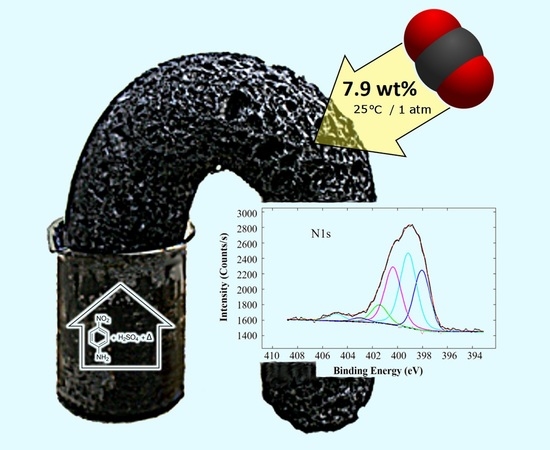
CO2 Adsorption by para-Nitroaniline Sulfuric Acid-Derived Porous Carbon Foam
Abstract
:1. Introduction
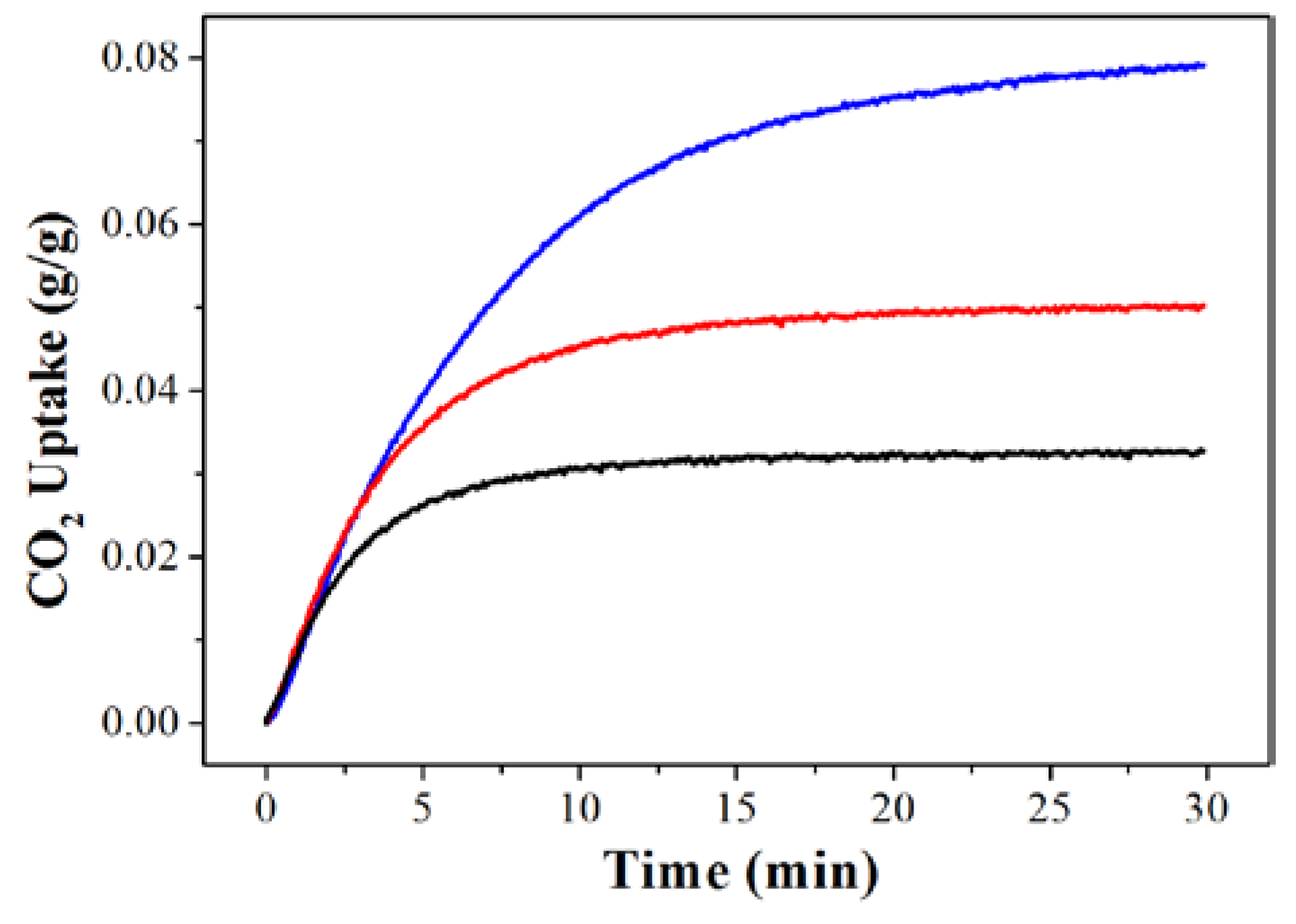
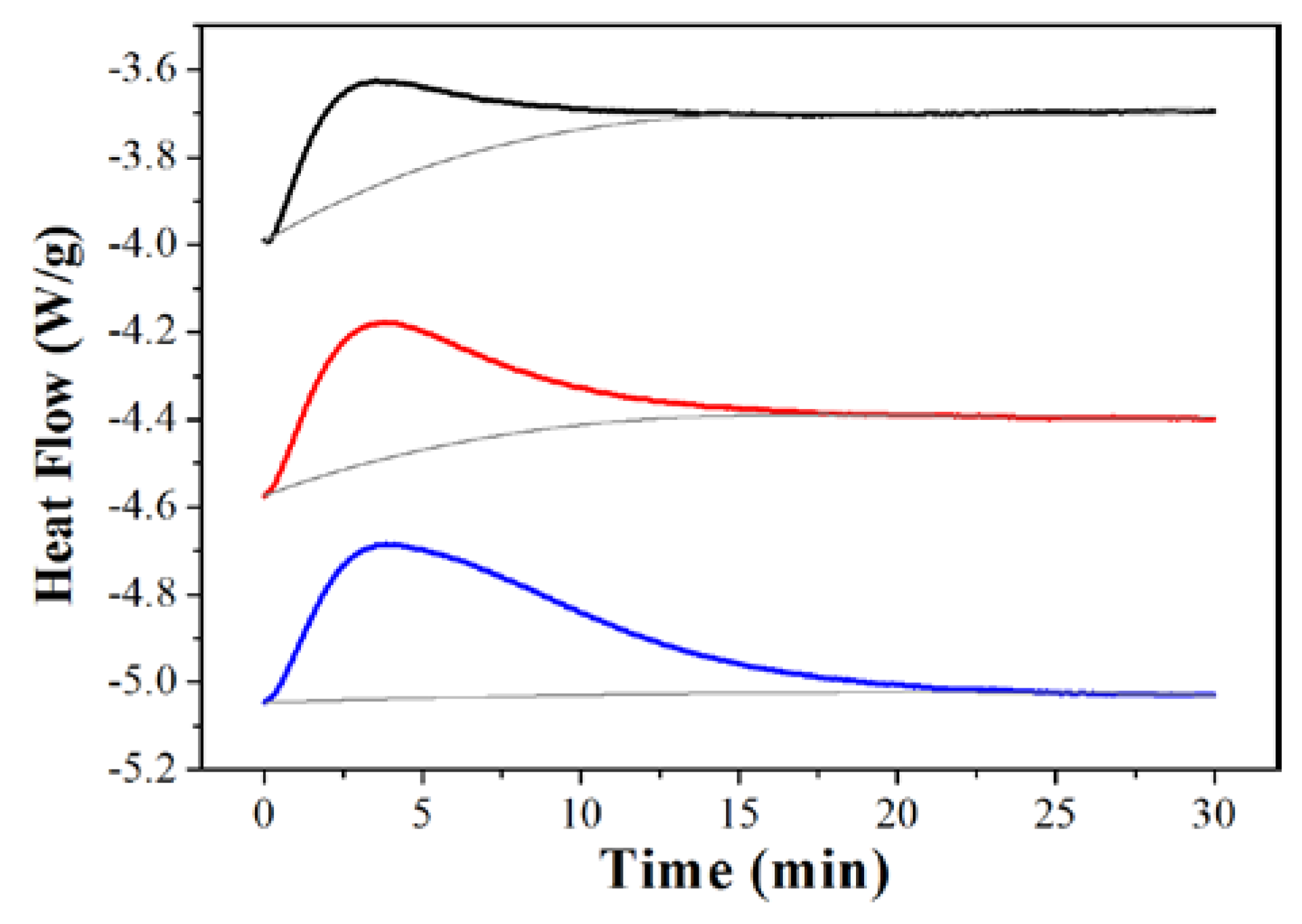
2. Results and Discussion
3. Experimental
3.1. Materials and Characterization
3.2. Synthesis of N/S-Doped Porous Carbon Foam (NSPC)
3.3. CO2 Adsorption
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Saravanamurugan, S.; Kunov-Kruse, A.J.; Fehrmann, R.; Riisager, A. Amine-functionalized amino acid-based ionic liquids as efficient and high-capacity absorbents for CO2. ChemSusChem 2014, 7, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, S.; Ma, X.; Gong, J. Recent advances in capture of carbon dioxide using alkali-metal-based oxides. Energy Environ. Sci. 2011, 4, 3805–3819. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Zu, J.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Chen, C.; Lee, Y.-R.; Ahn, W.-S. CO2 adsorption over metal-organic frameworks: A mini review. J. Nanosci. Nanotechnol. 2016, 16, 4291–4301. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Cheng, W.; Shen, X.; Fan, M.; Russell, A.; Wu, Z.; Yi, X. Mesoporous amine-modified SiO2 aerogel: A potential CO2 sorbent. Energy Environ. Sci. 2011, 4, 2070–2074. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Arencibia, A.; Sanz, R.; Calleja, G. New developments on carbon dioxide capture using amine-impregnated silicas. Adsorption 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Andreoli, E.; Dillon, E.P.; Cullum, L.; Alemany, L.B.; Barron, A.R. Cross-linking amine-rich compounds into high performing selective CO2 absorbents. Sci. Rep. 2014, 4, 7304. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, E.; Barron, A.R. Activation effect of fullerene C60 on the carbon dioxide absorption performance of amine-rich polypropylenimine dendrimers. ChemSusChem 2015, 8, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, T.A.; Soares, V.P.; Andreoli, E. Pentaethylenehexamine-C60 for temperature consistent carbon capture. C 2015, 1, 16–26. [Google Scholar] [CrossRef]
- Ghosh, S.; Sevilla, M.; Fuertes, A.B.; Andreoli, E.; Barron, A.R. Defining a performance map of porous carbon sorbents for high-pressure carbon dioxide uptake and carbon dioxide-methane selectivity. J. Mater. Chem. A 2016, 4, 14739–14751. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Tour, J.J.; Kittrell, C.; Espinal, L.; Alemany, L.B.; Tour, J.M. Capturing carbon dioxide as a polymer from natural gas. Nat. Commun. 2014, 5, 3961. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Parra, J.B.; Fuertes, A.B. Assessment of the role of micropore size and N-doping in CO2 capture by porous carbons. ACS Appl. Mater. Interfaces 2013, 5, 6360–6368. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, B.; Mokaya, R. Is N-doping in porous carbons beneficial for CO2 storage? Experimental demonstration of the relative effects of pore size and N-doping. Chem. Mater. 2016, 28, 994–1001. [Google Scholar] [CrossRef]
- Ghosh, S.; Barron, A.R. Is the formation of poly-CO2 stabilized by Lewis base moieties in N- and S-doped Porous carbon? C 2016, 2, 5. [Google Scholar] [CrossRef]
- Khatri, R.A.; Chuang, S.S.C.; Soong, Y.; Gray, M. Thermal and chemical stability of regenerable solid amine sorbent for CO2 capture. Energy Fuels 2006, 20, 1514–1520. [Google Scholar] [CrossRef]
- Dell’Amico, D.B.; Calderazzo, F.; Labella, L.; Marchetti, F.; Pampaloni, G. Converting carbon dioxide into carbamato derivatives. Chem. Rev. 2003, 103, 3857–3898. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, T.L.; Nguyen, Y.N. Carbon dioxide reaction kinetics and transport in aqueous amine membranes. Ind. Eng. Chem. Fundam. 1980, 19, 260–266. [Google Scholar] [CrossRef]
- Mebane, D.S.; Kress, J.D.; Storlie, C.B.; Fauth, D.J.; Gray, M.L.; Li, K. Transport, zwitterions, and the role of water for CO2 adsorption in mesoporous silica-supported amine sorbents. J. Phys. Chem C 2013, 117, 26617–26627. [Google Scholar] [CrossRef]
