Abstract
To address the need for reducing carbon emissions and enhancing the sustainable utilization of non-fossil resources, a one-step calcination strategy has been developed to fabricate hierarchical carbon aerogels from balsa wood. The resulting wood-derived carbon aerogels (WCA) were functionalized with Mg(OH)2 to boost their environmental remediation potential. Comprehensive characterization using XRD, FT-IR, XPS, and SEM confirmed that the optimized WCA/Mg(OH)2 composite (WCAMg) retained a three-dimensional hierarchical porous structure, and Mg(OH)2 nanosheets were attached to it. The adsorption performance of WCAMg composites towards Cd2+ was systematically investigated through controlled experiments, which focused on three critical variables (Mg(OH)2 loading content, initial Cd2+ concentration and solution ionic strength). The functionalized WCAMg demonstrated a maximum Cd2+ adsorption capacity of 351.1 mg g−1—a tenfold improvement over pristine WCA. Combined with exceptional adsorption efficiency, this biomass-derived composite offers an eco-friendly, cost-effective solution for heavy metal ion remediation. Its scalable fabrication from renewable resources aligns with sustainable water treatment objectives, presenting the advantage of pollution mitigation.
1. Introduction
With the rapid advancement of industrialization, the widespread use of heavy metal ions has emerged as a global environmental challenge [1,2]. Among these contaminants, cadmium ions (Cd2+) have gained significant attention due to their extensive utilization in battery manufacturing, electroplating processes, and pigment production [3,4], owing to their favorable electrochemical properties. However, cadmium’s high toxicity and bioaccumulative potential in the human body pose severe ecological and public health risks [5]. Chronic exposure to cadmium-contaminated environments has been linked to renal dysfunction, osteoporosis, carcinogenesis, and various systemic disorders. Effective wastewater treatment prior to discharge is critically required to safeguard ecological integrity and public health safety.
To address Cd2+ contamination, researchers have devoted themselves to various conventional remediation strategies, including chemical precipitation [6,7], ion exchange [8,9], membrane separation [10,11], and adsorption [12,13]. Adsorption has become a particularly promising solution due to its operational simplicity, cost-effectiveness, and high removal efficiency. Various materials have been applied as adsorbents in treating water, such as activated carbon [14,15,16,17], zeolites [18,19], and clay minerals [20,21]. Of them, carbon materials are widely used because of their low preparation cost and good adsorption property. For example, Xu and his co-workers prepared three-dimensional N-doped carbon (NC) polyhedrons through pyrolysis carbonization and KOH activation of zeolitic imidazolate framework-8 (ZIF-8) precursors. The resulting NC material exhibited an ultrahigh specific surface area of 3041 m2 g−1 and achieved a maximum adsorption capacity of 370.2 mg g−1 for Cd2+ [22]. In a separate study, Wei and his colleagues synthesized magnetic carbon nanopolymers (Fe3O4/C@PM) using suspension polymerization; these materials demonstrated a maximum Cd2+ adsorption capacity of 250.7 mg g−1 [23].
Since most carbon-based materials exist in powder form, their recovery after adsorption is challenging, limiting their practical applications. To solve this problem, researchers have developed magnetic–carbon composites for efficient post-treatment recovery [24]. Carbon aerogels (CAs) with three-dimensional (3D) porous architectures present another remediation strategy. Their inherent structural integrity enables bulk removal from aqueous systems post-adsorption, significantly simplifying separation processes. Many materials, such as MOF, graphene, carbon nanotubes, polyacrylonitrile, polyimide, and chitosan have been applied to engineer CAs. For instance, Zhang’s group prepared a modified graphene oxide composite aerogel with a saturated Cd2+ adsorption capacity of 174.85 mg g−1 [25].
Being a natural material, wood possesses vertical channels and a cellulose skeleton that can form multistage channels after carbonization. These characteristics render itself a highly suitable raw material candidate for the synthesis of CA. Due to its renewable property and 3D–porous structure, wood-derived carbon aerogel (WCA) serves as an excellent substrate for combining with other functional materials. The resulted composite aerogel can be utilized as binder-free supercapacitor electrode materials [26], flexible electrode materials [27], advanced packaging materials [28], electromagnetic interference shielding materials, and flame-retardant materials [29].
In this study, wood-derived carbon aerogel (WCA) was synthesized via balsa wood calcination. With it as a substrate, Mg(OH)2 nanoparticles were loaded. The WCA/Mg(OH)2 composite was fabricated (designated as WCAMg). The Cd2+ adsorption performance was thoroughly investigated. The influence of Mg(OH)2 loading amount on adsorption was tested, and the adsorption mechanism was proposed. As the cadmium complexes were adsorbed and embedded into the 3D structure of WCAMg, it enabled facile separation and recovery from wastewater. These WCAMg materials provide a novel water treatment strategy that eliminates secondary pollution risks, and offer potential solutions for heavy metal pollution remediation and recovery.
