Chestnut Waste-Derived Fe-Based Photocatalyst for Diclofenac Degradation
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Biochar-Supported Iron Catalyst
2.3. Materials Characterisation
2.4. Adsorption and Photocatalytic Experiments
3. Results and Discussion
3.1. Materials Characterisation
3.1.1. Pristine Biochar
3.1.2. Fe-Based Catalysts
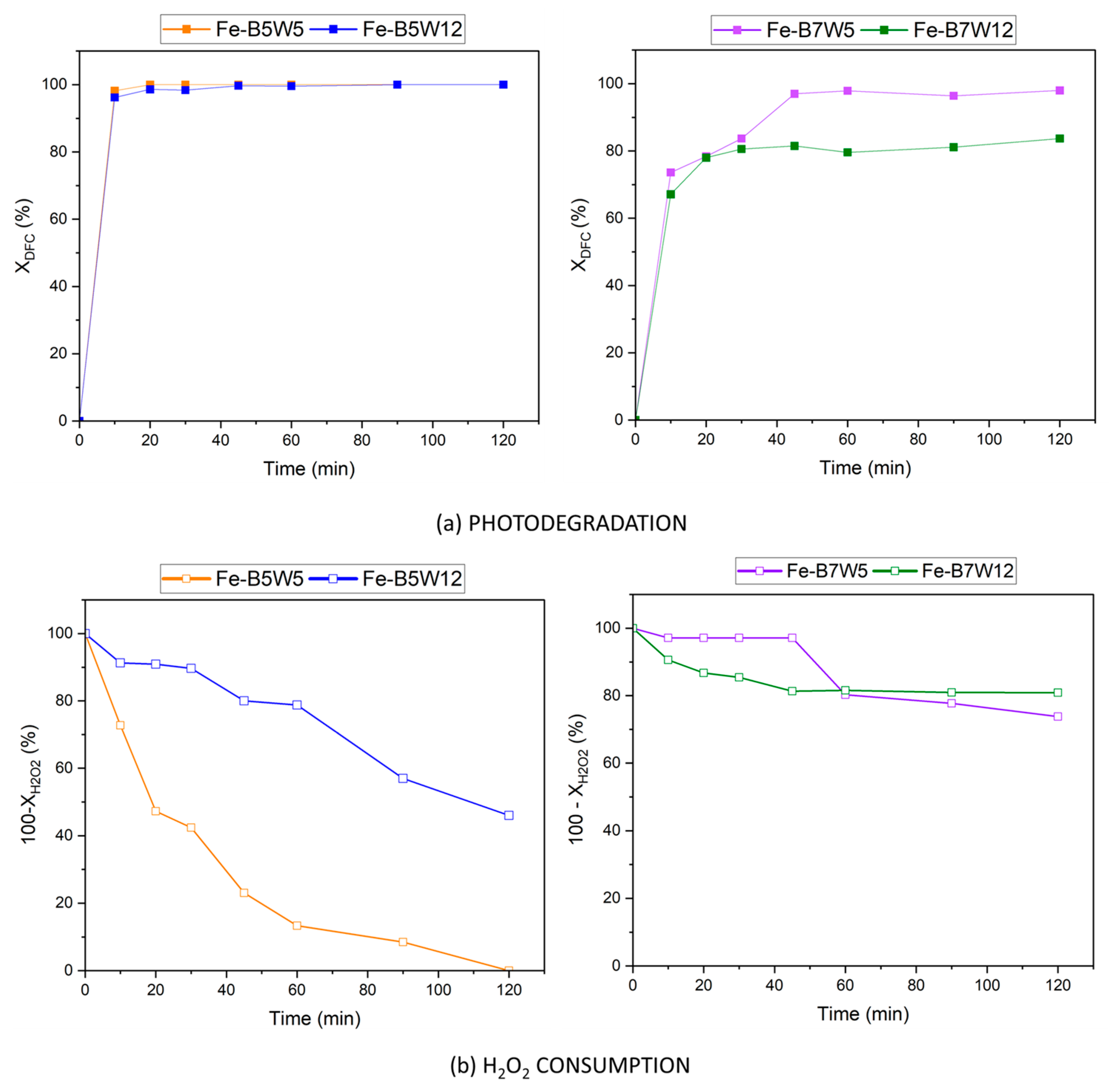
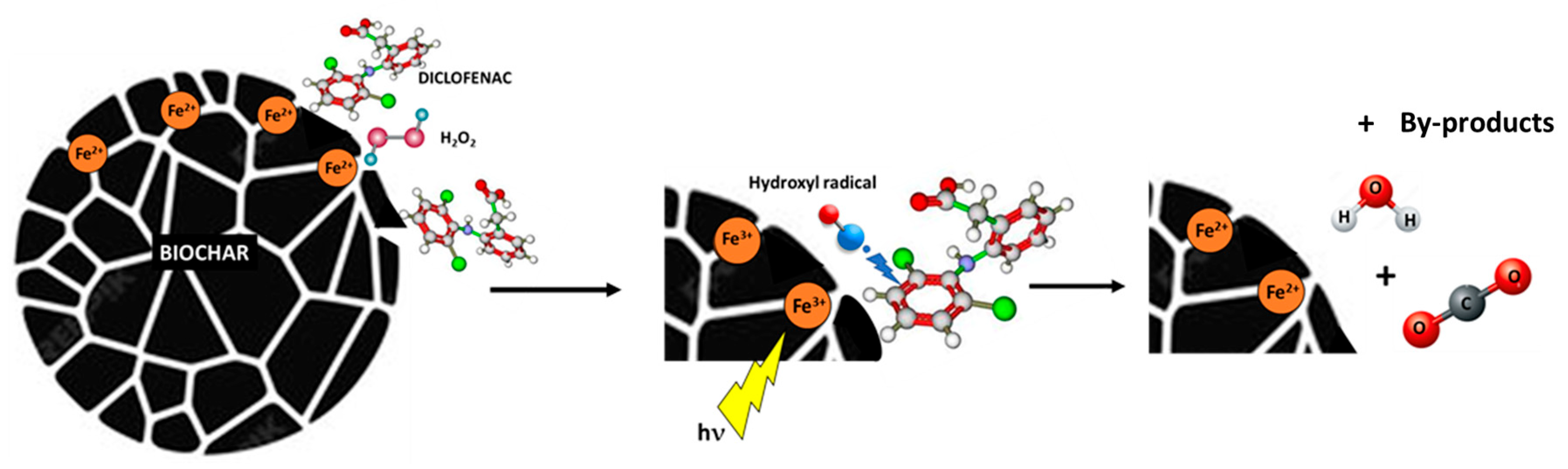
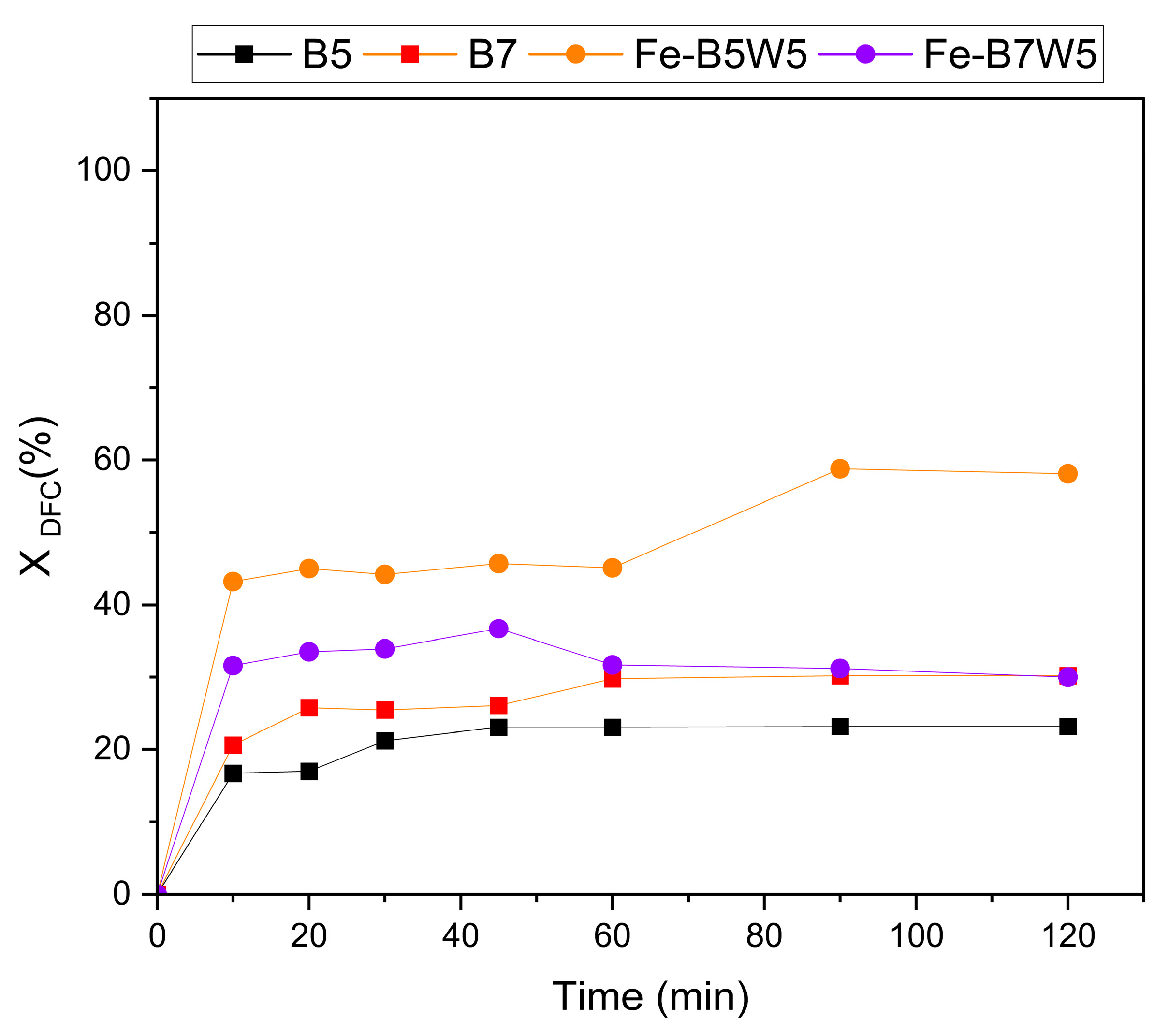
3.2. Photocatalytic and Adsorption Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B5 | Pristine biochar pyrolysed at 500 °C |
| B7 | Pristine biochar pyrolysed at 700 °C |
| Fe-B5W5 | Fe-biochar catalyst pyrolysed at 500 °C and washed 5 times |
| Fe-B5W12 | Fe-biochar catalyst pyrolysed at 500 °C and washed 12 times |
| Fe-B7W5 | Fe-biochar catalyst pyrolysed at 700 °C and washed 5 times |
| Fe-B7W12 | Fe-biochar catalyst pyrolysed at 700 °C and washed 12 times |
| EA | Elemental Analysis |
| ICP-OES | Inductively Coupled Plasma–Optical Emission Spectroscopy |
| TOC | Total Organic Carbon |
| XRPD | X-Ray Powder Diffraction |
| TG-DTG | Thermogravimetric analyses |
| SEM-EDX | Scanning Electron Microscopy and Energy Dispersive X-Ray |
| AOP | Advanced Oxidation process |
| NSAID | Non-steroidal Anti-inflammatory Drug |
| DFC | Diclofenac |
References
- Lakshmi, S.D.; Geetha, B.V.; Vibha, M. From Prescription to Pollution: The Ecological Consequences of NSAIDs in Aquatic Ecosystems. Toxicol. Rep. 2024, 13, 101775. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions—A Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and Removal of Pharmaceuticals in a Municipal Sewage Treatment System in the South of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Kermia, A.E.B.; Fouial-Djebbar, D.; Trari, M. Occurrence, Fate and Removal Efficiencies of Pharmaceuticals in Wastewater Treatment Plants (WWTPs) Discharging in the Coastal Environment of Algiers. Comptes Rendus Chim. 2016, 19, 963–970. [Google Scholar] [CrossRef]
- Sun, Q.; Li, M.; Ma, C.; Chen, X.; Xie, X.; Yu, C.-P. Seasonal and Spatial Variations of PPCP Occurrence, Removal and Mass Loading in Three Wastewater Treatment Plants Located in Different Urbanization Areas in Xiamen, China. Environ. Pollut. 2016, 208, 371–381. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in Wastewater Treatment Plants in Greece: Occurrence, Removal and Environmental Risk Assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Nokwethemba Mahlambi, P.; Chimuka, L.; Madikizela, L.M. Adsorbents and Removal Strategies of Non-Steroidal Anti-Inflammatory Drugs from Contaminated Water Bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Gautam, M.K.; Mondal, T.; Nath, R.; Mahajon, B.; Chincholikar, M.; Bose, A.; Das, D.; Das, R.; Mondal, S. Harnessing Activated Hydrochars: A Novel Approach for Pharmaceutical Contaminant Removal. C 2024, 10, 8. [Google Scholar] [CrossRef]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of Diclofenac from Wastewater: A Comprehensive Review of Detection, Characteristics and Tertiary Treatment Techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A Review of Recent