Thermochemistry of a Biomimetic and Rubisco-Inspired CO2 Capture System from Air
Abstract
:1. Introduction
2. Results and Discussion
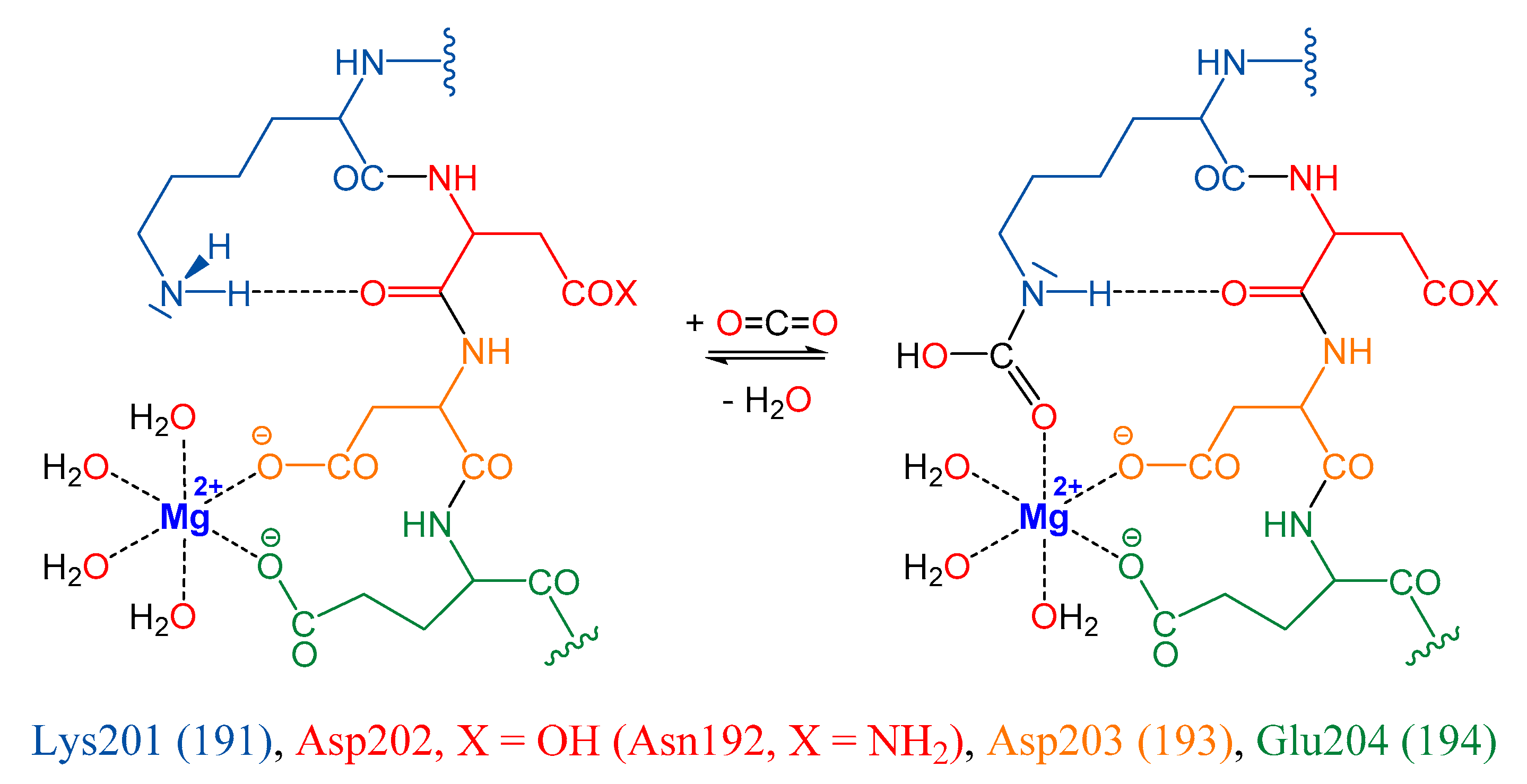
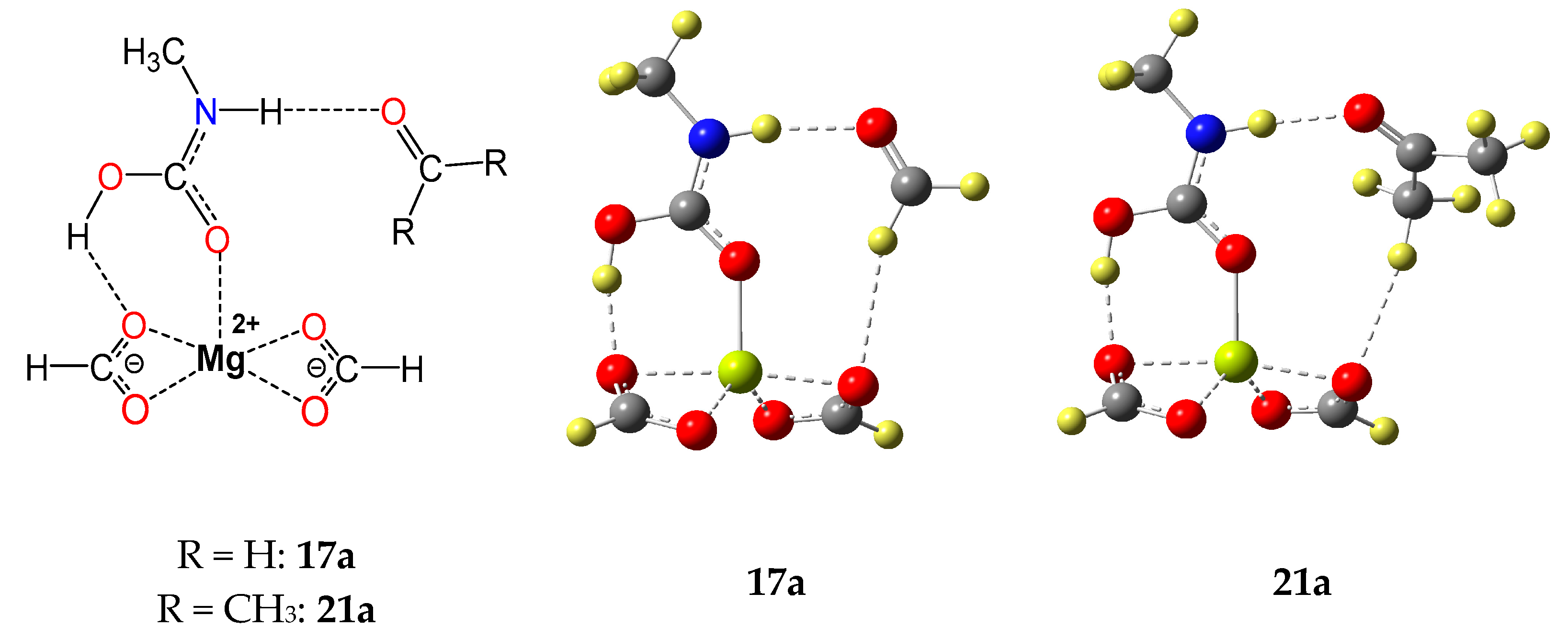
2.1. Capture Reaction Systems Studied
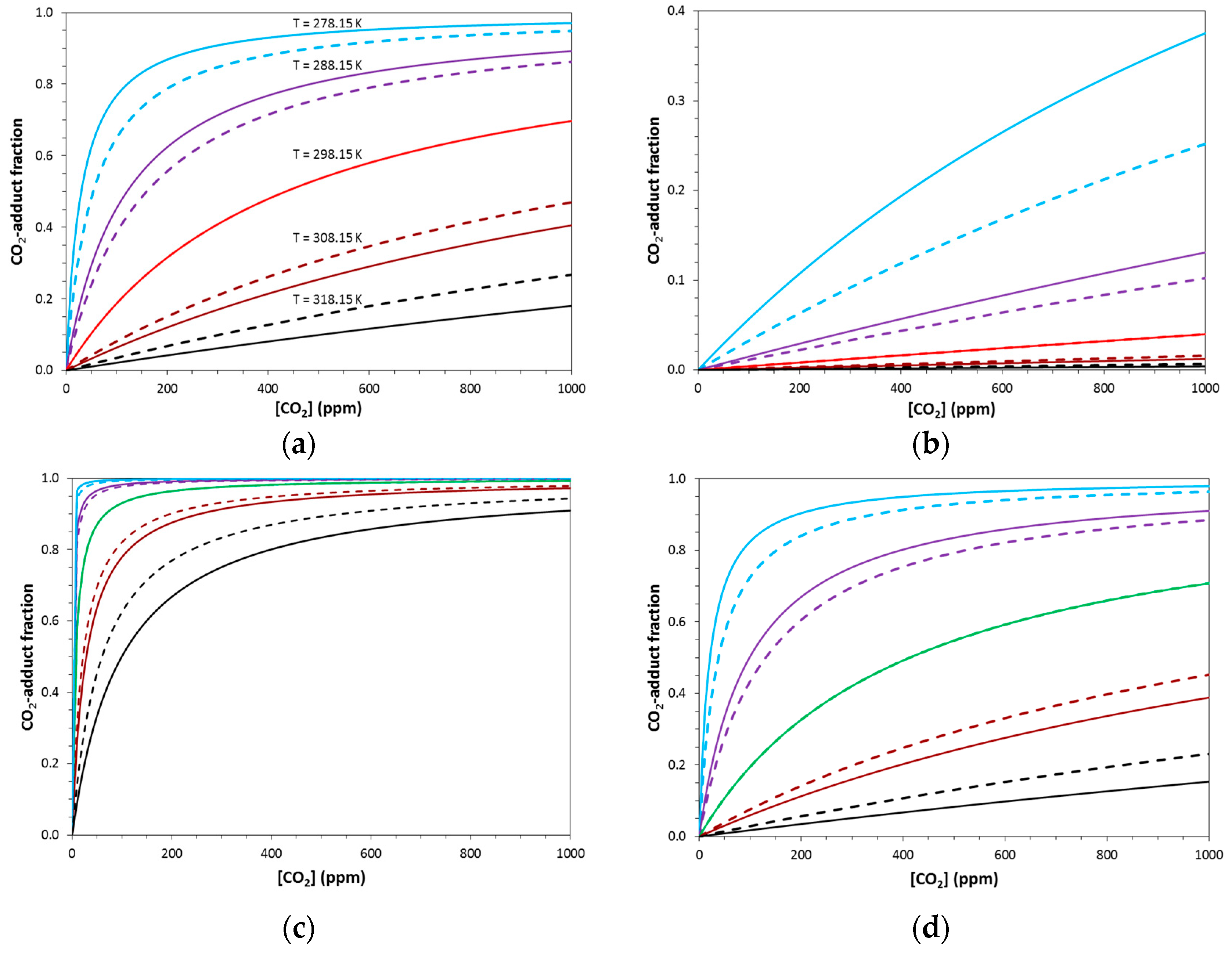
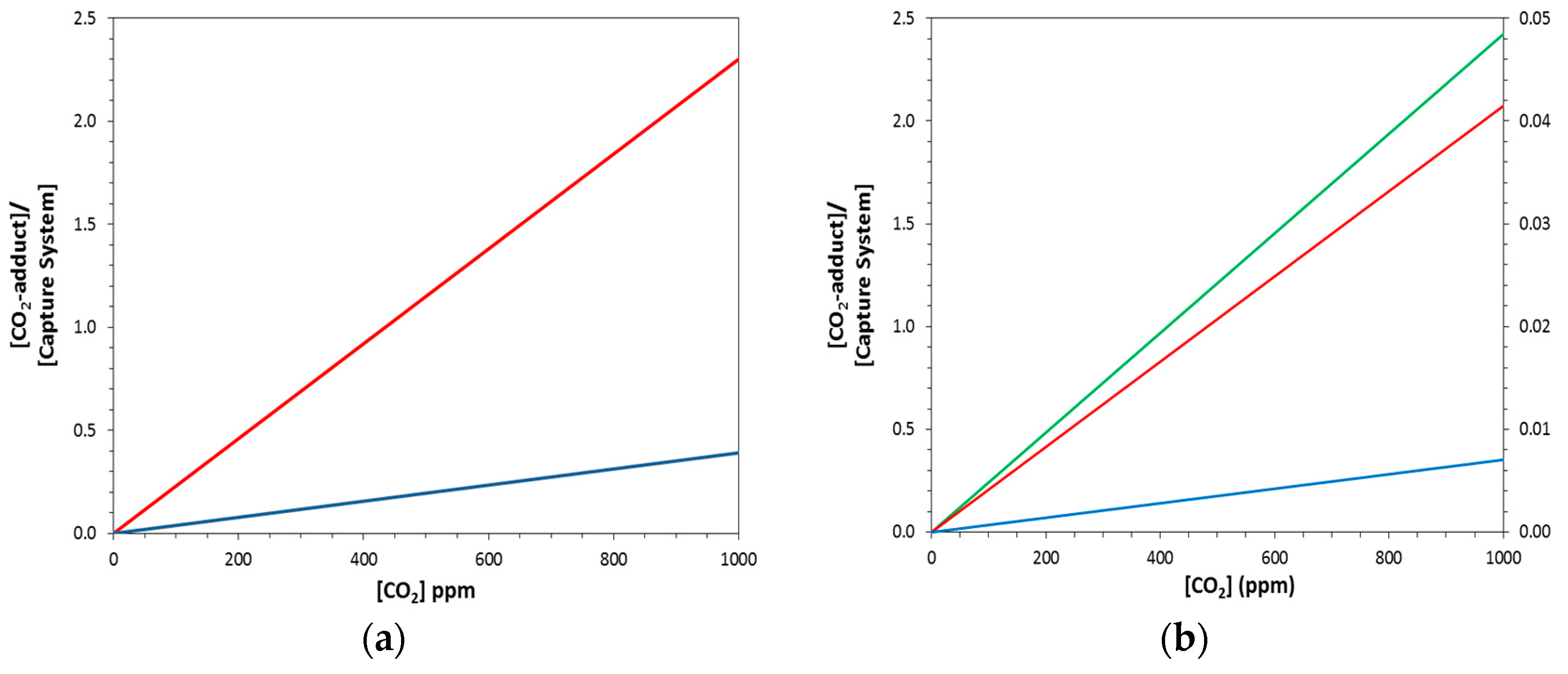
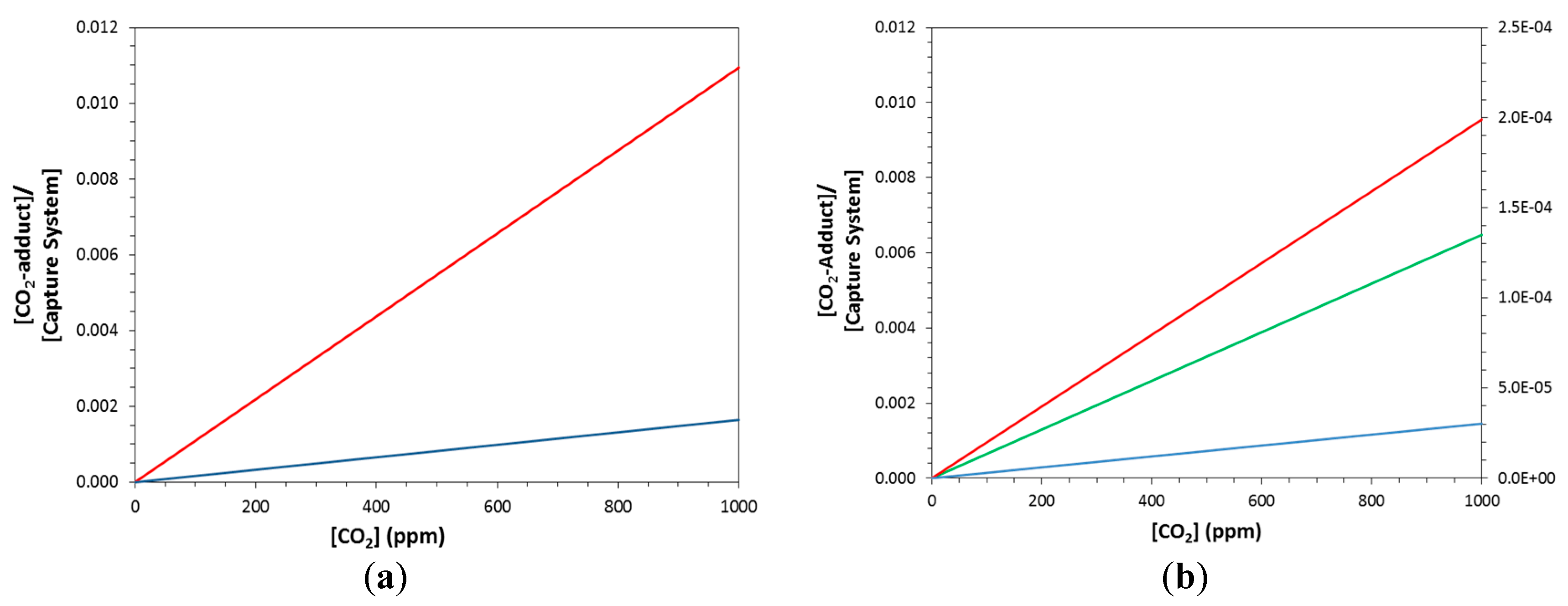
2.2. Equilibrium Concentrations with Non-Stoichiometric Conditions
