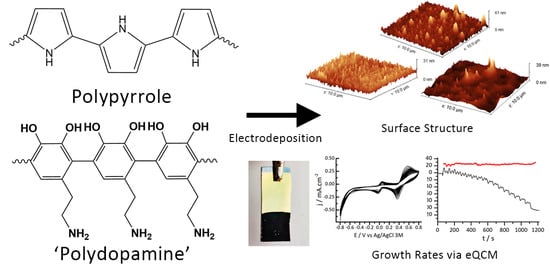
Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrochemical and Electrochemical Quartz Crystal Microbalance Studies
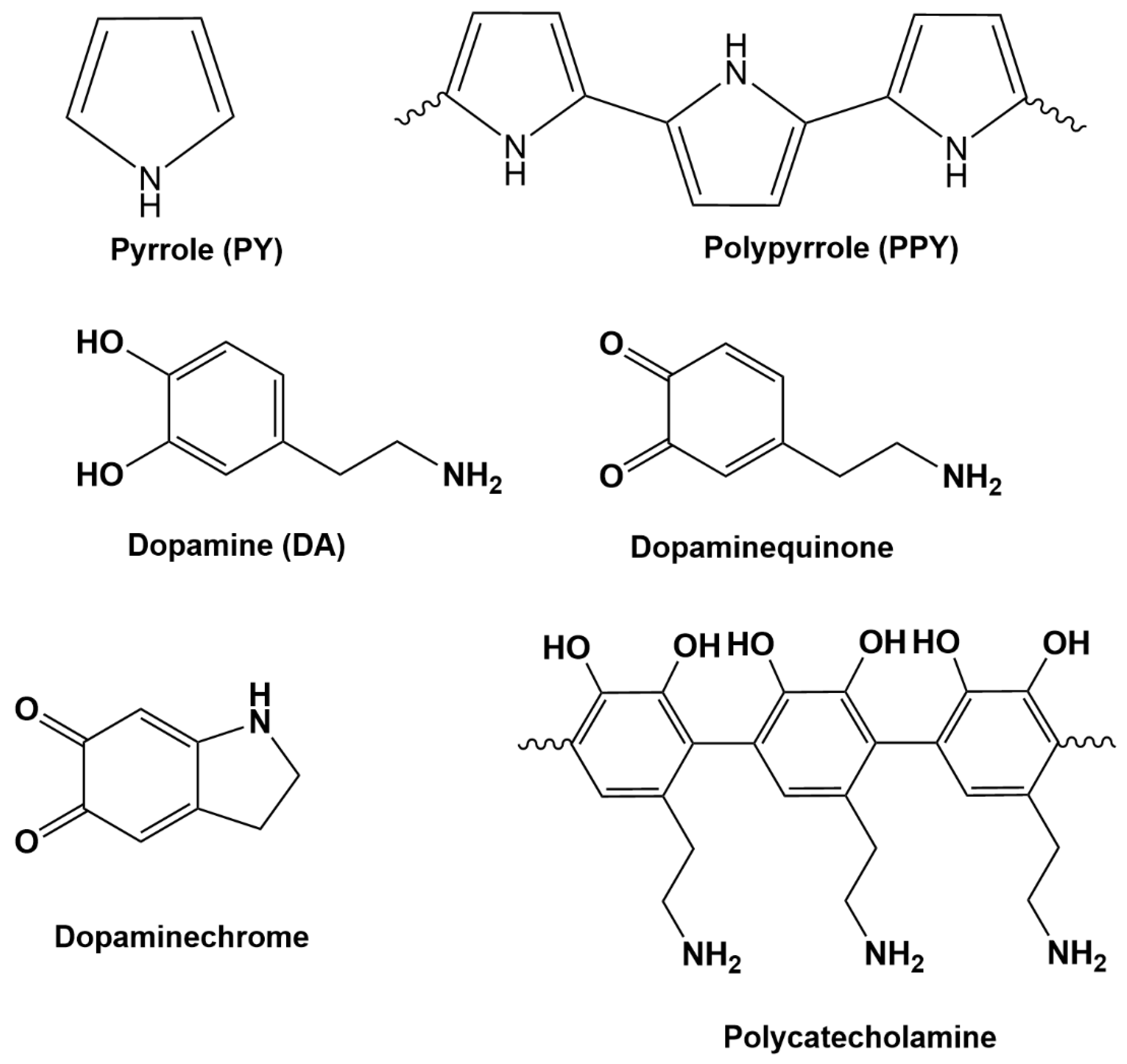
2.3. Preparation of PPY, PDA and PPY-PDA Films
2.4. Characterisation of PPY, PDA and PPY-PDA Films
2.5. Estimation of Film Thicknesses from eQCM Frequency Data
3. Results
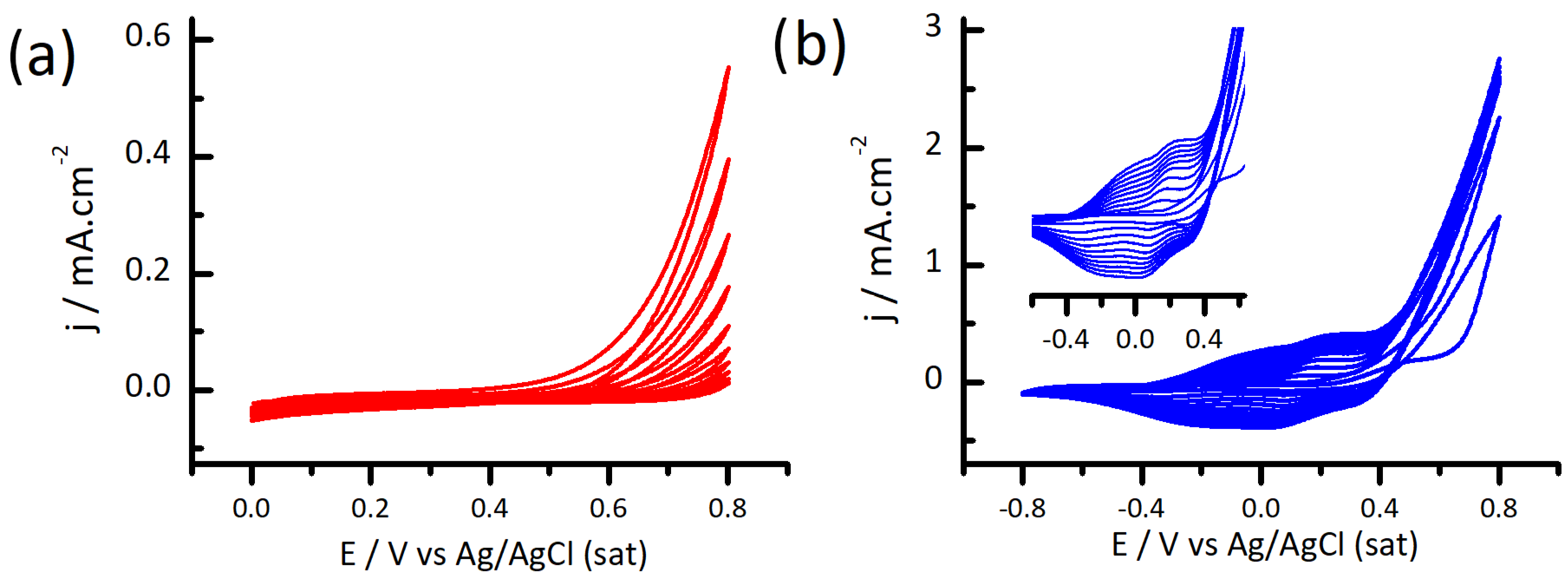
3.1. Electrodeposition of Polydopamine-Polypyrrole Films by Cyclic Voltammetry
3.2. Electrochemical Quartz Crystal Microbalance (eQCM) Studies of PPY-PDA Deposition
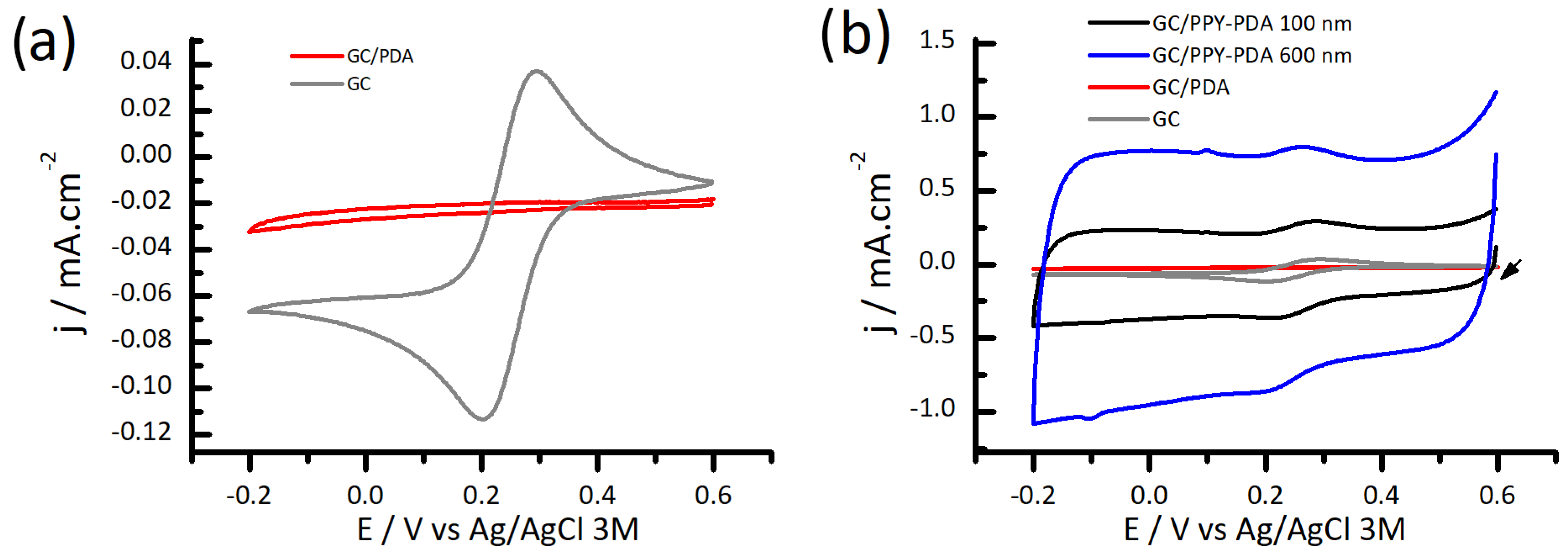
3.3. Electrochemical Performance of PPY-PDA Coatings
3.4. Morphology and Roughness of PPY and PPY-PDA Films by AFM and Water Contact Angle Measurements
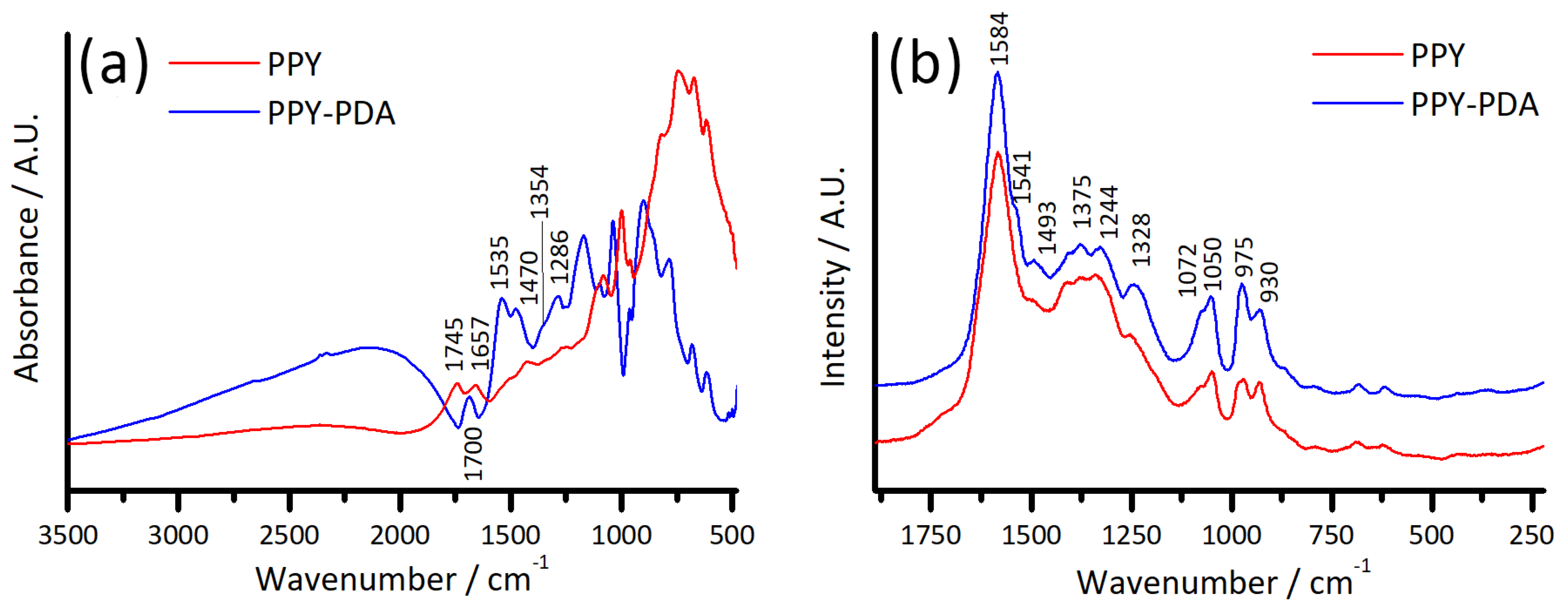
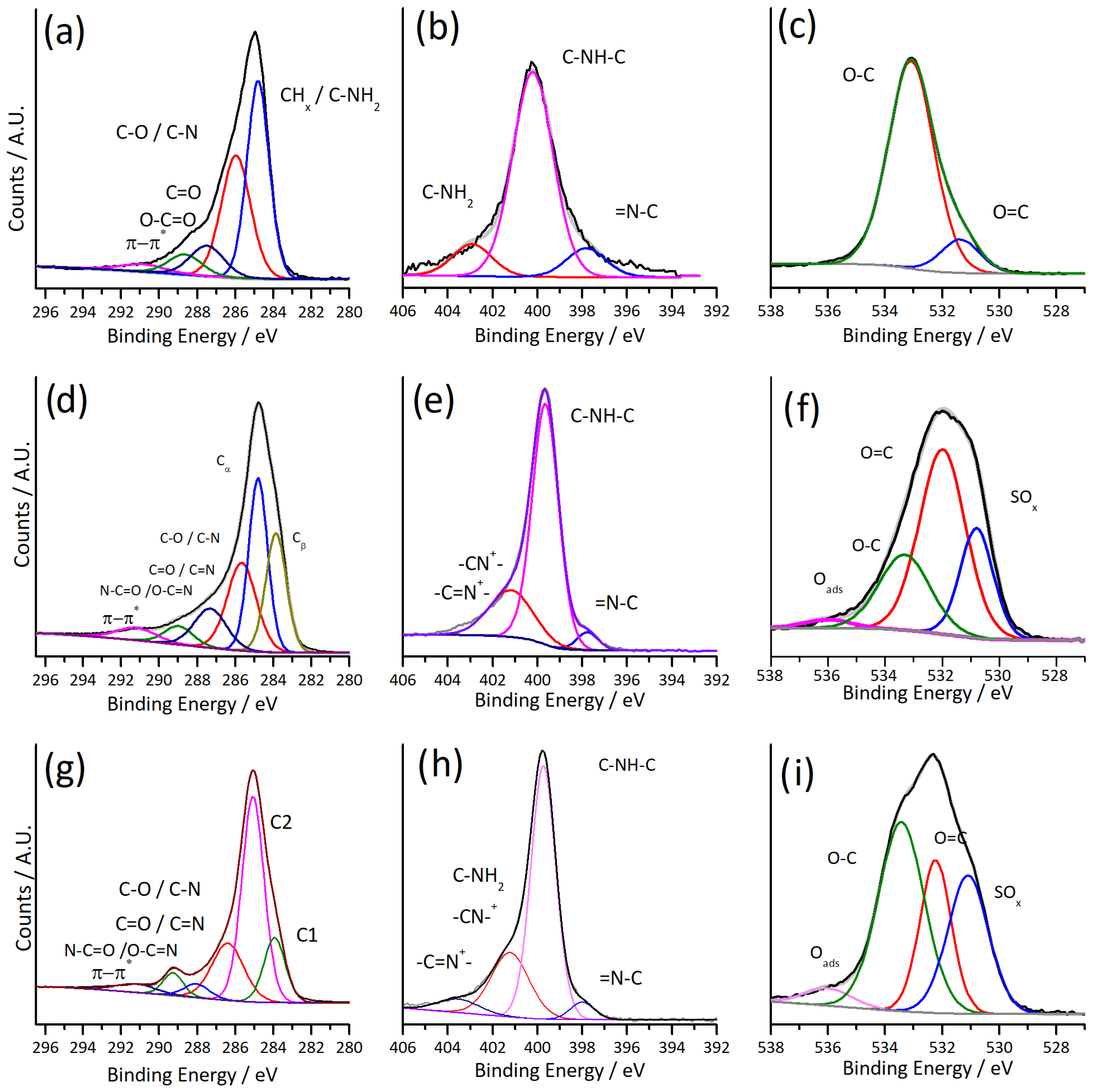
3.5. Spectroscopic Characterisation of PPY and PPY-PDA Films
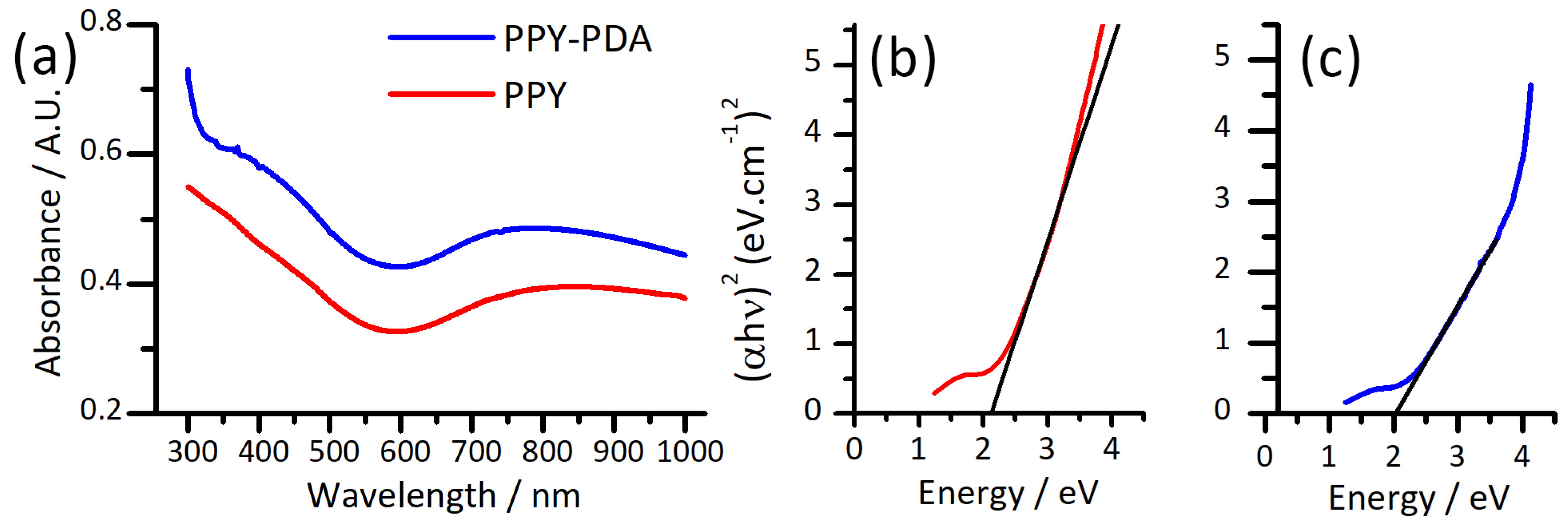
3.6. Optoelectronic Properties of PPY and PPY-PDA Films
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asavapiriyanont, S.; Chandler, G.K.; Gunawardena, G.A.; Pletcher, D. The Electrodeposition of Polypyrrole Films from Aqueous Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1984, 177, 229–244. [Google Scholar] [CrossRef]
