Abstract
This study aims to demonstrate the feasibility of the use of chestnut waste as a green and circular material for developing iron-based photocatalysts for non-steroidal anti-inflammatory drug (NSAID) photodegradation. Four Fe-based catalysts and two pristine biochars were obtained upon a pyrolysis process at 500 and 700 °C and fully characterised. Due to the applied synthesis, iron is present in the form of isotropic grains of magnetite (Fe3O4), quite homogeneously dispersed onto the biochar. The textural properties of all the materials are mainly determined by the pyrolytic temperature, which results in macroporous materials at 500 °C and microporous ones at 700 °C. Fe-based catalysts were tested in Diclofenac (DFC) photodegradation. DFC removal was the result of both adsorption and photocatalytic reactions. Despite the good yield in DFC removal (80–100%), the formation of degradation by-products can partially invalidate the good effectiveness of this approach. However, the encouraging results of this study represent a step forward for the possible development of waste-derived biochar-based catalysts for in-field application.
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are extensively used due to their antipain effect in various diseases and their availability without a medical prescription. However, NSAID consumption is followed by excretion through faecal matter and urine, causing adverse effects on the environment and the ecosystem, if not properly removed from the water before its reintroduction in the aquatic environment [1]. As they are so widespread, the efficient removal of NSAIDs from contaminated waters is a global problem [2], as demonstrated by the numerous and diverse approaches proposed by researchers from several countries [3,4,5,6]. Different techniques have been proposed for the efficient removal of NSAIDs, for instance, electrochemical methods, sonochemical processes, and photocatalytic degradation; however, their total elimination is still an open point [7]. Among others, adsorption by different sorbents has been reported to be able to remove large amounts of NSAIDs from contaminated water, and different sorbents have been studied, such as activated carbon, ligninolytic enzymes, graphene-based adsorbents, and molecularly imprinted polymers [8,9].
Diclofenac (DFC), one of the NSAIDs, is often detected in wastewater and surface water, due to its high consumption by both humans and animals, its improper use, and its disposal. European Commission regulations stated 0.1–0.01 μg L−1 and 75–7.5 μg L−1 as the maximum DFC concentrations in order to avoid chronic and acute toxicity for inland and coastal surface waters, respectively [10]; therefore, the degradation of DFC is one of the most extensively debated and assessed problems within the scientific community [10].
Different strategies are available for DFC removal, such as adsorption, membrane separation, and advanced oxidation processes (AOPs) [10]. Among these, AOPs represent an emerging strategy to remove organic contaminants from wastewater [10]. They consist of non-selective processes that exploit the action of radical species, such as hydroxyl radicals (OH•), superoxide radical anions (O2−•), a hydroperoxyl radical (HO2•), or a sulphate radical (SO4−•), to degrade and mineralize the organic molecules into CO2, H2O, and inorganic elements [11]. Typically, the formation of radicals is facilitated by the activation of an oxidant agent, such as H2O2 or persulphate, in the presence of a metal oxide that acts as a catalyst (e.g., iron, copper, zinc, cobalt, manganese) [12,13].
The photo-Fenton reaction, which occurs in the presence of Fe2+, H2O2, and UV light in an acidic condition (pH = 3), has been reported as a good strategy [14]. Despite the high level of effectiveness of this methodology, it presents some drawbacks, as it is performed in a homogeneous phase, harsh conditions, such as strong acid pH, with the formation of Fe-containing sludges [15].
To overcome these limitations, the use of heterogeneous catalysts, such as metal-supported ones, has been proposed [15,16]. For this purpose, several materials have been reported as supports, for instance, clays, Layered Double Hydroxides (LDHs), zeolites, graphene, Metal–Organic Frameworks (MOFs), and activated carbon [15]. Although these supports are valid and efficient, new, low-cost, and “greener” materials, often generated by residual waste, are highly requested to achieve the sustainability goals and the transition to circularity. Accordingly, biochar, being the by-product of bio-energy production [17,18], is gaining a lot of attention.
Biochar’s characteristics are diverse, including a high surface area, micro-, macro-, and meso-porosity, as well as the presence of oxygenated functional groups at its surface, which make biochar an exceptionally versatile material. This versatility includes the possibility of catalytic applications [11,19,20]; indeed, the morphological and surface properties of biochar are proper for its efficient functioning as a catalytic support [20]. In this context, efforts are reported in the literature for the development of biochar-based catalysts for different applications [11,21,22,23], including biodiesel production, removal of tar, electrochemical reactions, microbial fuel cell electrodes, photocatalytic degradation, and advanced oxidation processes. In this regard, in the literature, there is still a debate about the catalytic activity that pristine biochar may present due to its diverse functional structural characteristics, such as its relatively high surface area, its pore structure, its inorganic components, and the presence of Persistent Free Radicals (PFRs) [24,25,26,27,28].
The characteristics of biochar strongly depend on the original biomass and the applied thermochemical process used to create it. Therefore, different biomass sources have been experimented with, such as sugarcane, maize straw, rice straw, peanut shells, herb residues, rice hulls, and Taihu Blue Algae, mainly by adopting pyrolysis conditions [12,29,30,31,32,33]. Similarly, several preparation routes have been used to synthetize biochar-based catalysts, such as co-precipitation, hydrothermal and sol/gel reactions, and self-assembly methods [28]. Furthermore, to improve the catalyst performances, the combination of different metals has also been proposed, to obtain, for instance, bi-metallic-supported catalysts, such as ZnFe2O4/biochar [34], CuFeO2/biochar [35], MnFe2O4/chitosan modified biochar [36], biochar decorated with a core–shell of nano-Zero Valent Iron (nZVI)/Fe3O4 [37], and LaFeO3/lignin biochar [38].
These catalysts were proposed for the degradation of organic molecules such as antibiotics (tetracycline [35], ofloxacin [38]), and dyes (rhodamine B, methyl orange, methylene blue [34,36,37]). The main parameters that can affect the degradation of the organic molecules were ascribed to the dose of catalyst and H2O2, the pH of the medium, and the light source [35,36,37]. For example, a higher dosage of catalyst and H2O2 could result in a decreased degradation of the pollutant probably due to the high resistance for light to penetrate the solution, and to the generation of undesirable decomposition of active radicals [35,37].
Castanea sativa is considered a valuable tree in the agricultural and forestry economy. Indeed, in 2023, about 382,916 tons of chestnuts were produced and processed in South Europe (France, Greece, Italy, Spain, Portugal, and Turkey), with Italy and Spain providing almost 64% of chestnut production [39]. The agro-residue resulting from chestnut processing (removal of outer shells and peeling of inner shells) generates large quantities of wastes, which are usually burned [40]. According to EUROSTAT data, in 2021, primary production and manufacturing of food products accounted for 9% and 21% of food wastes, respectively [41], and the disposal of these wastes represents a global issue, since the majority is currently incinerated or landfilled in soil, causing the generation of greenhouse gases (GHGs) like carbon dioxide (CO2), nitrous oxide (N2O), and methane (CH4), which poses a threat to the human and the natural environment [42]. An efficient valorisation of these copious wastes, and, more generally, of lignocellulosic agricultural residues, is represented by their thermochemical conversion, namely pyrolysis and/or gasification [43]. However, to the best of the authors’ knowledge, there is a limited number of studies regarding the utilisation of chestnut shells to produce biochar for catalytic application, and it is a relatively unexplored field within the scientific community [44,45,46].
The aim and the novelty of this work rely on the possibility of reusing and re-evaluating a waste material, produced in large amounts, i.e., chestnut waste, in the perspective of circular economy and sustainability, rather than in the need of replacing biochars derived from other different biomasses.
In this context, the main goal of this work was to investigate the behaviour of iron-based catalysts, prepared by the simply mixing of the chestnut shells (outer pericarp) with an aqueous solution of the iron precursor (FeSO4·7H2O), subject them directly to a pyrolysis process, and apply them in the degradation process.
Both the adsorption and the photocatalytic degradation of Diclofenac was evaluated for a DFC initial concentration of 10 mg L−1; this initial concentration was selected to exploit both the adsorption and the photodegradation processes.
The physico-chemical properties, the adsorption capacity, the photodegradation activity, as well as the stability of the Fe-based catalysts were analysed by considering the iron content and the temperatures of heat treatment (i.e., 500 °C and 700 °C).
2. Experimental
2.1. Materials
Diclofenac (DFC), C14H11Cl2NO2, (CAS 15307-86-5), a pure commercial powder from Sigma-Aldrich (St. Louis, MO, USA), was chosen as representative of Non-steroidal Anti-inflammatory Drug (NSAID) molecules [47]. DFC is a weak acid (pka = 4.5), characterised by two aromatic rings, two chloride (Cl) atoms, and a negative charge for pH > pKa [48].
FeSO4 7 H2O was supplied by Sigma-Aldrich (St. Louis, MO, USA).
Ultrapure water (Type I) was used to prepare all solutions.
2.2. Preparation of the Biochar-Supported Iron Catalyst
Fe-chestnut-biochar-based catalysts were synthesised according to Muelas-Ramos et al. [49]. Briefly, washed chestnut shells were dried at 60 °C in an oven (Digitheat—TFT, J.P. Selecta, Abrera (Barcelona), Spain) for 12 h. To separate the outer and the inner pericarp, the chestnut shells were treated in a blender (MC300, Moulinex, Bagnolet, France), and the pericarps were grounded (<1 mm) before the activation with Fe [50]. Then, 24.89 g of FeSO4 7 H2O were dissolved in 80 mL of ultrapure water, and 10 g of outer pericarps were added to the solution to obtain the desired 1/2 Fe/pericarps ratio. The suspension was kept at 80 °C for 2 h, continuously mixing, and, finally, dried at 105 °C.
