Design and Preparation of Biomass-Derived Carbon Materials for Supercapacitors: A Review
Abstract
1. Introduction
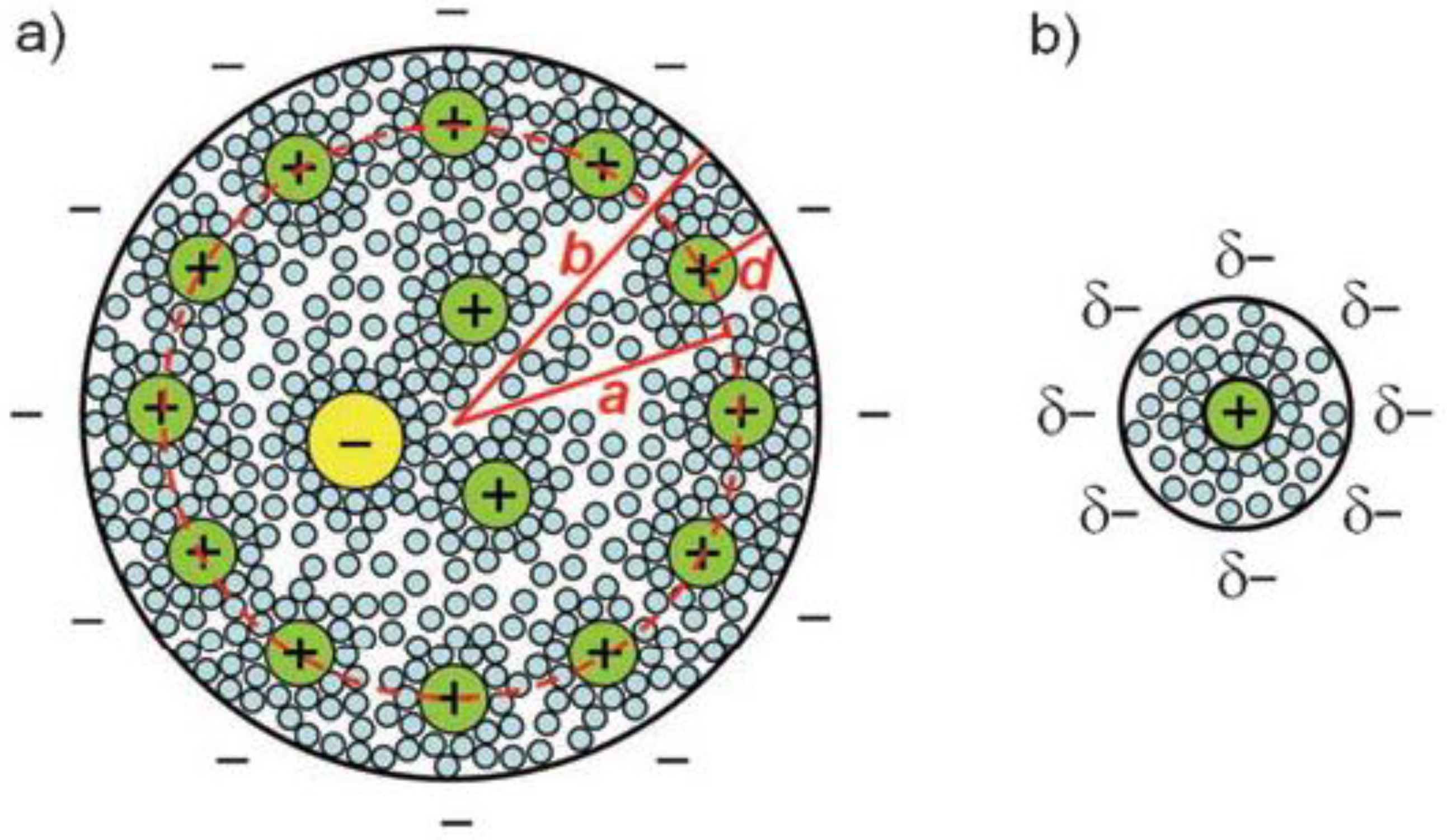
- (a)
- Cost-effectiveness: the precursors of biomass-derived carbons are cheap and abundant, which are mostly from plant organs, food and animal wastes, and microorganisms.
- (b)
- In-situ nanoporous structure formation: the skeleton of the biomacromolecules is preserved as they are converted to carbon under inert gas protection, forming interconnected conductive carbon structures with nanopores generated in-situ.
- (c)
- Versatility in products and processing: various kinds of bioprecursors can be converted into biomass-derived carbons through similar processing steps, including carbonization, activation, and purification. On the other hand, different chemicals, i.e., metallic compounds, can be introduced to the conversion process which further endow the biomass-derived carbons with exceptional electrochemical capacitive and catalytic properties.
- (d)
- Environmentally friendly: compared with the synthesis processes of CNT and graphene, the fabrication of biomass-derived carbons does not require high-pressure conditions and harsh chemicals, therefore it is more energy-saving and environmentally friendly. On the other hand, the utilization of biowastes as precursors to fabricate high-performance biomass-derived carbons also represents the state-of-the-art green pathway to obtain functional carbon materials.
2. Precursors
2.1. Plant-Based Biomass
2.2. Fruit-Based Biomass
2.3. Microorganism Based Biomass
2.4. Animal-Based Precursors
2.5. Principles for the Precursor Selection of Biomass-Derived Carbon
- (i)
- The precursor biomass should contain high contents of highly crosslinked, high molecular weight, and thermally stable biomacromolecules, such as lignin, chitin and keratin, for higher rate of aromatic carbon formation and higher yield of carbon during the thermal carbonization process.
- (ii)
- The precursor biomass should contain low contents of non-crosslinked, low molecular weight, and aliphatic compounds, which provide insignificant contribution to the yield of carbon and impede the formation of aromatic carbon by generating volatile compounds that prevent fusion and flow.
- (iii)
- The precursor biomass should contain low elemental contents of oxygen for the oxygen would impede the aromatic carbon formation and increase the biochemical oxygen demand (BOD); while it should contain high elemental contents of nitrogen for the in-situ generation of nitrogen-doped carbon with higher conductivity.
3. Structures and Properties
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nover, G.; Stoll, J.B.; Gonna, J. Promotion of graphite formation by tectonic stress—A laboratory experiment. Geophys. J. Int. 2005, 160, 1059–1067. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W. Formation of diamond in the Earth’s mantle. J. Phys. Condens. Matter 2009, 21, 364206. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Balu, A.M.; Waal, J.C.; Luque, R. Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Jung, S.; Myung, Y.; Kim, B.N.; Kim, I.G.; You, I.K.; Kim, T.Y. Activated Biomass-derived Graphene-based Carbons for Supercapacitors with High Energy and Power Density. Sci. Rep. 2018, 8, 1915. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Charge storage mechanism in nanoporous carbons and its consequence for electrical double layer capacitors. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Olivier, J.; Vaxelaire, J.; Hoadley, A.F.A. Electrical field: A historical review of its application and contributions in wastewater sludge dewatering. Water Res. 2010, 44, 2381–2407. [Google Scholar] [CrossRef] [PubMed]
- Madhu, R.; Veeramani, V.; Chen, S. Heteroatom-enriched and renewable banana-stem-derived porous carbon for the electrochemical determination of nitrite in various water samples. Sci. Rep. 2014, 4, 4679. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, S.; Sun, Y.; Wang, G.; Chen, H.; Yu, X.; Su, Y.; Chen, G. Preparation of a Highly Porous Carbon Material Based on Quinoa Husk and Its Application for Removal of Dyes by Adsorption. Materials 2018, 11, 1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, F.; Qi, X. A corn stalk-derived porous carbonaceous adsorbent for adsorption of ionic liquids from aqueous solution. RSC Adv. 2016, 6, 32505. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising Porous Carbon Derived from Celtuce Leaves with Outstanding Supercapacitance and CO2 Capture Performance. ACS Appl. Mater. Interfaces 2012, 4, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Jalilov, A.S.; Ruan, G.; Hwang, C.C.; Schipper, D.E.; Tour, J.J.; Li, Y.; Fei, H.; Samuel, E.L.G.; Tour, J.M. Asphalt-Derived High Surface Area Activated Porous Carbons for Carbon Dioxide Capture. ACS Appl. Mater. Interfaces 2015, 7, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Chen, C.; Li, X.; Zhang, J. Popcorn-Derived Porous Carbon for Energy Storage and CO2 Capture. Langmuir 2016, 32, 8042–8049. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, L.; Ren, J.; Zhang, M.; Luo, X.; Li, B.; Song, Z.; Zhou, X. Wheat Straw-Derived N-, O-, and S-Tri-doped Porous Carbon with Ultrahigh Specific Surface Area for Lithium-Sulfur Batteries. Materials 2018, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, J.; Nagaraju, D.H.; Jiang, L.; Marinov, V.R.; Lubineau, G.; Alshareef, H.N.; Oh, M. Flexible, Highly Graphitized Carbon Aerogels Based on Bacterial Cellulose/Lignin: Catalyst-Free Synthesis and its Application in Energy Storage Devices. Adv. Funct. Mater. 2015, 25, 3193–3202. [Google Scholar] [CrossRef]
- Wahid, M.; Puthusseri, D.; Phase, D.; Ogale, S. Enhanced Capacitance Retention in a Supercapacitor Made of Carbon from Sugarcane Bagasse by Hydrothermal Pretreatment. Energy Fuels 2014, 28, 4233–4240. [Google Scholar] [CrossRef]
- Karnan, M.; Subramani, K.; Sudhan, N.; Ilayaraja, N.; Sathish, M. Aloe vera Derived Activated High-Surface-Area Carbon for Flexible and High-Energy Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Subramani, K.; Sathish, M.; Gautam, U.K. Soya Derived Heteroatom Doped Carbon as a Promising Platform for Oxygen Reduction, Supercapacitor and CO2 Capture. Carbon 2017, 114, 679–689. [Google Scholar] [CrossRef]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379. [Google Scholar] [CrossRef]
- Chen, C.; Yu, D.; Zhao, G.; Du, B.; Tang, W.; Sun, L.; Sun, Y.; Besenbacher, F.; Yu, M. Three-dimensional scaffolding framework of porous carbon nanosheets derived from plant wastes for high-performance supercapacitors. Nano Energy 2016, 27, 377–389. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Orange-Peel-Derived Carbon: Designing Sustainable and High-Performance Supercapacitor Electrodes. J. Carbon Res. 2017, 3, 25. [Google Scholar] [CrossRef]
- Dang, Y.Q.; Ren, S.Z.; Liu, G.; Cai, J.; Zhang, Y.; Qiu, J. Electrochemical and Capacitive Properties of Carbon Dots/Reduced Graphene Oxide Supercapacitors. Nanomaterials 2016, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.R.; Choi, J.W.; Yang, Y.; Mantia, F.L.; Zhang, Y.; Cui, Y. Carbon Nanofiber Supercapacitors with Large Areal Capacitances. Appl. Phys. Lett. 2009, 95, 243109. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, J.; Hur, J. Stretchable Supercapacitors Based on Carbon Nanotubes-Deposited Rubber Polymer Nanofibers Electrodes with High Tolerance against Strain. Nanomaterials 2018, 8, 541. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Amirkhiz, B.S.; Tan, X.; Xu, Z.; Wang, H.; Olsen, B.C.; Holt, C.M.B.; Mitlin, D. Carbonized Chicken Eggshell Membranes with 3D Architectures as High-Performance Electrode Materials for Supercapacitors. Adv. Energy Mater. 2012, 2, 431–437. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.; Liu, S.; Wang, Y.; Qiu, J. Sustainable Synthesis and Assembly of Biomass-Derived B/N Co-Doped Carbon Nanosheets with Ultrahigh Aspect Ratio for High-Performance Supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. [Google Scholar] [CrossRef]