2. Experimental
2.1. Materials and Chemicals
The balsa wood comes from Papua New Guinea. Magnesium chloride hexahydrate (MgCl2∙6H2O, AR, purity ≥ 99.0%, and CAS 7791-18-6), nitric acid (HNO3, AR, 65–68% w/w, and CAS 7697-37-2), and sodium hydroxide (NaOH, AR, purity ≥ 99.0%, and CAS 1310-73-2) were purchased from Tianli Chemical Reagent Manufacturing Co., Ltd. (Tianjin, China). Acetic acid (CH3COOH, AR, 99.5% w/w, and CAS 64-19-7) was purchased from Tianjin Ruijinte Chemical Co., Ltd., Tianjin, China. Calcium chloride (CaCl2, AR, purity ≥ 99.0%, and CAS 10043-52-4), sodium chloride (NaCl, AR, purity ≥ 99.0%, and CAS 7647-14-5), and potassium chloride (KCl, AR, purity ≥ 99.0%, and CAS 7447-40-7) were purchased from Tianjin Comin Chemical Reagent Co., Ltd., Tianjin, China. Cadmium nitrate tetrahydrate (Cd(NO3)2∙4H2O, AR, purity ≥ 99.0%, and CAS 10325-94-7) was purchased from Aladdin Biochemical Technology Co., Ltd., Shanghai, China. All the solutions were prepared using deionized water.
2.2. Preparation of WCA
Balsa wood was firstly cut into pieces with the size of 10 mm × 10 mm × 20 mm, and dried at 102 ± 3 °C for 24 h. Then, the wood pieces were put into a beaker with 70 mL of 5% NaClO2 solution for delignification at 80 °C for 10 h. After that, delignified wood pieces were transferred into a beaker with deionized water. After heating at 90 °C for 5 h, the delignified wood pieces were taken out and frozen at −18 °C for 4 h in a polystyrene cup. Then, they were frozen dry in a freeze dryer for 48 h to obtain delignified wood aerogel. The same method was also adopted in references [30,31,32].
The delignified wood aerogel was calcined at 600 °C in nitrogen atmosphere for 3 h and WCA was prepared.
2.3. Preparation of WCAMg
MgCl2∙6H2O served as the precursor in the synthesis of Mg(OH)2. Two pieces of WCA (≈0.08 g) were immersed into 10 mL 1.5 mol L−1 MgCl2 solution for ultrasonic treatment at 80 °C (40 KHz, ultrasonic power of 360 W). After 30 min, the WCAs were taken out and vacuumed in a vacuum dryer at −0.1 MPa for 1 h, and then put into 10 mL 1 M L−1 NaOH solution for 30 min at 80 °C. The WCA samples were removed from the solution, and evacuated to −0.1 MPa vacuum pressure.
After repeating the procedure three times, the WCAMg composite was collected, subsequently rinsed with deionized water, and vacuum-dried at 50 °C for 6 h. The loaded content is calculated by the change of mass of WCA before and after Mg(OH)2 loading according to Equation (1), as follows:
R (%) is the loading rate of the prepared sample; M2 (g) is the mass of the WCAMg sample after the loading of Mg(OH)2; and M1 (g) is the mass of the WCA sample before the loading of Mg(OH)2.
The MgCl2 concentration variations (0.75, 1.5, and 3.0 mol L−1) were systematically evaluated as critical experimental parameters for Mg(OH)2 loading. Accordingly, the synthesized WCAMg composites were labeled WCAMg-1, -2, and -3 to reflect their respective MgCl2 precursor concentrations.
2.4. Adsorption Experiments
Adsorption experiments were conducted using 200 mL cadmium solutions (200 mg L−1 Cd2+, pH 6) with WCAMg specimens (≈0.09 g) under ambient conditions. At predetermined intervals, the solutions were extracted for a cadmium concentration analysis via atomic absorption spectroscopy.
The Langmuir (Equation (2)) and Freundlich (Equation (3)) models were applied to analyze the adsorption mechanism [33], as follows:
where qe (mg g–1) represents the adsorption capacity at equilibrium, and ce (mg L–1) represents equilibrium concentration. The binding energy of the whole system is generally expressed by Langmuir’s constant KL (L g–1), and the qmax is the maximum adsorption capacity. The Freundlich constant is KF (mg g–1 L1/n mg–1/n) and n is the adsorption strength.
The adsorption kinetics were modeled using three classical approaches: pseudo-first-order (PFO, Equation (4)), pseudo-second-order (PSO, Equation (5)), and intra-particle diffusion (IPD, Equation (6)), with their respective formulations defined as follows [34]:
PFO model:
PSO model:
IPD model:
where k1 (h–1) and k2 (g mg–1 h–1) are the PFO and PSO adsorption rate constant; Kid (mg g−1 h−1/2) is the IPD rate constant; qe (mg g–1) and qt (mg g–1) are the adsorption capacities at equilibrium and time t; and C (mg g−1) is the boundary layer thickness correlation constant for the IPD model.
2.5. Characterizations
A Fourier transform infrared spectrometer (FT-IR, PerkinElmer, Waltham, MA, USA) was used to test the corresponding functional groups of the samples. X-ray diffraction (XRD, Rigaku D/Max-RC, Tokyo, Japan) with Cu Kα radiation (wavelength of 1.5418 Å) was used to analyze the structure of the samples. Scanning electron microscopy (SEM, Hitachi S-4800, Tokyo, Japan) was applied to observe the morphology of the composites. The chemical compositions of the samples were measured by energy dispersive X-ray spectroscopy (EDS, Oxford Instruments ULTIM Max 65, Abingdon, UK) and X-ray photoelectron spectroscopy (XPS, K-Alpha, XPS, Thermo Fisher Scientific K-Alpha, Waltham, MA, USA). Cd2+ was quantitatively analyzed by an atomic adsorption spectrometer (AAS, PerkinElmer PinAAcle 900H, Waltham, MA, USA).
