Secreted Non-Coding RNAs: Functional Impact on the Tumor Microenvironment and Clinical Relevance in Triple-Negative Breast Cancer
Abstract
:1. Introduction
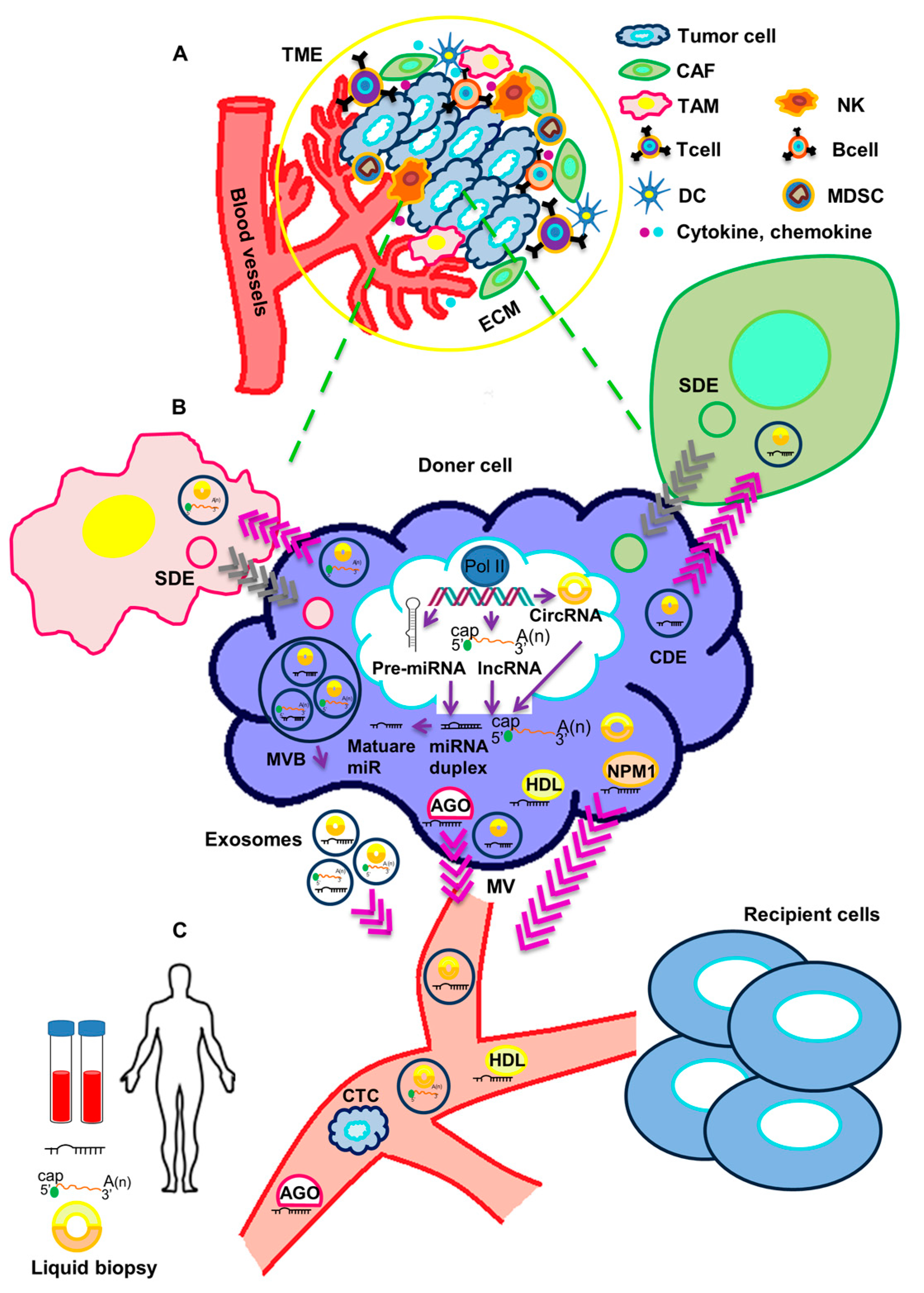
2. Functional Impact of Secreted ncRNAs on Surrounding Stromal Cells and at Metastatic Sites in TNBC
3. Circulating Non-Coding RNAs as Biomarkers in TNBC
3.1. Circulating microRNAs in TNBC
3.2. Circulating lncRNAs in TNBC
3.3. Circulating Circular RNAs in TNBC
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Lee, A.; Djamgoz, M.B.A. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat. Rev. 2018, 62, 110–122. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, M.; Damjanovic, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.J.; Tay, Y. Noncoding RNA:RNA Regulatory Networks in Cancer. Int. J. Mol. Sci. 2018, 19, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhang, J.; Diao, L.X.; Han, L. Small non-coding RNAs in human cancer: Function, clinical utility, and characterization. Oncogene 2021, 40, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Lam, S.; Nagahara, M.; Hoon, D.S.B. Circulating microRNA Biomarkers as Liquid Biopsy for Cancer Patients: Pros and Cons of Current Assays. J. Clin. Med. 2015, 4, 1890–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical utility of circulating non-coding RNAs—An update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers 2019, 11, 1170. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.L.; Tang, X.Q.; Mendu, V.; Tang, X.H.; Jia, X.Y.; Chen, Q.J.; He, L.H. The art of microRNA: Various strategies leading to gene silencing via an ancient pathway. Bba-Gene Regul. Mech. 2008, 1779, 655–662. [Google Scholar] [CrossRef]
- Takahashi, R.; Prieto-Vila, M.; Kohama, I.; Ochiya, T. Development of miRNA-based therapeutic approaches for cancer patients. Cancer Sci. 2019, 110, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Tessitore, A.; Cicciarelli, G.; Mastroiaco, V.; Del Vecchio, F.; Capece, D.; Verzella, D.; Fischietti, M.; Vecchiotti, D.; Zazzeroni, F.; Alesse, E. Therapeutic Use of MicroRNAs in Cancer. Anti-Cancer Agent Me 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Fazi, F.; Donzelli, S.; Kedmi, M.; Sas-Chen, A.; Muti, P.; Strano, S.; Yarden, Y. Tumor suppressor microRNAs: A novel non-coding alliance against cancer. Febs. Lett. 2014, 588, 2639–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Geng, X.C.; Jia, Y.C.; Zhang, Y.H.; Shi, L.; Li, Q.; Zang, A.M.; Wang, H. Circular RNA: Biogenesis, degradation, functions and potential roles in mediating resistance to anticarcinsuogens. Epigenomics 2020, 12, 267–283. [Google Scholar] [CrossRef]