2.3. Temperature Dependence of Capture Systems: Loading and Unloading
2.4. Variations of Thermodynamic Parameters—Guidelines for Capture System Design
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keeling, R.F. Recording Earth’s Vital Signs. Science 2008, 319, 1771–1772. [Google Scholar] [CrossRef] [PubMed]
- Trends in Atmospheric Carbon Dioxide, US Department of Commerce, National Oceanic & Atmospheric Administration (NOAA), Earth System Research Laboratory, Global Monitoring Division. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends (accessed on 28 April 2016).
- CarbonTracker CT2015, US Department of Commerce, National Oceanic & Atmospheric Administration (NOAA), Earth System Research Laboratory, Global Monitoring Division. Available online: http://www.esrl.noaa.gov/gmd/ccgg/carbontracker (accessed on 28 April 2016).
- Peters, W.; Jacobson, A.R.; Sweeney, C.; Andrews, A.E.; Conway, T.J.; Masarie, K.; Miller, J.B.; Bruhwiler, L.M.P.; Pétron, G.; Hirsch, A.I.; et al. An atmospheric perspective on North American carbon dioxide exchange: CarbonTracker. Proc. Natl. Acad. Sci. USA 2007, 104, 18925–18930. [Google Scholar] [CrossRef] [PubMed]
- CarbonTracker Documentation, CT2015 Release, CarbonTracker Team. 17 March 2016. Available online: http://www.esrl.noaa.gov/gmd/ccgg/carbontracker/CT2015_doc.pdf (accessed on 28 April 2016).
- United States Carbon Cycle Science Program. Available online: http://www.carbon cyclescience.gov (accessed on 28 April 2016).
- OCO-2, Orbiting Carbon Observatory, NASA, Jet Propulsion Laboratory. Available online: http://oco.jpl.nasa.gov (accessed on 28 April 2016).
- Intergovernmental Panel on Climate Change (IPCC). Available online: http://www.ipcc.ch (accessed on 28 April 2016).