- Long, C.; Chen, X.; Jiang, L.; Zhi, L.; Fan, Z. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 2015, 12, 141–151. [Google Scholar] [CrossRef]
- Duan, B.; Gao, X.; Yao, X.; Fang, Y.; Huang, L.; Zhou, J.; Zhang, L. Unique elastic N-doped carbon nanofibrous microspheres with hierarchical porosity derived from renewable chitin for high rate supercapacitors. Nano Energy 2016, 27, 482–491. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Li, L.; Wei, X. A versatile biomass derived carbon material for oxygen reduction reaction, supercapacitors and oil/water separation. Nano Energy 2017, 33, 334–342. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wei, T.; Fan, Z.; Yan, P. Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Activated Carbon from Biomass Transfer for High-Energy Density Lithium-Ion Supercapacitors. Adv. Energy Mater. 2016, 6, 1600802. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, F.; Smith, R.L.; Yan, L.; Li, L.; Qi, X. Black liquor-derived porous carbons from rice straw for high-performance supercapacitors. Chem. Eng. J. 2017, 316, 770–777. [Google Scholar] [CrossRef]
- Liu, W.; Mei, J.; Liu, G.; Kou, Q.; Yi, T.; Xiao, S. Nitrogen-Doped Hierarchical Porous Carbon from Wheat Straw for Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 11595–11605. [Google Scholar] [CrossRef]
- Hu, C.C.; Wang, C.C.; Wu, F.C.; Tseng, R.L. Characterization of Pistachio Shell-derived Carbons Activated by a Combination of KOH and CO2 for Electric Double-layer Capacitors. Electrochim. Acta 2007, 52, 2498–2505. [Google Scholar] [CrossRef]
- Su, X.; Cheng, M.; Fu, L.; Yang, J.; Zheng, X.; Guan, X. Superior Supercapacitive Performance of Hollow Activated Carbon Nanomesh with Hierarchical Structure Derived from Poplar Catkins. J. Power Sources 2017, 362, 27–38. [Google Scholar] [CrossRef]
- Dai, C.; Wan, J.; Geng, W.; Song, S.; Ma, F.; Shao, J. KOH direct treatment of kombucha and in situ activation to prepare hierarchical porous carbon for high-performance supercapacitor electrodes. J. Solid State Electrochem. 2017, 21, 2929–2938. [Google Scholar] [CrossRef]
- Zhu, H.; Yin, J.; Wang, X.; Wang, H.; Yang, X. Microorganism-Derived Heteroatom-Doped Carbon Materials for Oxygen Reduction and Supercapacitors. Adv. Funct. Mater. 2013, 23, 1305–1312. [Google Scholar] [CrossRef]
- Li, F.; Qin, F.; Zhang, K.; Fang, J.; Lai, Y.; Li, J. Hierarchically porous carbon derived from banana peel for lithium sulfur battery with high areal and gravimetric sulfur loading. J. Power Sources 2017, 362, 160–167. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Nonaqueous Electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Karnan, M.; Subramani, K.; Srividhya, P.K.; Sathish, M. Electrochemical Studies on Corncob Derived Activated Porous Carbon for Supercapacitors Application in Aqueous and Non-aqueous Electrolytes. Electrochim. Acta 2017, 228, 586–596. [Google Scholar] [CrossRef]
- Berenguer, R.; Garcia-Mateos, F.J.; Ruiz-Rosas, R.; Cazorla-Amoros, D.; Morallon, E.; Rodriguez-Mirasol, J.; Cordero, T. Biomass-derived Binderless Fibrous Carbon Electrodes for Ultrafast Energy Storage. Green Chem. 2016, 18, 1506. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Deng, S.; Zhan, J.; Fang, R.; Xia, Y.; Wang, X.; Zhang, Q.; Tu, J. Popcorn Inspired Porous Macrocellular Carbon: Rapid Puffing Fabrication from Rice and Its Applications in Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1701110. [Google Scholar] [CrossRef]
- Cheng, P.; Gao, S.; Zang, P.; Yang, X.; Bai, Y.; Xu, H.; Liu, Z.; Lei, Z. Hierarchically porous carbon by activation of shiitake mushroom for capacitive energy storage. Carbon 2015, 93, 315–324. [Google Scholar] [CrossRef]
- Hao, R.; Yang, Y.; Wang, H.; Jia, B.; Ma, G.; Yu, D.; Guo, L. Direct chitin conversion to N-doped amorphous carbon nanofibers for high-performing full sodium-ion batteries. Nano Energy 2018, 45, 220–228. [Google Scholar] [CrossRef]
- Rath, N.C.; Makkar, S.; Packialakshmi, B.; Donoghue, A.M. Egg Shell Membrane Improves Immunity of Post Hatch Poultry: A Paradigm for Nutritional Immunomodulation. United States Department of Agriculture. Available online: https://www.ars.usda.gov/alternativestoantibiotics/Symposium2016/includes/Oral%20Presentations/4%20-%20Immune%20Related%20Products/pdfs/3%20RathATA%20symposiumFinal.pdf (accessed on 17 August 2018).
- Yang, T.; Qian, T.; Wang, M.; Shen, X.; Xu, N.; Sun, Z.; Yan, C. A Sustainable Route from Biomass Byproduct Okara to High Content Nitrogen-Doped Carbon Sheets for Efficient Sodium Ion Batteries. Adv. Mater. 2016, 28, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, F.; Bai, T.; Long, B.; Liao, Q.; Ren, Y.; Yang, J. Interconnected Highly Graphitic Carbon Nanosheets Derived from Wheat Stalk as High Performance Anode Materials for Lithium Ion Batteries. Green Chem. 2016, 18, 2078. [Google Scholar] [CrossRef]
- Arsene, M.A.; Bilba, K.; Savastano, H.; Ghavami, K. Treatments of Non-wood Plant Fibres Used as Reinforcement in Composite Materials. Mater. Res. 2013, 16, 903–923. [Google Scholar] [CrossRef]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet Explosion: A Universal and Efficient Pretreatment Process for Lignocellulosic Biorefineries. Bioenergy Res. 2015, 8, 1101–1116. [Google Scholar] [CrossRef]
- Daud, W.M.A.; Ali, W.S.W. Comparison on pore development of activated carbon produced from palm shell and coconut shell. Bioresour. Technol. 2004, 93, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zanzi, R.; Sjostrom, K.; Bjornbom, E. Rapid pyrolysis of agricultural residues at high temperature. Biomass Bioenergy 2002, 23, 357–366. [Google Scholar] [CrossRef]
- Reed, A.R.; Williams, P.T. Thermal processing of biomass natural fibre wastes by pyrolysis. Int. J. Energy Res. 2004, 28, 131–145. [Google Scholar] [CrossRef]
- Raisanen, T.; Athanassiadis, D. Basic Chemical Composition of the Biomass Components of Pine, Spruce and Birch. Forest Refine. 2013. Available online: http://biofuelregion.se/wp-content/uploads/2017/01/1_2_IS_2013-01-31_Basic_chemical_composition.pdf (accessed on 14 August 2018).
- Nurmi, J. Heating values of mature trees. Acta For. Fennica 1997, 256, 28. [Google Scholar] [CrossRef]
- Chauvet, E. Changes in the chemical composition of alder, poplar and willow leaves during decomposition in a river. Hydrobiologia 1987, 148, 35–44. [Google Scholar] [CrossRef]
- Corbett, D.B.; Kohan, N.; Machado, G.; Jing, C.; Nagardeolekar, A.; Bujanovic, B.M. Chemical Composition of Apricot Pit Shells and Effect of Hot-Water Extraction. Energies 2015, 8, 9640–9654. [Google Scholar] [CrossRef]
- Curvetto, N.R.; Figlas, D.; Matute, R.G.; Delmastro, S. Shiitake bag cultivation on sunflower seed hulls. In Shiitake Mushroom Growers’ Handbook; MushWorld: Brighton, UK, 2005; Chapter 4; pp. 100–104. [Google Scholar]
- Saka, S. Chemical composition and distribution. In Wood and Cellulosic Chemistry; Marcel Dekker, Inc.: New York, NY, USA, 1991; Chapter 2; pp. 59–88. [Google Scholar]
- Nascimento, M.S.; Santana, A.L.; Maranhao, C.A.; Oliveira, L.S.; Bieber, L. Phenolic Extractives and Natural Resistance of Wood. In Biodegradation–Life of Science; Intech: London, UK, 2013; Chapter 13; pp. 349–370. [Google Scholar]
- Rutherford, D.W.; Wershaw, R.L.; Cox, L.G. Changes in Composition and Porosity Occurring during the Thermal Degradation of Wood and Wood Components; Scientific Investigations Report; U.S. Geological Survey: Reston, VA, USA, 2004.