3. Results and Discussion
3.1. Structure and Morphology of WCAMg
The structure of WCAMg and WCA were characterized by XRD, and the results are shown in Figure 1a. For WCA, there is a broad peak located at 20~30° [35], indicating the amorphous structure of calcined WCA. The XRD plot for WCAMg shows some differences: diffraction peaks were observed at 2θ values of 18.5°, 37.9°, 50.8°, and 58.6°, which correspond to the (001), (101), (102), and (110) lattice planes of hexagonal Mg(OH)2 (JCPDS# 44-1482). The XRD analysis confirms that WCAMg comprises two distinct crystalline phases: WCA and Mg(OH)2. It demonstrates successful Mg(OH)2 attachment into the WCA matrix.
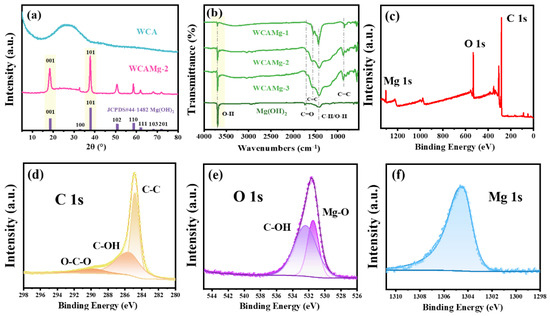
Figure 1.
(a) XRD patterns of WCA, WCAMg-2, and Mg(OH)2, (b) FT-IR spectra of Mg(OH)2 and WCAMg-n, and XPS spectra of the composite WCAMg-2, (c) survey, (d) C 1s, (e) O1s, and (f) Mg 1s.
The FT-IR spectra of WCAMg samples are shown in Figure 1b. For Mg(OH)2, a distinct and sharp peak was observed at 3699 cm−1, attributed to the -OH stretching vibration in Mg(OH)2. Meanwhile, the peak at 1433 cm−1 was associated with the O-H bending vibration of adsorbed water. Once loaded with Mg(OH)2, the samples of WCAMg also show these two adsorption peaks. However, the intensity of the -OH stretching vibration at 3699 cm−1 exhibits a distinct concentration-dependent trend: an increased MgCl2 concentration correlates directly with an enhanced peak intensity. This indicated that elevated MgCl2 concentrations significantly enhanced Mg(OH)2 loading efficiency on WCA. Meanwhile, the peak at 1433 cm−1 in WCAMg shows significant enhancement compared to pure Mg(OH)2. Two key effects are indicated: (i) improved water adsorption amount, and (ii) the emergence of additional functional groups (e.g., C-O) within the composite aerogel structure. Moreover, the characteristic peaks observed at 860, 1562, and 1704 cm−1 are related to the C-H bending vibration, C=C stretching vibration, and C=O stretching vibration of WCA, respectively [36].
One can note that WCAMg-3 exhibits the largest loaded amount of Mg(OH)2. However, the loaded amount does not linearly correlate with the initial feed ratio. So, WCAMg-2 was chosen for further experiments due to its optimal balance between performance and cost-effectiveness.
The XPS was applied to analyze the elemental environment of WCAMg. The XPS survey of the composite aerogel (Figure 1c) shows three main peaks, which correspond to C 1s, O 1s, and Mg 1s. Figure 1d is the C 1s spectrum, and three peaks at 284.8, 285.6, and 289.8 eV can be fitted. They are binding energies of C-C/C-H, C-OH, and C-O-C originated from WCA. The spectrum for O 1s in Figure 1e can be fitted into two peaks at 531.3 and 532.4 eV. They are attributed to the binding energies of Mg-O in Mg(OH)2 and C-O/C-OH in WCA, respectively. The Mg 1s spectrum was depicted in Figure 1f, and a distinct peak was observed at a binding energy of 1303.0 eV, which is related to the binding energy of Mg-O in Mg(OH)2 [37]. The XPS results further confirm the successful up-loading of Mg(OH)2 into the WCA.
The morphology of WCA and WCAMg samples were examined using SEM. As illustrated in Figure 2a, WCA exhibits an irregular fibrous architecture containing interconnected cavities and channels. Morphological features are characteristic of biomass-derived carbon aerogels. However, observations of WCAMg samples (Figure 2b–d) find that surfaces of WCAs were covered. After the deposition of Mg(OH)2 nanoparticles, plate-like nanostructures with the size of about 200 nm were determined via SEM (Figure 2e–g). This result not only confirms the successful in situ crystallization of Mg(OH)2, but also demonstrates its effective anchoring on the WCA surface through interfacial bonding. WCAMg-1 displays aggregated Mg(OH)2 nanosheets that distribute across the WCA. While increasing the MgCl2 concentration, the prepared WCAMg-2 and WCAMg-3 show a morphology of progressive surface coverage by vertically aligned Mg(OH)2 nanosheets. These results also give the evidence that a high concentration of MgCl2 would result in a high load capacity of Mg(OH)2, agreeing with the discussion above.
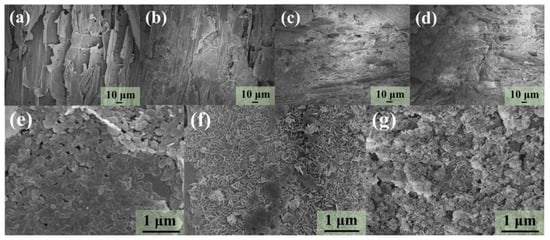
Figure 2.
SEM images of (a) WCA, (b,e) WCAMg-1, (c,f) WCAMg-2, and (d,g) WCAMg-3.