- IPCC, 2014: Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team; Pachauri, R.K.; Meyer, L.A. (Eds.) IPCC: Geneva, Switzerland, 2016; p. 151. Available online: http://www.ipcc.ch/report/ar5 (accessed on 28 April 2016).
- Coal—Current Issues and Trends. EIA, U.S. Energy Information Agency. Available online: https://www.eia.gov (accessed on 28 April 2016).
- Gore, A. An Inconvenient Truth: The Planetary Emergency of Global Warming and What We Can Do about It; Rodale Books: Emmaus, PA, USA, 2006. [Google Scholar]
- Alliance for Climate Protection. Available online: https://www.change.org/organizations/climate_project (accessed on 28 April 2016).
- 350.org. Available online: https://350.org (accessed on 28 April 2016).
- The Carbon Bathtub. National Geographic Magazine. 2009. Available online: http://ngm.nationalgeographic.com/big-idea/05/carbon-bath (accessed on 28 April 2016).
- Bringing CO2 Monitoring to You: Communicating Atmospheric Chemistry. Available online: http://www.acs.org/content/acs/en/acs-webinars/professional-development/communicating-carbon.html (accessed on 28 April 2016).
- Victor, D.G. The Collapse of the Kyoto Protocol and the Struggle to Slow Global Warming; Princeton University Press: New Jersey, NJ, USA, 2004. [Google Scholar]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; pp. 1342–1343. [Google Scholar]
- First Successful Demonstration of Carbon Dioxide Air Capture Technology Achieved. By Earth Institute, Columbia University. Available online: physorg.com/news96732819.html (accessed on 28 April 2016).
- Keith, D.W.; Ha-Duong, M.; Stolaroff, J.K. Climate strategy with CO2 capture from the air. Clim. Chang. 2006, 74, 17–45. [Google Scholar] [CrossRef]
- Lackner, K.S. Capture of carbon dioxide from ambient air. Eur. Phys. J. Special Topics 2009, 176, 93–106. [Google Scholar] [CrossRef]
- Socolow, R.; Desmond, M.; Aines, R.; Blackstock, J.; Bolland, O.; Kaarsberg, T.; Lewis, N.; Mazzotti, M.; Pfeffer, A.; Sawyer, K.; et al. Direct Air Capture of CO2 with Chemicals: A Technology Assessment for the APS Panel on Public Affairs; American Physical Society: Washington, DC, USA, 1 June 2011; Available online: http://www.aps.org/ policy/reports/assessments/upload/dac2011.pdf (accessed on 28 April 2016).
- Zeman, F.S.; Lackner, K.S. Capturing carbon dioxide directly from the atmosphere. World Resour. Rev. 2004, 16, 62–68. [Google Scholar]
- Lackner, K.S.; Brennan, S. Envisioning carbon capture and storage: Expanded possibilities due to air capture, leakage insurance, and C-14 monitoring. Clim. Chang. 2009, 96, 357–378. [Google Scholar] [CrossRef]
- Lackner, K.S.; Brennan, S.; Matter, J.M.; Alissa Park, A.H.; Wright, A.; van der Zwaan, B. The urgency of the development of CO2 capture from ambient air. Proc. Natl. Acad. Sci. USA 2012, 109, 13156–13162. [Google Scholar] [CrossRef] [PubMed]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; MacDowell, N.; Fernandez, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Murphy, L.J.; Robertson, K.N.; Kemp, R.A.; Tuononen, H.M.; Clyburne, J.A.C. Structurally simple complexes of CO2. Chem. Commun. 2015, 51, 3942–3956. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fan, M.; Argyle, M. Supported monoethanolamine for CO2 separation. Ind. Eng. Chem. Res. 2011, 50, 11343–11349. [Google Scholar] [CrossRef]
- Kuntz, J.; Aroonwilas, A. Performance of spray column for CO2 capture application. Ind. Eng. Chem. Res. 2008, 47, 145–153. [Google Scholar] [CrossRef]