- Sabouraud, G.; Sadki, S.; Brodie, N. The Mechanisms of Pyrrole Electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Application of Polypyrrole for the Creation of Immunosensors. Crit. Rev. Anal. Chem. 2002, 32, 245–252. [Google Scholar] [CrossRef]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-Based Conducting Polymers and Interactions with Biological Tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a Flexible Electrode Material of Supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Ansari, S.; Purty, B. Robust Electrochemical Performance of Polypyrrole (PPy) and Polyindole (PIn) Based Hybrid Electrode Materials for Supercapacitor Application: A Review. J. Energy Storage 2020, 29, 101302. [Google Scholar] [CrossRef]
- Fabregat, G.; Córdova-Mateo, E.; Armelin, E.; Bertran, O.; Alemán, C. Ultrathin Films of Polypyrrole Derivatives for Dopamine Detection. J. Phys. Chem. C 2011, 115, 14933–14941. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole Based next Generation Electrochemical Sensors and Biosensors: A Review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Andriukonis, E.; Reinikovaite, V.; Ramanavicius, A. Comparative Study of Polydopamine and Polypyrrole Modified Yeast Cells Applied in Biofuel Cell Design. Sustain. Energy Fuels 2022, 6, 4209–4217. [Google Scholar] [CrossRef]