Developments in Catalytic Applications of Biochar-Based Materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New Notion of Biochar: A Review on the Mechanism of Biochar Applications in Advannced Oxidation Processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S.; et al. Fabrication of Novel Magnetic MnFe2O4/Bio-Char Composite and Heterogeneous Photo-Fenton Degradation of Tetracycline in near Neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, D.; Cortijo, M.; Huang, C.P. The Role of Iron on the Degradation and Mineralization of Organic Compounds Using Conventional Fenton and Photo-Fenton Processes. Chem. Eng. J. 2009, 155, 637–646. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton Catalysts: A Review of Recent Advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Gou, Y.; Chen, P.; Yang, L.; Li, S.; Peng, L.; Song, S.; Xu, Y. Degradation of Fluoroquinolones in Homogeneous and Heterogeneous Photo-Fenton Processes: A Review. Chemosphere 2021, 270, 129481. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Abdelkader-Fernández, V.K.; Matos, R.; Peixoto, A.F.; Fernandes, D.M. Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review. Catalysts 2022, 12, 207. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. A Comprehensive Assessment of the Method for Producing Biochar, Its Characterization, Stability, and Potential Applications in Regenerative Economic Sustainability—A Review. Clean. Mater. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Okiemute Akpasi, S.; Michael Smarte Anekwe, I.; Adedeji, J.; Lewis Kiambi, S. Biochar Development as a Catalyst and Its Application. In Biochar—Productive Technologies, Properties and Applications; Bartoli, M., Giorcelli, M., Tagliaferro, A., Eds.; IntechOpen: London, UK, 2023; ISBN 978-1-80356-251-3. [Google Scholar]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of Biochar and Its Composites in Catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, Y.; Li, J.; Patel, A.K.; Dong, C.-D.; Jin, X.; Gu, C.; Yip, A.C.K.; Tsang, D.C.W.; Ok, Y.S. Recent Advancements and Challenges in Emerging Applications of Biochar-Based Catalysts. Biotechnol. Adv. 2023, 67, 108181. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Dalai, A.K.; Chaurasia, S.P. Activity and Stability of Biochar in Hydrogen Peroxide Based Oxidation System for Degradation of Naphthenic Acid. Chemosphere 2020, 241, 125007. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Wang, Y.; Dionysiou, D.D.; Zhou, D. Photogeneration of Reactive Oxygen Species from Biochar Suspension for Diethyl Phthalate Degradation. Appl. Catal. B Environ. 2017, 214, 34–45. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Z.; Zeng, G.; Lai, C.; Xiao, R.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. Insight into the Mechanism of Persulfate Activated by Bone Char: Unraveling the Role of Functional Structure of Biochar. Chem. Eng. J. 2020, 401, 126127. [Google Scholar] [CrossRef]