- Idem, R.; Wilson, M.; Tontiwachwuthikul, P.; Chakma, A.; Veawab, A.; Aroonwilas, A.; Gelowitz, D. Pilot plant studies of the CO2 capture performance of aqueous MEA and mixed MEA/MDEA solvents at the University of Regina CO2 capture technology development plant and the Boundary Dam CO2 capture demonstration plant. Ind. Eng. Chem. Res. 2006, 45, 2414–2420. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; May, R.B.; Prakash, G.K.S.; Olah, G.A.; Narayanan, S.R. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J. Am. Chem. Soc. 2011, 133, 20164–20167. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, E.; Barron, A.R. Effect of spray-drying and cryo-milling on the CO2 absorption performance of C60 cross-linked polyethyleneimine. J. Mater. Chem. A 2015, 3, 4323–4329. [Google Scholar] [CrossRef]
- McDonald, T.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Poloni, R.; Smit, B.; Neaton, J.B. Ligand-assisted enhancement of CO2 capture in metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 6714–6719. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.M.; Mason, J.A.; Kong, X.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocella, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2015, 519, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Song, Q.-W.; He, L.-N. Capture and Utilization of Carbon Dioxide with Poly-Ethylene Glycol, SpringerBriefs in Molecular Science; Springer: New York, NY, USA, 2012. [Google Scholar]
- Glaser, R.; Castello-Blindt, P.O.; Yin, J. Biomimetic Approaches to Reversible CO2 Capture from Air. N-Methylcarbaminic Acid Formation in Rubsico-Inspired Models. In New and Future Developments in Catalysis: Activation of Carbon Dioxide, 1st ed.; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 501–534. [Google Scholar]
- Glaser, R. RuBisCO-Inspired Biomimetic Approaches to Reversible CO2 Capture from Air. Metal Dependence of the H2O/CO2 Replacement Penalty. In Advances in CO2 Capture, Sequestration and Conversion; Jin, F., He, L.-N., Hu, Y.H., Eds.; ACS Books: Washington, DC, USA, 2015; pp. 501–534. [Google Scholar]
- Shively, J.M.; Van Keulen, G.; Meijer, W.G. Something about almost nothing: CO2 fixation in chemautotrophs. Ann. Rev. Microbiol. 1998, 52, 191–230. [Google Scholar] [CrossRef] [PubMed]
- Hugler, M.; Sievert, S.M. Beyond the Calvin cycle: Autotrophic carbon fixation in the ocean. Ann. Rev. Marine Sci. 2011, 3, 89–261. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.C.; Andersson, I. Structure of a product complex of spinach ribulose-1,5-bisphosphate carboxylase/oxygenase. Biochemistry 1997, 36, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.C.; Andersson, I. Structural transitions during activation and ligand binding in hexadecameric Rubisco inferred from the crystal structure of the activated unliganded spinach enzyme. Nat. Struct. Biol. 1996, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, S.; Newman, J.; Herrmann, C.; Rhoades, D. The crystal structures of Rubisco and opportunities for manipulating photosynthesis. J. Exp. Botany 1995, 46, 1261–1267. [Google Scholar] [CrossRef]
- Lundqvist, T.; Schneider, G. Structure of the ternary complex of ribulose 1,5-bisphosphate carboxylase, magnesium(II) and activator carbon dioxide at 2.3 Å resolution. Biochemistry 1991, 30, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Hartman, F.C.; Harpel, M.R. Structure, function, regulation, and assembly of d-ribulose-1,5-bisphosphate carboxylase/oxygenase. Annu. Rev. Biochem. 1994, 63, 197–234. [Google Scholar] [CrossRef] [PubMed]
- Cleland, W.W.; Andrews, T.J.; Gutteridge, S.; Hartman, F.C.; Lorimer, G.H. Mechanism of Rubisco: The carbamate as general base. Chem. Rev. 1998, 98, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Earth System Research Laboratory Global Monitoring Division. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 28 April 2016).