The dried solids were pyrolysed at two different temperatures, 500 and 700 °C, for a dwelling time of 2 h, and a heating rate of 10 °C·min−1 (AAF 11/3 Muffle, Carbolite, Hope, Hope Valley, Derbyshire, UK), according to a modified Burbano et al. [51] protocol, previously developed for lab-scale application. The pyrolysed powders were divided into two batches and washed with ultrapure water five or twelve times by resuspending and centrifuging (Didacen II, Orto Alresa, Spain) the suspensions for any washing step. The washing steps are required to avoid iron leaching during the photocatalytic reactions [49]; five and twelve washing steps were applied, considering them representative of a smaller and larger number of washings, respectively.
At the end of the washing process, the materials were dried at 105 °C before testing. Iron leaching upon washing was determined by applying the 1,10-phenantroline colorimetric method [52].
For the sake of comparison, pristine biochar, i.e., without Fe addition and washing, was also prepared following the same pyrolytic procedure reported above.
Four catalysts and two pristine biochars were prepared in six different batches. The acronyms of the samples, as well as the parameters of the synthesis, are summarised in Table 1.

Table 1.
Acronyms and synthesis operating paraments of pristine biochar and Fe-biochar catalysts.
2.3. Materials Characterisation
The textures of the biochar and of the catalysts were analysed using N2 adsorption–desorption isotherms at −196 °C (Micromeritics ASAP 2420 apparatus (Norcross, GA, USA)); the samples were previously outgassed overnight at 120 °C to a residual pressure lower than 10−4 Pa. The specific surface area (SBET) was calculated according to the Brunauer–Emmett–Teller (BET) equation [53], the micropore volume (Vmicro) and the external surface (Sext) were calculated according to the t-plot method.
Meso- and macropores volume was determined by mercury intrusion porosimetry (Micromeritics Autopore IV apparatus, Norcross, GA, USA), which also allows for the materials’ skeletal density determination [41].
Elemental Analysis (EA) was performed by Costech ECS mod.4010 instrument (Avenue Stanford, Santa Clarita, CA, USA), which allows for the detection of carbon (C), nitrogen (N), hydrogen (H), and sulphur (S) simultaneously.
Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES) was performed with a PerkinElmer OPTIMA 7000 DV spectrometer (PerkinElmer, Waltham, MA, USA). The average of three measurements is reported, and the estimated error is within 3%.
Total Organic Carbon (TOC) was determined using the Shimadzu TOC-V CSH model (Kyoto, Japan) with a Non-purgeable Organic Carbon (NPOC) method.
X-Ray Powder Diffraction (XRPD) patterns were collected in the 4–90 2θ° range, 0.02°·s−1 scan rate, and 50 s of counting time, by a PANalytical X’Pert PRO X-ray polycrystal diffractometer (Almelo, The Netherlands), using a Ni-filtered Cu Kα radiation and an ultrafast X’Celerator detector. The patterns were analysed via the Rietveld method to derive the microstructural parameters of the magnetite phase from the measured line broadening. The instrumental line broadening has been determined via X-ray tracing of the instrumental optical path, considering the characteristics of the source and all the present optical elements. The Profex software 5.4.1 [54] has been used for the analysis.
Thermogravimetric (TG-DTG) analyses were performed in nitrogen in the temperature range of 30–800 °C at a heating rate of 5 °C/min (Perkin Elmer Q600 DTA-TG apparatus, Waltham, MA, USA).
FTIR-ATR analysis of the sample powders has been performed (100 scans, resolution 4 cm−1, background air) using a Thermo Nicolet 380 Instrument (Waltam, MA, USA) equipped with a diamond window for Attenuated Total Reflectance (ATR) analysis.
Scanning Electron Microscopy and Energy Dispersive X-ray (SEM-EDX) analyses were performed with a Zeiss EVO 50 EP (Zeiss, Jena, Germany) combined with an Oxford INCA energy 2000 spectrometer (Oxford Instruments, Abingdon, UK). The SEM-EDX equipment was operated at a high electron tension (EHT) voltage of 15 and 20 kV, a probe current of 120 and 300 pA, and at high vacuum (about 10−4 Pa) conditions.
2.4. Adsorption and Photocatalytic Experiments
The photocatalytic activity tests of Fe-B5W5, Fe-B5W12, Fe-B7W5, and Fe-B7W12 were performed in batch, using 250 mL glass bottles, shaking in a Digital Orbital Shaker at 150 rpm (Edmund Buhler KL 2, Bodelshausen, Tübingen, Germany) at room temperature and atmospheric pressure.
In a typical experiment, 0.1 g of catalyst was suspended in 100 mL of deionised water at constant pH 4–4.5. A 10 mg·L−1 DFC solution was added to the suspension, together with the stoichiometric amount of H2O2 (35.2 mg L−1) for the complete oxidation of DFC to CO2 and H2O.
Subsequently, the photoreactor was illuminated with UV light at 365 nm (I = 250 mA; total photon flux of about 1.0 × 1021 photon·m−2·s−1)a custom assembled lamp (10 LEDs CUN6GB1A supplied by Seoul Vyosis, Republic of Korea) was used to perform the photocatalytic process. The advancement of the reaction was followed by collecting samples of the suspension at different reaction times, namely 10, 20, 30, 45, 60, 90, and 120 min by a syringe equipped with a 0.22 μm PTFE filter (Branchia, Labbox, Spain).
DFC concentration was analysed by High-performance Liquid Chromatography (HPLC) (12600II QuatAgilent 1260 Infinity II, Agilent Technologies (Santa Clara, CA, USA) equipped with a C18 column (120 EC-C18, Poroshell, Santa Clara, CA, USA) with an estimated error within 4.5–5.5%. A mixture of 80% HPLC-grade methanol (Sigma-Aldrich, St. Louis, MO, USA) and 20% Ultrapure Type I H2O (Wasserlab, Spain) was used as the mobile phase; a constant flow of 0.8 mL·min−1 was applied.
The DFC detection wavelength was fixed at 222 nm, i.e., the maximum absorption value for DFC.
The evolution of Diclofenac degradation was expressed as molar conversion (X), calculated according to Equation (1):
where C0 (mg L−1) is the DFC initial concentration and Ct (mg L−1) is DFC concentration measured at time t during the photocatalytic experiment.
The consumption of H2O2 was analysed according to the titanium sulphate spectrophotometric Pobiner’s method [55].
The consumption of hydrogen peroxide is calculated according to Equation (2):
where C0, H202 (mg L−1) is the H2O2 initial concentration and Ct, H202 (mg L−1) is the concentration of H2O2 at time t during the photocatalytic experiment.
Lixiviated iron, at 60 and 120 min of irradiation times, was determined according to Tamura et al. [52].
Adsorption tests were performed applying the same protocol as the photocatalytic tests, without the addition of H2O2 and without illumination; the amount of adsorbed DFC was expressed as molar conversion (X), calculated according to Equation (1).
3. Results and Discussion
3.1. Materials Characterisation
3.1.1. Pristine Biochar
Nitrogen adsorption–desorption isotherms of the pristine biochar pyrolysed at 500 °C (B5) and 700 °C (B7), respectively, are shown in Figure S1.
Both materials present a type IV isotherm, and an H3 hysteresis loop, characteristics of adsorbents with a narrow distribution of uniform pores [56]. The textural parameters of the samples are summarised in Table 2 (recalling that SBET: BET surface area; Sext and Smicro: external and micropores’ surface; VTot: total pores’ volume; Vmicro, Vmeso, and Vmacro: pores’ volume; dmeso and dmacro: meso- and macropores diameters).

Table 2.
Textural Properties of B5 and B7 biochar.
Noteworthy differences can be observed between B5 and B7 textures (Table 2). B5 shows a very low SBET (3.5 m2 g−1), which is the result of the presence of large macropores (1030 nm) accounting for 94.1% of the total volume. Moreover, macropores are accompanied by a very limited number of mesopores, whose dimensions could not be measured, accounting for 5.8% of the total volume.
On the contrary, B7 is characterised by a very high SBET value (343.2 m2 g−1), where the contribution of the micropores (37.6%) determines both the high surface area and the high total pore volume (0.355 (cm3 g−1). Furthermore, a large number (59.9%) of macropores (894 nm) and a very limited number (2.5%) of mesopores (3.8 nm) contribute to the complex texture of this sample. According to the literature [57,58], the high surface area of B7 is the result of the pyrolysis temperature applied to this sample (700 °C), enough to decompose the organic matter, thus leading to the formation of micropores [59]. Moreover, in the B7 sample, the removal of the pore-blocking substances also promotes the accessibility to the external surface area (4.3 m2 g−1 for B7 vs. 0.3 m2 g−1 for B5) [60].
The skeletal density of the materials was also determined: 1.263 g mL−1 was found for B5, while 1.244 g mL−1 was found for B7, and despite the different textures, B5 and B7 evidenced very close skeletal density; therefore, this finding suggests that only a modest amount of residual undecomposed biomass should be remained in B5 following decomposition.
Pristine biochars were also characterised by X-Ray Powder Diffraction (XRPD) (Figure 1).
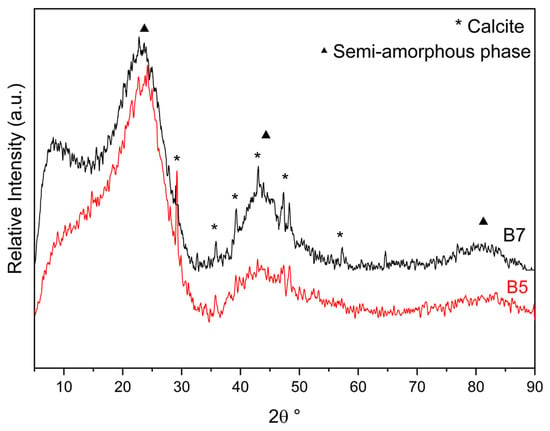
Figure 1.