- Li, K.; Kress, J.D.; Mebane, D.S. The mechanism of CO2 adsorption under dry and humid conditions in mesoporous silica-supported amine sorbents. J. Phys. Chem. C 2016, 120, 23683–23691. [Google Scholar] [CrossRef]
- Andreoli, E.; Barron, A.R. Correlating carbon dioxide capture and chemical changes in pyrolyzed polyethylenimine-C60. Energy Fuels 2015, 29, 4479–4487. [Google Scholar] [CrossRef]
- Davis, T.L. Pyrotechnic snakes. J. Chem. Educ. 1940, 17, 268–269. [Google Scholar] [CrossRef]
- Poshkus, A.C.; Parker, J.A. Studies on nitroaniline–sulfuric acid compositions: Aphrogenic pyrostats. J. Appl. Polym. Sci. 1970, 14, 2049–2064. [Google Scholar] [CrossRef]
- Hao, G.-P.; Li, W.-C.; Qian, D.; Lu, A.-H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mat. 2010, 22, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Bolis, V. Fundamentals in adsorption at the solid-gas interface. Concepts and thermodynamics. In Calorimetry and Thermal Methods in Catalysis; Auroux, A., Ed.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Maiti, A.; Bourcier, W.L.; Aines, R.D. Atomistic modelling of CO2 capture in primary and tertiary amines—Heat of absorption and density changes. Chem. Phys. Lett. 2011, 509, 25–28. [Google Scholar] [CrossRef]