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Kumar, P.S.; Hogendoorn, J.A.; Feron, P.H.M.; Versteeg, G.F. New absorption liquids for the removal of CO2 from dilute gas streams using membrane contactors. Chem. Eng. Sci. 2002, 57, 1639–1651. [Google Scholar] [CrossRef]
- Dindore, V.Y.; Brilman, D.W.F.; Geuzebroek, F.H.; Versteeg, G.F. Membrane-solvent selection for CO2 removal using membrane gas-liquid contactors. Sep. Purif. Technol. 2004, 40, 133–145. [Google Scholar] [CrossRef]
- Atchariyawut, S.; Feng, C.; Wang, R.; Jiraratananon, R.; Liang, D.T. Effect of membrane Structure on mass-transfer in the membrane gas-liquid contacting process using microporous PVDF hollow fibers. J. Membr. Sci. 2006, 285, 272–281. [Google Scholar] [CrossRef]
- Simons, K.; Nijmeijer, K.; Wessling, M. Gas-liquid membrane contactors for CO2 removal. J. Membr. Sci. 2009, 340, 214–220. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Climatological Data for St. Louis, Columbia, and Quincy—National Weather Service. Available online: http://www.weather.gov/lsx/cli_archive (accessed on 28 April 2016).

| Reaction | ΔG° | ΔH° | ΔS° | Fraction CO2-Adduct at [CO2] = 400 ppm | ||
|---|---|---|---|---|---|---|
| T° | T° + 20 | T° + 40 | ||||
| R1 | 4.09 | −2.02 | −20.49 | |||
| R14a | −6.59 | −17.26 | −35.79 | 0.0163 | 0.00159 | 0.000202 |
| R14b | −5.54 | −17.72 | −40.85 | 0.00281 | 0.000257 | 0.0000313 |
| R14h | −9.00 | −19.67 | −35.79 | 0.492 | 0.0676 | 0.00737 |
| HR1a | −6.59 | −15.00 | −28.21 | 0.0163 | 0.00204 | 0.000320 |
| HR1b | −6.59 | −20.00 | −44.98 | 0.0163 | 0.00120 | 0.000118 |
| HR1c | −6.59 | −25.00 | −61.75 | 0.0163 | 0.000705 | 0.0000435 |
| HR2a | −9.00 | −15.00 | −20.12 | 0.492 | 0.106 | 0.0184 |
| HR2b | −9.00 | −20.00 | −36.89 | 0.492 | 0.0654 | 0.00685 |
| HR2c | −9.00 | −25.00 | −53.66 | 0.492 | 0.0395 | 0.00254 |
| HR3a | −11.00 | −17.00 | −20.12 | 0.966 | 0.738 | 0.269 |
| HR3b | −11.00 | −22.00 | −36.89 | 0.966 | 0.623 | 0.119 |
| HR3c | −11.00 | −27.00 | −53.66 | 0.966 | 0.493 | 0.0499 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muelleman, A.; Schell, J.; Glazer, S.; Glaser, R. Thermochemistry of a Biomimetic and Rubisco-Inspired CO2 Capture System from Air. C 2016, 2, 18. https://doi.org/10.3390/c2030018
Muelleman A, Schell J, Glazer S, Glaser R. Thermochemistry of a Biomimetic and Rubisco-Inspired CO2 Capture System from Air. C. 2016; 2(3):18. https://doi.org/10.3390/c2030018
Chicago/Turabian StyleMuelleman, Andrew, Joseph Schell, Spencer Glazer, and Rainer Glaser. 2016. "Thermochemistry of a Biomimetic and Rubisco-Inspired CO2 Capture System from Air" C 2, no. 3: 18. https://doi.org/10.3390/c2030018
APA StyleMuelleman, A., Schell, J., Glazer, S., & Glaser, R. (2016). Thermochemistry of a Biomimetic and Rubisco-Inspired CO2 Capture System from Air. C, 2(3), 18. https://doi.org/10.3390/c2030018