- Zhu, N.; Li, C.; Bu, L.; Tang, C.; Wang, S.; Duan, P.; Yao, L.; Tang, J.; Dionysiou, D.D.; Wu, Y. Bismuth Impregnated Biochar for Efficient Estrone Degradation: The Synergistic Effect between Biochar and Bi/Bi2O3 for a High Photocatalytic Performance. J. Hazard. Mater. 2020, 384, 121258. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.N.; Wu, Z.; Goh, P.S.; Zhou, S. The State-of-the-Art Development of Biochar Based Photocatalyst for Removal of Various Organic Pollutants in Wastewater. J. Clean. Prod. 2023, 429, 139487. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Xiao, R.; Tafti, N.; DeLaune, R.D.; Seo, D.-C. Degradation of Orange G by Fenton-like Reaction with Fe-Impregnated Biochar Catalyst. Bioresour. Technol. 2018, 249, 368–376. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, M.; Deng, Y.; Khan, Z.H.; Liu, X.; Song, Z.; Qiu, W. Efficient Oxidation and Adsorption of As(III) and As(V) in Water Using a Fenton-like Reagent, (Ferrihydrite)-Loaded Biochar. Sci. Total Environ. 2020, 715, 136957. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Pyrolysis of Different Biomass Pre-Impregnated with Steel Pickling Waste Liquor to Prepare Magnetic Biochars and Their Use for the Degradation of Metronidazole. Bioresour. Technol. 2019, 289, 121613. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Zhao, H.; Yan, Q. Adsorption and Fenton-like Removal of Chelated Nickel from Zn-Ni Alloy Electroplating Wastewater Using Activated Biochar Composite Derived from Taihu Blue Algae. Chem. Eng. J. 2020, 379, 122372. [Google Scholar] [CrossRef]
- Yan, J.; Qian, L.; Gao, W.; Chen, Y.; Ouyang, D.; Chen, M. Enhanced Fenton-like Degradation of Trichloroethylene by Hydrogen Peroxide Activated with Nanoscale Zero Valent Iron Loaded on Biochar. Sci. Rep. 2017, 7, 43051. [Google Scholar] [CrossRef] [PubMed]
- Welter, N.; Leichtweis, J.; Silvestri, S.; Sánchez, P.I.Z.; Mejía, A.C.C.; Carissimi, E. Preparation of a New Green Composite Based on Chitin Biochar and ZnFe2O4 for Photo-Fenton Degradation of Rhodamine B. J. Alloys Compd. 2022, 901, 163758. [Google Scholar] [CrossRef]
- Xin, S.; Ma, B.; Liu, G.; Ma, X.; Zhang, C.; Ma, X.; Gao, M.; Xin, Y. Enhanced Heterogeneous Photo-Fenton-like Degradation of Tetracycline over CuFeO2/Biochar Catalyst through Accelerating Electron Transfer under Visible Light. J. Environ. Manag. 2021, 285, 112093. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xie, X.; Wang, Z. Bifunctional MnFe2O4/Chitosan Modified Biochar Composite for Enhanced Methyl Orange Removal Based on Adsorption and Photo-Fenton Process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126104. [Google Scholar] [CrossRef]
- Bashir, A.; Pandith, A.H.; Qureashi, A.; Malik, L.A.; Gani, M.; Perez, J.M. Catalytic Propensity of Biochar Decorated with Core-Shell nZVI@Fe3O4: A Sustainable Photo-Fenton Catalysis of Methylene Blue Dye and Reduction of 4-Nitrophenol. J. Environ. Chem. Eng. 2022, 10, 107401. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Qin, H.; Zhou, J.; Shen, Q.; Wang, K.; Chen, W.; Liu, M.; Li, N. Synergy Effect between Adsorption and Heterogeneous Photo-Fenton-like Catalysis on LaFeO3/Lignin-Biochar Composites for High Efficiency Degradation of Ofloxacin under Visible Light. Sep. Purif. Technol. 2022, 280, 119751. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 November 2024).