- Zhao, W.S.; Dong, M.; Pan, J.R.; Wang, Y.J.; Zhou, J.Y.; Ma, J.J.; Liu, S.Y. Circular RNAs: A novel target among non-coding RNAs with potential roles in malignant tumors. Mol. Med. Rep. 2019, 20, 3463–3474. [Google Scholar] [CrossRef]
- Fontemaggi, G.; Turco, C.; Esposito, G.; Di Agostino, S. New Molecular Mechanisms and Clinical Impact of circRNAs in Human Cancer. Cancers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Bullock, M.D.; Silva, A.M.; Kanlikilicer-Unaldi, P.; Filant, J.; Rashed, M.H.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. Exosomal Non-Coding RNAs: Diagnostic, Prognostic and Therapeutic Applications in Cancer. Noncoding RNA 2015, 1, 53–68. [Google Scholar] [CrossRef]
- Cui, M.Y.; Wang, H.D.; Yao, X.X.; Zhang, D.; Xie, Y.J.; Cui, R.J.; Zhang, X.W. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.Q.; Xie, Y.H.; Yang, F.; Hua, Y.J.; Zeng, T.Y.; Sun, C.X.; Yang, M.Z.; Huang, X.; Wu, H.; Fu, Z.Y.; et al. Comprehensive description of the current breast cancer microenvironment advancements via single-cell analysis. J. Exp. Clin. Canc. Res. 2021, 40, 142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.F.; Liu, Y.Q.; Xu, W.L.; Zhu, X.L. Exosomal Non-coding RNAs-Mediated Crosstalk in the Tumor Microenvironment. Front. Cell Dev. Biol 2021, 9, 646864. [Google Scholar] [CrossRef] [PubMed]
- Baghba, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.E. Extracellular Vesicles and Metastasis. Csh. Perspect. Med. 2020, 10, a037275. [Google Scholar] [CrossRef]
- Pigati, L.; Yaddanapudi, S.C.S.; Iyengar, R.; Kim, D.J.; Hearn, S.A.; Danforth, D.; Hastings, M.L.; Duelli, D.M. Selective Release of MicroRNA Species from Normal and Malignant Mammary Epithelial Cells. PLoS ONE 2010, 5, e13515. [Google Scholar] [CrossRef] [Green Version]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef] [Green Version]
- Madhavan, D.; Zucknick, M.; Wallwiener, M.; Cuk, K.; Modugno, C.; Scharpff, M.; Schott, S.; Heil, J.; Turchinovich, A.; Yang, R.X.; et al. Circulating miRNAs as Surrogate Markers for Circulating Tumor Cells and Prognostic Markers in Metastatic Breast Cancer. Clin. Cancer Res. 2012, 18, 5972–5982. [Google Scholar] [CrossRef] [Green Version]
- Teplyuk, N.M.; Mollenhauer, B.; Gabriely, G.; Giese, A.; Kim, E.; Smolsky, M.; Kim, R.Y.; Saria, M.G.; Pastorino, S.; Kesari, S.; et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro-Oncology 2012, 14, 689–700. [Google Scholar] [CrossRef] [Green Version]