- Yuan, X.; Ding, X.-L.; Wang, C.-Y.; Ma, Z.-F. Use of Polypyrrole in Catalysts for Low Temperature Fuel Cells. Energy Environ. Sci. 2013, 6, 1105–1124. [Google Scholar] [CrossRef]
- Kim, M.; Li, S.; Kong, D.S.; Song, Y.E.; Park, S.-Y.; Kim, H.; Jae, J.; Chung, I.; Kim, J.R. Polydopamine/Polypyrrole-Modified Graphite Felt Enhances Biocompatibility for Electroactive Bacteria and Power Density of Microbial Fuel Cell. Chemosphere 2023, 313, 137388. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical Deposition of Conductive and Adhesive Polypyrrole-Dopamine Films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef]
- Hemmatpour, H.; De Luca, O.; Crestani, D.; Stuart, M.C.A.; Lasorsa, A.; van der Wel, P.C.A.; Loos, K.; Giousis, T.; Haddadi-Asl, V.; Rudolf, P. New Insights in Polydopamine Formation via Surface Adsorption. Nat. Commun. 2023, 14, 664. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Park, J.; Lee, J.Y. Electrochemical Co-Deposition of Polydopamine/Hyaluronic Acid for Anti-Biofouling Bioelectrodes. Front. Chem. 2019, 7, 262. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.; Park, C.B. Polydopamine as a Biomimetic Electron Gate for Artificial Photosynthesis. Angew. Chem. Int. Ed. 2014, 53, 6364–6368. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-Step Modification of Superhydrophobic Surfaces by a Mussel-Inspired Polymer Coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Lupton, E.J.; Ma, Y.; Sun, R.; Grigsby, C.L.; Brachi, G.; Li, X.; Zhou, K.; Stuckey, D.J.; Stevens, M.M. Hybrid Polypyrrole and Polydopamine Nanosheets for Precise Raman/Photoacoustic Imaging and Photothermal Therapy. Adv. Healthc. Mater. 2023, 12, e2301148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, F.K.; Pan, Z.; Zhang, J.; Zhao, B. Bio-Inspired Dopamine Functionalization of Polypyrrole for Improved Adhesion and Conductivity. Macromol. Rapid Commun. 2014, 35, 350–354. [Google Scholar] [CrossRef]
- Behan, J.A.; Grajkowski, F.; Jayasundara, D.R.; Vilella-Arribas, L.; García-Melchor, M.; Colavita, P.E. Influence of Carbon Nanostructure and Oxygen Moieties on Dopamine Adsorption and Charge Transfer Kinetics at Glassy Carbon Surfaces. Electrochim. Acta 2019, 304, 221–230. [Google Scholar] [CrossRef]
- Ait-Itto, F.-Z.; Behan, J.A.; Martinez, M.; Barrière, F. Development of Bioanodes Rich in Exoelectrogenic Bacteria Using Iron-Rich Palaeomarine Sediment Inoculum. Bioelectrochemistry 2024, 156, 108618. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Q.; Pan, W.; Yao, Y. Advances in the Mass Sensitivity Distribution of Quartz Crystal Microbalances: A Review. Sensors 2022, 22, 5112. [Google Scholar] [CrossRef]