- Kim, I.; Svendsen, H.F. Heat of absorption of carbon dioxide (CO2) in monoethanolamine (MEA) and 2-(aminoethyl) ethanolamine (AEEA) solutions. Ind. Eng. Chem. Res. 2007, 46, 5803–5809. [Google Scholar] [CrossRef]
- Kessel, R.; Schultze, J.W. Surface analytical and photoelectrochemical investigations of conducting polymers. Surf. Interface Anal. 1990, 16, 401–406. [Google Scholar] [CrossRef]
- Hashemi, T.; Hogarth, C.A. The mechanism of corrosion inhibition of copper in NaCl solution by benzotriazole studied by electron spectroscopy. Electrochim. Acta 1988, 33, 1123–1127. [Google Scholar] [CrossRef]
- Peeling, J.; Hruska, F.E.; McKinnon, D.M.; Chauhan, M.S.; McIntyre, N.S. ESCA studies of the uracil base. The effect of methylation, thionation, and ionization on charge distribution. Can. J. Chem. 1978, 56, 2405–2411. [Google Scholar] [CrossRef]
- Andreoli, E.; Cullum, L.; Barron, A.R. Carbon dioxide absorption by polyethylenimine-functionalized nanocarbons: A kinetic study. Ind. Eng. Chem. Res. 2015, 54, 878–889. [Google Scholar] [CrossRef]
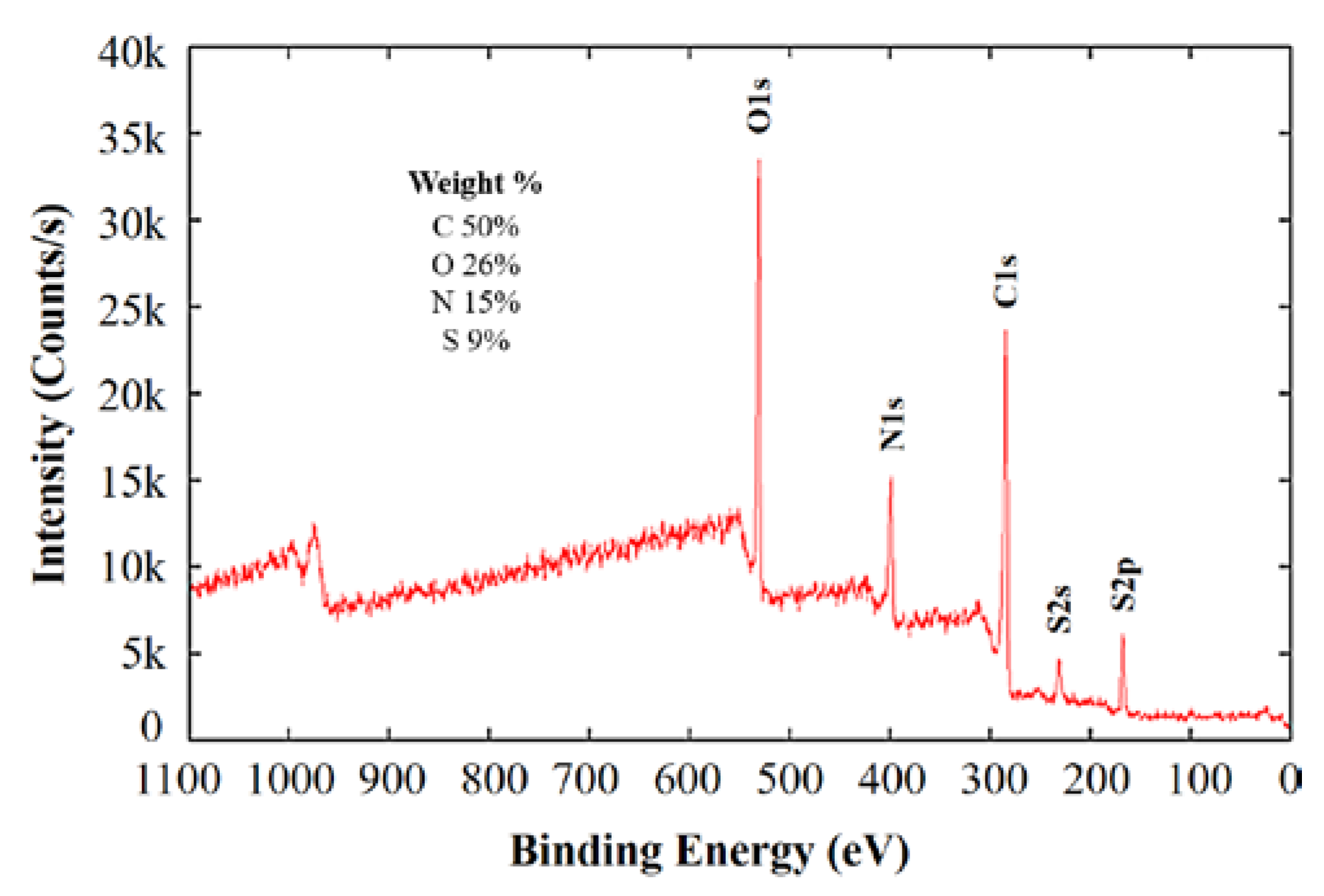
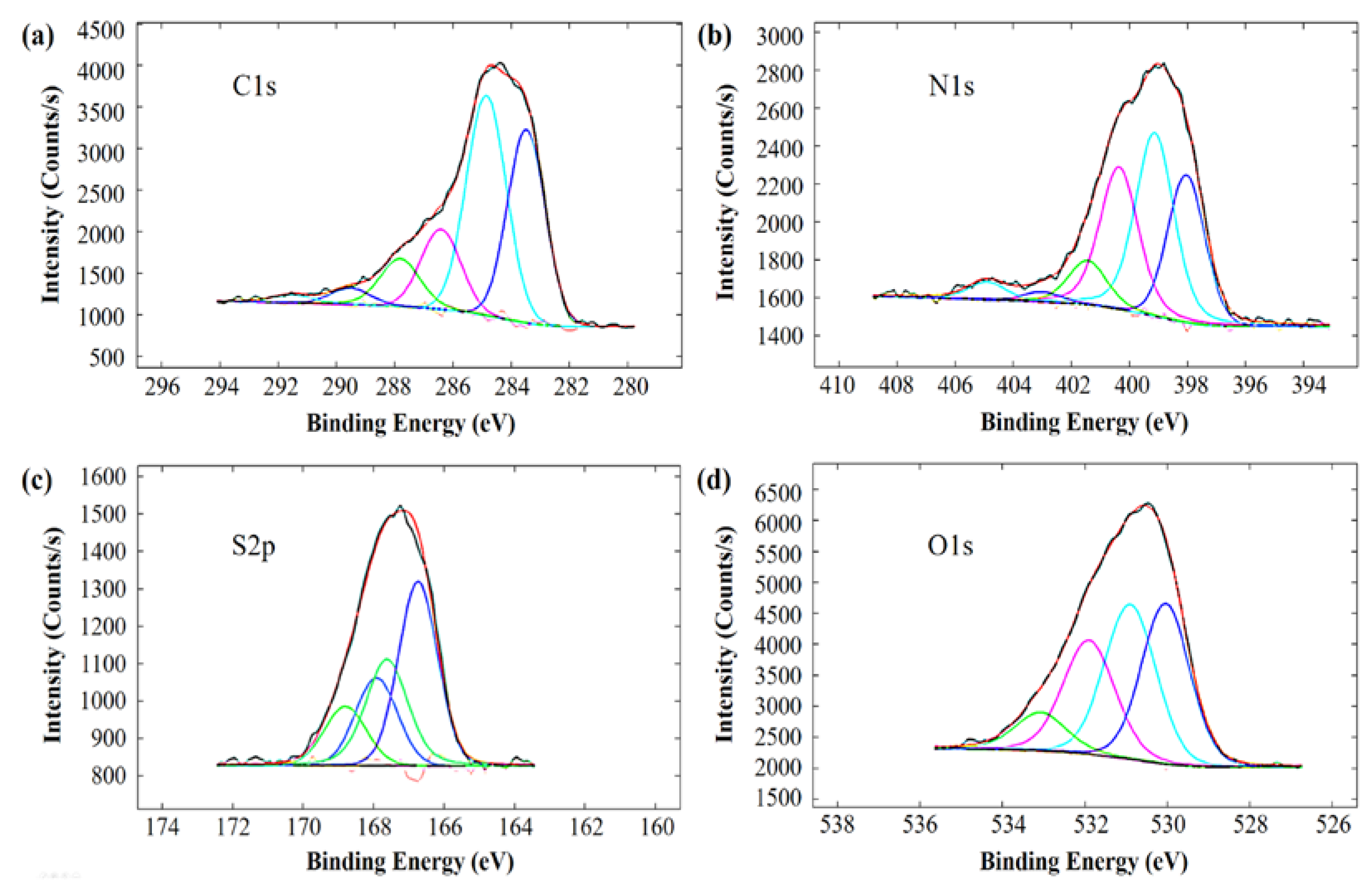
| Signal | Peak (eV) | atom % | Assignment |
|---|---|---|---|
| C1s | 283.5 | 32.4 | oxidized conjugated C |
| 284.9 | 39.3 | aromatic C | |
| 286.4 | 14.3 | C–OH, C–NR2, –C≡N | |
| 287.8 | 9.2 | C=O, C(O)NH | |
| 289.5 | 3.1 | C(O)OH | |