- De Vasconcelos, M.C.; Bennett, R.N.; Rosa, E.A.; Ferreira-Cardoso, J.V. Composition of European Chestnut (Castanea sativa Mill.) and Association with Health Effects: Fresh and Processed Products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Eurostat. Food Waste and Food Waste Prevention-Estimates. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 15 January 2024).
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of Biomass Waste to Engineered Activated Biochar by Microwave Pyrolysis: Progress, Challenges, and Future Directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Singh, A.; Himanshu, M.; Verma, B.; Singh, R.; Lal, B.; Syed, A.; Elgorban, A.M.; Wong, L.S.; Srivastava, N. Evaluation of Sustainability of Fabrication Process and Characterization Studies of Activated Carbon Nanocatalyst from Waste Chestnut Peels. J. Mol. Struct. 2025, 1321, 139810. [Google Scholar] [CrossRef]
- Nguyen, T.-K.-T.; Nguyen, T.-B.; Chen, C.-W.; Chen, W.-H.; Bui, X.-T.; Lam, S.S.; Dong, C.-D. Boosting Acetaminophen Degradation in Water by Peracetic Acid Activation: A Novel Approach Using Chestnut Shell-Derived Biochar at Varied Pyrolysis Temperatures. Environ. Res. 2024, 252, 119143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Dai, Z.; Zhang, A.-Y.; Yin, J.; Peng, S.; Liang, H. Recycling Chestnut Shell for Superior Peroxymonosulfate Activation in Contaminants Degradation via the Synergistic Radical/Non-Radical Mechanisms. J. Hazard. Mater. 2022, 430, 128471. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Milbeau, C.L.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of Diclofenac onto Organoclays: Effects of Surfactant and Environmental (pH and Temperature) Conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef]
- Muelas-Ramos, V.; Gascó, A.; Salvatierra, M.; De Los Ríos, C.; Jiménez-Bautista, K.; Merayo, N.; Bahamonde, A.; Hermosilla, D. Assessing the Application of a Biochar-Supported Iron Oxide Catalyst to the Treatment of Imidacloprid by Photo-Fenton Technologies. Catal. Today 2024, 438, 114782. [Google Scholar] [CrossRef]
- Shen, T.; Zhang, F.; Yang, S.; Wang, Y.; Liu, H.; Wang, H.; Hu, J. Comprehensive Study on the Pyrolysis Process of Chestnut Processing Waste (Chestnut Shells): Kinetic Triplet, Thermodynamic, in-Situ Monitoring of Evolved Gasses and Analysis Biochar. Fuel 2023, 331, 125944. [Google Scholar] [CrossRef]
- Burbano, A.A.; Medina, G.A.M.; Sánchez, F.H.; Lassalle, V.L.; Horst, M.F.; Gascó, G.; Méndez, A. Influence of Post-Pyrolysis Treatment on Physicochemical Properties and Acid Medium Stability of Magnetic Carbon Nanocomposites. Biomass Conv. Bioref. 2024, 14, 27871–27884. [Google Scholar] [CrossRef]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Pobiner, H. Determination of Hydroperoxides in Hydrocarbon by Conversion to Hydrogen Peroxide and Measurement by Titanium Complexing. Anal. Chem. 1961, 33, 1423–1426. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, Y.; Sun, W.; Qin, Z.; Liu, H.; Ma, Y.; Wang, X. Cellulose Derived Biochar: Preparation, Characterization and Benzo[a]Pyrene Adsorption Capacity. Grain Oil Sci. Technol. 2021, 4, 182–190. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of Naphthalene and 1-Naphthol by Biochars of Orange Peels with Different Pyrolytic Temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef]
- Katyal, S.; Thambimuthu, K.; Valix, M. Carbonisation of Bagasse in a Fixed Bed Reactor: Influence of Process Variables on Char Yield and Characteristics. Renew. Energy 2003, 28, 713–725. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R. Influence of Pyrolysis Temperature on Physico-Chemical Properties of Corn Stover (Zea mays L.) Biochar and Feasibility for Carbon Capture and Energy Balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef]
- Da Luz Corrêa, A.P.; Bastos, R.R.C.; Rocha Filho, G.N.D.; Zamian, J.R.; Conceição, L.R.V.D. Preparation of Sulfonated Carbon-Based Catalysts from Murumuru Kernel Shell and Their Performance in the Esterification Reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ruiz, B.; Martínez-Blanco, D.; Sánchez-Arenillas, M.; Diez, M.A.; Suárez-Ruiz, I.; Marco, J.F.; Blanco, J.; Fuente, E. Sustainable Thermochemical Single-Step Process To Obtain Magnetic Activated Carbons from Chestnut Industrial Wastes. ACS Sustain. Chem. Eng. 2019, 7, 17293–17305. [Google Scholar] [CrossRef]
- Rahman, M.A.; Oomori, T. Structure, Crystallization and Mineral Composition of Sclerites in the Alcyonarian Coral. J. Cryst. Growth 2008, 310, 3528–3534. [Google Scholar] [CrossRef]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-Photoacoustic Spectroscopy for Phosphorus Speciation Analysis of Biochars. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 29–36. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Korolenko, E.A.; Korolik, E.V.; Zhbankov, R.G. IR Spectrum of Cellulose. J. Appl. Spectrosc. 1989, 51, 847–851. [Google Scholar] [CrossRef]
- Yang, R.; Liu, G.; Li, M.; Zhang, J.; Hao, X. Preparation and N2, CO2 and H2 Adsorption of Super Activated Carbon Derived from Biomass Source Hemp (Cannabis sativa L.) Stem. Microporous Mesoporous Mater. 2012, 158, 108–116. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Dhaveethu Raja, J.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A Complete Review on Biochar: Production, Property, Multifaceted Applications, Interaction Mechanism and Computational Approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- De Lima, R.S.; De Paiva E Silva Zanta, C.L.; Meili, L.; Dos Santos Lins, P.V.; De Souza Dos Santos, G.E.; Tonholo, J. Fenton-Based Processes for the Regeneration of Biochar from Syagrus Coronata Biomass Used as Dye Adsorbent. Desalin. Water Treat. 2019, 162, 391–398. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Thermal Decomposition of Calcium Carbonate (Calcite Polymorph) as Examined by in-Situ High-Temperature X-Ray Powder Diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of Biochar: Biological Properties. In Biochar for Environmental Management; Routledge: London, UK, 2012. [Google Scholar]