- Diaz, A.F.; Castillo, J.I.; Logan, J.A.; Lee, W.-Y. Electrochemistry of Conducting Polypyrrole Films. J. Electroanal. Chem. Interfacial Electrochem. 1981, 129, 115–132. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Ball, V.; Del Frari, D.; Michel, M.; Buehler, M.J.; Toniazzo, V.; Singh, M.K.; Gracio, J.; Ruch, D. Deposition Mechanism and Properties of Thin Polydopamine Films for High Added Value Applications in Surface Science at the Nanoscale. BioNanoScience 2012, 2, 16–34. [Google Scholar] [CrossRef]
- Tabačiarová, J.; Mičušík, M.; Fedorko, P.; Omastová, M. Study of Polypyrrole Aging by XPS, FTIR and Conductivity Measurements. Polym. Degrad. Stab. 2015, 120, 392–401. [Google Scholar] [CrossRef]
- Khadem, F.; Pishvaei, M.; Salami-Kalajahi, M.; Najafi, F. Morphology Control of Conducting Polypyrrole Nanostructures via Operational Conditions in the Emulsion Polymerization. J. Appl. Polym. Sci. 2017, 134, 44697. [Google Scholar] [CrossRef]
- Mallinson, D.; Mullen, A.B.; Lamprou, D.A. Probing Polydopamine Adhesion to Protein and Polymer Films: Microscopic and Spectroscopic Evaluation. J. Mater. Sci. 2018, 53, 3198–3209. [Google Scholar] [CrossRef]
- Nguyen Thi Le, H.; Bernard, M.C.; Garcia-Renaud, B.; Deslouis, C. Raman Spectroscopy Analysis of Polypyrrole Films as Protective Coatings on Iron. Synth. Met. 2004, 140, 287–293. [Google Scholar] [CrossRef]