XRPD patterns of B5 (red) and B7 (black) (stars: CaCO3 calcite [JCPDS 05-0586], triangles: semi-amorphous phase).
B5 and B7 materials showed similar patterns (Figure 1), characterised by broad reflections at about 25 (main one), 45, and 85 2θ° of a semi-amorphous phase (Figure 1, triangles), accompanied by the reflections (Figure 1, stars) of a crystalline phase. The semi-amorphous features are consistent with a carbon structure containing randomly oriented aromatic carbon layers [61,62], which, in the case of B7, apparently presents a more ordered organisation with a high spacing distance. The crystalline phase is associated with a polycrystalline CaCO3 calcite phase [JCPDS 05-0586] formed during the pyrolysis process from the natural inorganic components of the biomass cells [63]. In view of the higher pyrolytic temperature, the calcite phase is more evident in the B7 sample, as it is better crystallised.
Infra-red analysis in Attenuated Total Reflectance mode (FT-IR-ATR) of the pristine biochar is compared in Figure 2 in the region of 3500–500 cm−1. Indeed, the two materials show completely different features, which can be related to the different thermal treatments.
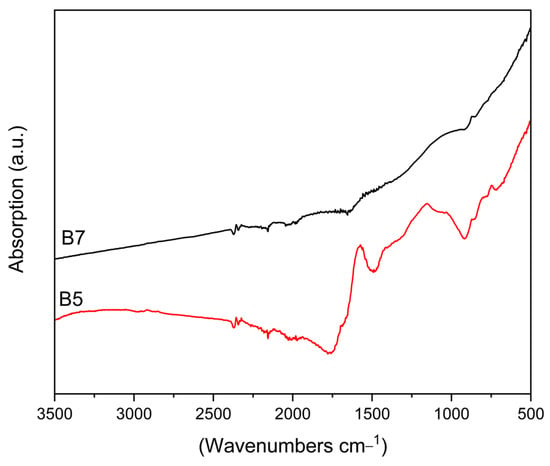
Figure 2.
FT-IR ATR spectra of pristine biochar B5 (red), B7 (black).
Bands at 751, 874, and 1571 cm−1 in the spectrum of sample B5 are assigned to carbonate species [64], as evidenced by XRPD analysis. The broad envelope of bands in the spectral region of 1200–1000 cm−1 can be attributed to the overlap of CC and CO stretching modes, in addition to ring vibration and COH deformation modes, which are characteristics of the residual cellulosic material [65], and lignin matrix. Clearly, this material is still characterised by a high amount of oxygen-containing functional groups arising from biomass degradation. In the spectrum of sample B7, all these features disappear, and, only very weak bands, if any, due to residual carbonate species, are detected. The overall spectrum is flattened and consistent with the spectrum of carbonaceous materials reported in the literature, showing that the carbon matrix and the surface structure have been settled following pyrolysis at 700 °C [66].
As expected, a higher pyrolysis temperature leads to the removal of most of the oxygen-containing functional groups.
Thermogravimetric (TG) analysis, performed in N2, on B5 and B7 are reported in Figure 3.
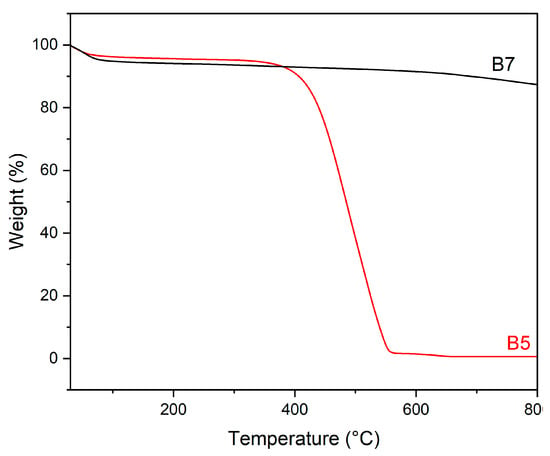
Figure 3.
TG curves in N2 of B5 (red) and B7 (black).
Once more, a markedly different behaviour has been found for B5 and B7 (Figure 3); in fact, in the B5 sample, a large weight loss, accounting for 100% of the mass, is observed between 400 and 550 °C. According to the literature, such a weight loss could be ascribed to a further pyrolysis process of the residual lignocellulosic material, not fully decomposed during the pyrolytic treatment at 500 °C of this sample [67,68]. The absence of calcite decomposition, expected between 700 and 800 °C (790 °C for highly pure and crystalline material [69]), can be due either to the limited amount of this phase, below the detection limit of the technique, or to an anticipation of the decomposition, due to the presence of polycrystals and impurities. Calcite decomposition, thus, could be overlapped by the strong thermal phenomenon between 400 and 550 °C.
On the contrary, no weight losses associated with biomass decomposition are manifest in B7, confirming that the pyrolysis temperature of this sample was high enough to fully decompose the biomass [57]. Moreover, in B7, no calcite decomposition was detected, thus suggesting that the low content of this phase, in agreement with IR data, is the most probable explanation for the findings.
The morphology of the samples was assessed by Scanning Electron Microscopy (SEM) (Figure 4).

Figure 4.
SEM images, at different magnifications (250 and 5000×), of (a) B5 and (b) B7. (Yellow arrows: fibres).
B5 and B7 exhibit quite similar morphology, characterised by an irregular and coarse surface, irrespective of the pyrolysis temperature (Figure 4a,b). The typical biochar porous structure, characterised by quite regular channels [70], is not clearly discernible in this case, even at high magnification (Figure 4a,b, left); moreover, the presence of fibres is clearly detectable. These fibres may correspond to lignocellulosic components of the original biomass only partially decomposed [71], suggesting that, even if quite effective, the pyrolysis at 500 and 700° is not enough to totally decompose all the biomass components, in particular, lignin [72]. These results are in line with TG analysis of pristine biochar, where a further biomass decomposition was observed during the analysis; however, these residues are present in a very limited amount and do not change the bulk morphology, texture, or composition.
The elemental composition of B5 and B7 was evaluated by both Elemental Analysis (EA) and Energy Dispersive X-ray spectroscopy (EDX) (Table 3).

Table 3.
Elemental and EDX analysis of B5 and B7 (* by EA, ** by EDX).
The higher carbon content of B7 (82%) compared to B5 (72%) is due to the higher degree of carbonisation of the first sample, in view of the higher pyrolysis temperature. This picture is also confirmed by the lower H/C and O/C ratio of B7 (Table 3).
The higher C/N ratio of CB7 (109.3) as compared to CB5 (92.3) still confirms the effect of the different employed temperatures; indeed, it is reported that N diminishes at a faster rate than C with the increase in pyrolysis temperature [73].
Other elements, mainly Mg, Al, K, Ca, and P, natural components of the pyrolysed biomass cells, were also detected by EDX analysis (not reported), but in a very limited amount (0.1–0.8%); moreover, no sulphur or iron were detected in these samples.
3.1.2. Fe-Based Catalysts
A similar characterisation has been performed on the Fe-supported catalysts, Fe-B5W5, Fe-B5W12, Fe-B7W5, and Fe-B7W12 (recalling that W5 and W12 correspond to samples washed 5 and 12 times).
After the introduction of iron, the texture of all the samples became more complex than the original biochar, suggesting that either the synthesis process or the presence of iron may exert some effects on the texture of the pristine systems.
In the case of B5-based catalysts, a considerable increase in the surface area and in the total pore volume occurred. Such an increase is mainly related to both micro- and mesopores formation upon the catalysts’ synthesis and the washing process (Table 4), and it can be explained once more with B5 pyrolysis temperature (500 °C).

Table 4.
Textural properties of the Fe-based catalysts, pristine B5 and B7 reported for comparison. (Abbreviations as in Table 2).
As already discussed, this temperature is not enough to totally decompose the biomass, whose residues remain in the pores. When the iron solution is present in the mixture, an acid environment is generated, that, together with the pyrolytic process, could favour biomass decomposition. This could result in the emptying of the pores, thereby generating a new porosity in Fe-B5W5. The further pores’ volume and surface area increase, observed in Fe-B5W12, could be the result of the larger number of washing steps, which, additionally, favour the pores’ emptying (Table 4). Accordingly, Fe-B5W12 texture appears to be more the result of emptying the already existing pores’ than the formation of new ones.
On the contrary, in B7-based catalysts, the textural properties can be explained by considering two concurrent opposite effects. The decreased surface area in Fe-B7W5, with respect to pristine B7, can be the result of the occupation of the pores, mainly the micropores, by the iron phase (Table 4). This hypothesis is supported by the halving of the micropores’ volume in Fe-B7W5 and Fe-B7W12 (Table 4). On the contrary, the increase in surface area after 12 washes could be the consequence of the partial emptying of the pores due to the removal of the iron during washing.
Iron release upon washing has been qualitatively checked by colorimetric determination [52], and the presence of iron was found in the washing water both after 5 and 12 washing steps. However, considering the textural evolution of B5-based catalysts, it can be assumed that the effect of the pores emptying, due to biomass removal, may be predominant over the effect of iron removal during washing.
Irrespective of the pyrolysis temperature and the number of washing cycles, all catalysts are characterised by very close skeletal densities, in the range 1.703–1.837 g mL−1, and, in any case, higher than those of pristine B5 and B7 due to the presence of iron. Considering that the pristine biochars and the Fe-based catalysts have been prepared in four different batches, the constancy of skeletal densities can be taken as an indication of the reproducibility of the preparation procedure.
XRPD patterns of the catalysts in the 2 θ° range of 10–90 are plotted in Figure 5.
