Eng. 2017, 3, 2090–2100. [Google Scholar] [CrossRef]
- Danmaliki, G.I.; Saleh, T.A. Influence of conversion parameters of waste tires to activated carbon on adsorption of dibenzothiophene from model fuels. J. Clean. Prod. 2016, 117, 50–55. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Sun, Y.; Zhu, L. Preparation, characterization and application of lignin-based activated carbon from black liquor lignin by steam activation. Chem. Eng. J. 2013, 228, 1074–1082. [Google Scholar] [CrossRef]
- Chen, F.; Hu, Y.; Dou, X.; Chen, D.; Dai, X. Chemical forms of heavy metals in pyrolytic char of heavy metal-implanted sewage sludge and their impacts on leaching behaviors. J. Anal. Appl. Pyrolysis 2015, 116, 152–160. [Google Scholar] [CrossRef]
- Jiang, L.; Hua, S.; Sun, L.; Su, S.; Xu, K.; He, L.; Xiang, J. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef]
- Chaala, A.; Darmstadt, H.; Roy, C. Acid-base method for the demineralization of pyrolytic carbon black. Fuel Process. Technol. 1996, 46, 1–15. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309. [Google Scholar] [CrossRef]
| Sample | Description |
|---|---|
| Ar | Carbonized and activated as received |
| D | Oven-dried before carbonization and activation |
| Sw | Sawdust activated untreated |
| Ex | Sawdust acetic acid-extracted and activated |
| Sample | Total Carbon Content (%) | Yield (%) | Humidity (%) |
|---|---|---|---|
| Spruce_2 cm_Ar | 71.1 | 5 | 50 |
| Spruce 6 mm_Ar | 94.5 | 4.9 | - |
| Spruce_2 cm_D | - | 12 | - |
| Spruce_2 mm_Ar | - | 5 | - |
| Birch_2 cm_Ar | 87.6 | 4 | 52 |
| Birch_6 mm_Ar | 89.9 | 2.5 | - |
| Birch_2 cm_D | - | 14 | - |
| Birch_2 mm_Ar | - | 4.5 | - |
| Calculation Method | Unit | Spruce 2 cm_Ar | Spruce 2 cm_D | Spruce 6 mm_Ar | Spruce 2 mm_Ar | Birch 2 cm_Ar | Birch 2 cm_D | Birch 6 mm_Ar | Birch 2 mm_Ar |
|---|---|---|---|---|---|---|---|---|---|
| BET | |||||||||
| SSA | m2/g | 1066 | 983 | 1048 | 990 | 906 | 833 | 1063 | 1029 |
| Pore volume | cm3/g | 0.848 | 0.710 | 0.800 | 0.700 | 0.625 | 0.590 | 0.765 | 0.740 |
| C value | 1438 | 2229 | 1646 | 2783 | 2702 | 2371 | 934 | 3123 | |
| Langmuir surface area (c.corr. > 0.999) | m2/g | 1323 | 1217 | 1287 | 1227 | 1121 | 1040 | 1280 | 1262 |
| t-plot | |||||||||
| micropore volume | cm3/g | 0.221 | 0.208 | 0.205 | 0.210 | 0.186 | 0.165 | 0.225 | 0.199 |
| micropore area | m2/g | 499 | 398 | 496 | 509 | 364 | 322 | 551 | 472 |
| External surface area | m2/g | 555 | 505 | 576 | 498 | 472 | 473 | 538 | 575 |
| DFT | |||||||||
| pore volume | cm3/g | 0.736 | 0.627 | 0.689 | 0.623 | 0.535 | 0.544 | 0.656 | 0.647 |
| µpores | cm3/g | 0.289 | 0.276 | 0.289 | 0.279 | 0.256 | 0.236 | 0.298 | 0.282 |
| mesopores | cm3/g | 0.446 | 0.348 | 0.397 | 0.343 | 0.27 | 0.305 | 0.355 | 0.355 |
| macropores | cm3/g | 0.001 | 0.003 | 0.003 | 0.001 | 0.009 | 0.003 | 0.003 | 0.010 |
| µpores | % | 39 | 44 | 42 | 45 | 48 | 43 | 45 | 44 |
| mesopores | % | 61 | 56 | 58 | 55 | 50 | 56 | 54 | 55 |
| macropores | % | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 |
| Calculation Method | Unit | Spruce_ Sw | Birch_ Sw | Pine_ Sw | Spruce_ Ex | Birch_ Ex | Pine_ Ex |
|---|---|---|---|---|---|---|---|
| BET | |||||||
| SSA | m2/g | 1302 | 1190 | 1296 | 1125 | 1109 | 1141 |
| Pore volume | cm3/g | 1.669 | 1.053 | 1.260 | 0.538 | 0.476 | 0.511 |
| C value | 3611 | 1415 | 1657 | 1095 | 3812 | 4043 | |
| Langmuir surface area (c.corr. > 0.999) | m2/g | 1407 | 1393 | 1464 | 1530 | 1335 | 1462 |
| t-plot | |||||||
| micropore volume | cm3/g | 0.090 | 0.160 | 0.138 | 0.397 | 0.321 | 0.396 |
| micropore area | m2/g | 155 | 346 | 275 | 993 | 824 | 990 |
| External surface area | m2/g | 1147 | 843 | 1020 | 231 | 284 | 151 |
| DFT | |||||||
| pore volume | cm3/g | 1.370 | 0.973 | 1.280 | 0.455 | 0.395 | 0.427 |
| µpores | cm3/g | 0.290 | 0.310 | 0.330 | 0.408 | 0.376 | 0.392 |
| Mesopores | cm3/g | 1.050 | 0.640 | 0.900 | 0.046 | 0.016 | 0.033 |
| Macropores | cm3/g | 0.030 | 0.023 | 0.05 | 0.001 | 0.003 | 0.002 |
| µpores | % | 21 | 32 | 26 | 90 | 95 | 92 |
| Mesopores | % | 76 | 66 | 70 | 10 | 4 | 7 |
| Macropores | % | 2 | 2 | 4 | 0 | 1 | 1 |
| Sample | Total Carbon Content (%) | Yield (%) |
|---|---|---|
| Spruce_Ex | 89.5 | 14 |
| Birch_Ex | 87.8 | 17 |
| Pine_Ex | 84.1 | 12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergna, D.; Romar, H.; Lassi, U. Physical Activation of Wooden Chips and the Effect of Particle Size, Initial Humidity, and Acetic Acid Extraction on the Properties of Activated Carbons. C 2018, 4, 66. https://doi.org/10.3390/c4040066
Bergna D, Romar H, Lassi U. Physical Activation of Wooden Chips and the Effect of Particle Size, Initial Humidity, and Acetic Acid Extraction on the Properties of Activated Carbons. C. 2018; 4(4):66. https://doi.org/10.3390/c4040066
Chicago/Turabian StyleBergna, Davide, Henrik Romar, and Ulla Lassi. 2018. "Physical Activation of Wooden Chips and the Effect of Particle Size, Initial Humidity, and Acetic Acid Extraction on the Properties of Activated Carbons" C 4, no. 4: 66. https://doi.org/10.3390/c4040066