3.2. Absorption Performance
The adsorption performance of WCAMg for Cd2+ was fully studied. The systematic evaluation of Cd2+ adsorption efficiency revealed a significant enhancement in WCAMg samples (Figure 3a). It can be observed that pristine WCA alone has an adsorption capacity of 33.30 mg g−1. Interestingly, the three composites WCAMg-n (n = 1, 2, and 3) give sequentially increasing capacities of 225.8, 351.1, and 355.9 mg g−1. As shown in Figure 3b, their Mg(OH)2 loading ratios were calculated to be 42.38% (±1.09 wt%), 56.82% (±1.38 wt%), and 60.27% (±0.87 wt%) according to Equation (1), respectively. It further confirms that the adsorption capacity depends on the loaded amount of Mg(OH)2. Notably, WCAMg-2 has the best cost–benefit ratio. It achieves a 10-times-higher adsorption capacity than WCA. This suggests that 1.5 mol L−1 MgCl2 is the optimal concentration for loading Mg(OH)2.
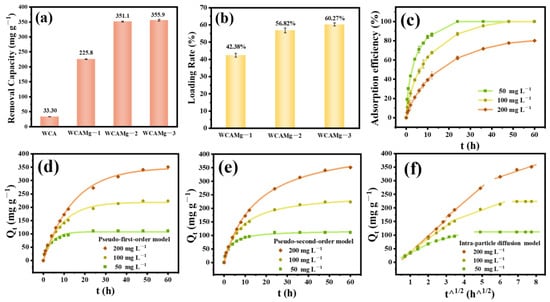
Figure 3.
(a) Cd2+ adsorption capacity of WCAMg, (b) Mg(OH)2 loading ratio in WCAMg, (c) adsorption kinetic curves of WCAMg-2 at different Cd2+ concentrations, together with plots fitted by (d) PFO, (e) PSO, and (f) IPD models. All experimental procedures were performed in triplicate under independently controlled conditions, with quantitative data expressed as mean values.
Table 1 presents adsorption capacities of carbon aerogels reported in previous studies and in this work. Compared to conventional easily recoverable adsorbents, our composite WCAMg not only exhibits a superior Cd2+ adsorption capacity, but also demonstrates significant cost-effectiveness.

Table 1.
Comparison of Cd2+ adsorption capacity of different carbon aerogels.
The kinetic curves of WCAMg-2 to various Cd2+ concentrations are shown in Figure 3c. To 50 and 100 mg L−1 Cd2+ solution, adsorption equilibriums were reached within 12 h and 24 h, respectively, and the adsorption efficiencies are almost 99.9%. Whereas, for the 200 mg L−1 Cd2+ solution, it took 48 h to get to the adsorption equilibrium, and the adsorption efficiency is 80.1%. The qe were determined as 110.9, 223.6, and 351.1 mg g−1 at initial Cd2+ concentrations of 50, 100, and 200 mg L−1, respectively (Table 2). For systems with a concentration less than 200 mg L−1, the post-adsorption analysis confirmed a cadmium concentration reduction to <0.1 mg L−1 (Cd2+ removal efficiency > 99.9%). This meets the Class I discharge standards per China’s Integrated Wastewater Discharge Standard (GB 8978-1996) [41]. These findings indicate the exceptional adsorption efficiency of WCAMg for industrial wastewater remediation.

Table 2.
The parameters fitted by PF/PSO models for Cd2+ adsorption.
To study the adsorption mechanism, PFO and PSO models were applied to fit adsorption kinetic curves (Figure 3d,e). The relevant fitting parameters are shown in Table 2. Both the PFO and PSO models fit the adsorption process well. The PSO model has higher R2 values (0.9965~0.9984). This indicates that the process is predominantly governed by the chemical adsorption [42]. The calculated k2 of Cd2+ exhibits a clear concentration-dependent pattern, measuring 0.002890 g mg−1 h−1 at 50 mg L−1 Cd2+, 0.0004935 g mg−1 h−1 at 100 mg L−1 Cd2+, and 0.0001457 g mg−1 h−1 at 200 mg L−1 Cd2+. These values suggest an inverse correlation between initial cadmium concentration and adsorption kinetics, where lower Cd2+ concentrations correspond to significantly faster contaminant removal rates.
To elucidate the rate-limiting mechanism that governs cadmium adsorption, we analyzed the kinetic data using the Weber–Morris IPD model. As illustrated in Figure 3f, the Cd2+ adsorption profile on WCAMg exhibits triphasic behavior. Three sequential regimes were characterized: (I) instantaneous surface adsorption, (II) gradual pore diffusion, and (III) adsorptive equilibrium. The Kp (Table 3) indicates a consistent concentration-dependent progression: Kp1 > Kp2 > Kp3. It finds that (1) boundary layer diffusion dominates initial rapid capture, (2) gradual penetration through mesopores controls mid-term kinetics, and (3) final saturation occurs through micropore filling. Notably, elevated Cd2+ concentrations accelerate diffusion rates across all the phases (Kp increased 65.93% in Phase 1 and 229.3% in Phase 2 at 200 mg L−1 vs. 50 mg L−1). This confirms that the concentration gradient-driven mass transfer serves as the principal transport mechanism.

Table 3.
The parameters fitted by IPD model for Cd2+ adsorption.
To investigate the effect of initial concentration on adsorption capacity, experiments were carried out. They started with different initial concentrations of Cd2+ solutions by WCAMg-2. The results are shown in Figure 4 along with the fitted plots.
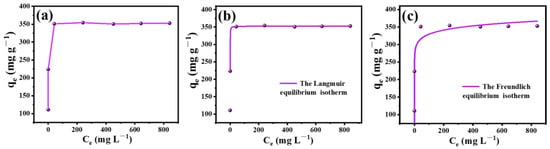
Figure 4.