| 291.5 | 1.7 | π → π* transition of the aromatic ring | |
| N1s | 398.0 | 24.5 | azole/triazole N |
| 399.1 | 34.8 | –NH2, aromatic N, –C≡N | |
| 400.4 | 27.0 | C(O)NH, amino acid, carbazole, uracil | |
| 401.4 | 8.4 | ammonium, amino benzenesulfonic acid | |
| 403.0 | 1.9 | polyaniline, N-oxide | |
| 404.9 | 3.4 | –NO2, –N=O | |
| S2p | 166.75 and 167.93 | 59.6 | sulfite |
| 167.62 and 168.80 | 40.4 | sulfate, sulfonic, sulfonate | |
| O1s | 530.0 | 32.2 | sulfinyl (organic) |
| 530.9 | 33.3 | amino acid CO2H | |
| 531.9 | 25.4 | methacrylate, terephthalate, sulfone | |
| 533.1 | 9.1 | H2O, CO2H |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreoli, E.; Barron, A.R. CO2 Adsorption by para-Nitroaniline Sulfuric Acid-Derived Porous Carbon Foam. C 2016, 2, 25. https://doi.org/10.3390/c2040025
Andreoli E, Barron AR. CO2 Adsorption by para-Nitroaniline Sulfuric Acid-Derived Porous Carbon Foam. C. 2016; 2(4):25. https://doi.org/10.3390/c2040025
Chicago/Turabian StyleAndreoli, Enrico, and Andrew R. Barron. 2016. "CO2 Adsorption by para-Nitroaniline Sulfuric Acid-Derived Porous Carbon Foam" C 2, no. 4: 25. https://doi.org/10.3390/c2040025
APA StyleAndreoli, E., & Barron, A. R. (2016). CO2 Adsorption by para-Nitroaniline Sulfuric Acid-Derived Porous Carbon Foam. C, 2(4), 25. https://doi.org/10.3390/c2040025