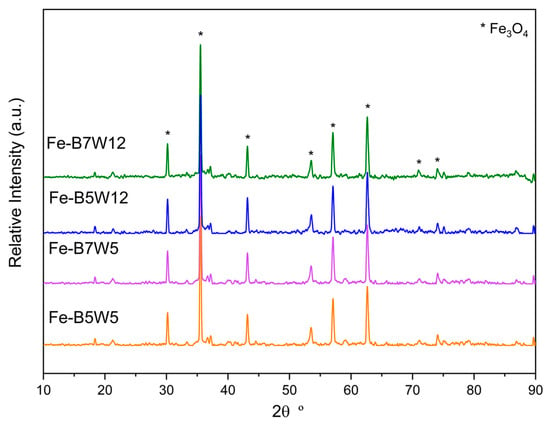
Figure 5.
XRPD patterns of Fe-B5W5 (orange), Fe-B5W12 (blue), Fe-B7W5 (violet), and Fe-B7W12 (green). (Stars: Fe3O4, magnetite, [JCPDS 19–629]).
The Fe-supported catalysts, irrespective of the number of washing cycles, presented mainly the reflections corresponding to Fe3O4, magnetite [JCPDS 19–629], as confirmed by the magnetic properties of the samples (Figure S2); moreover, the partial reduction of Fe(III) to Fe(II) is a confirmation of the effectiveness of the applied pyrolytic process.
The reflections attributed to the calcite phase, present in the pristine biochar, are no longer detected in the catalysts’ samples. The disappearance of calcite is possibly due to the formation of CaSO4 during the impregnation, via a double exchange reaction with FeSO4, followed by the CaSO4 dissolution when the washing process is applied. This behaviour is justified by the higher strength of the sulphuric acid, compared with the carbonic one, and by the higher CaSO4 solubility (2.4 gL−1 at 25 °C), compared with that of CaCO3 (0.015 g L−1 at 25 °C) [74]. Furthermore, the acid environment could also have decomposed the calcite phase.
XRPD patterns have been analysed by Rietveld analysis (plots not reported), and results are summarised in Table 5.

Table 5.
Rietveld analysis of the catalysts (* calculated on (1, 1, 1) and (1, 0, 0) directions).
Except for the Fe-B5W5 sample, all the catalysts present very close features, with cell parameters in line with reference Fe3O4 [JCPDS 19–629] (Table 5). The difference observed in both lattice parameters and grain size for sample Fe-B5W5 can be related to the presence of Fe vacancies in the structure [75], associated with incomplete pyrolysis and different redox conditions during Fe-B5W5 preparation. Both iron vacancies and preparation conditions can account for the low grain size of this sample (Table 5); on the contrary, Fe-B5W12, Fe-B7W5, and Fe-B7W12 showed isotropic grains of similar dimensions, in the range of 150–170 nm. For all the samples, in view of the very limited microstrains, one can suppose that the Fe3O4 phase is not perturbated when interacting with the biochar structure.
Moreover, despite the fact that no direct comparison can be performed among the samples, as they have been prepared in different batches, the slight grain size increase, observed in the samples upon 12 washing, irrespective of the pyrolytic temperature (Table 5), can be related to a sort of ripening effect, due to the dissolution and recrystallisation of the active phase during the washing process.
Iron content in the samples was determined by Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES). Iron contents of 24.6 and 22.5% (w/w) were found for Fe-B7W5 and Fe-B7W12, respectively, and of 14.6 and 13.3% (w/w) were detected for Fe-B5W5 and Fe-B5W12, respectively.
Since the samples were prepared from the same initial iron content, the differences in iron content of the catalysts after washing, can be explained by referring to the morphology of the systems. In fact, the higher iron content of the sample pyrolysed at 700° seems to be linked to the presence of Fe3O4 possibly allocated in the smaller pores, such as the micropores, which can trap the active phase, thus limiting the leaching during washing. Instead, in the case of the B5-based systems, Fe3O4 is accommodated in the macropores, therefore, more accessible, thus its removal is favoured during washing. The slightly lower percentage of iron in the samples washed 12 times, compared to those washed only 5 times, highlights how the number of washes, in addition to the texture, becomes a determining factor in establishing the content of the active phase in the catalyst.
In Figure 6, the FT-IR spectra in the ATR mode of the Fe-supported catalysts are reported.
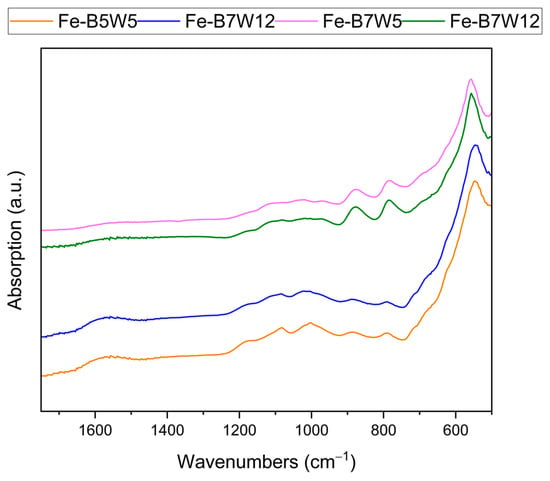
Figure 6.
FT-IR ATR spectra of Fe-B5W5 (orange), Fe-B5W12 (blue), Fe-B7W5 (violet), and Fe-B7W12 (green).
All four samples exhibit a band at approximately 574 cm−1, indicative of Fe-O bonding in Fe3O4 [76]. As reported by Gadgeel et al. [77], the other characteristic bands of Fe3O4 at 694, 627, and 896 cm−1 are no longer observed in our spectra; otherwise, two bands at 781 and 878 cm−1, more intense in B7 sample, are evident. These bands, close to those of magnetite but not exactly corresponding, suggest a possible interaction of Fe3O4 with the substrate, hence, the consequent bands’ perturbation.
Several weak bands in the region of 1200–900 cm−1 can be assigned to residual oxygen-containing groups and, mainly, to SO vibrational modes of sulphur-containing species, namely ionic bulklike sulphates, with a possible contribution of sulphites formed by the decomposition of sulphates [78].
TG analysis (Figure S3) showed, for all the samples, a limited weight loss of about 20–25% w/w, where specific decomposition phenomena are hardly distinguishable; for this reason, DTG curves have also been analysed (Figure 7).
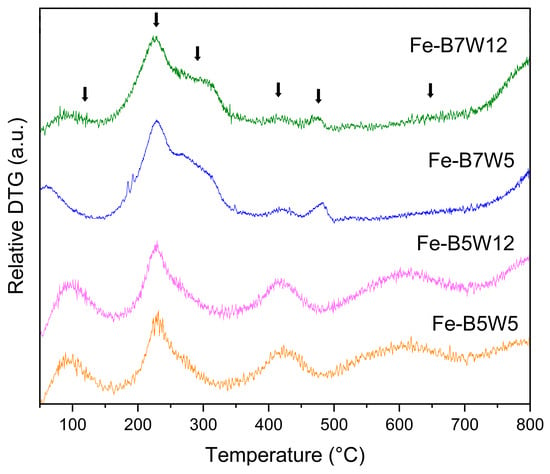
Figure 7.
DTG analysis of Fe-B5W5 (orange), Fe-B5W12 (blue), Fe-B7W5 (violet), and Fe-B7W12 (green). (Arrows: thermal phenomena).
The broad peaks of the DTG curves can be associated with dehydration and thermal phenomena occurring in both Fe3O4 and residual FeSO4 7H2O.
The features observed below 150 °C may account for water loss in Fe3O4 [79], while those in the range of 200–400 °C could be related to water release from residual FeSO4, better evident in B5-based samples [80]. This hypothesis is also confirmed by the presence of a further thermal phenomenon at higher temperatures in B5-based samples. Indeed, FeSO4 decomposition to give Fe2O3 is reported as a two-step process in the temperature range of 525–710 °C [79]; thus, DTG findings are in line with the incomplete pyrolysis observed in B5-based catalysts. Finally, the phenomenon observed starting from 650 °C could be associated with the oxidation of Fe(II) to Fe(III) in Fe3O4 to give Fe2O3 [81].
The morphology and the elemental composition of the Fe-supported catalysts were assessed by SEM-EDX (Figure 8a,b).
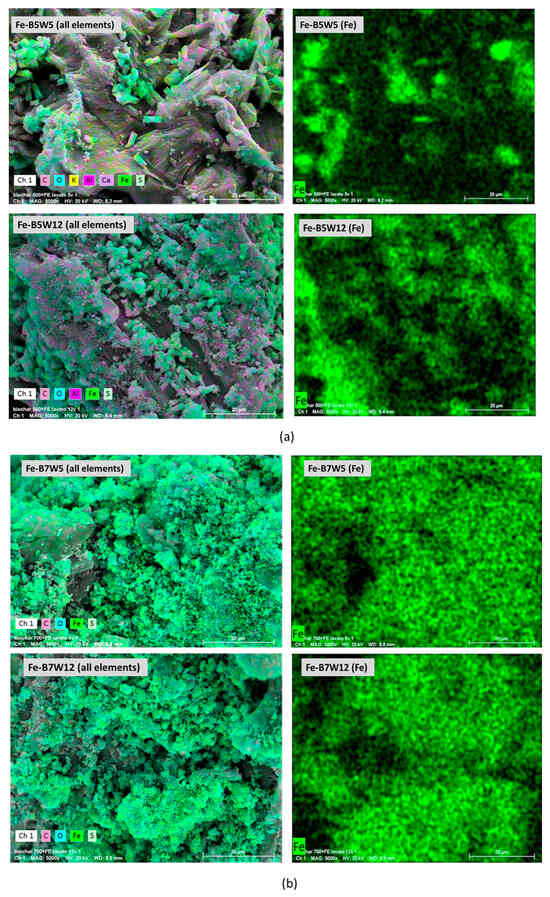
Figure 8.
SEM-EDX analysis of (a) Fe-B5W5 and Fe-B5W12 and (b) Fe-B7W5 and Fe-B7W12.