- McDonald-Wharry, J.; Manley-Harris, M.; Pickering, K. A comparison of the charring and carbonisation of oxygen-rich precursors with the thermal reduction of graphene oxide. Philos. Mag. 2015, 95, 4054–4077. [Google Scholar] [CrossRef]
- Hao, R.; Lan, H.; Kuang, C.; Wang, H.; Guo, L. Superior potassium storage in chitin-derived natural nitrogen-doped carbon nanofibers. Carbon 2018, 128, 224–230. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ro, K.S.; Libra, J.A.; Kammann, C.I.; Lima, I.; Berge, N.; Li, L.; Li, Y.; Chen, N.; Yang, J.; et al. Effects of Biomass Types and Carbonization Conditions on the Chemical Characteristics of Hydrochars. J. Agric. Food Chem. 2013, 61, 9401–9411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Choi, Y.S.; Yoo, C.G.; Kim, T.H.; Brown, R.C.; Shanks, B.H. Cellulose–Hemicellulose and Cellulose–Lignin Interactions during Fast Pyrolysis. ACS Sustain. Chem. Eng. 2015, 3, 293–301. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.J.; Tuskan, G.A. Poplar as a feedstock for biofuels: A review of compositional characteristics. Biofuels Bioprod. Biorefin. 2010, 4, 209–226. [Google Scholar] [CrossRef]
- Huang, H.J.; Ramaswamy, S.; Al-Dajani, W.; Tschirner, U.; Cairncross, R.A. Effect of biomass species and plant size on cellulosic ethanol: A comparative process and economic analysis. Biomass Bioenerg. 2009, 33, 234–246. [Google Scholar] [CrossRef]
- USDOE-Office of Energy Efficiency and Renewable Energy. Biomass Feedstock and Composition Database. 2006. Available online: http://www1.eere.energy.gov/biomass/printable _versions/feedstock_databases.html (accessed on 4 September 2009).
- Brown, R.C. Biorenewable Resources: Engineering New Products from Agriculture; Iowa State Press: Ames, IA, USA, 2003. [Google Scholar]
- Hosseinaei, O.; Harper, D.P.; Bozell, J.J.; Rials, T.G. Improving Processing and Performance of Pure Lignin Carbon Fibers through Hardwood and Herbaceous Lignin Blends. Int. J. Mol. Sci. 2017, 18, 1410. [Google Scholar] [CrossRef] [PubMed]
- Faris, A.H.; Ibrahim, M.N.M.; Rahim, A.A.; Hussin, M.H.; Brosse, N. Preparation and characterization of lignin polyols from the residues of oil palm empty fruit bunch. BioResources 2015, 10, 7339–7352. [Google Scholar] [CrossRef]
- Hassan, S.N.A.M.; Ishak, M.A.M.; Ismail, K.; Ali, S.N.; Yusop, M.F. Comparison Study of Rubber Seed Shell and Kernel (Hevea brasiliensis) as Raw Material for Bio-Oil Production. Energy Procedia 2014, 52, 610–617. [Google Scholar] [CrossRef]
- Latshaw, W.L.; Miller, E.C. Elemental Composition of the Corn Plant. J. Agric. Res. 1924, 27, 845–861. [Google Scholar]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Romelle, F.D.; Rani, P.A.; Manohar, R.S. Chemical Composition of Some Selected Fruit Peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Kasarda, D.D.; Black, D.R. Thermal Degradation of Proteins Studied by Mass Spectrometry. Biopolymers 1968, 6, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Nawar, W.W. Thermal Degradation of Lipids: A Review. J. Agric. Food Chem. 1969, 17, 18–21. [Google Scholar] [CrossRef]
- Orozco, R.S.; Hernandez, P.B.; Morales, G.R.; Nunez, F.U.; Villafuerte, J.O.; Lugo, V.L.; Ramirez, N.F.; Diaz, C.E.B.; Vazquez, P.C. Characterization of Lignocellulosic Fruit Waste as an Alternative Feedstock for Bioethanol Production. Bioresources 2014, 9, 1873–1885. [Google Scholar]
- Bandara, A.R.; Karunarathna, S.C.; Mortimer, P.E.; Hyde, K.D.; Khan, S.; Kakumyan, P.; Xu, J. First successful domestication and determination of nutritional and antioxidant properties of the red ear mushroom Auricularia thailandica (Auriculariales, Basidiomycota). Mycol. Prog. 2017, 16, 1029–1039. [Google Scholar] [CrossRef]
- Sunner, J.; Avci, R.; Richards, L.; Groenewold, G.; Ingram, J.; Arthun, M. Preservation of yeast cell morphology for scanning electron microscopy using 3.28-µm IR laser irradiation. J. Microbiol. Methods 2003, 54, 285–287. [Google Scholar] [CrossRef]
- Tsujikawa, K.; Kanamori, T.; Iwata, Y.; Ohmae, Y.; Sugita, R.; Inoue, H.; Kishi, T. Morphological and chemical analysis of magic mushrooms in Japan. Forens. Sci. Int. 2003, 138, 85–90. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Li, Q.; Shang, J.K. Real time, in situ observation of the photocatalytic inactivation of Saccharomyces cerevisiae cells. Mater. Sci. Eng. C 2015, 49, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Abou Raya, M.A.; Shalaby, M.T.; Hafez, S.A.; Alshimaa, M.H. Chemical Composition and Nutritional Potential of Some Mushroom Varieties Cultivated in Egypt. J. Food Dairy Sci. 2014, 5, 421–434. [Google Scholar]