- Le, M.T.N.; Hamar, P.; Guo, C.Y.; Basar, E.; Perdigao-Henriques, R.; Balaj, L.; Lieberman, J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Investig. 2014, 124, 5109–5128. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Pochampally, R.; Watabe, K.; Lu, Z.H.; Mo, Y.Y. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol. Cancer 2014, 13, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Young, J.; Prabhala, H.; Pan, E.; Mestdagh, P.; Muth, D.; Teruya-Feldstein, J.; Reinhardt, F.; Onder, T.T.; Valastyan, S.; et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010, 12, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroni, S.; Romero-Cordoba, S.; Plantamura, I.; Dugo, M.; D’Ippolito, E.; Cataldo, A.; Cosentino, G.; Angeloni, V.; Rossini, A.; Daidone, M.G.; et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016, 7, e2312. [Google Scholar] [CrossRef]
- Cosentino, G.; Romero-Cordoba, S.; Plantamura, I.; Cataldo, A.; Iorio, M.V. miR-9-Mediated Inhibition ofEFEMP1Contributes to the Acquisition of Pro-Tumoral Properties in Normal Fibroblasts. Cells 2020, 9, 2143. [Google Scholar] [CrossRef]
- Yan, W.; Wu, X.W.; Zhou, W.Y.; Fong, M.Y.; Cao, M.H.; Liu, J.; Liu, X.J.; Chen, C.H.; Fadare, O.; Pizzo, D.P.; et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat. Cell Biol. 2018, 20, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.Y.; Fong, M.Y.; Min, Y.F.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted miR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017, 384, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lotvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.X.; Cao, M.H.; Palomares, M.; Wu, X.W.; Li, A.; Yan, W.; Fong, M.Y.; Chan, W.C.; Wang, S.E. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res. 2018, 20, 127. [Google Scholar] [CrossRef]
- Fong, M.Y.; Zhou, W.Y.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.S.; Chin, A.R.; et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef] [Green Version]
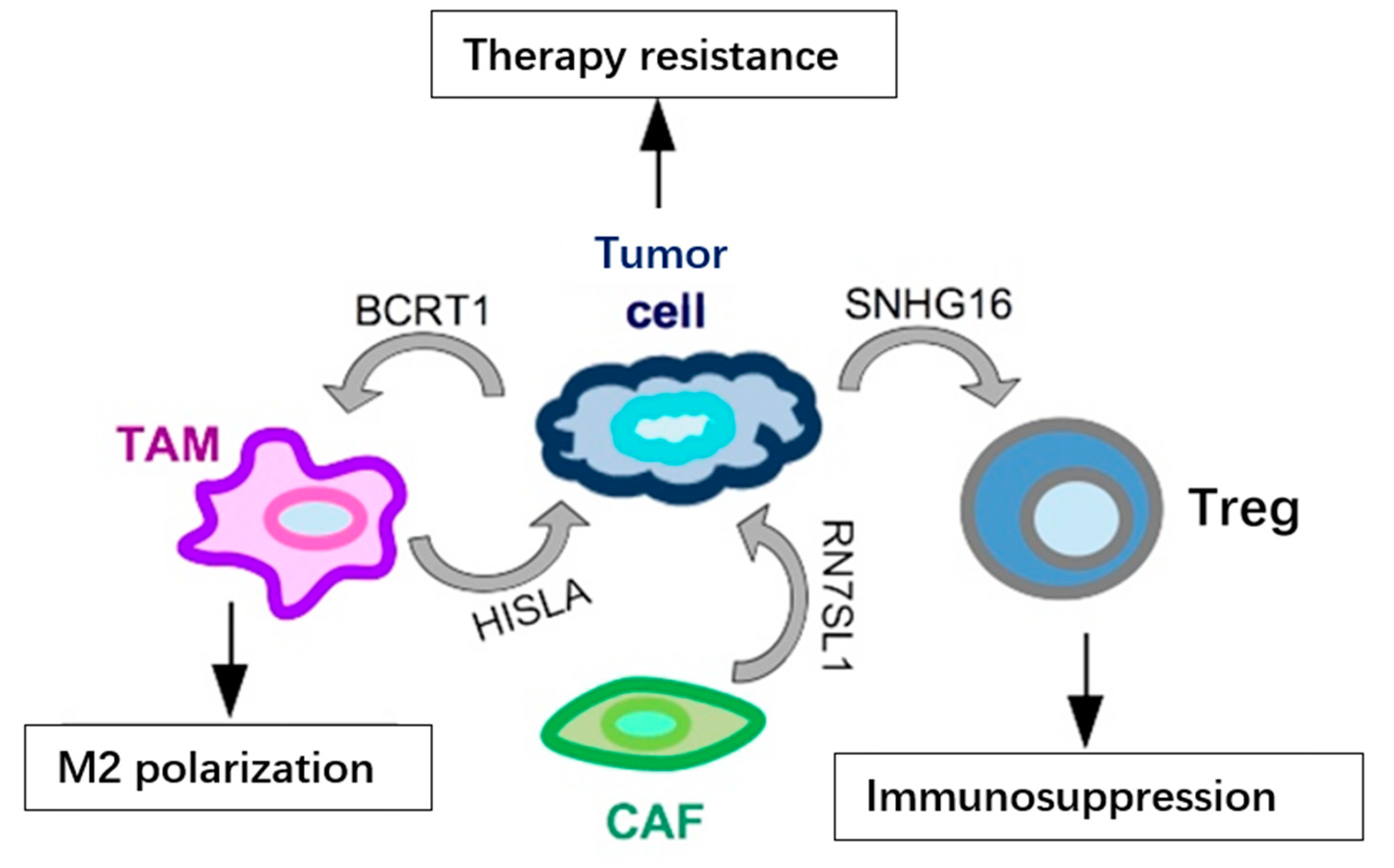
- Ni, C.; Fang, Q.Q.; Chen, W.Z.; Jiang, J.X.; Jiang, Z.; Ye, J.; Zhang, T.; Yang, L.; Meng, F.B.; Xia, W.J.; et al. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+gamma delta 1 Treg cells. Signal. Transduct Tar. 2020, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.R.; Song, X.J.; Li, Y.M.; Chen, B.; Zhao, W.J.; Wang, L.J.; Zhang, H.W.; Liu, Y.; Han, D.W.; Zhang, N.; et al. LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol. Cancer 2020, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Nabet, B.Y.; Qiu, Y.; Shabason, J.E.; Wu, T.J.; Yoon, T.; Kim, B.C.; Benci, J.L.; DeMichele, A.M.; Tchou, J.; Marcotrigiano, J.; et al. Exosome RNA Unshielding Couples Stromal Activation to Pattern Recognition Receptor Signaling in Cancer. Cell 2017, 170, 352–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Chen, J.N.; Yang, L.B.; Liu, J.; Zhang, X.Q.; Zhang, Y.; Tu, Q.Q.; Yin, D.; Lin, D.C.; Wong, P.P.; et al. Extracellular vesicle-packaged HIF-1 alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019, 21, 498–510. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, H.X.; Lu, K.F.; Lu, Y.N.; Wang, Y.; Feng, T.B. Exosome-mediated delivery of MALAT1 induces cell proliferation in breast cancer. Oncotargets Ther. 2018, 11, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Amodio, N.; Raimondi, L.; Juli, G.; Stamato, M.A.; Caracciolo, D.; Tagliaferri, P.; Tassone, P. MALAT1: A druggable long non-coding RNA for targeted anti-cancer approaches. J. Hematol. Oncol. 2018, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Shaath, H.; Vishnubalaji, R.; Elango, R.; Khattak, S.; Alajez, N.M. Single-cell long noncoding RNA (lncRNA) transcriptome implicates MALAT1 in triple-negative breast cancer (TNBC) resistance to neoadjuvant chemotherapy. Cell Death Discov. 2021, 7, 23. [Google Scholar] [CrossRef]
- Pruszko, M.; Milano, E.; Forcato, M.; Donzelli, S.; Ganci, F.; Di Agostino, S.; De Panfilis, S.; Fazi, F.; Bates, D.O.; Bicciato, S.; et al. The mutant p53-ID4 complex controls VEGFA isoforms by recruiting lncRNA MALAT1. Embo. Rep. 2017, 18, 1331–1351. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramowicz, A.; Story, M.D. The Long and Short of It: The Emerging Roles of Non-Coding RNA in Small Extracellular Vesicles. Cancers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Babin, P.J.; Gibbons, G.F. The evolution of plasma cholesterol: Direct utility or a “spandrel” of hepatic lipid metabolism? Prog Lipid Res. 2009, 48, 73–91. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, G.Z.; Liang, X.L.; Wang, D.G.; Gao, H.Q.; Wang, L.; Wang, L.L.; Liu, J.J.; Du, Z.H. High expression of miR-21 in triple-negative breast cancers was correlated with a poor prognosis and promoted tumor cell in vitro proliferation. Med. Oncol. 2014, 31, 57. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, M.; Stribinskis, V.; Klinge, C.M.; Ramos, K.S.; Colburn, N.H.; Li, Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 2008, 27, 4373–4379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickramasinghe, N.S.; Manavalan, T.T.; Dougherty, S.M.; Riggs, K.A.; Li, Y.; Klinge, C.M. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009, 37, 2584–2595. [Google Scholar] [CrossRef]
- Feng, Y.H.; Tsao, C.J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.X.; Huang, X.F.; Shao, Q.; Huang, M.Y.; Deng, L.; Wu, Q.L.; Zeng, Y.X.; Shao, J.Y. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. Rna 2008, 14, 2348–2360. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.; Mirza, M.; Nandi, K.; Jain, S.K.; Kaza, R.C.M.; Khurana, N.; Ray, P.C.; Saxena, A. Serum microRNA-21 expression as a prognostic and therapeutic biomarker for breast cancer patients. Tumor Biol. 2016, 37, 15275–15282. [Google Scholar] [CrossRef]