Despite changes observed in textural properties, all the materials show a morphology close to that of the pristine biochar, suggesting that neither the catalyst preparation nor the washing process apparently modified it, and the main difference with respect to the pristine B5 and B7 is the presence of iron, accompanied by sulphur.
Iron is more homogenously distributed in the Fe-B7-based samples, irrespective of the number of washing cycles (Figure 8b); on the contrary, in Fe-B5W5 and Fe-B5W12, Fe-containing clusters, clearly evident in the figure, are allocated in specific areas of the sample (Figure 8a). However, at the eye’s inspection, these clusters appear to be a mere aggregation of smaller particles than large crystals formation.
The different dispersion of iron in the catalysts may be related to both the catalysts’ synthetic procedure and the different textural properties obtained during the thermal treatment. As a matter of fact, the pyrolysis of the biomass and the catalyst production occurs one pot; thus, to understand the reason for the different Fe dispersion in B5- and B7-based samples, the textural evolution of pristine B5 and B7 must be recalled.
As already discussed, very different surface areas (3.5 and 343.2 m2 g−1 for B5 and B7, respectively) and porosity (such as, 94.1% of macropores and 5.8 % of mesopores in B5, and 59.9% of macropores and 37.6% of micropore in B7) were found when the biomass was pyrolysed without the presence of Fe (Table 4).
When the biomass and the iron sulphate mixture are pyrolysed together, the texture of the biochar and the Fe-containing phase are contemporary formed; therefore, Fe3O4, while forming, could be allocated in the pores.
In the case of B7-based catalysts, where pores of smaller dimensions are present, the iron-containing phase, “trapped” inside the pores, is not free to move, and aggregate; thus, it gives rise to a more homogeneous iron distribution (Figure 8b). Instead, in the Fe-B5-based catalysts, in view of the macroporosity of the system, the iron phase, being allocated in larger pores, is free to move and aggregate (Figure 8a), so that a lower iron dispersion is found.
Moreover, in the case of the samples pyrolysed at 500 °C, the residual functional surface groups, observed by FTIR analysis, are available to preferentially interact with the iron phase, thus explaining iron clustering in preferential areas of Fe-B5W5 and Fe-B5W12 (Figure 8a). In B7-based systems, such a preferential interaction could hardly occur in view of the limited number of functional groups retained at 700 °C.
Finally, considering the very low microstrain of Fe3O4, calculated by Rietveld analysis for all the catalysts (Table 5), it can be speculated that the iron-containing clusters are only weakly interacting with the biochar structure.
3.2. Photocatalytic and Adsorption Tests
The photocatalytic activities of Fe-B5W5, Fe-B5W12, Fe-B7W5, and Fe-B7W12 were first investigated as a strategy for the removal of Diclofenac (DFC) from a solution with an initial concentration of 10 mg L−1. Experiments were performed according to the methodological approach described in Section 2.4. The outcomes of the photocatalytic activity of the Fe-supported catalysts are reported in Figure 9a, while the corresponding H2O2 decomposition reaction is plotted in Figure 9b.
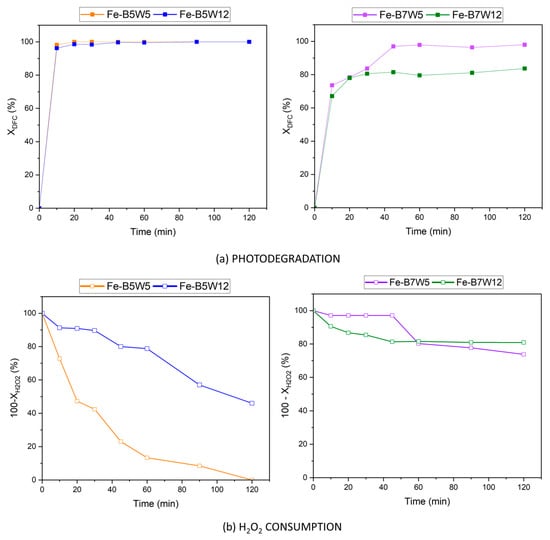
Figure 9.
DFC removal by Fe-B5 and Fe-B7-based catalysts: (a) photocatalytic activity and (b) H2O2 decomposition. (Operating conditions: 10 mg L−1 DFC, 10–120 min, 0.1 g catalyst, UV light at 365 nm).
Total DFC removal was achieved by all the catalysts, except for Fe-B7W12, whose yield was not higher than 80% (Figure 9a). However, the total conversion of hydrogen peroxide was observed only for the Fe-B5W5 sample (Figure 9b), suggesting that the other Fe-based catalysts are less efficient in hydrogen peroxide decomposition to hydroxyl radicals; as a matter of fact, hydroxyls are directly involved in the photocatalytic process; thus the H2O2 conversion is an indication of the photocatalytic reaction efficiency [82].
The photodegradation reaction was irrespective of both iron content and surface areas, since the better performing catalysts (Fe-B5W5 and Fe-B5W12) retained the lower Fe content and were not characterized by the higher surface areas.
Instead, the photocatalytic activity seems related to the temperature of pyrolysis; indeed, the higher efficiency was obtained for the samples pyrolysed at 500 °C, and it could be related to the presence of the surface functional groups, as suggested by the FTIR analysis of these samples.
Notably, in the case of Fe-B7W12, the iron loss upon washing, could be partially compensated by the high dispersion of the iron phase, thereby preventing a potential marked decrease in photoactivity.
Moreover, iron leaching was experimentally verified at the end of the photocatalytic experiments. A very limited, practically negligible, iron leaching of 0–0.005 mg L−1 was measured; therefore, if can be concluded that all the iron loss has occurred during the washing process.
Furthermore, in the samples obtained at 500 °C, the photodegradation is faster (100% in 10 min) than in the samples obtained at 700° (80–100% in 40 min), maybe due to the different catalysts’ texture.
TOC analysis was also performed to evaluate whether DFC was completely mineralised to CO2 and H2O, or if any by-products were generated during the photocatalytic process. A residual TOC of about 5–5.7 mg L−1 was observed at the end of the process, indicating that part of the DFC was not fully degraded, and some organic by-products remained in the medium, contributing to the detected TOC.
The degradation mixtures can be highly complex and dependent on the catalysts’ nature. The degradation products have been fully studied in the literature, both in homogeneous [83] and heterogeneous [84] processes. The by-products can be, for instance, the result of hydroxylation, decarboxylation, and oxidation processes, as well as those resulting from the degradation of recalcitrant organic acids, acetic, maleic, oxalic, and formic [84].
The potential application of biochar as a catalyst in advanced oxidation processes (AOPs) is closely linked to its physico-chemical characteristics. The oxygen-containing functional groups on the surface of biochar—primarily carboxyl, carbonyl, hydroxyl, and ester groups—play a key role in determining the catalytic, adsorptive, and redox properties. Indeed, these functional groups can activate the oxidising agents, the hydrogen peroxide in this work, by donating electrons. This electron transfer leads to the formation of reactive species, including hydroxyl radicals [85].
In addition to oxidant activation, the oxygen-containing groups at the biochar surface can adsorb pharmaceutical contaminants like Diclofenac; thus increasing the probability of interactions between this pollutant and the reactive radicals, thereby enhancing the degradation efficiency of the process [86].
Furthermore, as a carbon-based material, biochar can contain structural defects such as curvatures, vacancies, and edge defects. These defects also facilitate the activation of the oxidant and the subsequent formation of the radical species. Indeed, the adsorption of the oxidant onto the biochar surface, via sigma (σ) bonds and interactions with delocalised π-electrons, enables electron transfer from the biochar to the oxidant, resulting in the generation of reactive radicals, such as hydroxyl (•HO).
Finally, the iron active species present at the catalyst surface also can generate different reactive species, including, •HO and O2•−, which contribute to the Diclofenac degradation in the presence of light and hydrogen peroxide.
Figure 10 presents a simplified mechanism for Diclofenac degradation by the use of Fe-biochar-based catalysts proposed on the basis of the literature. When photons interact with the active phase at the catalyst surface, they photoexcite electrons, promoting them from the valence band to the conduction band, thus generating positively charged holes (h+). Both the excited electrons (e−) and the holes (h+) can activate hydrogen peroxide, dissolved oxygen, hydroxyl ions, and/or the iron active phase present at the catalyst surface, leading to the formation of reactive species. Such species, for instance hydroxyl radicals (•OH) or superoxide radicals (O2•−), are able to degrade Diclofenac, possibly until its complete mineralisation.
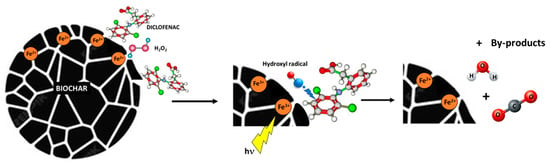
Figure 10.
Simplified reaction pathway of the Diclofenac degradation by the Fe-biochar catalysts.
On the samples with the best photocatalytic performances, namely, Fe-B5W5 and Fe-B7W5, the adsorption capacity towards a DFC solution at an initial concentration of 10 mg L−1 was also investigated, as adsorption is the most probable reaction occurring in the system, other than photocatalysis.
As a matter of fact, it is difficult to distinguish between these two processes, and certain considerations should be carried out regarding the H2O2 decomposition reaction, given that the adsorption process occurs in the absence of H2O2.
For sake of comparison, the adsorption capability of pristine B5 and B7 was also assessed, and the results are reported in Figure 11.