- Morávková, Z.; Taboubi, O.; Minisy, I.M.; Bober, P. The Evolution of the Molecular Structure of Polypyrrole during Chemical Polymerization. Synth. Met. 2021, 271, 116608. [Google Scholar] [CrossRef]
- Rella, S.; Mazzotta, E.; Caroli, A.; De Luca, M.; Bucci, C.; Malitesta, C. Investigation of Polydopamine Coatings by X-Ray Photoelectron Spectroscopy as an Effective Tool for Improving Biomolecule Conjugation. Appl. Surf. Sci. 2018, 447, 31–39. [Google Scholar] [CrossRef]
- Idla, K.; Talo, A.; Niemi, H.E.-M.; Forsén, O.; Yläsaari, S. An XPS and AFM Study of Polypyrrole Coating on Mild Steel. Surf. Interface Anal. 1997, 25, 837–854. [Google Scholar] [CrossRef]
- Behan, J.A.; Stamatin, S.N.; Hoque, M.K.; Ciapetti, G.; Zen, F.; Esteban-Tejeda, L.; Colavita, P.E. Combined Optoelectronic and Electrochemical Study of Nitrogenated Carbon Electrodes. J. Phys. Chem. C 2017, 121, 6596–6604. [Google Scholar] [CrossRef]
- Zen, F.; Karanikolas, V.D.; Behan, J.A.; Andersson, J.; Ciapetti, G.; Bradley, A.L.; Colavita, P.E. Nanoplasmonic Sensing at the Carbon-Bio Interface: Study of Protein Adsorption at Graphitic and Hydrogenated Carbon Surfaces. Langmuir 2017, 33, 4198–4206. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Paiva, R.S.; Ramírez, A.M.R.; Mwanda, J.A.; Pereira, E.C.; Cuesta, A. Mapping the Electronic Structure of Polypyrrole with Image-Based Electrochemical Scanning Tunneling Spectroscopy. Electrochem. Sci. Adv. 2022, 2, e2100028. [Google Scholar] [CrossRef]