- Bull, A.T. Chemical Composition of Wild-type and Mutant Aspergillus nidulans Cell Walls. The Nature of Polysaccharide and Melanin Constituents. J. Gen. Microbiol. 1970, 63, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.X.; Yin, J.Z. Yunnan wild edible Boletus nutrition analysis and evaluation. Edible Fungi 2008, 4, 61–62. [Google Scholar]
- Zhang, B.Q.; Chen, J. Determination and evaluation of the Craterellus aureus nutrients. Edible Fungi 2012, 4, 58–60. [Google Scholar]
- Yin, J.Z.; Zhou, L.X. Analysis of nutritional components of 4 kinds of wild edible fungi in Yunnan. Food Res. Dev. 2008, 29, 133–136. [Google Scholar]
- Zhu, X.Q.; Wang, X.J.; Xiong, Z. Nutrient analysis of the wild Lentinula edodes. For. By-Prod. Spec. China 2007, 2, 9–11. [Google Scholar]
- Zhang, B.Q.; Chen, J. Determination and analysis of nutrition components in Sarcodon aspratus. Food Sci. 2011, 32, 299–302. [Google Scholar]
- Liu, G.; Wang, H.; Zhou, B.H.; Guo, X.X.; Hu, X.M. Compositional analysis and nutritional studies of Tricholoma matsutake collected from southwest China. J. Med. Plants Res. 2010, 4, 1222–1227. [Google Scholar]
- Diez, A.A.; Alvarez, A. Compositional and nutritional studies on two wild edible mushrooms from northwest Spain. Food Chem. 2001, 75, 417–422. [Google Scholar] [CrossRef]
- Arroyo, J.; Farkas, V.; Sanz, A.B.; Cabib, E. Strengthening the fungal cell wall through chitin–glucan cross-links: Effects on morphogenesis and cell integrity. Cell. Microbiol. 2016, 18, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Peat, S. Plant Carbohydrates. Annu. Rev. Biochem. 1946, 15, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Kalac, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Pineda-Insuasti, J.A.; Soto-Arroyave, C.P.; Beltran, L. Stoichiometry equation to describe the growth of the Pleurotus ostreatus ceba-gliie-po-010606 strain. Biotecnol. Apl. 2014, 31, 48–52. [Google Scholar]
- Sales-Campos, C.; Araujo, L.M.; Minhoni, M.T.; Andrade, M.C.N. Physiochemical analysis and centesimal composition of Pleurotus ostreatus mushroom grown in residues from the Amazon. Cienc. Tecnol. Aliment. Camp. 2011, 31, 456–461. [Google Scholar] [CrossRef]
- Moda, E.M.; Horii, J.; Spoto, M.H.F. Edible Mushroom Pleurotus sajor-caju Production on Washed and Supplemented Sugarcane Bagasse. Sci. Agric. 2005, 62, 27–132. [Google Scholar] [CrossRef]
- Latge, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Moussian, B. Electron-microscopic and genetic dissection of arthropod cuticle Differentiation. Mod. Res. Educ. Top. Microsc. 2007, 3, 316–325. [Google Scholar]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Rodde, R.H.; Einbu, A.; Varum, K.M. A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr. Polym. 2008, 71, 388–393. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and Characterization of Chitin from the Beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Ahyat, N.M.; Mohamad, F.; Ahmad, A.; Azmi, A.A. Chitin and Chitosan Extraction from Portunus pelagicus. Malays. J. Anal. Sci. 2017, 21, 770–777. [Google Scholar]
- Kaya, M.; Lelesius, E.; Nagrockaite, R.; Sargin, I.; Arslan, G.; Mol, A.; Baran, T.; Can, E.; Bitim, B. Differentiations of Chitin Content and Surface Morphologies of Chitins Extracted from Male and Female Grasshopper Species. PLoS ONE 2015, 10, e0115531. [Google Scholar] [CrossRef] [PubMed]
- Percot, A.; Viton, C.; Domard, A. Optimization of Chitin Extraction from Shrimp Shells. Biomacromole 2003, 4, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, E.; Pestov, A.; Stepanova, D.; Molochnikov, L. Characterization of chitin and its complexes extracted from natural raw sources. AIP Conf. Proc. 2016, 1772, 050007. [Google Scholar]
- Majtan, J.; Bilikova, K.; Markovic, O.; Grof, J.; Kogan, G.; Simuth, J. Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 2007, 40, 237–241. [Google Scholar] [CrossRef] [PubMed]
- McKittrick, J.; Chen, P.Y.; Bodde, S.G.; Yang, W.; Novitskaya, E.E.; Meyers, M.A. The Structure, Functions, and Mechanical Properties of Keratin. JOM 2012, 64, 449–468. [Google Scholar] [CrossRef]
- Robbins, C.R. Chemical and Physical Behavior of Human Hair; Springer: Berlin, Germany, 2012; Chapter 2; pp. 105–176. [Google Scholar]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
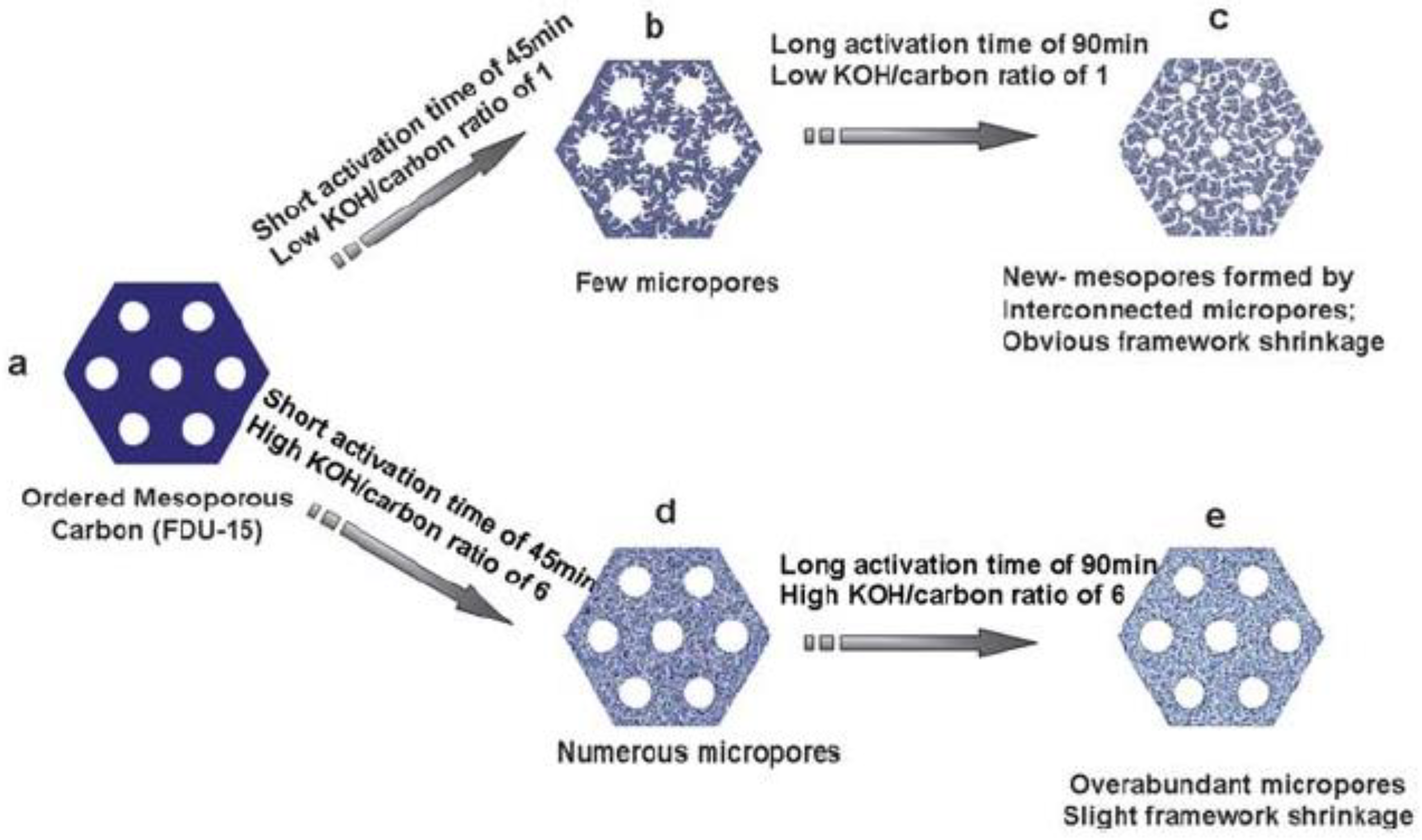