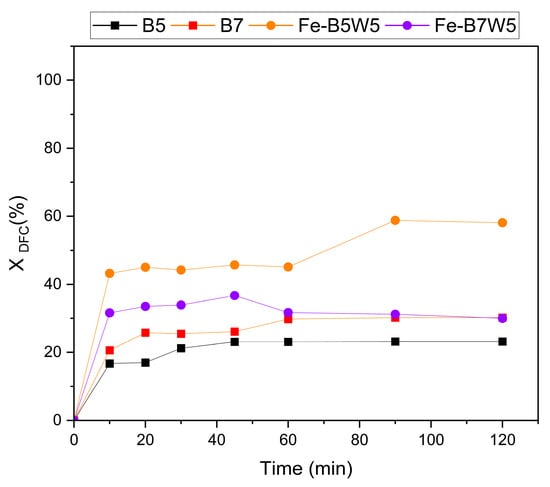
Figure 11.
Absorption of DFC by B5, B7, Fe-B5W5, and Fe-B7W5. (Operating conditions: 10 mg L−1 DFC, 10–120 min, 0.1 g catalyst).
DFC removal by adsorption in the range of 20–60% was achieved by all the samples. However, Fe-B5W5 (Figure 11) showed the highest removal efficiency (60%), while B5 the lowest one (20%) at the end of the experiment (120 min). On the contrary, B7 and Fe-B7W12 achieved comparable DFC adsorptions of about 30% (Figure 11).
These results suggest that the adsorption capacity of B5 and B7, and of the corresponding catalysts, is not solely related to their textural properties, as B7, the biochar with the highest SBET (343 m2 g−1), did not give the highest DFC adsorption. Instead, the high DFC adsorption observed in Fe-B5W5 could be explained with the presence of a larger number of surface groups, for instance, hydroxyls or aromatic rings (detected by FTIR) able to coordinate DFC better [87]. Similarly, Bano et al. [88] found a maximum DFC adsorption of 40.2% when the initial DFC concentration was set at 20 mg L−1 and the adsorbent dose at 0.1 g L−1, and, a maximum DFC removal efficiency equal to 97.8% was obtained by increasing the adsorbent dose.
The improvement in pharmaceutical removal by biochar-based photocatalysts was attributed to the synergistic effect between adsorption and photodegradation, as the photodegradation degrade the pollutants more effectively in comparison to adsorbent and photocatalyst used separately. Biochar, thanks to its porous structure and adsorption capacity, facilitates the distribution of the active phase, iron in this case, thus improving the contact between reagents and contaminants. Moreover, biochar acting as a support for iron improves the stability and the efficiency of the catalyst [89].
A possible mechanism of interaction of DFC, while adsorbed, can be hypothesised only on the bases of the literature report, as data here reported were not performed targeted to mechanistic studies, and devoted experiments would be required on purpose. However, on the basis of literature indications, it can be assumed that the formation of π-π interactions or hydrogen bonds, proposed in the literature, are also active in our samples [87,90]. As a matter of fact, π-π interactions should be favoured by the presence of the observed aromatic rings in the layers of both B5 and B7 biochars, being them able to interact with the DFC molecules. Moreover, at the catalyst surfaces Fe ions could also behave as Lewis acid sites, coordinating the electron-rich regions of the DFC molecule, and finally H-bonds between the sorbent and DFC could be responsible for the above interaction. Finally, pH value during adsorption may also exert an influence on the adsorption process [91,92], by favouring interaction among species with opposite charges [91,93].
It can be concluded that, in the case of both Fe-B5W5 and Fe-B7W12, the observed total DFC removal could be mainly attributed to the photodegradation process, while in the case of Fe-B5W12 and Fe-B7W12, the observed DFC removal could be the combined effect of both adsorption and photocatalysis. In any case, the occurrence of the photodegradation process is fundamental for DFC total removal, because no removal higher than 60% can be reached by adsorption.
4. Conclusions
The feasibility of using chestnut waste as a green and circular material for developing iron-based photocatalysts has been demonstrated in this study. Specifically, chestnut waste-derived materials were used to produce iron-based biochar catalysts capable of removing a pharmaceutical pollutant, such as Diclofenac, through a hybrid adsorption/photocatalytic process.
Four Fe-based catalysts, namely Fe-B5W5, Fe-B5W12, Fe-B7W5, and Fe-B7W12 along with two pristine biochars, B5 and B7, were prepared via pyrolysis at 500 °C and 700 °C, respectively, and fully characterised.
The physico-chemical properties of the materials were primarily determined by the thermal treatment, leading to very different textural properties, such as, in the case of pristine biochar (B5 and B7) for which very different surface areas of 3.5 m2 g−1 (B5) and 343.2 m2 g−1 (B7) were measured. Differences in textural properties were still preserved by the catalysts, for which a broad surface area range, 55–162 m2 g−1, was found depending on both pyrolysis and washing conditions.
After the synthesis and the repeated washing cycles, the Fe-based catalysts retained an iron content in the range of 15–20%, determined by EDX analysis. Iron was incorporated as isotropic magnetite (Fe3O4) grains, characterised by the presence of Fe vacancies in the structure. Iron is more homogenously distributed in the Fe-B7-based samples, irrespective of the number of washing cycles. This different dispersion of iron was associated with the synthesis route of the materials, where the Fe3O4 formation is prone to the microporosity of the biochar. Finally, considering the very low microstrain of Fe3O4, calculated by Rietveld analysis, it can be speculated that the iron-containing clusters are only weakly interacting with the biochar structure.
The pyrolysis temperature also influences the number of the surface sites, thus resulting in a larger number of them when the samples were obtained at the lower temperature (i.e., 500 °C); as a result, carbonates, CO, CC, and COH, are the groups available for DFC adsorption.
All the samples exhibited good adsorption and photocatalytic efficiencies, in the range of 84–100%, and the synergistic effect between these two mechanisms enabled a high—often complete—removal of Diclofenac (DFC).
Finally, the adsorption and photocatalytic properties appeared to be irrespective of the surface area of the materials, since the more active catalyst, Fe-B5W5, was characterised by the lower surface area (55 m2 g−1); therefore, the nature and the number of the residual functional groups play a pivotal role in the DFC removal efficiency.
Despite the high DFC removal efficiency, the formation of degradation by-products was observed, which may partially compromise the overall effectiveness of the process. Nevertheless, the promising results of this study highlight the potential for further development and possible in-field application of these systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/c11020038/s1, Figure S1: N2 adsorption–desorption isotherms of biochar materials (B5, B7) and their corresponding Fe-based catalysts, Figure S2: Fe-B5W5 catalyst with magnetic properties, Figure S3: TG analysis of Fe-B5W5 (orange line), Fe-B5W12 (blue line) Fe-B7W5 (violet line), and F-B7W12 (green line) catalysts.
Author Contributions
Conceptualisation, A.G., D.H. and A.B.; methodology, M.G., C.d.l.R. and K.J.-B.; validation, M.G., A.G. and D.H.; formal analysis, M.G., C.d.l.R. and K.J.-B.; investigation, M.G., C.C., M.B., E.F. and A.B.; resources, A.B., E.F., M.B. and C.C.; data curation, M.G.; writing—original draft preparation, M.G., C.C, A.B., M.B. and E.F.; writing—review and editing, M.G., A.B., E.F., M.B., A.G., D.H., K.J.-B., V.M.-R., C.d.l.R. and C.C.; visualisation, M.G. and C.C.; supervision, A.G. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
The research activities were possible thanks to the financial support received under Project PID2021-124021OB-I00 (URBRAINTREAT), funded by MCIN/AEI/10.13039/501100011033 and “ERDF: A way of making Europe”.
Data Availability Statement
Data are available upon request from the authors.
Acknowledgments
The research activities were possible thanks to the financial support received under the European project PON FSE REACT-EU, and the resources allocated by Ministerial Decree No. 1061 of 10 August 2021.