- Hong, X.; Fang, C.; Hui, K.S.; Hui, K.N.; Zhuang, H.; Liu, W.; Shan, S. Influence of Interfering Anions on Cu2+ and Zn2+ Ions Removal on Chestnut Outer Shell-Derived Hydrochars in Aqueous Solution. RSC Adv. 2017, 7, 51199–51205. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes 2021, 9, 1154. [Google Scholar] [CrossRef]
- Nkomo, N.; Odindo, A.O.; Musazura, W.; Missengue, R. Optimising Pyrolysis Conditions for High-Quality Biochar Production Using Black Soldier Fly Larvae Faecal-Derived Residue as Feedstock. Heliyon 2021, 7, e07025. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill Handbooks; McGraw-Hill: New York, NY, USA, 2003; ISBN 978-0-07-049439-8. [Google Scholar]
- Cervellino, A.; Frison, R.; Cernuto, G.; Guagliardi, A.; Masciocchi, N. Lattice Parameters and Site Occupancy Factors of Magnetite–Maghemite Core–Shell Nanoparticles. A Critical Study. J. Appl. Crystallogr. 2014, 47, 1755–1761. [Google Scholar] [CrossRef]
- Safavi, F.S.; Ebrahimipour, S.Y.; Fatemi, S.J.; Mohammadi, P.; Shamspur, T. Green Synthesis of Silver Nanoparticles and Their Immobilization on Magnetic Biochar for the Removal of Tetracycline and Enrofloxacin. Biomass Conv. Biorefin. 2025. [Google Scholar] [CrossRef]
- Gadgeel, A.A.; Mhaske, S.T.; Duerr, C.; Liu, K.L. In-Situ Preparation and Characterization of Aconitic Acid Capped Fe3O4 Nanoparticle by Using Citric Acid as a Reducing Agent. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1688–1700. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, S.; Pei, J.; Li, X.; Yu, R.; Zhao, J.; Martyniuk, C.J. Adsorption Performance of SO2 over ZnAl2O4 Nanospheres. J. Ind. Eng. Chem. 2016, 41, 151–157. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, B.P.; Ningthoujam, R.S. Confirmation of Highly Stable 10 Nm Sized Fe3O4 Nanoparticle Formation at Room Temperature and Understanding of Heat-Generation under AC Magnetic Fields for Potential Application in Hyperthermia. AIP Adv. 2020, 10, 105033. [Google Scholar] [CrossRef]
- Masset, P.; Poinso, J.Y.; Poignet, J.C. TG/DTA/MS Study of the Thermal Decomposition of FeSO4·6H2O. J. Therm. Anal. Calorim. 2006, 83, 457–462. [Google Scholar] [CrossRef]
- Chaklader, A.C.D.; Blair, G.R. Differential thermal study of FeO and Fe3O4. J. Therm. Anal. 1970, 2, 165–179. [Google Scholar] [CrossRef]
- Gallego-Ramírez, C.; Chica, E.; Rubio-Clemente, A. Combination of Biochar and Advanced Oxidation Processes for the Sustainable Elimination of Pharmaceuticals in Water. Sustainability 2024, 16, 10761. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Canzano, S.; Prisciandaro, M.; Musmarra, D. Photodegradation of Diclofenac in Wastewaters. Desalination Water Treat. 2017, 61, 293–297. [Google Scholar] [CrossRef]