- Lv, Y.; Zhang, F.; Dou, Y.; Zhai, Y.; Wang, J.; Liu, H.; Xia, Y.; Tu, B.; Zhao, D. A comprehensive study on KOH activation of ordered mesoporous carbons and their supercapacitor application. J. Mater. Chem. 2012, 22, 93. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710. [Google Scholar] [CrossRef]
- Ajuria, J.; Redondo, E.; Arnaiz, M.; Mysyk, R.; Rojo, T.; Goikolea, E. Lithium and sodium ion capacitors with high energy and power densities based on carbons from recycled olive pits. J. Power Source 2017, 359, 17–26. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Lee, J.H.; Je, S.H.; Buyukcakir, O.; Kwon, T.; Polychronopoulou, K.; Choi, J.W.; Coskun, A. Chemical Blowing Approach for Ultramicroporous Carbon Nitride Frameworks and Their Applications in Gas and Energy Storage. Adv. Funct. Mater. 2017, 27, 1604658. [Google Scholar] [CrossRef]
- Cha, W.S.; Talapaneni, S.N.; Kempaiah, D.M.; Joseph, S.; Lakhi, K.S.; Al-Enizi, A.M.; Park, D.H.; Vinu, A. Excellent supercapacitance performance of 3-D mesoporous carbon with large pores from FDU-12 prepared using a microwave method. RSC Adv. 2018, 8, 17017. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Kim, J.; Je, S.H.; Buyukcakir, O.; Oh, J.; Coskun, A. Bottom-up synthesis of fully sp2 hybridized three dimensional microporous graphitic frameworks as metal-free catalysts. J. Mater. Chem. A 2017, 5, 12080. [Google Scholar] [CrossRef]
- Hibbett, D.S. After the gold rush, or before the flood? Evolutionary morphology of mushroom-forming fungi (Agaricomycetes) in the early 21st century. Mycol. Res. 2007, 111, 1001–1018. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Sudhan, N.; Karnan, M.; Sathish, M. Orange Peel Derived Activated Carbon for Fabrication of High-Energy and High-Rate Supercapacitors. ChemistrySelect 2017, 2, 11384–11392. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, Z.; Zheng, L.; Teng, F.; Hu, L.; Fang, X. A Novel Sustainable Flour Derived Hierarchical Nitrogen-Doped Porous Carbon/Polyaniline Electrode for Advanced Asymmetric Supercapacitors. Adv. Energy Mater. 2016, 6, 1601111. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132. [Google Scholar] [CrossRef]
- Zhou, M.; Gomez, J.; Li, B.; Jiang, Y.B.; Deng, S. Oil tea shell derived porous carbon with an extremely large specific surface area and modification with MnO2 for high-performance supercapacitor electrodes. Appl. Mater. Today 2017, 7, 47–54. [Google Scholar] [CrossRef]
- Ba, H.; Wang, W.; Pronkin, S.; Romero, T.; Baaziz, W.; Nguyen-Dinh, L.; Chu, W.; Ersen, O.; Pham-Huu, C. Biosourced Foam-Like Activated Carbon Materials as High-Performance Supercapacitors. Adv. Sustain. Syst. 2018, 2, 1700123. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Qi, P.; Zhu, M.; Wang, G.; Ma, Y.; Guo, X.; Chen, H.; Zhang, B.; Zhao, Z.; Dai, B.; Yu, F. Nitrogen-Doped Banana Peel–Derived Porous Carbon Foam as Binder-Free Electrode for Supercapacitors. Nanomaterials 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Yang, F.; Yang, X. Promising Carbons for Supercapacitors Derived from Fungi. Adv. Mater. 2011, 23, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Poyraz, S.; Zhang, C.; Xin, J.H. One-Step Synthesis of Multifunctional Zinc-Iron-Oxide Hybrid Carbon Nanowires by Chemical Fusion for Supercapacitors and Interfacial Water Marbles. ChemNanoMat 2018, 4, 546–556. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.M. 3D Aperiodic Hierarchical Porous Graphitic Carbon Material for High-Rate Electrochemical Capacitive Energy Storage. Angew. Chem. Int. Ed. 2008, 47, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Tipler, P.A. Physics for Scientists and Engineers; Worth Publishers: New York, NY, USA, 1976; p. 769. [Google Scholar]
- Huang, J.; Sumpter, B.G.; Meunier, V. A Universal Model for Nanoporous Carbon Supercapacitors Applicable to Diverse Pore Regimes, Carbon Materials, and Electrolytes. Chem. Eur. J. 2008, 14, 6614–6626. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E. Carbon Materials for Supercapacitor Application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Fang, R.; Tian, P.; Yang, X.; Luque, R.; Li, Y. Encapsulation of ultrafine metal-oxide nanoparticles within mesopores for biomass-derived catalytic applications. Chem. Sci. 2018, 9, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, Y.; Hu, Y.; Kong, B.; Simon, G.P.; Zhang, J.; Jiang, S.P.; Wang, H. A Versatile Iron–Tannin-Framework Ink Coating Strategy to Fabricate Biomass-Derived Iron Carbide/Fe-N-Carbon Catalysts for Efficient Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