(a) Adsorption isotherm of WCAMg-2 for Cd2+, and experimental data with (b) Langmuir fitting curve and (c) Freundlich fitting curve.
From the adsorption isotherm (Figure 4a), one can see that qmax of 351.1 mg g−1 was obtained when the concentration of Cd2+ was 200 mg L−1. The adsorption isotherms were fitted using Langmuir and Freundlich models (Figure 4b,c). The results are shown in Table 4, and the R2 values for these two models are 0.9994 and 0.7417, respectively. Clearly, the Langmuir model describes the removal process better, indicating a monolayer adsorption mechanism. The qmax calculated by fitting results from the Langmuir model is about 352.5 mg g−1, which is consistent with the experimentally determined result.

Table 4.
The parameters fitted by Langmuir and Freundlich models for Cd2+ removal isotherm.
3.3. Adsorption Mechanism
To comprehensively elucidate the adsorption mechanism, the sample of WCAMg after adsorbing Cd2+ was recovered and characterized. The spent sample is marked as WCAMg-Cd. Its XRD patterns in Figure 5a show that recovered Cd2+ exists as Cd(OH)2 (JCPDS# 31-0228) and CdCO3 (JCPDS# 42-1342). It agrees with the previous studies [37,43].
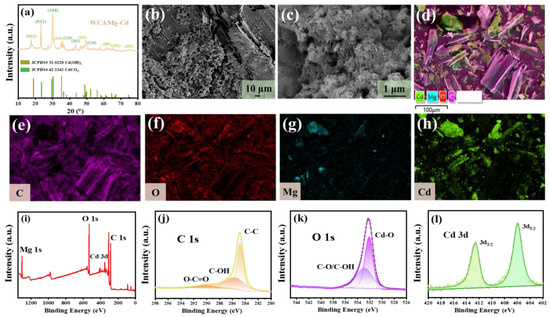
Figure 5.
(a) XRD patterns, (b,c) SEM images, (d–h) EDS mapping images, and (i–l) XPS spectra of WCAMg-Cd.
From SEM images (Figure 5b,c), one can observe particles instead of nanosheets on the surface of WCAMg-Cd. The EDS mapping (Figure 5d–h) evidences that Cd2+ has been adsorbed onto WCAMg. The XPS was applied to analyze the elemental environment of WCAMg-Cd. It is found in Figure 5i that Cd element appears in the survey spectrum other than Mg, C, and O. It further indicates the successful adsorption of Cd2+ by WCAMg. For the Cd 3d spectrum (Figure 5l), a doublet peak appears at binding energies of 405.9 eV and 412.6 eV. This corresponds to the 3d5/2 and 3d3/2 states of Cd2+ in CdCO3/Cd(OH)2 [44,45]. In the XPS spectrum of C 1s (Figure 5j), a peak at 289.9 eV was fitted, which is the binding energy of CO32− [46]. This result also indicates the formation of CdCO3 post-adsorption.
According to these results, the adsorption mechanism is summarized. Weak basic Mg(OH)2 is dissolved into Mg2+ and OH− in solution, and Cd2+ would precipitate by the released OH− to give Cd(OH)2 [47]. It is described by the reaction in Equation (7), as follows:
Mg(OH)2 + Cd2+ → Cd(OH)2 + Mg2+
Meanwhile, CO2 from air dissolves in water to form H2CO3. The released OH− ions subsequently react with H2CO3 to generate CO32−, which would precipitate Cd2+ as CdCO3. The involved reactions are shown in Equations (8)–(12). Notably, the pH of solution is kept at 6 during the adsorption.
CO2 (air) + H2O → H2CO3
H2CO3 → CO32– + 2H+
Mg(OH)2 → Mg2+ + 2OH−
H+ + OH− → H2O
Cd2+ + CO32− → CdCO3↓
3.4. Competitive Ion Interference Effects and Environmental Performance in Natural Water
Since industrial effluents typically contain high salinity that may compromise the adsorption efficiency of WCAMg, we have systematically investigated the interference effects of four common co-existing ions (K+, Na+, Ca2+, and Mg2+). The results are presented in Figure 6a–d. Distinct structure–activity relationships are revealed. Monovalent cations (K+/Na+) exhibit no significant inhibitory effects on Cd2+ adsorption across the tested concentration range (100–800 mg L−1). In contrast, divalent cations (Ca2+/Mg2+) show concentration-dependent inhibitory effects, where the adsorption capacity decreases proportionally as their concentration increases.
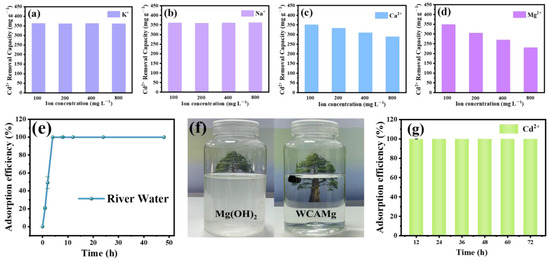
Figure 6.
The effects of (a) K+, (b) Na+, (c) Ca2+, and (d) Mg2+ on the adsorption capacity of Cd2+ by WCAMg-2, (e) the adsorption efficiency of WCAMg for treating river water, (f) comparison diagram of pure Mg(OH)2 and WCAMg after adsorption, and (g) durability test for WCAMg-2.