- Li, S.C.; Yang, X.R.; Yang, J.M.; Zhen, J.S.; Zhang, D.C. Serum microRNA-21 as a potential diagnostic biomarker for breast cancer: A systematic review and meta-analysis. Clin. Exp. Med. 2016, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zelli, V.; Compagnoni, C.; Capelli, R.; Cannita, K.; Sidoni, T.; Ficorella, C.; Capalbo, C.; Zazzeroni, F.; Tessitore, A.; Alesse, E. Circulating MicroRNAs as Prognostic and Therapeutic Biomarkers in Breast Cancer Molecular Subtypes. J. Pers. Med. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Sahlberg, K.K.; Bottai, G.; Naume, B.; Burwinkel, B.; Calin, G.A.; Borresen-Dale, A.L.; Santarpia, L. A Serum MicroRNA Signature Predicts Tumor Relapse and Survival in Triple-Negative Breast Cancer Patients. Clin. Cancer Res. 2015, 21, 1207–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.G.; Romagnoli, M.; Mineva, N.D.; Barille-Nion, S.; Jezequel, P.; Campone, M.; Sonenshein, G.E. miR-720 is a downstream target of an ADAM8-induced ERK signaling cascade that promotes the migratory and invasive phenotype of triple-negative breast cancer cells. Breast Cancer Res. 2016, 18, 40. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gong, X.X.; Zhang, W.; Zhang, J.; Ding, L.; Li, H.; Tu, D.Y.; Tang, J.H. Inhibition of miRNA-34a promotes triple negative cancer cell proliferation by promoting glucose uptake. Exp. Ther. Med. 2019, 18, 3936–3942. [Google Scholar] [CrossRef]
- Liu, W.J.; Xu, Y.M.; Guan, H.L.; Meng, H.W. Clinical potential of miR-940 as a diagnostic and prognostic biomarker in breast cancer patients. Cancer Biomark 2018, 22, 487–493. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Liu, B.Q.; Su, F.; Chen, M.W.; Li, Y.; Qi, X.Y.; Xiao, J.B.; Li, X.M.; Liu, X.C.; Liang, W.T.; Zhang, Y.F.; et al. Serum miR-21 and miR-125b as markers predicting neoadjuvant chemotherapy response and prognosis in stage II/III breast cancer. Hum. Pathol. 2017, 64, 44–52. [Google Scholar] [CrossRef]
- Gu, X.; Xue, J.Q.; Han, S.J.; Qian, S.Y.; Zhang, W.H. Circulating microRNA-451 as a predictor of resistance to neoadjuvant chemotherapy in breast cancer. Cancer Biomark 2016, 16, 395–403. [Google Scholar] [CrossRef]
- Al-Khanbashi, M.; Caramuta, S.; Alajmi, A.M.; Al-Haddabi, I.; Al-Riyami, M.; Lui, W.O.; Al-Moundhri, M.S. Tissue and Serum miRNA Profile in Locally Advanced Breast Cancer (LABC) in Response to Neo-Adjuvant Chemotherapy (NAC) Treatment. PLoS ONE 2016, 11, e0152032. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Hirschfeld, M.; Berner, K.; Rucker, G.; Jager, M.; Weiss, D.; Medl, M.; Nothling, C.; Gassner, S.; Asberger, J.; et al. Circulating non-coding RNA-biomarker potential in neoadjuvant chemotherapy of triple negative breast cancer? Int. J. Oncol. 2020, 56, 47–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, J.; Hossein, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Zhang, J.H.; Zhao, J.; Mu, K.; Zhang, J.; Jin, Z.; Yu, J.P.; Liu, J.T. Precision medicine based on tumorigenic signaling pathways for triple-negative breast cancer. Oncol. Lett. 2018, 16, 4984–4996. [Google Scholar] [CrossRef] [Green Version]