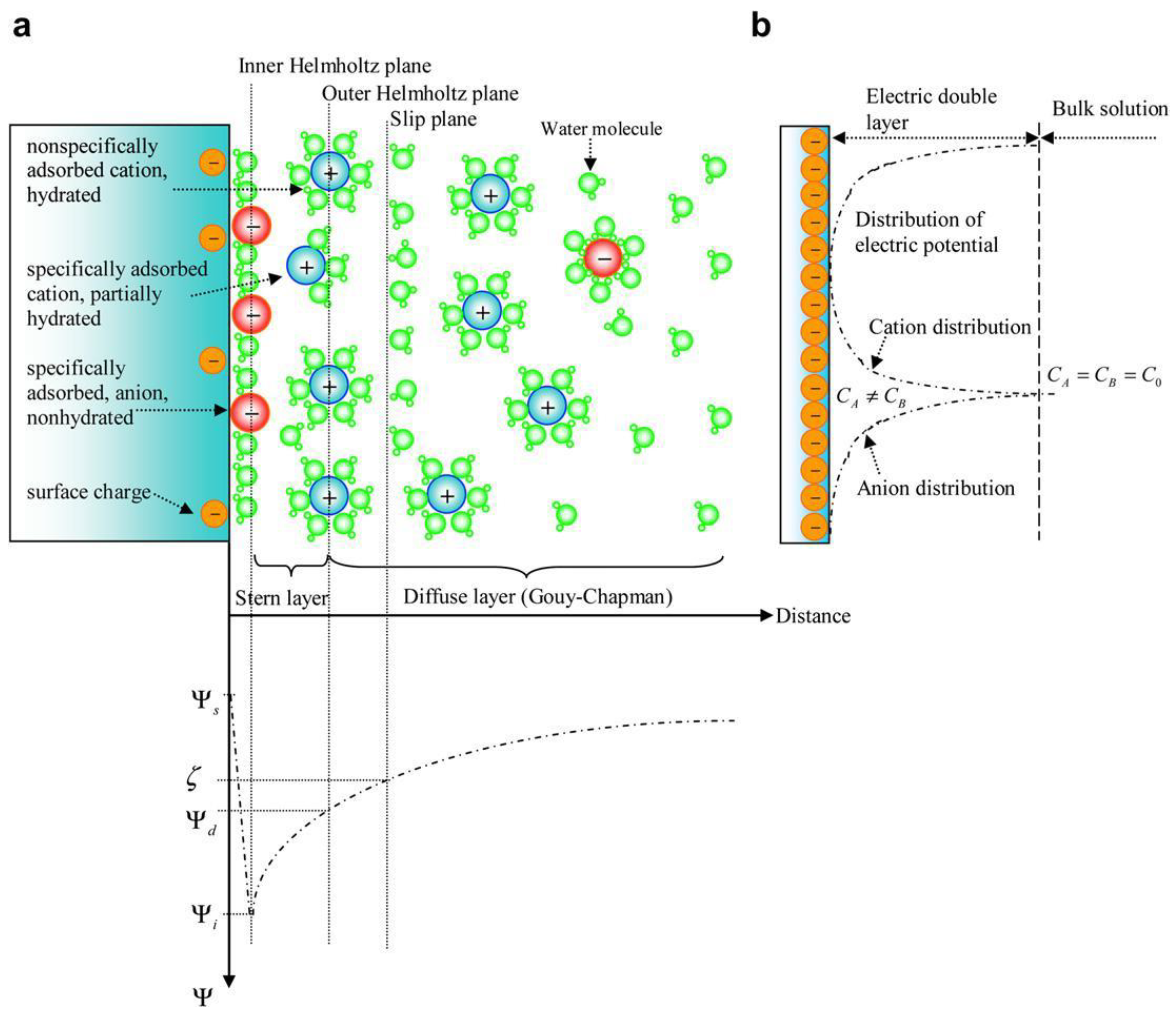
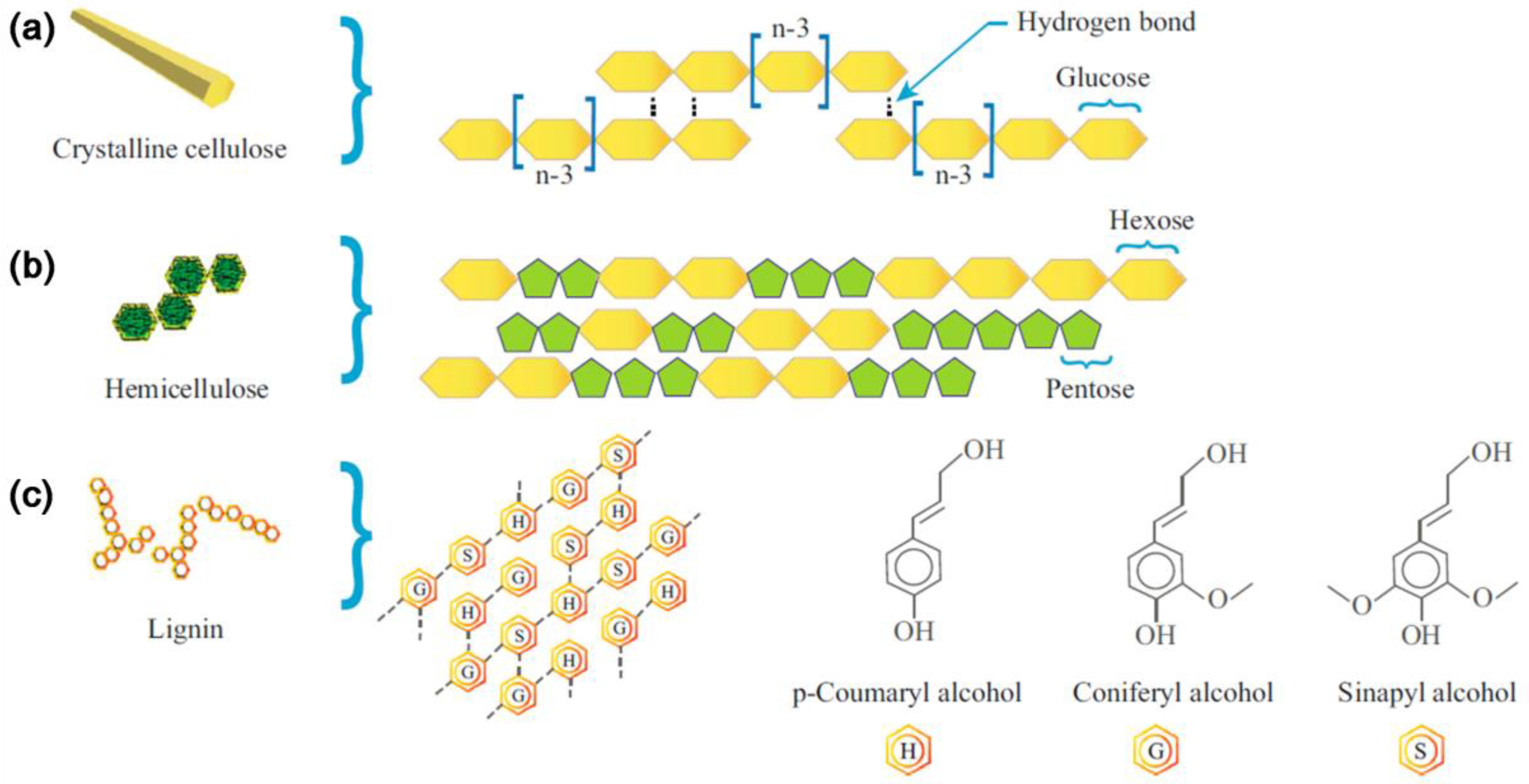

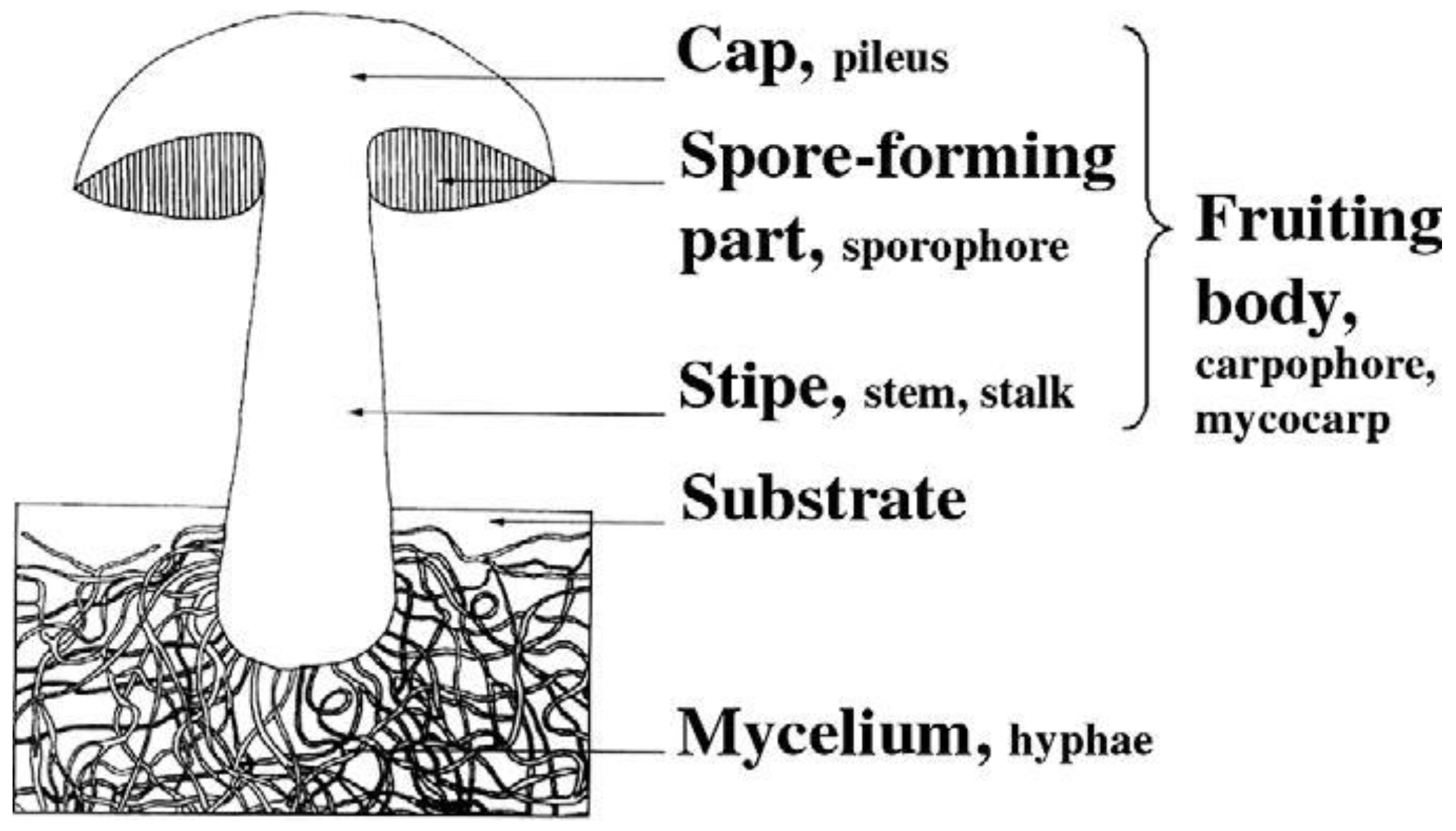
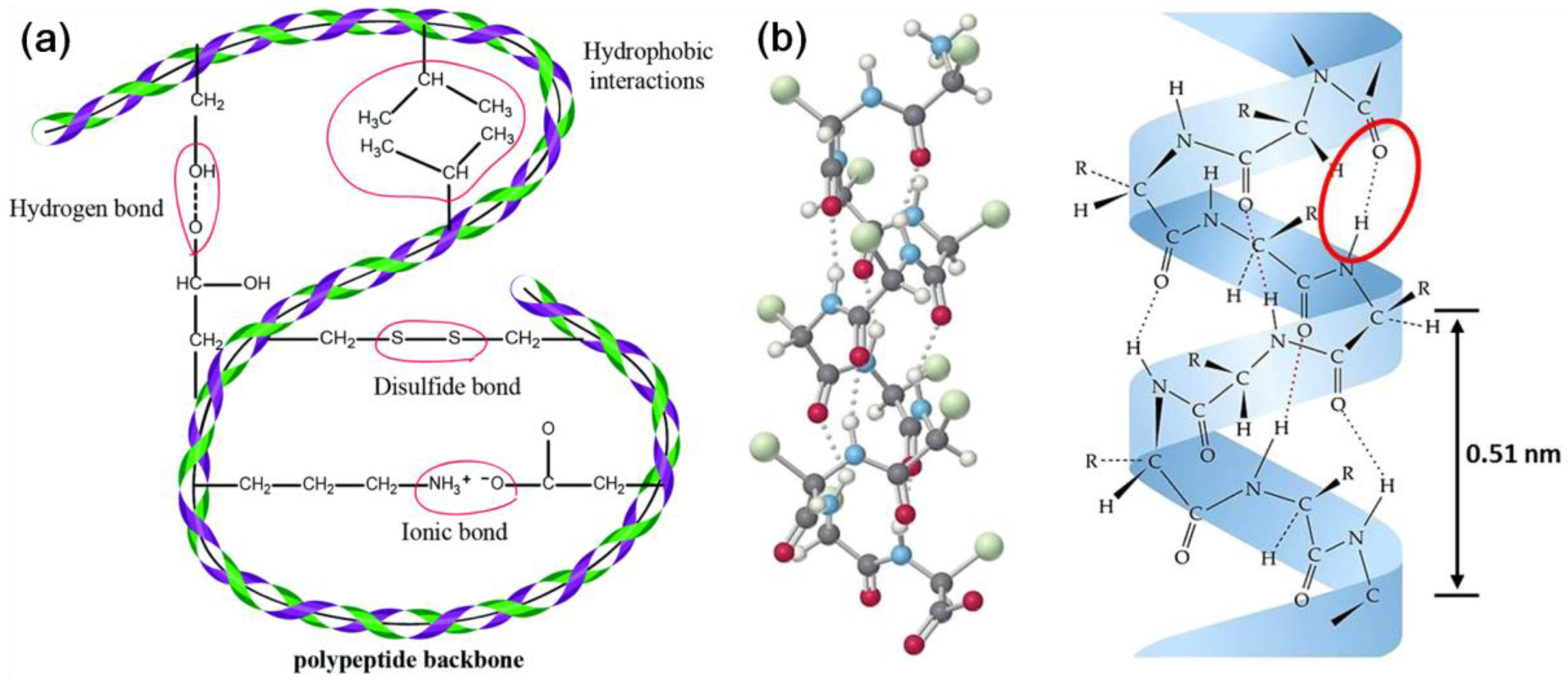
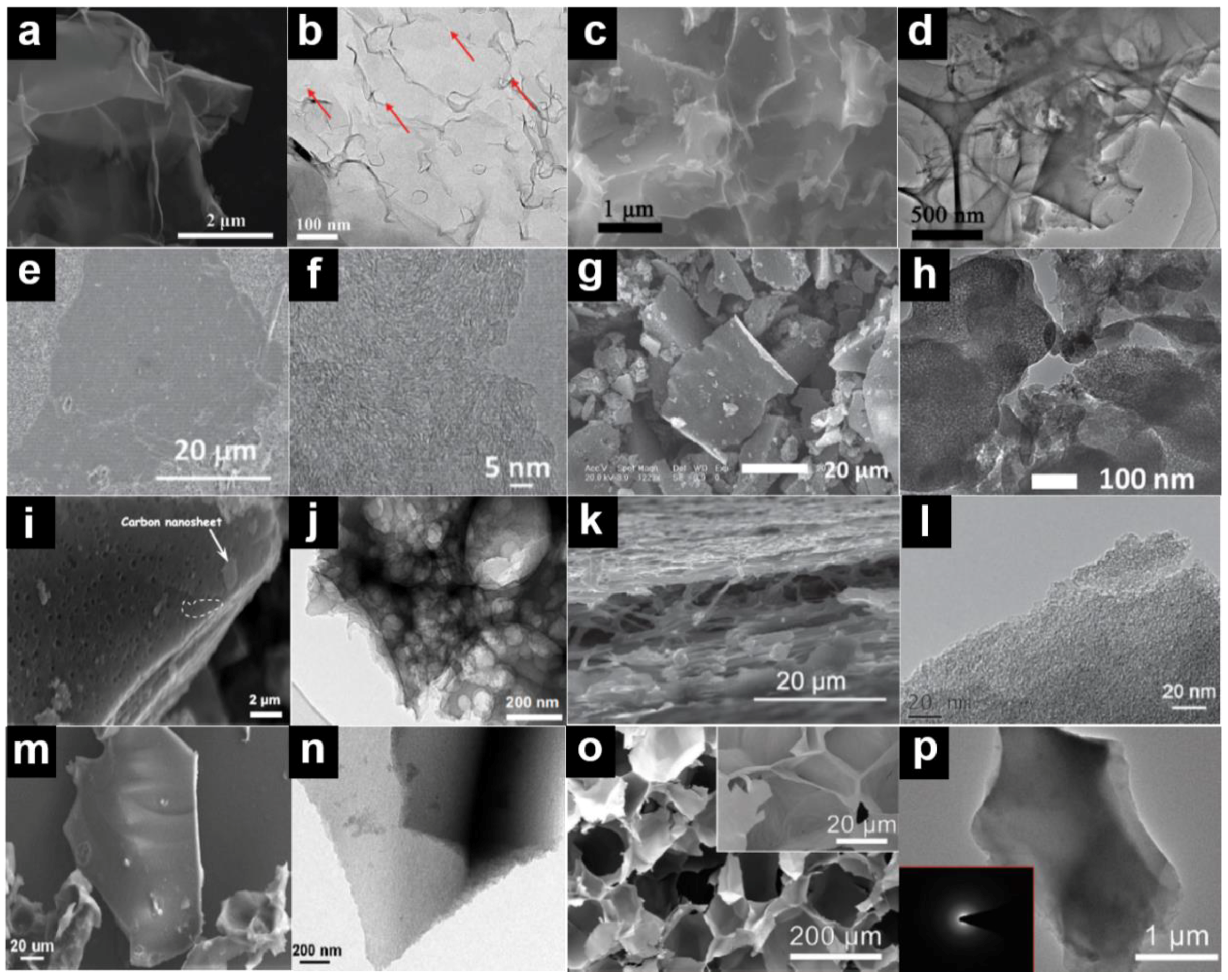
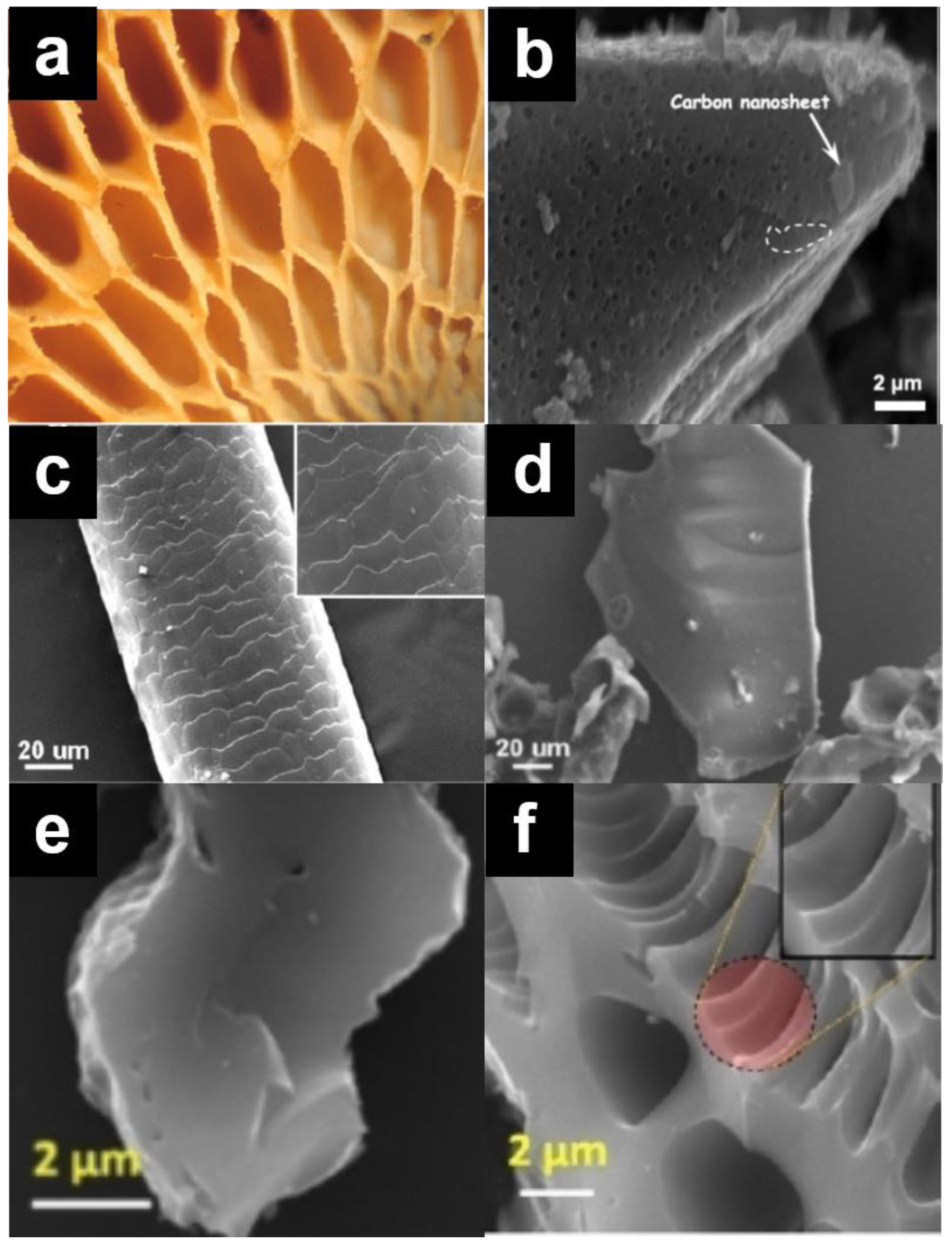
| Biomass | Moisture (%) | Lignin (%) | Cellulose (%) | Hemicellulose (%) | Extractives (%) | References |
|---|---|---|---|---|---|---|
| Coconut coir | 13.68 | 46.48 | 21.46 | 12.36 | 8.77 | [47] |
| Coconut sheath | 5.90 | 29.7 | 31.05 | 19.22 | 1.74 | [47] |
| Bagasse | 5.64 | 22.56 | 39.45 | 26.97 | 4.33 | [47] |
| Banana leaf | 11.69 | 24.84 | 25.65 | 17.04 | 9.84 | [47] |
| Sisal | - | 7.6–9.2 | 43–56 | 21–24 | - | [47] |
| Corn stover | - | 18–22 | 37–42 | 20–28 | - | [48] |
| Wheat straw | - | 16–24 | 31–44 | 22–24 | - | [49] |
| Rice straw | - | 10–18 | 32–41 | 15–24 | - | [49] |
| Barley straw | - | 8–17 | 33–40 | 20–35 | - | [49] |
| Switchgrass | - | 12–23 | 33–46 | 22–32 | - | [49] |
| Palm shell | - | 53.4 | 29.7 | - | - | [50] |
| Olive waste | - | 28.0 | 44.8 | - | - | [51] |
| Jute | - | 11.8 | 64.4 | - | - | [52] |
| Abaca | - | 5.1 | 63.2 | - | - | [52] |
| Flax | - | 2.5 | 56.5 | - | - | [52] |
| Hemp | - | 3.3 | 67.0 | - | - | [52] |
| Scots pine stem wood | - | 27.0 | 40.7 | 26.9 | 5.0 | [53] |
| Scots pine bark | - | 13.1 | 22.2 | 8.1 | 25.2 | [53] |
| Scots pine branches | - | 21.5 | 32.0 | 32.0 | 16.6 | [53] |
| Scots pine needles | - | 6.9 | 29.1 | 24.9 | 39.6 | [53] |
| Scots pine stump | - | 19.5 | 36.4 | 28.2 | 18.7 | [54] |
| Scots pine roots | - | 29.8 | 28.6 | 18.9 | 13.3 | [54] |
| Poplar leaves | - | 23.2 | 22.3 | 12.8 | 40.0 | [55] |
| Alder leaves | - | 12.4 | 15.0 | 13.3 | 44.7 | [55] |
| Willow leaves | - | 20.0 | 18.5 | 14.7 | 43.4 | [55] |
| Apricot pit shell | - | 31.91 | 34.31 | - | - | [56] |
| Sunflower seed hull | 11.8 | 28.7 | 31.3 | 25.2 | - | [57] |
| Biomass | C (%) | H (%) | O (%) | N (%) | S (%) |
|---|---|---|---|---|---|
| Coconut coir [47] | 46.22 | 5.44 | 40.47 | 0.36 | - |
| Coconut sheath [47] | 42.23 | 5.69 | 45.57 | 0.44 | - |
| Bagasse [47] | 48.6 | 6.3 | 45.1 | - | - |
| Banana leaf [47] | 44.01 | 6.10 | 38.84 | 1.36 | - |
| Hybrid poplar [66] | 48.45 | 5.85 | 43.69 | 0.47 | 0.01 |
| Poplar, DN 34 [67] | 50.02 | 6.28 | 42.17 | 0.19 | 0.02 |
| Hybrid poplar, DN 34 [68] | 51.73 | 4.47 | 35.11 | 0.24 | 0.03 |
| Corn stover [69] | 43.65 | 5.56 | 43.31 | 0.61 | 0.11 |
| Switchgrass [69] | 47.75 | 5.75 | 42.37 | 0.74 | 0.08 |
| Wheat straw [69] | 43.20 | 5.00 | 39.40 | 0.61 | 0.11 |
| Ponderosa pine [69] | 49.25 | 5.99 | 44.36 | 0.06 | 0.03 |
| Yellow poplar [70] | 64.5 | 5.89 | 29.2 | 0.26 | - |
| Kraft lignin [71] | 57.83 | 3.42 | 33.66 | 0.47 | 4.62 |
| Rubber seed shell [72] | 48.8 | 5.9 | 43.7 | 1.5 | 0.1 |
| Rubber seed kernel [72] | 64.5 | 8.2 | 23.4 | 3.6 | 0.3 |
| Saline corn stem [73] | 44.51 | 5.90 | 43.90 | 0.84 | 0.16 |
| Saline corn leaves [73] | 41.27 | 5.86 | 43.86 | 1.30 | 0.24 |
| Biomass | Lignin (Lc %) | Cellulose (Cc %) | HemicelluLose (Hc %) | Calculated Yield (%) | Experimental Yield (%) | Standard Deviation (%) |
|---|---|---|---|---|---|---|
| Coconut shell | 66.9 | 8.7 | 24.4 | 30.8 | 25.6 | 2.6 |
| Apple pulp | 58.0 | 18.8 | 23.1 | 16.2 | 25.7 | 4.8 |
| Plum pulp | 79.0 | 5.6 | 15.4 | 22.2 | 25.9 | 1.9 |
| Plum stones | 70.8 | 14.1 | 15.1 | 31.1 | 24.6 | 3.3 |
| Olive stones | 75.3 | 10.7 | 14.1 | 25.1 | 30.9 | 2.9 |
| Sulphuric acid treated olive stones | 64.8 | 19.0 | 16.2 | 29.1 | 27.4 | 0.9 |
| Soft wood | 55.0 | 27.6 | 17.4 | 24.9 | 21.3 | 1.8 |
| Synthetic coconut shell | 66.9 | 8.5 | 24.5 | 33.5 | 32.7 | 0.4 |
| Microorganisms | Carbohydrates | Crude Fiber | Crude Protein | Crude Fat | Ash | References |
|---|---|---|---|---|---|---|
| Agaricus bisporus | 42.56 | 13.21 | 33.85 | 2.41 | 7.97 | [83] |
| Auricularia thailandica | 17.36 | 4.62 | 12.99 | 2.93 | 4.30 | [79] |
| Aspergillus nidulans | 60.5 | - | 10.0–10.4 | 9.5 | - | [84] |
| B. aereus | 34.0 | 17.0 | 26.9 | 2.1 | 8.5 | [85] |
| B. edulis | 30.6 | 15.3 | 28.7 | 4.1 | 9.2 | [86] |
| B. speciosus | 28.6 | 21.0 | 28.1 | 2.9 | 7.6 | [86] |
| C. aureus | 61.5 | 5.2 | 14.1 | 4.0 | 9.2 | [87] |
| Lactarius deliciosus | 25.0 | 36.3 | 20.2 | 2.5 | 7.5 | [88] |
| Lactarius hatsudake | 38.2 | 31.8 | 15.3 | 1.0 | 7.3 | [88] |
| Lactarius volemus | 15.0 | 40.0 | 17.6 | 6.7 | 13.3 | [88] |
| L. crocipodium | 12.8 | 37.9 | 29.3 | 1.0 | 5.8 | [86] |