Conflicts of Interest
Author Maurizio Bellotto was employed by the company Opigeo s.r.l. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| B5 | Pristine biochar pyrolysed at 500 °C |
| B7 | Pristine biochar pyrolysed at 700 °C |
| Fe-B5W5 | Fe-biochar catalyst pyrolysed at 500 °C and washed 5 times |
| Fe-B5W12 | Fe-biochar catalyst pyrolysed at 500 °C and washed 12 times |
| Fe-B7W5 | Fe-biochar catalyst pyrolysed at 700 °C and washed 5 times |
| Fe-B7W12 | Fe-biochar catalyst pyrolysed at 700 °C and washed 12 times |
| EA | Elemental Analysis |
| ICP-OES | Inductively Coupled Plasma–Optical Emission Spectroscopy |
| TOC | Total Organic Carbon |
| XRPD | X-Ray Powder Diffraction |
| TG-DTG | Thermogravimetric analyses |
| SEM-EDX | Scanning Electron Microscopy and Energy Dispersive X-Ray |
| AOP | Advanced Oxidation process |
| NSAID | Non-steroidal Anti-inflammatory Drug |
| DFC | Diclofenac |
References
- Lakshmi, S.D.; Geetha, B.V.; Vibha, M. From Prescription to Pollution: The Ecological Consequences of NSAIDs in Aquatic Ecosystems. Toxicol. Rep. 2024, 13, 101775. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions—A Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and Removal of Pharmaceuticals in a Municipal Sewage Treatment System in the South of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Kermia, A.E.B.; Fouial-Djebbar, D.; Trari, M. Occurrence, Fate and Removal Efficiencies of Pharmaceuticals in Wastewater Treatment Plants (WWTPs) Discharging in the Coastal Environment of Algiers. Comptes Rendus Chim. 2016, 19, 963–970. [Google Scholar] [CrossRef]
- Sun, Q.; Li, M.; Ma, C.; Chen, X.; Xie, X.; Yu, C.-P. Seasonal and Spatial Variations of PPCP Occurrence, Removal and Mass Loading in Three Wastewater Treatment Plants Located in Different Urbanization Areas in Xiamen, China. Environ. Pollut. 2016, 208, 371–381. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in Wastewater Treatment Plants in Greece: Occurrence, Removal and Environmental Risk Assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Nokwethemba Mahlambi, P.; Chimuka, L.; Madikizela, L.M. Adsorbents and Removal Strategies of Non-Steroidal Anti-Inflammatory Drugs from Contaminated Water Bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Gautam, M.K.; Mondal, T.; Nath, R.; Mahajon, B.; Chincholikar, M.; Bose, A.; Das, D.; Das, R.; Mondal, S. Harnessing Activated Hydrochars: A Novel Approach for Pharmaceutical Contaminant Removal. C 2024, 10, 8. [Google Scholar] [CrossRef]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of Diclofenac from Wastewater: A Comprehensive Review of Detection, Characteristics and Tertiary Treatment Techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A Review of Recent Developments in Catalytic Applications of Biochar-Based Materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New Notion of Biochar: A Review on the Mechanism of Biochar Applications in Advannced Oxidation Processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S.; et al. Fabrication of Novel Magnetic MnFe2O4/Bio-Char Composite and Heterogeneous Photo-Fenton Degradation of Tetracycline in near Neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, D.; Cortijo, M.; Huang, C.P. The Role of Iron on the Degradation and Mineralization of Organic Compounds Using Conventional Fenton and Photo-Fenton Processes. Chem. Eng. J. 2009, 155, 637–646. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton Catalysts: A Review of Recent Advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Gou, Y.; Chen, P.; Yang, L.; Li, S.; Peng, L.; Song, S.; Xu, Y. Degradation of Fluoroquinolones in Homogeneous and Heterogeneous Photo-Fenton Processes: A Review. Chemosphere 2021, 270, 129481. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Abdelkader-Fernández, V.K.; Matos, R.; Peixoto, A.F.; Fernandes, D.M. Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review. Catalysts 2022, 12, 207. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. A Comprehensive Assessment of the Method for Producing Biochar, Its Characterization, Stability, and Potential Applications in Regenerative Economic Sustainability—A Review. Clean. Mater. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Okiemute Akpasi, S.; Michael Smarte Anekwe, I.; Adedeji, J.; Lewis Kiambi, S. Biochar Development as a Catalyst and Its Application. In Biochar—Productive Technologies, Properties and Applications; Bartoli, M., Giorcelli, M., Tagliaferro, A., Eds.; IntechOpen: London, UK, 2023; ISBN 978-1-80356-251-3. [Google Scholar]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of Biochar and Its Composites in Catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, Y.; Li, J.; Patel, A.K.; Dong, C.-D.; Jin, X.; Gu, C.; Yip, A.C.K.; Tsang, D.C.W.; Ok, Y.S. Recent Advancements and Challenges in Emerging Applications of Biochar-Based Catalysts. Biotechnol. Adv. 2023, 67, 108181. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Dalai, A.K.; Chaurasia, S.P. Activity and Stability of Biochar in Hydrogen Peroxide Based Oxidation System for Degradation of Naphthenic Acid. Chemosphere 2020, 241, 125007. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Wang, Y.; Dionysiou, D.D.; Zhou, D. Photogeneration of Reactive Oxygen Species from Biochar Suspension for Diethyl Phthalate Degradation. Appl. Catal. B Environ. 2017, 214, 34–45. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Z.; Zeng, G.; Lai, C.; Xiao, R.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. Insight into the Mechanism of Persulfate Activated by Bone Char: Unraveling the Role of Functional Structure of Biochar. Chem. Eng. J. 2020, 401, 126127. [Google Scholar] [CrossRef]
- Zhu, N.; Li, C.; Bu, L.; Tang, C.; Wang, S.; Duan, P.; Yao, L.; Tang, J.; Dionysiou, D.D.; Wu, Y. Bismuth Impregnated Biochar for Efficient Estrone Degradation: The Synergistic Effect between Biochar and Bi/Bi2O3 for a High Photocatalytic Performance. J. Hazard. Mater. 2020, 384, 121258. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.N.; Wu, Z.; Goh, P.S.; Zhou, S. The State-of-the-Art Development of Biochar Based Photocatalyst for Removal of Various Organic Pollutants in Wastewater. J. Clean. Prod. 2023, 429, 139487. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Xiao, R.; Tafti, N.; DeLaune, R.D.; Seo, D.-C. Degradation of Orange G by Fenton-like Reaction with Fe-Impregnated Biochar Catalyst. Bioresour. Technol. 2018, 249, 368–376. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, M.; Deng, Y.; Khan, Z.H.; Liu, X.; Song, Z.; Qiu, W. Efficient Oxidation and Adsorption of As(III) and As(V) in Water Using a Fenton-like Reagent, (Ferrihydrite)-Loaded Biochar. Sci. Total Environ. 2020, 715, 136957. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Pyrolysis of Different Biomass Pre-Impregnated with Steel Pickling Waste Liquor to Prepare Magnetic Biochars and Their Use for the Degradation of Metronidazole. Bioresour. Technol. 2019, 289, 121613. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Zhao, H.; Yan, Q. Adsorption and Fenton-like Removal of Chelated Nickel from Zn-Ni Alloy Electroplating Wastewater Using Activated Biochar Composite Derived from Taihu Blue Algae. Chem. Eng. J. 2020, 379, 122372. [Google Scholar] [CrossRef]
- Yan, J.; Qian, L.; Gao, W.; Chen, Y.; Ouyang, D.; Chen, M. Enhanced Fenton-like Degradation of Trichloroethylene by Hydrogen Peroxide Activated with Nanoscale Zero Valent Iron Loaded on Biochar. Sci. Rep. 2017, 7, 43051. [Google Scholar] [CrossRef] [PubMed]
- Welter, N.; Leichtweis, J.; Silvestri, S.; Sánchez, P.I.Z.; Mejía, A.C.C.; Carissimi, E. Preparation of a New Green Composite Based on Chitin Biochar and ZnFe2O4 for Photo-Fenton Degradation of Rhodamine B. J. Alloys Compd. 2022, 901, 163758. [Google Scholar] [CrossRef]
- Xin, S.; Ma, B.; Liu, G.; Ma, X.; Zhang, C.; Ma, X.; Gao, M.; Xin, Y. Enhanced Heterogeneous Photo-Fenton-like Degradation of Tetracycline over CuFeO2/Biochar Catalyst through Accelerating Electron Transfer under Visible Light. J. Environ. Manag. 2021, 285, 112093. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xie, X.; Wang, Z. Bifunctional MnFe2O4/Chitosan Modified Biochar Composite for Enhanced Methyl Orange Removal Based on Adsorption and Photo-Fenton Process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126104. [Google Scholar] [CrossRef]
- Bashir, A.; Pandith, A.H.; Qureashi, A.; Malik, L.A.; Gani, M.; Perez, J.M. Catalytic Propensity of Biochar Decorated with Core-Shell nZVI@Fe3O4: A Sustainable Photo-Fenton Catalysis of Methylene Blue Dye and Reduction of 4-Nitrophenol. J. Environ. Chem. Eng. 2022, 10, 107401. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Qin, H.; Zhou, J.; Shen, Q.; Wang, K.; Chen, W.; Liu, M.; Li, N. Synergy Effect between Adsorption and Heterogeneous Photo-Fenton-like Catalysis on LaFeO3/Lignin-Biochar Composites for High Efficiency Degradation of Ofloxacin under Visible Light. Sep. Purif. Technol. 2022, 280, 119751. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 November 2024).
- De Vasconcelos, M.C.; Bennett, R.N.; Rosa, E.A.; Ferreira-Cardoso, J.V. Composition of European Chestnut (Castanea sativa Mill.) and Association with Health Effects: Fresh and Processed Products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Eurostat. Food Waste and Food Waste Prevention-Estimates. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 15 January 2024).