- Holubekova, V.; Kolkova, Z.; Grendar, M.; Brany, D.; Dvorska, D.; Stastny, I.; Jagelkova, M.; Zelinova, K.; Samec, M.; Liskova, A.; et al. Pathway Analysis of Selected Circulating miRNAs in Plasma of Breast Cancer Patients: A Preliminary Study. Int. J. Mol. Sci. 2020, 21, 7288. [Google Scholar] [CrossRef]
- Qattan, A.; Al-Tweigeri, T.; Alkhayal, W.; Suleman, K.; Tulbah, A.; Amer, S. Clinical Identification of Dysregulated Circulating microRNAs and Their Implication in Drug Response in Triple Negative Breast Cancer (TNBC) by Target Gene Network and Meta-Analysis. Genes 2021, 12, 549. [Google Scholar] [CrossRef]
- Thomopoulou, K.; Papadaki, C.; Monastirioti, A.; Koronakis, G.; Mala, A.; Kalapanida, D.; Mavroudis, D.; Agelaki, S. MicroRNAs Regulating Tumor Immune Response in the Prediction of the Outcome in Patients With Breast Cancer. Front. Mol. Biosci. 2021, 8, 668534. [Google Scholar] [CrossRef]
- Batista, P.J.; Chang, H.Y. Long Noncoding RNAs: Cellular Address Codes in Development and Disease. Cell 2013, 152, 1298–1307. [Google Scholar] [CrossRef] [Green Version]
- Hansji, H.; Leung, E.Y.; Baguley, B.C.; Finlay, G.J.; Askarian-Amiri, M.E. Keeping abreast with long non-coding RNAs in mammary gland development and breast cancer. Front. Genet. 2014, 5, 379. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R.; Feng, F.Y.; Chinnaiyan, A.M. The bright side of dark matter: lncRNAs in cancer. J. Clin. Investig. 2016, 126, 2775–2782. [Google Scholar] [CrossRef] [Green Version]
- Klinge, C.M. Non-Coding RNAs in Breast Cancer: Intracellular and Intercellular Communication. Noncoding RNA 2018, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.F.; He, H.F.; Chen, Q. Estradiol induces HOTAIR levels via GPER-mediated miR-148a inhibition in breast cancer. J. Transl. Med. 2015, 13, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef]
- Wu, Z.H.; Wang, X.L.; Tang, H.M.; Jiang, T.; Chen, J.; Lu, S.; Qiu, G.Q.; Peng, Z.H.; Yan, D.W. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol. Rep. 2014, 32, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.Y.; Liu, W.Y.; Kong, Y.G. Roles and expression profiles of long non-coding RNAs in triple-negative breast cancers. J. Cell Mol. Med. 2018, 22, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Collina, F.; Aquino, G.; Brogna, M.; Cipolletta, S.; Buonfanti, G.; De Laurentiis, M.; Di Bonito, M.; Cantile, M.; Botti, G. LncRNA HOTAIR up-regulation is strongly related with lymph nodes metastasis and LAR subtype of Triple Negative Breast Cancer. J. Cancer 2019, 10, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.Z.; Zhang, J.; Zhang, W.; Huang, Y.H.; Wang, N.X.; Zhang, Q.; Qu, S.H. Circulating HOTAIR expression predicts the clinical response to neoadjuvant chemotherapy in patients with breast cancer. Cancer Biomark 2018, 22, 249–256. [Google Scholar] [CrossRef]