It may be caused by the following reason. Mg2+ induces competitive precipitation through Mg(OH)2 formation (reducing its solubility equilibrium), thereby suppressing Cd2+ up-taken efficiency. Conversely, Ca2+ interference primarily originates from OH− depletion effects, where elevated cation concentrations progressively diminish adsorption capacity via hydroxyl ion competition.
To evaluate the environmental applicability of WCAMg as a water purification material, a field-simulated adsorption experiment was carried out using Songhua River water (Harbin, China). It was amended with 1 mg L−1 Cd2+ through the controlled addition of cadmium nitrate tetrahydrate (Cd(NO3)2·4H2O). As demonstrated in Figure 6e, WCAMg achieves exceptional remediation efficiency over 99.99% Cd2+ removal within 4 h. Crucially, the treated water maintains visually clear (Figure 6f), whereas parallel tests with conventional Mg(OH)2 produces turbid suspensions due to uncontrolled Cd(OH)2/CdCO3 precipitation. This marked visual contrast confirms WCAMg’s unique microstructural advantage in effectively encapsulating cadmium complexes within its carbon aerogel architecture. Thereby, it enables the prevention of secondary particulate pollution and simplifies the recovery.
To assess the durability of the composite WCAMg, six consecutive adsorption cycles were conducted using 100 mL of 20 mg L−1 Cd2+ aqueous solution. Upon completing each adsorption cycle, the adsorbent was taken out with tweezers and re-immersed in fresh Cd2+ solution for subsequent remediation phases. Remarkably, the composite maintains almost 100% retention efficiency throughout six cycles (Figure 6g). This demonstrates the exceptional structural stability and confirms its potential as a long-lasting reagent for heavy metal removal in water purification applications.
4. Conclusions
A novel balsa wood-derived carbon aerogel (WCA) has been prepared in this study. Further functionalization with Mg(OH)2 nanosheets enabled us to construct a WCAMg water treatment reagent. It is demonstrated that WCAMg achieves an exceptional cadmium immobilization performance. Representatively, the best composite sample exhibits a maximum adsorption capacity of 351.1 mg g−1 and high removal efficiency (>99.9%) towards Cd2+ at concentrations ≤ 100 mg L−1. The treated water utterly satisfies the Class I discharge limits of China’s GB 8978-1996 standard. The removal mechanism involves dual pathways: (1) the Mg(OH)2 component enables Cd2+ chemisorption through Cd(OH)2/CdCO3 precipitation, and (2) the three-dimensional carbon aerogel network effectively immobilizes the formed cadmium complexes. This unique architecture prevents the secondary sludge formation and facilitates straightforward separation. The synergistic design positions WCAMg as a sustainable water remediation agent that provides an economically viable solution for heavy metal contamination management.
Author Contributions
Conceptualization, Y.G.; data curation, Y.Y.; formal analysis, J.L.; investigation, R.A. and H.M.; supervision, Q.P. and S.L.; writing—original draft, R.A.; and writing—review and editing, Y.G., Q.P. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (22276046).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The Analyzing and Testing Center of Northeast Forestry University is acknowledged for the SEM tests.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Piwowarska, D.; Kiedrzyńska, E.; Jaszczyszyn, K. A global perspective on the nature and fate of heavy metals polluting water ecosystems, and their impact and remediation. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1436–1458. [Google Scholar] [CrossRef]
- Liu, K.; Guan, X.; Li, C.; Zhao, K.; Yang, X.; Fu, R.; Li, Y.; Yu, F. Global perspectives and future research directions for the phytoremediation of heavy metal-contaminated soil: A knowledge mapping analysis from 2001 to 2020. Front. Environ. Sci. Eng. 2021, 16, 73. [Google Scholar] [CrossRef]
- Rahman, M.M.; Karim, M.R.; Alharbi, H.F.; Aldokhayel, B.; Uzzaman, T.; Zahir, H. Cadmium Selenide Quantum Dots for Solar Cell Applications: A Review. Chem. Asian. J. 2021, 16, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Mavazzan, A.; Mendhe, A.C.; Bayannavar, P.K.; Sankapal, B.R.; Kamble, R.R.; Madar, S.F.; KM, M.P.; Bheemayya, L. Design of Metal Free Fluorescent Pyridine Dyes Anchored on Cadmium Sulfide Nanowires: Optical, Electrochemical and Photovoltaic Applications. J. Fluoresc. 2023, 34, 2405–2414. [Google Scholar] [CrossRef]
- Cirovic, A.; Denic, A.; Clarke, B.L.; Vassallo, R.; Cirovic, A.; Landry, G.M. A hypoxia-driven occurrence of chronic kidney disease and osteoporosis in COPD individuals: New insights into environmental cadmium exposure. Toxicology 2022, 482, 153355–153364. [Google Scholar] [CrossRef]