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of Biomass Waste to Engineered Activated Biochar by Microwave Pyrolysis: Progress, Challenges, and Future Directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Singh, A.; Himanshu, M.; Verma, B.; Singh, R.; Lal, B.; Syed, A.; Elgorban, A.M.; Wong, L.S.; Srivastava, N. Evaluation of Sustainability of Fabrication Process and Characterization Studies of Activated Carbon Nanocatalyst from Waste Chestnut Peels. J. Mol. Struct. 2025, 1321, 139810. [Google Scholar] [CrossRef]
- Nguyen, T.-K.-T.; Nguyen, T.-B.; Chen, C.-W.; Chen, W.-H.; Bui, X.-T.; Lam, S.S.; Dong, C.-D. Boosting Acetaminophen Degradation in Water by Peracetic Acid Activation: A Novel Approach Using Chestnut Shell-Derived Biochar at Varied Pyrolysis Temperatures. Environ. Res. 2024, 252, 119143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Dai, Z.; Zhang, A.-Y.; Yin, J.; Peng, S.; Liang, H. Recycling Chestnut Shell for Superior Peroxymonosulfate Activation in Contaminants Degradation via the Synergistic Radical/Non-Radical Mechanisms. J. Hazard. Mater. 2022, 430, 128471. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Milbeau, C.L.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of Diclofenac onto Organoclays: Effects of Surfactant and Environmental (pH and Temperature) Conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef]
- Muelas-Ramos, V.; Gascó, A.; Salvatierra, M.; De Los Ríos, C.; Jiménez-Bautista, K.; Merayo, N.; Bahamonde, A.; Hermosilla, D. Assessing the Application of a Biochar-Supported Iron Oxide Catalyst to the Treatment of Imidacloprid by Photo-Fenton Technologies. Catal. Today 2024, 438, 114782. [Google Scholar] [CrossRef]
- Shen, T.; Zhang, F.; Yang, S.; Wang, Y.; Liu, H.; Wang, H.; Hu, J. Comprehensive Study on the Pyrolysis Process of Chestnut Processing Waste (Chestnut Shells): Kinetic Triplet, Thermodynamic, in-Situ Monitoring of Evolved Gasses and Analysis Biochar. Fuel 2023, 331, 125944. [Google Scholar] [CrossRef]
- Burbano, A.A.; Medina, G.A.M.; Sánchez, F.H.; Lassalle, V.L.; Horst, M.F.; Gascó, G.; Méndez, A. Influence of Post-Pyrolysis Treatment on Physicochemical Properties and Acid Medium Stability of Magnetic Carbon Nanocomposites. Biomass Conv. Bioref. 2024, 14, 27871–27884. [Google Scholar] [CrossRef]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Pobiner, H. Determination of Hydroperoxides in Hydrocarbon by Conversion to Hydrogen Peroxide and Measurement by Titanium Complexing. Anal. Chem. 1961, 33, 1423–1426. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, Y.; Sun, W.; Qin, Z.; Liu, H.; Ma, Y.; Wang, X. Cellulose Derived Biochar: Preparation, Characterization and Benzo[a]Pyrene Adsorption Capacity. Grain Oil Sci. Technol. 2021, 4, 182–190. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of Naphthalene and 1-Naphthol by Biochars of Orange Peels with Different Pyrolytic Temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef]
- Katyal, S.; Thambimuthu, K.; Valix, M. Carbonisation of Bagasse in a Fixed Bed Reactor: Influence of Process Variables on Char Yield and Characteristics. Renew. Energy 2003, 28, 713–725. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R. Influence of Pyrolysis Temperature on Physico-Chemical Properties of Corn Stover (Zea mays L.) Biochar and Feasibility for Carbon Capture and Energy Balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef]
- Da Luz Corrêa, A.P.; Bastos, R.R.C.; Rocha Filho, G.N.D.; Zamian, J.R.; Conceição, L.R.V.D. Preparation of Sulfonated Carbon-Based Catalysts from Murumuru Kernel Shell and Their Performance in the Esterification Reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ruiz, B.; Martínez-Blanco, D.; Sánchez-Arenillas, M.; Diez, M.A.; Suárez-Ruiz, I.; Marco, J.F.; Blanco, J.; Fuente, E. Sustainable Thermochemical Single-Step Process To Obtain Magnetic Activated Carbons from Chestnut Industrial Wastes. ACS Sustain. Chem. Eng. 2019, 7, 17293–17305. [Google Scholar] [CrossRef]
- Rahman, M.A.; Oomori, T. Structure, Crystallization and Mineral Composition of Sclerites in the Alcyonarian Coral. J. Cryst. Growth 2008, 310, 3528–3534. [Google Scholar] [CrossRef]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-Photoacoustic Spectroscopy for Phosphorus Speciation Analysis of Biochars. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 29–36. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Korolenko, E.A.; Korolik, E.V.; Zhbankov, R.G. IR Spectrum of Cellulose. J. Appl. Spectrosc. 1989, 51, 847–851. [Google Scholar] [CrossRef]
- Yang, R.; Liu, G.; Li, M.; Zhang, J.; Hao, X. Preparation and N2, CO2 and H2 Adsorption of Super Activated Carbon Derived from Biomass Source Hemp (Cannabis sativa L.) Stem. Microporous Mesoporous Mater. 2012, 158, 108–116. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Dhaveethu Raja, J.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A Complete Review on Biochar: Production, Property, Multifaceted Applications, Interaction Mechanism and Computational Approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- De Lima, R.S.; De Paiva E Silva Zanta, C.L.; Meili, L.; Dos Santos Lins, P.V.; De Souza Dos Santos, G.E.; Tonholo, J. Fenton-Based Processes for the Regeneration of Biochar from Syagrus Coronata Biomass Used as Dye Adsorbent. Desalin. Water Treat. 2019, 162, 391–398. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Thermal Decomposition of Calcium Carbonate (Calcite Polymorph) as Examined by in-Situ High-Temperature X-Ray Powder Diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of Biochar: Biological Properties. In Biochar for Environmental Management; Routledge: London, UK, 2012. [Google Scholar]
- Hong, X.; Fang, C.; Hui, K.S.; Hui, K.N.; Zhuang, H.; Liu, W.; Shan, S. Influence of Interfering Anions on Cu2+ and Zn2+ Ions Removal on Chestnut Outer Shell-Derived Hydrochars in Aqueous Solution. RSC Adv. 2017, 7, 51199–51205. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes 2021, 9, 1154. [Google Scholar] [CrossRef]
- Nkomo, N.; Odindo, A.O.; Musazura, W.; Missengue, R. Optimising Pyrolysis Conditions for High-Quality Biochar Production Using Black Soldier Fly Larvae Faecal-Derived Residue as Feedstock. Heliyon 2021, 7, e07025. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill Handbooks; McGraw-Hill: New York, NY, USA, 2003; ISBN 978-0-07-049439-8. [Google Scholar]
- Cervellino, A.; Frison, R.; Cernuto, G.; Guagliardi, A.; Masciocchi, N. Lattice Parameters and Site Occupancy Factors of Magnetite–Maghemite Core–Shell Nanoparticles. A Critical Study. J. Appl. Crystallogr. 2014, 47, 1755–1761. [Google Scholar] [CrossRef]
- Safavi, F.S.; Ebrahimipour, S.Y.; Fatemi, S.J.; Mohammadi, P.; Shamspur, T. Green Synthesis of Silver Nanoparticles and Their Immobilization on Magnetic Biochar for the Removal of Tetracycline and Enrofloxacin. Biomass Conv. Biorefin. 2025. [Google Scholar] [CrossRef]
- Gadgeel, A.A.; Mhaske, S.T.; Duerr, C.; Liu, K.L. In-Situ Preparation and Characterization of Aconitic Acid Capped Fe3O4 Nanoparticle by Using Citric Acid as a Reducing Agent. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1688–1700. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, S.; Pei, J.; Li, X.; Yu, R.; Zhao, J.; Martyniuk, C.J. Adsorption Performance of SO2 over ZnAl2O4 Nanospheres. J. Ind. Eng. Chem. 2016, 41, 151–157. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, B.P.; Ningthoujam, R.S. Confirmation of Highly Stable 10 Nm Sized Fe3O4 Nanoparticle Formation at Room Temperature and Understanding of Heat-Generation under AC Magnetic Fields for Potential Application in Hyperthermia. AIP Adv. 2020, 10, 105033. [Google Scholar] [CrossRef]
- Masset, P.; Poinso, J.Y.; Poignet, J.C. TG/DTA/MS Study of the Thermal Decomposition of FeSO4·6H2O. J. Therm. Anal. Calorim. 2006, 83, 457–462. [Google Scholar] [CrossRef]
- Chaklader, A.C.D.; Blair, G.R. Differential thermal study of FeO and Fe3O4. J. Therm. Anal. 1970, 2, 165–179. [Google Scholar] [CrossRef]
- Gallego-Ramírez, C.; Chica, E.; Rubio-Clemente, A. Combination of Biochar and Advanced Oxidation Processes for the Sustainable Elimination of Pharmaceuticals in Water. Sustainability 2024, 16, 10761. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Canzano, S.; Prisciandaro, M.; Musmarra, D. Photodegradation of Diclofenac in Wastewaters. Desalination Water Treat. 2017, 61, 293–297. [Google Scholar] [CrossRef]
- Pérez-Estrada, L.A.; Malato, S.; Gernjak, W.; Agüera, A.; Thurman, E.M.; Ferrer, I.; Fernández-Alba, A.R. Photo-Fenton Degradation of Diclofenac: Identification of Main Intermediates and Degradation Pathway. Environ. Sci. Technol. 2005, 39, 8300–8306. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, B.; Gao, B.; Cheng, N.; Feng, Q.; Chen, M.; Wang, S. Degradation of Organic Pollutants from Water by Biochar-Assisted Advanced Oxidation Processes: Mechanisms and Applications. J. Hazard. Mater. 2023, 442, 130075. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The Potential Role of Biochar in the Removal of Organic and Microbial Contaminants from Potable and Reuse Water: A Review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C. Adsorption of Organic Molecules from Aqueous Solutions on Carbon Materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Bano, A.; Aziz, M.K.; Mishra, R.; Dave, H.; Prasad, B.; Kumari, M.; Dubey, D.; Meili, L.; Shah, M.P.; Prasad, K.S. Response Surface Methodology–Based Optimisation of Adsorption of Diclofenac and Treatment of Pharmaceutical Effluent Using Combined Coagulation-Adsorption onto nFe2O3 Decorated Water Chestnut Shells Biochar. Environ. Sci. Pollut. Res. 2024, 31, 55317–55335. [Google Scholar] [CrossRef]
- Zahid, M.; Khan, Z.U.H.; Sun, J.; Muhammad, N.; Sabahat, S.; Shah, N.S.; Iqbal, J. Biochar-Derived Photocatalysts for Pharmaceutical Waste Removal, a Sustainable Approach to Water Purification. Appl. Surf. Sci. Adv. 2025, 26, 100721. [Google Scholar] [CrossRef]
- Chauhan, S.; Shafi, T.; Dubey, B.K.; Chowdhury, S. Biochar-Mediated Removal of Pharmaceutical Compounds from Aqueous Matrices via Adsorption. Waste Dispos. Sustain. Energy 2023, 5, 37–62. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Kaur Brar, S.; Verma, M.; Surampalli, R.Y. An Insight into the Adsorption of Diclofenac on Different Biochars: Mechanisms, Surface Chemistry, and Thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, Y.; Xu, M.; Cheng, F.; Zhang, H.; Li, Z. Photo-Fenton Degradation of Carbamazepine and Ibuprofen by Iron-Based Metal-Organic Framework under Alkaline Condition. J. Hazard. Mater. 2022, 424, 127698. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, R.; Kaur, H. A Systematic Review of Lignocellulosic Biomass for Remediation of Environmental Pollutants. Appl. Surf. Sci. Adv. 2024, 19, 100547. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).