| Lentinula edodes | 30.2 | 39.4 | 17.1 | 1.9 | 4.3 | [89] |
| Pleurotus ostreatus | 57.05 | 8.25 | 26.05 | 2.79 | 5.86 | [83] |
| R. virescens | 13.4 | 32.8 | 28.3 | 1.5 | 11.9 | [88] |
| S. aspratus | 64.6 | 5.1 | 12.0 | 2.8 | 10.4 | [90] |
| T. matsutake | 36.7 | 29.1 | 14.3 | 5.0 | 8.9 | [91] |
| Tricholoma portentosum | 34.6 | 30.1 | 19.6 | 5.8 | 9.9 | [92] |
| Tricholoma terreum | 31.1 | 30.1 | 20.1 | 6.6 | 12.1 | [92] |
| Crustaceans | Chitin (%) | References | Insects | Chitin (%) | References | Mollusks | Chitin (%) | References |
|---|---|---|---|---|---|---|---|---|
| Cancer (crab) | 72.1 | [101] | Periplaneta (cockroach) | 2.0 | [101] | Clam | 6.1 | [101] |
| Carcinus (crab) | 64.2 | [101] | Blatella (cockroach) | 18.4 | [101] | Shell oysters | 3.6 | [101] |
| Paralithodes (king crab) | 35.0 | [101] | Coleoptera (ladybird) | 27–35 | [101] | Squid pen | 41.0 | [101] |
| Callinectes (blue crab) | 14.0 | [101] | Diptera pupae | 54.8 | [101] | Krill, deproteinized shells | 40.2 | [101] |
| Crangon and Pandalus (shrimp) | 17–40 | [101] | Pieris pupae (butterfly) | 64.0 | [101] | - | - | - |
| Alaska shrimp | 28.0 | [101] | Bombyx (silk worm) | 44.2 | [101] | - | - | - |
| Pandalus borealis (shrimp) | 17–20 | [102] | Galleria (wax worm) | 33.7 | [101] | - | - | - |
| Nephro (lobster) | 69.8 | [101] | Holotrichia parallela (beetle) | 15 | [103] | - | - | - |
| Homarus (lobster) | 60–75 | [101] | Brachytrupes portentosus (house cricket) | 4.3–7.1 | [104] | - | - | - |
| Lepas (goose barnacle) | 58.3 | [101] | Cicada sloughs | 36.6 | [105] | - | - | - |
| Portunus pelagicus (crab) | 20.24 | [106] | Celes variabilis (grasshopper) | 9.93 | [107] | - | - | - |
| Biomass | Chitin C (%) | Chitin H (%) | Chitin N (%) | C/N | References |
|---|---|---|---|---|---|
| Pariplaneta americana linnaeus | 47.3 | 7.32 | 7.20 | 6.57 | [109] |
| Apis mellifera linneaus | 52.65 | 8.42 | 5.55 | 9.49 | [109] |
| Holotrichia parallela | 44.36 | 5.92 | 6.45 | 6.88 | [106] |
| Brachytrupes portentosus | 41.30 | - | 6.022 | 6.858 | [104] |
| Commercial shrimp | 43.61 | - | 4.794 | 9.10 | [104] |
| Portunus pelagicus | 77.67 | 12.71 | 9.62 | 8.07 | [103] |
| Activated Carbon | Unit Cell Size (nm) | BET Surface Area (cm2 g−1) | Pore Volume (cm3 g−1) | Micropore Surface Area (cm2 g−1) | Microporosity (%) | Micropore Volume (cm3 g−1) |
|---|---|---|---|---|---|---|
| FDU-15 | 10.2 | 660 | 0.44 | 180 | 27 | 0.07 |
| KF1-45 | 10.7 | 930 | 0.49 | 590 | 63 | 0.24 |
| KF1-60 | 10.2 | 1030 | 0.52 | 660 | 64 | 0.27 |
| KF1-90 | 9.8 | 1410 | 0.73 | 890 | 63 | 0.38 |
| KF4-45 | 10.5 | 1150 | 0.56 | 830 | 72 | 0.34 |
| KF4-60 | 10.3 | 1310 | 0.62 | 1030 | 79 | 0.43 |
| KF4-90 | 10.3 | 1240 | 0.59 | 970 | 78 | 0.40 |
| KF6-45 | 10.7 | 1280 | 0.59 | 990 | 77 | 0.41 |
| KF6-60 | 10.6 | 1400 | 0.69 | 1020 | 73 | 0.42 |
| KF6-90 | 10.6 | 1200 | 0.56 | 960 | 80 | 0.40 |
| Precursor | Biomass-Derived Carbon Produced | Activation Method | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Specific Capacitance | Current Density | Cycling Stability | Microstructure | Rate Performance & Conductivity |
|---|---|---|---|---|---|---|---|---|---|
| Wheat flour [123] | Hierarchically porous nitrogen-doped carbon (HPC) | KOH/C = 1:1, 800 °C, 2 h | 1294 | N/A | 383 F g−1 | 1 A g−1 | 91.6% after 5000 cycles |  | ~65% at 10 A g−1 Figure caption: (a,b) SEM images of HPC. |
| Poplar catkin [28] | Nitrogen and oxygen dual doped carbon (NODC) | ZnCl2/C = 3:1, 800 °C, 2 h | 1462.5 | 1.31 | 251 F g−1 | 0.5 A g−1 | ~100% after 1000 cycles |  | ~68% at 30 A g−1 (A) SEM and (B) TEM images of NODC-800. |
| Chicken egg white [30] | Egg white-derived activated carbon (eAC) | KOH/C = 3:1, 900 °C, 3 h | 3250 | 1.97 | 56 F g−1 | 12.8 A g−1 | 79.2% after 15,000 cycles |  | N/A (A) SEM and (B) TEM images of eAC-900. Scale bar: (A) 30 µm, (B) 20 nm. |
| Chicken eggshell membrane [24] | Carbonized eggshell membrane (CESM) | Activated in air at 300 °C, 2 h | 221.2 | 0.13 | 297 F g−1 | 0.2 A g−1 | 97% after 10,000 cycles |  | 66% at 20 A g−1, 8.9 × 10−4 Ω m (A,B) SEM images of activated CESM. |
| Bamboo char [124] | Porous graphitic biomass carbon (PGBC) | K2FeO4/C ≈ 2:1, 800 °C, 2 h | 1732 | 0.97 | 222.0 F g−1 | 0.5 A g−1 | 84% after 5000 cycles (solid-state) |  | 51.8% at 20 A g−1, 4.7 S cm−1 (a,b) SEM images of PGBC-1. Scale bar: (a) 50 µm, (b) 4 µm. |
| Bacillus subtilis [36] | Heteroatom-doped carbon (HDC) | KOH/C = 4:1, 800 °C, 2 h | 1578 | 1.092 | 310 F g−1 | 0.2 A g−1 | 96% after 1200 cycles |  | 64.5% at 10 A g−1 (A,B) SEM images of HDC activated by ZnCl2. |