- Zieliński, J.; Huculak-Mączka, M.; Kaniewski, M.; Nieweś, D.; Hoffmann, K.; Hoffmann, J. Kinetic modelling of cadmium removal from wet phosphoric acid by precipitation method. Hydrometallurgy 2019, 190, 105157–105163. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Zhang, X.; Luo, T.; Chen, Y. Removal of cadmium and arsenic from Cd-As-Pb-bearing dust based on peroxide leaching and coprecipitation. Hydrometallurgy 2022, 209, 105839–105851. [Google Scholar] [CrossRef]
- Dabrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Elfeghe, S.; Anwar, S.; Zhang, Y. Adsorption and removal studies of cadmium ion onto sulphonic/phosphonic acid functionalization resins. Can. J. Chem. Eng. 2022, 100, 3006–3014. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpää, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023–103037. [Google Scholar] [CrossRef]
- Maqbool, A.; Shahid, A.; Jahan, Z.; Bilal Khan Niazi, M.; Ali Inam, M.; Tawfeek, A.M.; Kamel, E.M.; Saeed Akhtar, M. Development of ZnO-GO-NiO membrane for removal of lead and cadmium heavy metal ions from wastewater. Chemosphere 2023, 338, 139622–139639. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, H.; Zheng, X.; Zhao, B.; Diao, J.; Jiang, Y. Enhanced adsorption of cadmium from aqueous solution by amino modification biochar and its adsorption mechanism insight. J. Environ. Chem. Eng. 2023, 11, 109747–109758. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Zhan, P.; Hu, F.; Ye, X. Removal of cadmium and copper from water by a magnetic adsorbent of PFM: Adsorption performance and micro-structural morphology. Sep. Purif. Technol. 2018, 206, 199–207. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Chan, J.C.; Hameed, B.H.; Lim, L.L.P. Adsorption behavior of cadmium ions onto phosphoric acid-impregnated microwave-induced mesoporous activated carbon. J. Water Process Eng. 2016, 14, 60–70. [Google Scholar] [CrossRef]
- Sufian, J.; Babaakbari Sari, M.; Marchelli, F.; Fiori, L.; Avanes, A.; Moradi, S. An Analysis of the Factors Influencing Cadmium Removal in Aquatic Environments by Chlorella vulgaris-Derived Solids. J. Carbon Res. 2023, 10, 2. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Maroulas, K.N.; Theologis, D.; Katsoyiannis, I.A.; Kyzas, G.Z. Comparison of Modified Peels: Natural Peels or Peels-Based Activated Carbons for the Removal of Several Pollutants Found in Wastewaters. J. Carbon Res. 2024, 10, 2. [Google Scholar] [CrossRef]
- Mulla, B.; Ioannou, K.; Kotanidis, G.; Ioannidis, I.; Constantinides, G.; Baker, M.; Hinder, S.; Mitterer, C.; Pashalidis, I.; Kostoglou, N.; et al. Removal of Crystal Violet Dye from Aqueous Solutions through Adsorption onto Activated Carbon Fabrics. J. Carbon Res. 2024, 10, 19. [Google Scholar] [CrossRef]
- Xu, H.; Ou, Z.; Li, W.; Hu, T.; Zhang, Y.; Xu, H.; Wang, J.; Li, Y. Cadmium(II) adsorption by recyclable Zeolite-Loaded Hydrogel: Extension to the removal of Cadmium(II) from contaminated soil. Chem. Eng. J. 2024, 492, 151842–151855. [Google Scholar] [CrossRef]
- Perfecto Barragán, P.; Guadalupe Macedo, M.M.; Olguín, M.T. Cadmium sorption by sodium and thiourea-modified zeolite-rich tuffs. J. Environ. Sci. 2017, 52, 39–48. [Google Scholar] [CrossRef]
- Xu, S.; Xing, Y.; Liu, S.; Luo, X.; Chen, W.; Huang, Q. Co-effect of minerals and Cd(II) promoted the formation of bacterial biofilm and consequently enhanced the sorption of Cd(II). Environ. Pollut. 2020, 258, 113774–113783. [Google Scholar] [CrossRef]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692–100706. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Shang, Y.; Li, B.; Yu, D.; Wang, Y. Highly efficient Cd(Ⅱ) removal using 3D N-doped carbon derived from MOFs: Performance and mechanisms. J. Hazard. Mater. 2022, 436, 129149–129161. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Huang, S.; Zhou, J.; Xiao, C.; Cao, J.; Xiao, J.; Xie, C. Magnetic Carbon Porous Polymer Prepared from a New Suspended Emulsion for the Absorption of Heavy Metal Ions. Polymers 2025, 3, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Liu, X.; Wang, Y.; Liu, Y.; Cheng, T.; Zada, A.; Ye, F.; Deng, W.; Sun, Y.; Zhao, T.; et al. High-efficient adsorption for versatile adsorbates by elastic reduced graphene oxide/Fe3O4 magnetic aerogels mediated by carbon nanotubes. J. Hazard. Mater. 2023, 457, 131846–131857. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Deng, H.; Qiao, N.; Zhang, D.; Lin, H.; Chen, Y. Modified graphene oxide composite aerogels for enhanced adsorption behavior to heavy metal ions. J. Environ. Chem. Eng. 2021, 9, 106008–106018. [Google Scholar] [CrossRef]
- Li, S.C.; Hu, B.C.; Ding, Y.W.; Liang, H.W.; Li, C.; Yu, Z.Y.; Wu, Z.Y.; Chen, W.S.; Yu, S.H. Wood-Derived Ultrathin Carbon Nanofiber Aerogels. Angew. Chem. Int. Ed. 2018, 57, 7085–7090. [Google Scholar] [CrossRef]
- Chen, Z.; Zhuo, H.; Hu, Y.; Lai, H.; Liu, L.; Zhong, L.; Peng, X. Wood-Derived Lightweight and Elastic Carbon Aerogel for Pressure Sensing and Energy Storage. Adv. Funct. Mater. 2020, 30, 1910292. [Google Scholar] [CrossRef]