- Pérez-Estrada, L.A.; Malato, S.; Gernjak, W.; Agüera, A.; Thurman, E.M.; Ferrer, I.; Fernández-Alba, A.R. Photo-Fenton Degradation of Diclofenac: Identification of Main Intermediates and Degradation Pathway. Environ. Sci. Technol. 2005, 39, 8300–8306. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, B.; Gao, B.; Cheng, N.; Feng, Q.; Chen, M.; Wang, S. Degradation of Organic Pollutants from Water by Biochar-Assisted Advanced Oxidation Processes: Mechanisms and Applications. J. Hazard. Mater. 2023, 442, 130075. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The Potential Role of Biochar in the Removal of Organic and Microbial Contaminants from Potable and Reuse Water: A Review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C. Adsorption of Organic Molecules from Aqueous Solutions on Carbon Materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Bano, A.; Aziz, M.K.; Mishra, R.; Dave, H.; Prasad, B.; Kumari, M.; Dubey, D.; Meili, L.; Shah, M.P.; Prasad, K.S. Response Surface Methodology–Based Optimisation of Adsorption of Diclofenac and Treatment of Pharmaceutical Effluent Using Combined Coagulation-Adsorption onto nFe2O3 Decorated Water Chestnut Shells Biochar. Environ. Sci. Pollut. Res. 2024, 31, 55317–55335. [Google Scholar] [CrossRef]
- Zahid, M.; Khan, Z.U.H.; Sun, J.; Muhammad, N.; Sabahat, S.; Shah, N.S.; Iqbal, J. Biochar-Derived Photocatalysts for Pharmaceutical Waste Removal, a Sustainable Approach to Water Purification. Appl. Surf. Sci. Adv. 2025, 26, 100721. [Google Scholar] [CrossRef]
- Chauhan, S.; Shafi, T.; Dubey, B.K.; Chowdhury, S. Biochar-Mediated Removal of Pharmaceutical Compounds from Aqueous Matrices via Adsorption. Waste Dispos. Sustain. Energy 2023, 5, 37–62. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Kaur Brar, S.; Verma, M.; Surampalli, R.Y. An Insight into the Adsorption of Diclofenac on Different Biochars: Mechanisms, Surface Chemistry, and Thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, Y.; Xu, M.; Cheng, F.; Zhang, H.; Li, Z. Photo-Fenton Degradation of Carbamazepine and Ibuprofen by Iron-Based Metal-Organic Framework under Alkaline Condition. J. Hazard. Mater. 2022, 424, 127698. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, R.; Kaur, H. A Systematic Review of Lignocellulosic Biomass for Remediation of Environmental Pollutants. Appl. Surf. Sci. Adv. 2024, 19, 100547. [Google Scholar] [CrossRef]


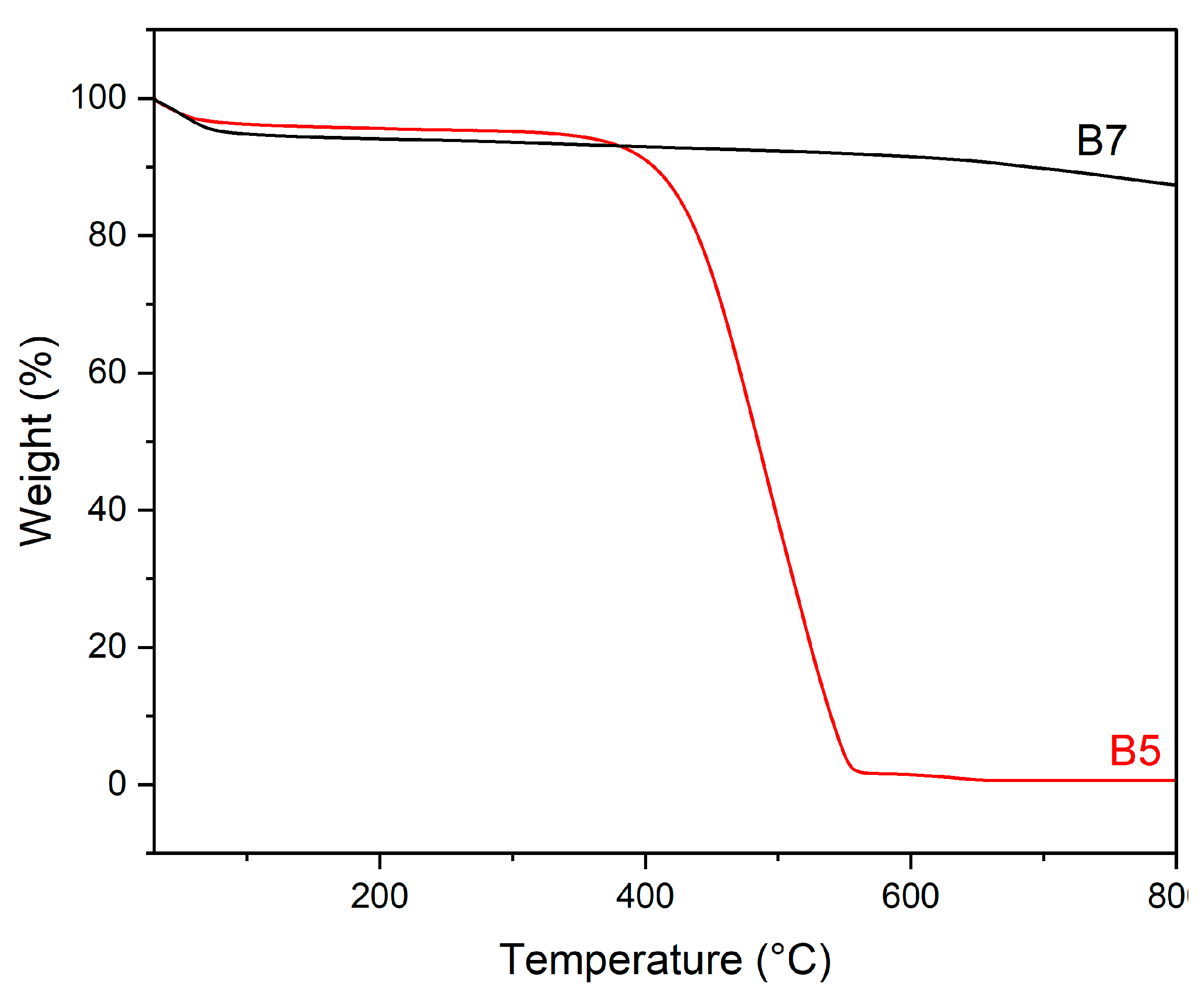
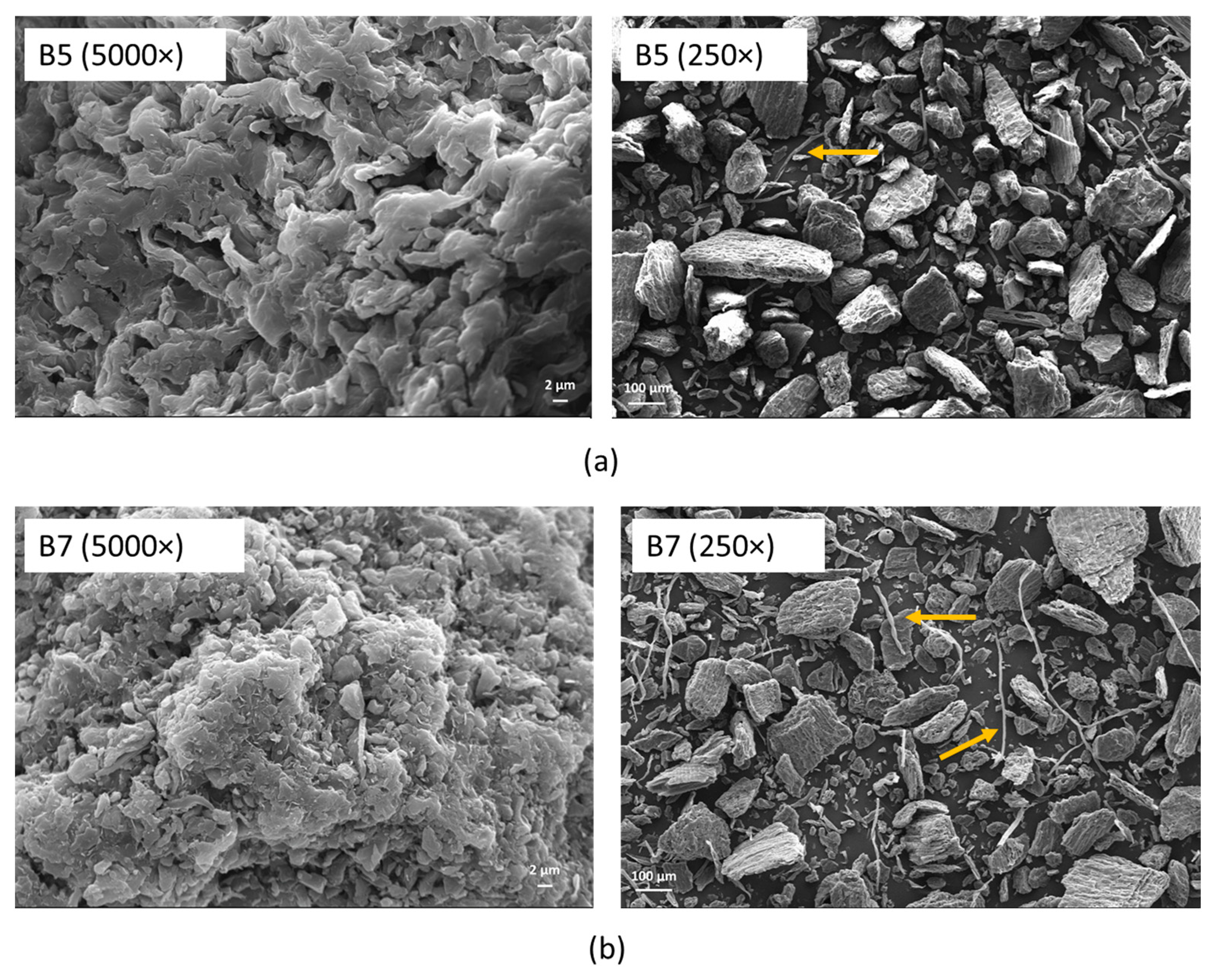
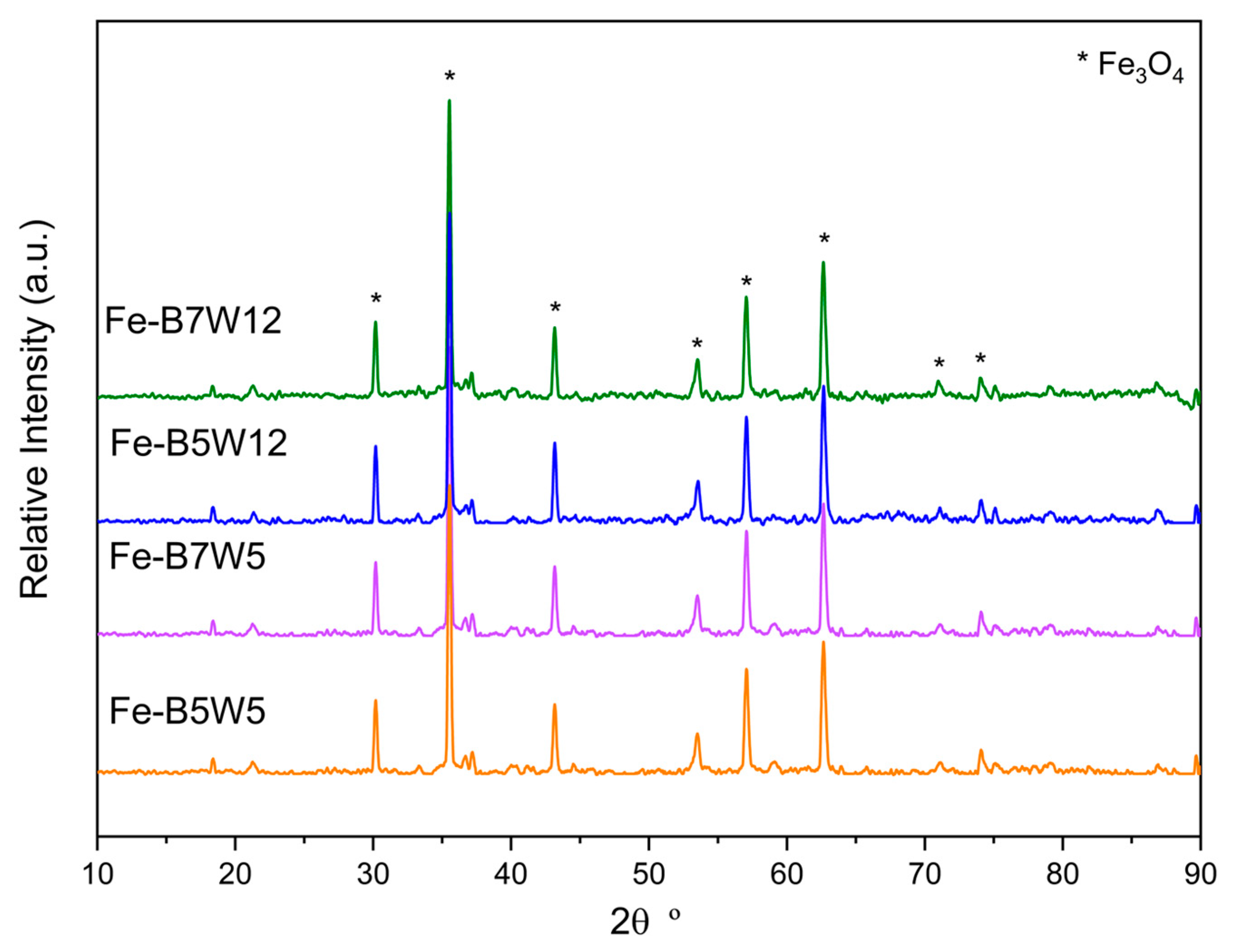


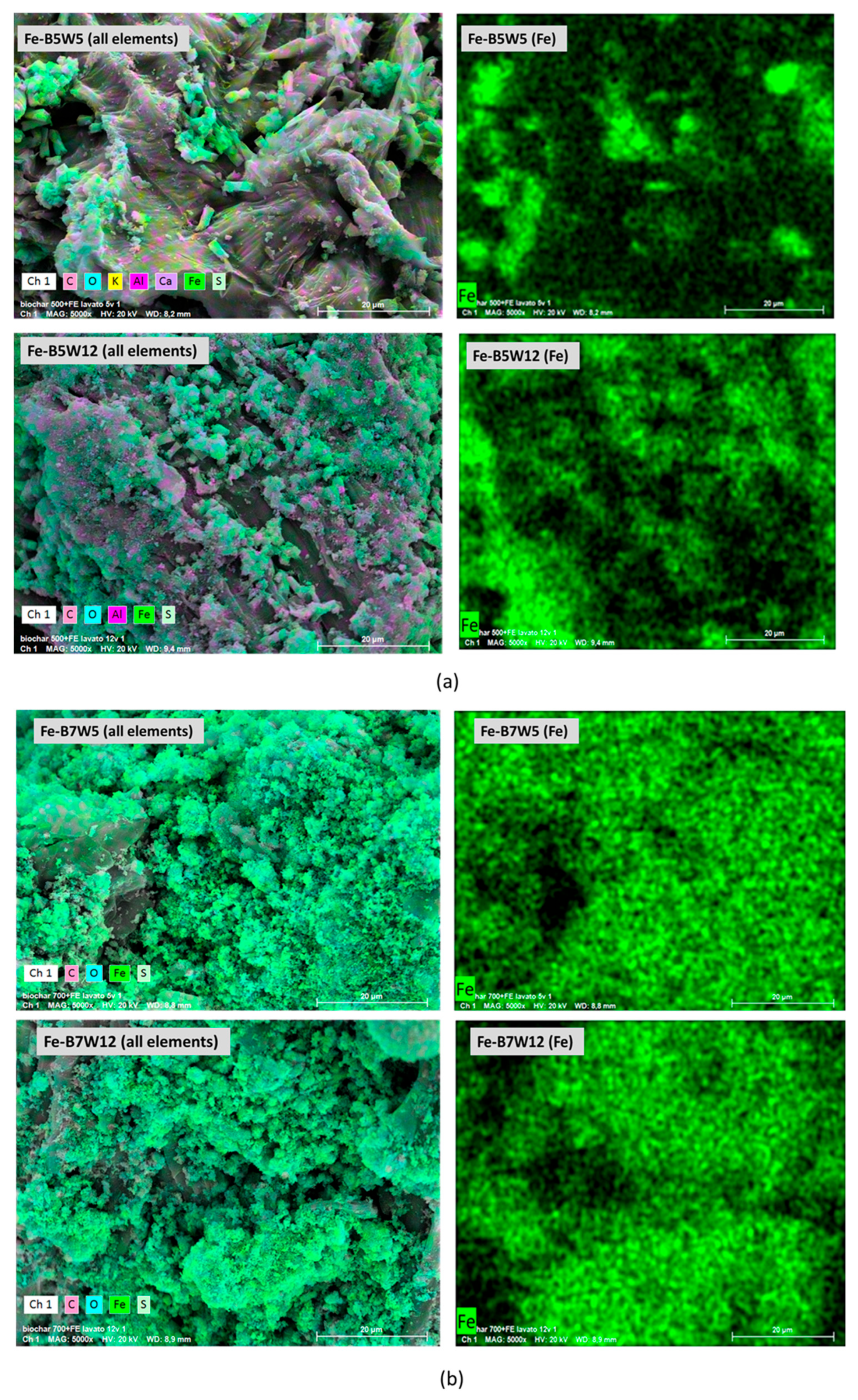
| Sample | Material | Temperature (°C) | Washing Number |
|---|---|---|---|
| B5 | Pristine biochar | 500 | none |