| Willow catkin [29] | N,S-co-doped porous carbon nanosheet (N,S-PCN) | KOH/willow catkin = 1:1, 400 °C for 3 h, and then 850 °C for 1 h, and then 300 °C in air for 1 h | 1533 | 0.92 | 298 F g−1 | 0.5 A g−1 | 98% after 10,000 cycles |  | 78.2% at 50 A g−1 (A) SEM and (B) TEM images of N,S-PCN. |
| Auricularia [26] | Porous graphene-like carbon (PGC) | One-pot hydrothermal carbonization and activation, KOH/fungus ≈ 0.14:1 | 1103 | 0.54 | 374 F g−1 | 0.5 A g−1 | 99% after 10,000 cycles | 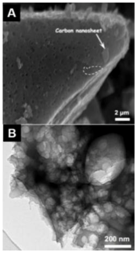 | 78% at 5 A g−1 (A) SEM and (B) TEM images of PGC. |
| Gelatin [25] | B/N co-doped carbon nanosheets (B/N-CS) | Not activated | 416 | 0.76 | 358 F g−1 | 0.1 A g−1 | 113% after 15,000 cycles |  | 74.6% at 30 A g−1, 9.8 S m−1 (A) SEM image of B/N-CS on AAO and (B) Cross-section TEM image of B/N-CS. |
| Dry elm samara [19] | Porous carbon nanosheets (PCNS) | One-pot carbonization and activation, KOH/dry elm samara ≈ 3.4:1, 700 °C, 2 h | 1947 | 1.33 | 470 F g−1 | 1 A g−1 | 98% after 50,000 cycles | 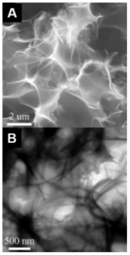 | 63.8% at 10 A g−1 (A) SEM and (B) TEM images of PCNS-6. |
| Chitin [27] | Nitrogen-doped nanofibrous carbon microspheres (NCM) | Not activated | 1141 | - | 219 F g−1 | 5 mV s−1 | 96% after 10,000 cycles | 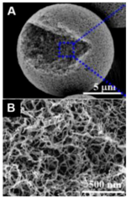 | 50% at 10,000 mV s−1 (A,B) SEM images of NCM-900 |
| Human hair [18] | Heteroatom-doped porous carbon flakes (HMC) | KOH/C = 2:1, 800 °C, 2 h | 1306 | 0.90 | 445 F g−1 | 0.5 A g−1 | 98% after 20,000 cycles |  | 51% at 10 A g−1, 47.3 S m−1 (A) SEM and (B) TEM images of HMC-800 |
| Oil tea shell [125] | Activated carbon (AC) | One-pot carbonization and activation, ZnCl2/oil tea shell = 3:1 | 2851 | 2.68 | 146 F g−1 | 0.5 A g−1 | 86% after 3000 cycles |  | 86.6% at 4 A g−1 (A) SEM and TEM images of AC. Scale bar: (A) 3 µm, (B) 100 nm. |
| Fig-fruit: inner part [126] | Highly porous foam-like carbon | One-pot carbonization and activation, KOH/fig-fruit = 3:1, 900 °C, 2 h | 2337 | 1.005 | 340 F g−1 | 0.5 A g−1 | >100% after 10,000 cycles | 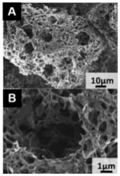 | 50% at 50 A g−1 (A,B) SEM images of the fig-fruit inner part after activation |
| Banana peel [127] | Nitrogen-doped porous carbon foam (N-BPPCF) | Not activated | 1357.6 | 0.77 | 210.6 F g−1 | 0.5 A g−1 | ~100% after 5000 cycles |  | 79.7% at 50 mV s−1 (A) SEM and (B) TEM images of N-BPPCF. |
| Orange peel [122] | 3D nanoporous carbon | KOH/C = 3:1, 600 °C, 1 h | 2160 | 0.779 | 460 F g−1 | 1 A g−1 | 98% after 10,000 cycles |  | 59% at 100 A g−1 (A,B) SEM images of the orange-peel derived activated carbon. |
| Orange peel [20] | Interconnected hollow-structured carbon (OPAC) | KOH/C = 1:1, 800 °C, 2 h | 1391 | 0.72 | 407 F g-1 | 0.5 A g−1 | 100% after 5000 cycles |  | ~37% at 20 A g−1 SEM images of OPAC-1(A) and OPAC-2 (B). Nos. 1 and 2 represent the KOH/C ratios. |
| Shiitake mushroom [42] | Hierarchically porous activated carbon (KPAC) | KOH/C = 3:1, 800 °C, 2 h | 2988 | 1.76 | 306 F g−1 | 1 A g−1 | 95.7% after 15,000 cycles |  | 78.4% at 30 A g−1 (A,B) SEM images of KPAC-800. |
| Kombucha [35] | Hierarchical porous carbon (KHPC) | KOH:C = 0.6:1, 700 °C, 1 h | 917 | 0.41 | 326 F g−1 | 1 A g−1 | 91.3% after 5000 cycles | 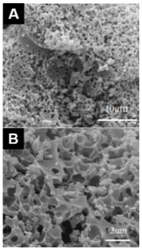 | 82% at 20 A g−1 (A,B) SEM images of KHPC. |
| Crude auricularia [128] | Pyrolyzed hydrothermally carbonized auricularia (P-HT-A) | Not activated | 80.08 | 0.496 | 196 F g−1 | 5 mV s−1 | 91.8% after 1000 cycles |  | ~31% at 200 mV s−1 SEM images of (A) HT-A and (B) P-HT-A. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, J.; Cui, B.; Yin, P.; Zhang, C. Design and Preparation of Biomass-Derived Carbon Materials for Supercapacitors: A Review. C 2018, 4, 53. https://doi.org/10.3390/c4040053
Liu Y, Chen J, Cui B, Yin P, Zhang C. Design and Preparation of Biomass-Derived Carbon Materials for Supercapacitors: A Review. C. 2018; 4(4):53. https://doi.org/10.3390/c4040053
Chicago/Turabian StyleLiu, Yang, Jiareng Chen, Bin Cui, Pengfei Yin, and Chao Zhang. 2018. "Design and Preparation of Biomass-Derived Carbon Materials for Supercapacitors: A Review" C 4, no. 4: 53. https://doi.org/10.3390/c4040053
APA StyleLiu, Y., Chen, J., Cui, B., Yin, P., & Zhang, C. (2018). Design and Preparation of Biomass-Derived Carbon Materials for Supercapacitors: A Review. C, 4(4), 53. https://doi.org/10.3390/c4040053