- Tellez-Gabriel, M.; Knutsen, E.; Perander, M. Current Status of Circulating Tumor Cells, Circulating Tumor DNA, and Exosomes in Breast Cancer Liquid Biopsies. Int. J. Mol. Sci. 2020, 21, 9457. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Zheng, K.; Tang, Y.Y.; Li, Z.; Zou, T.N.; Liu, D.Q. Overexpression of serum exosomal HOTAIR is correlated with poor survival and poor response to chemotherapy in breast cancer patients. J. Biosci. 2019, 44, 57. [Google Scholar] [CrossRef]
- Liu, M.; Xing, L.Q.; Liu, Y.J. A three-long noncoding RNA signature as a diagnostic biomarker for differentiating between triple-negative and non-triple-negative breast cancers. Medicine 2017, 96, e6222. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, S.; Xiao, H.Y.; Deng, X.Q. Serum lncRNA TINCR Serve as a Novel Biomarker for Predicting the Prognosis in Triple-Negative Breast Cancer. Technol Cancer Res. Treat. 2020, 19, 1533033820965574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Liu, W.W.; Li, W.D. Imbalance of Molecular Module of TINCR-miR-761 Promotes the Metastatic Potential of Early Triple Negative Breast Cancer and Partially Offsets the Anti-Tumor Activity of Luteolin. Cancer Manag. Res. 2021, 13, 1877–1886. [Google Scholar] [CrossRef]
- Bermejo, J.L.; Huang, G.M.Q.; Manoochehri, M.; Mesa, K.G.; Schick, M.; Silos, R.G.; Ko, Y.D.; Bruning, T.; Brauch, H.; Lo, W.Y.; et al. Long intergenic noncoding RNA 299 methylation in peripheral blood is a biomarker for triple-negative breast cancer. Epigenomics 2019, 11, 81–93. [Google Scholar] [CrossRef]
- Manoochehri, M.; Jones, M.; Tomczyk, K.; Fletcher, O.; Schoemaker, M.J.; Swerdlow, A.J.; Borhani, N.; Hamann, U. DNA methylation of the long intergenic noncoding RNA 299 gene in triple-negative breast cancer: Results from a prospective study. Sci. Rep. 2020, 10, 11762. [Google Scholar] [CrossRef]
- Brown, C.J.; Ballabio, A.; Rupert, J.L.; Lafreniere, R.G.; Grompe, M.; Tonlorenzi, R.; Willard, H.F. A Gene from the Region of the Human X-Inactivation Center Is Expressed Exclusively from the Inactive X-Chromosome. Nature 1991, 349, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Min, L.; Qiu, X.Y.; Wu, X.M.; Liu, C.Y.; Ma, J.X.; Zhang, D.Y.; Zhu, L.Y. Biological Function of Long Non-coding RNA (LncRNA) Xist. Front. Cell Dev. Biol. 2021, 9, 1447. [Google Scholar] [CrossRef]