- Abdi, M.M.; Ekramul Mahmud, H.N.M.; Abdullah, L.C.; Kassim, A.; Zaki Ab. Rahman, M.; Chyi, J.L.Y. Optical Band Gap and Conductivity Measurements of Polypyrrole-Chitosan Composite Thin Films. Chin. J. Polym. Sci. 2012, 30, 93–100. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. Structural Basis of Polydopamine Film Formation: Probing 5,6-Dihydroxyindole-Based Eumelanin Type Units and the Porphyrin Issue. ACS Appl. Mater. Interfaces 2018, 10, 7670–7680. [Google Scholar] [CrossRef]

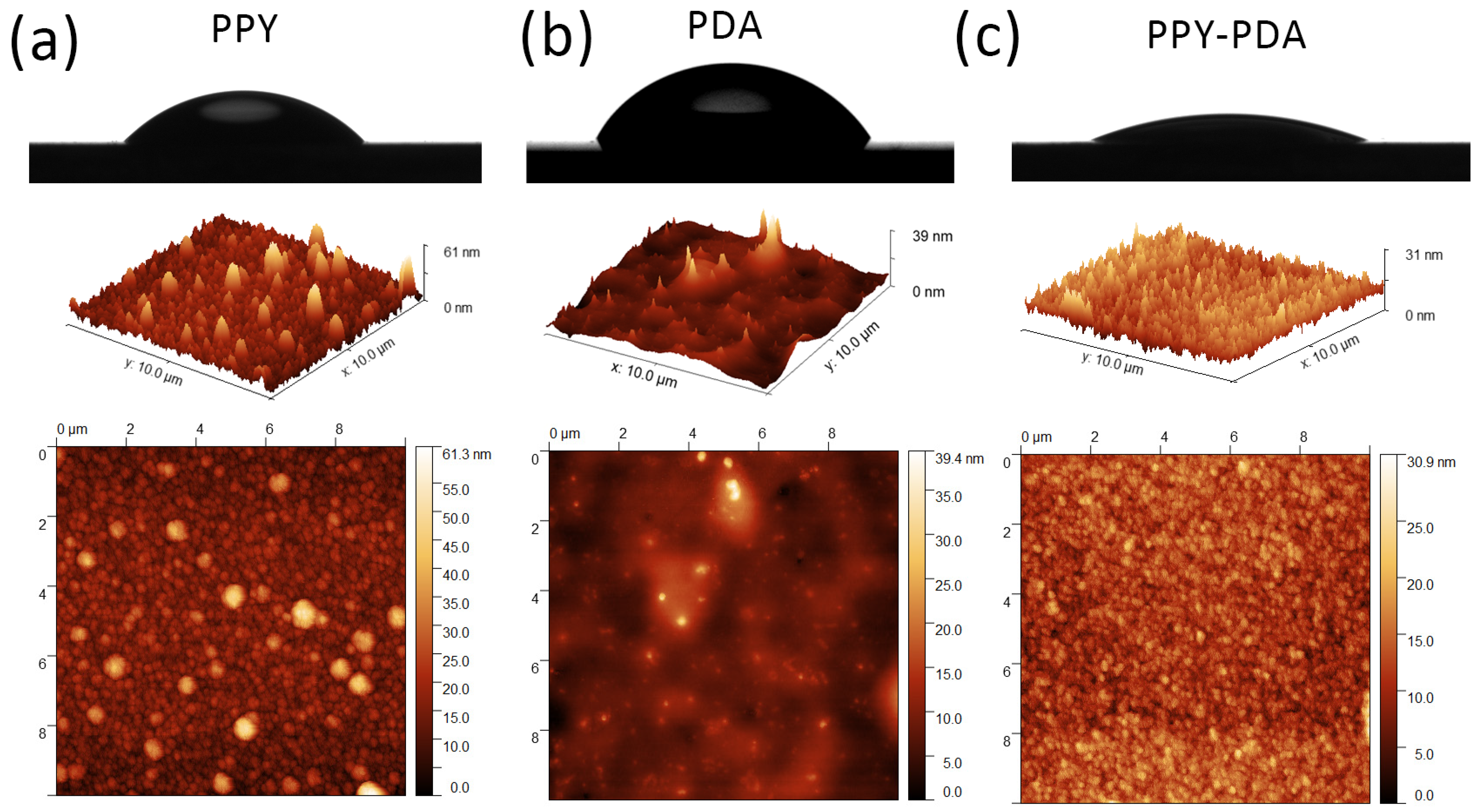
| Sample | Au | PPY | PDA | PPY-PDA |
|---|---|---|---|---|
| Roughness/nm | 1.7 ± 0.2 | 4.9 ± 0.9 | 2.4 ± 0.7 | 3.2 ± 0.1 |
| Water Contact Angle/° | >90 | 45 ± 1 | 54 ± 5 | 21.3 ± 0.9 |
| C 1s | N 1s | O 1s | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Contribution | Position | At.% | Contribution | Position | At.% | Contribution | Position | At.% |
| PDA | CHx | 284.8 | 43.81 | R3N/Ar-N | 397.8 | 10.86 | C=O | 531.36 | 12.19 |
| C-O/C-N | 285.9 | 35.65 | R2N-H | 400.2 | 77.06 | C-O | 533.09 | 87.81 | |
| C=O/C=N | 287.5 | 10.26 | RNH2 | 402.9 | 12.08 | ||||
| O-C=O | 288.6 | 6.9 | |||||||
| π-π* | 290.8 | 3.37 | |||||||
| PPY | Cβ | 283.9 | 24.05 | =N-C | 398.1 | 6.75 | SO42- | 530.79 | 22.11 |