| B7 | 700 | ||
| Fe-B5W5 | Fe-biochar catalyst | 500 | 5 |
| Fe-B5W12 | 12 | ||
| Fe-B7W5 | Fe-biochar catalyst | 700 | 5 |
| Fe-B7W12 | 12 |
| Sample | SBET (m2 g−1) | Sext (m2 g−1) | Smicro (m2 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Vmacro (cm3 g−1) | VTot (cm3 g−1) | dmeso (nm) | dmacro (nm) |
|---|---|---|---|---|---|---|---|---|---|
| B5 | 3.5 | 0.3 | 3.2 | 0.000 | 0.011 | 0.179 | 0.190 | - | 1030 |
| B7 | 343.2 | 4.3 | 338.9 | 0.134 | 0.009 | 0.213 | 0.355 | 3.8 | 894 |
| Sample | H/C * | C/N * | O/C ** |
|---|---|---|---|
| % | |||
| B5 | 0.04 | 92.3 | 0.3 |
| B7 | 0.01 | 109.3 | 0.1 |
| Sample | SBET (m2 g−1) | Sext (m2 g−1) | Smicro (m2 g−1) | Vmicro (cm3 g−1) | Vmeso. (cm3 g−1) | Vmacro (cm3 g−1) | VTot (cm3 g−1) | dmeso (nm) | dmacro (nm) |
|---|---|---|---|---|---|---|---|---|---|
| B5 | 3.5 | 0.3 | 3.2 | 0.000 | 0.011 | 0.179 | 0.190 | - | 1030 |
| Fe-B5W5 | 55.0 | 29.0 | 26.1 | 0.005 | 0.077 | 0.274 | 0.356 | 3.8 | 58, 3099 |
| Fe-B5W12 | 135.8 | 31.8 | 104.0 | 0.041 | 0.064 | 0.328 | 0.433 | 3.8 | 84, 2792 |
| B7 | 343.2 | 4.3 | 338.9 | 0.134 | 0.009 | 0.213 | 0.355 | 3.8 | 894 |
| Fe-B7W5 | 134.9 | 25.5 | 109.4 | 0.045 | 0.054 | 0.358 | 0.457 | 3.8 | 106, 2840 |
| Fe-B7W12 | 162.2 | 29.2 | 133.0 | 0.057 | 0.062 | 0.385 | 0.504 | 3.8 | 202, 2840 |
| Sample | a0 Fe3O4 (Å) | Microstrain | Grain Size (nm) * | Rwp (%) |
|---|---|---|---|---|
| Fe-B5W5 | 8.3905 (8) | 0.0024 (2) | 64 (5) | 2.82 |
| Fe-B7W5 | 8.3966 (4) | 0.00124 (8) | 151 (13) | 2.78 |
| Fe-B5W12 | 8.3964 (4) | 0.00133 (8) | 170 (16) | 2.80 |
| Fe-B7W12 | 8.3963 (4) | 0.00138 (9) | 173 (17) | 2.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guagliano, M.; Bahamonde, A.; Bellotto, M.; Cristiani, C.; Finocchio, E.; Gasco, A.; Muelas-Ramos, V.; Jiménez-Bautista, K.; de los Ríos, C.; Hermosilla, D. Chestnut Waste-Derived Fe-Based Photocatalyst for Diclofenac Degradation. C 2025, 11, 38. https://doi.org/10.3390/c11020038
Guagliano M, Bahamonde A, Bellotto M, Cristiani C, Finocchio E, Gasco A, Muelas-Ramos V, Jiménez-Bautista K, de los Ríos C, Hermosilla D. Chestnut Waste-Derived Fe-Based Photocatalyst for Diclofenac Degradation. C. 2025; 11(2):38. https://doi.org/10.3390/c11020038
Chicago/Turabian StyleGuagliano, Marianna, Ana Bahamonde, Maurizio Bellotto, Cinzia Cristiani, Elisabetta Finocchio, Antonio Gasco, Virginia Muelas-Ramos, Karla Jiménez-Bautista, Christian de los Ríos, and Daphne Hermosilla. 2025. "Chestnut Waste-Derived Fe-Based Photocatalyst for Diclofenac Degradation" C 11, no. 2: 38. https://doi.org/10.3390/c11020038
APA StyleGuagliano, M., Bahamonde, A., Bellotto, M., Cristiani, C., Finocchio, E., Gasco, A., Muelas-Ramos, V., Jiménez-Bautista, K., de los Ríos, C., & Hermosilla, D. (2025). Chestnut Waste-Derived Fe-Based Photocatalyst for Diclofenac Degradation. C, 11(2), 38. https://doi.org/10.3390/c11020038