- Xiong, C.; Fan, X.; Xiong, Q.; Zhang, Y.; Su, Y. Double hydrogen bonding force improves the performance of composite phase change materials by improving the carbon skeleton of wood aerogel. J. Energy Storage 2025, 114, 115752–115761. [Google Scholar] [CrossRef]
- Liang, C.; Qiu, H.; Song, P.; Shi, X.; Kong, J.; Gu, J. Ultra-light MXene aerogel/wood-derived porous carbon composites with wall-like “mortar/brick” structures for electromagnetic interference shielding. Sci. Bull. 2020, 65, 616–622. [Google Scholar] [CrossRef]
- Xu, K.; Jiao, Y.; Li, J.; Xiao, H.; Fu, Q. FeP nanoparticle embedded in N,P-doped 3D porous wood-derived carbon aerogel for oxygen reduction reaction. Carbon 2024, 228, 119408–119416. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Dong, H.; Yang, B.; Li, X.; Li, X.; Wu, Y.; Xu, K. Diatom-Inspired Nanoscale Heterogeneous Assembly Strategy for Constructing Thermal Insulating Wood-Based Aerogels with Exceptional Strength, Resilience, Degradability, and Flame Retardancy. ACS Nano 2025, 19, 6826–6839. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wei, L.; Pang, Z.; Wu, J.; Dong, Y.; Pan, X.; Hu, J.; Qu, J.; Li, J.; Tian, D.; et al. Multifunctional Wood Composite Aerogel with Integrated Radiant Cooling and Fog–Water Harvesting for All-Day Building Energy Conservation. Adv. Funct. Mater. 2024, 35, 2414590–2414601. [Google Scholar] [CrossRef]
- García-Zubiri, I.X.; González-Gaitano, G.; Isasi, J.R. Sorption models in cyclodextrin polymers: Langmuir, Freundlich, and a dual-mode approach. J. Colloid Interface Sci. 2009, 337, 11–18. [Google Scholar] [CrossRef]
- Ryszko, U.; Rusek, P.; Kołodyńska, D. Transforming industrial processes: Effective removal of Cd(II) ions from wet phosphoric acid streams. Chem. Eng. J. 2025, 505, 159696–159713. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, S.; Bi, C.; Peng, C.; Wang, Y.; Li, Y.; Tao, E. Modulation of ciprofloxacin adsorption conjugation effect by specific conformation of manganese-nitrogen co-doped biochar and its mechanistic study. Sep. Purif. Technol. 2025, 132304–132315. [Google Scholar] [CrossRef]
- Dai, H.; Li, N.; Cui, Y.; Gao, W.; Peng, X.; Peng, W.; Si, H.; Mu, L.; Shi, Y.; Cheng, Z.; et al. Indispensable Synergy between C=C and C=O Sites in Biochar for Peroxomonosulfate Activation and Sulfamethoxazole Degradation. ACS ES&T Eng. 2024, 4, 2888–2897. [Google Scholar] [CrossRef]
- Zhao, N.-D.; Wang, Y.; Zou, X.-H.; Yin, W.-M.; Wang, X.-Y.; Guo, Y.-R.; Pan, Q.-J. Fabrication of cellulose@Mg(OH)2 composite filter via interfacial bonding and its trapping effect for heavy metal ions. Chem. Eng. J. 2021, 426, 130812–130825. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Waterhouse, G.I.N.; Sun, J.; Shi, W.; Ai, S. Efficient removal of cadmium ions from water by adsorption on a magnetic carbon aerogel. Environ. Sci. Pollut. Res. 2021, 28, 5149–5157. [Google Scholar] [CrossRef]
- Jing, W.; Yang, C.; Lin, X.; Tang, M.; Lian, D.; Yu, Y.; Liu, D. MnFe2O4-loaded bamboo pulp carbon-based aerogel composite: Synthesis, characterization and adsorption behavior study for heavy metals removal. RSC Adv. 2024, 14, 39995–40005. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, D.; Liu, W.; Song, Z.; Shang, S.; Wang, Z.; Ren, J.; Cui, S. Graphene oxide/polydopamine modified montmorillonite/carboxymethyl chitosan composite aerogel for efficient removal of Pb2+, Cu2+, and Cd2+: Adsorption behavior, mechanism and DFT study. Sep. Purif. Technol. 2024, 339, 126585. [Google Scholar] [CrossRef]
- GB 8978-1996; Integrated Wastewater Discharge Standard. State Environmental Protection Administration (SEPA): Beijing, China, 1996.
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shuai, K.; Zhang, Y.; Jiao, G.; Zhou, H.; She, D. Removal of Cd2+ from wastewater to form a three-dimensional fiber network using Si-Mg doped industrial lignin-based carbon materials. Int. J. Biol. Macromol. 2023, 229, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Xu, M.; Ma, J.; Zhang, X.; Yang, G.; Long, L.; Chen, C.; Wu, J.; Song, C.; Xiao, Y. Improvement of cadmium immobilization in contaminated paddy soil by using ureolytic bacteria and rice straw. Sci. Total Environ. 2023, 874, 162594–162603. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Charan, S.; Patil, K.R.; Viswanath, A.K.; Khanna, P.K. Unusual formation of nano-particles of CdO and Cd(OH)2 from the reaction of dimethyl cadmium with DMF. Mater. Lett. 2006, 60, 3492–3498. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Yang, X.; Zhang, L. Effect and mechanisms of soil functional groups in bacterial-enhanced cadmium contaminated soil phytoremediation. Environ. Technol. Innov. 2024, 33, 103531–103545. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Hao, Y.; Zhao, H.-B.; Guo, Y.-R.; Pan, Q.-J. 2D-layered Mg(OH)2 material adsorbing cellobiose via interfacial chemical coupling and its applications in handling toxic Cd2+ and UO22+ ions. Chemosphere 2021, 279, 130617–130627. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).