| Cα | 284.9 | 29.65 | C-NH-C | 399.7 | 50.12 | C=O | 531.99 | 52 | |
| C-O and C-N | 285.8 | 24.67 | C-NH-C | 400.3 | 18.65 | C-O | 533.32 | 23.45 | |
| C=O and C=N | 287.4 | 11.56 | Polaron/Bipolaron | 401.2 | 21.66 | Oads | 536 | 2.44 | |
| O-C=O | 288.9 | 5.12 | 403.4 | 2.82 | |||||
| π-π* | 291.1 | 4.95 | |||||||
| PPY-PDA | CHx/Cβ | 283.9 | 15.61 | =N-C | 398 | 4.33 | SO42- | 530.84 | 29.16 |
| Cα | 285.1 | 51.19 | C-NH-C | 399.7 | 64.77 | C=O | 531.96 | 22.3 | |
| C-O/C-N | 286.4 | 20.4 | Polaron/Bipolaron | 401.2 | 25.13 | C-O | 533.14 | 44.26 | |
| C=O/C=N | 288.0 | 3.89 | 403.5 | 5.77 | Oads | 535.69 | 4.27 | ||
| O-C=O | 289.3 | 5.06 | |||||||
| π-π* | 291.2 | 3.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behan, J.A.; Barrière, F. Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films. C 2024, 10, 20. https://doi.org/10.3390/c10010020
Behan JA, Barrière F. Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films. C. 2024; 10(1):20. https://doi.org/10.3390/c10010020
Chicago/Turabian StyleBehan, James A., and Frédéric Barrière. 2024. "Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films" C 10, no. 1: 20. https://doi.org/10.3390/c10010020
APA StyleBehan, J. A., & Barrière, F. (2024). Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films. C, 10(1), 20. https://doi.org/10.3390/c10010020