- Yin, S.; Dou, J.Y.; Yang, G.F.; Chen, F.F. Long non-coding RNA XIST expression as a prognostic factor in human cancers: A meta-analysis. Int. J. Biol. Marker 2019, 34, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.M.; Zhang, X.D.; Li, H.B.; Yue, X.; Sun, Q.H. Serum exosomal lncRNA XIST is a potential non-invasive biomarker to diagnose recurrence of triple-negative breast cancer. J. Cell Mol. Med. 2021, 25, 7602–7607. [Google Scholar] [CrossRef] [PubMed]
- Salzman, J.; Chen, R.E.; Olsen, M.N.; Wang, P.L.; Brown, P.O. Cell-Type Specific Features of Circular RNA Expression. PLoS Genet. 2013, 9, e1003777. [Google Scholar] [CrossRef]
- Dong, R.; Ma, X.K.; Chen, L.L.; Yang, L. Increased complexity of circRNA expression during species evolution. Rna Biol. 2017, 14, 1064–1074. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yang, L.; Chen, L.L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef] [Green Version]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.L.; Bai, X.; Zeng, X.M.; Liu, J.R.; Liu, F.; Zhang, Z.W. circRNA-miRNA-mRNA in breast cancer. Clin. Chim Acta 2021, 523, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Chen, Z.H.; Hu, G.H.; Jiang, Y. Roles of circular RNA in breast cancer: Present and future. Am. J. Transl. Res. 2019, 11, 3945–3954. [Google Scholar]
- Chu, M.Y.; Fang, Y.Q.; Jin, Y.C. CircRNAs as promising biomarker in diagnosis of breast cancer: An updated meta-analysis. J. Clin. Lab. Anal. 2021, 35, 23934. [Google Scholar] [CrossRef]
- Liu, J.I.; Peng, X.Y.; Liu, Y.F.; Hao, R.; Zhao, R.M.; Zhang, L.; Zhao, F.Q.; Liu, Q.; Liu, Y.J.; Qi, Y.X. The Diagnostic Value of Serum Exosomal Has_circ_0000615 for Breast Cancer Patients. Int. J. Gen. Med. 2021, 14, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Ma, F.; Wu, L.G.; Zhang, X.; Tian, J.H.; Li, J.P.; Cao, J.; Ma, Y.F.; Zhang, L.; Wang, L.B. Identification of Hsa_circ_0104824 as a Potential Biomarkers for Breast Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820960745. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Chen, Z.H.; Hu, G.H.; Zhang, Y.; Feng, Y.L.; Jiang, Y.; Wang, J. Profiling and integrated analysis of differentially expressed circRNAs as novel biomarkers for breast cancer. J. Cell Physiol. 2020, 235, 7945–7959. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.B.; Yan, M.G.; Fang, X.; Guo, J.J.; Xiong, W.; Zhang, R.P. Circulating circular RNA hsa_circ_0001785 acts as a diagnostic biomarker for breast cancer detection. Clin. Chim Acta 2018, 487, 363–368. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhang, Q.; Zhou, S.Y.; Xu, H.Z.; Wang, D.D.; Feng, J.F.; Zhao, J.H.; Zhong, S.L. Circular RNA expression in exosomes derived from breast cancer cells and patients. Epigenomics 2019, 11, 411–421. [Google Scholar] [CrossRef]
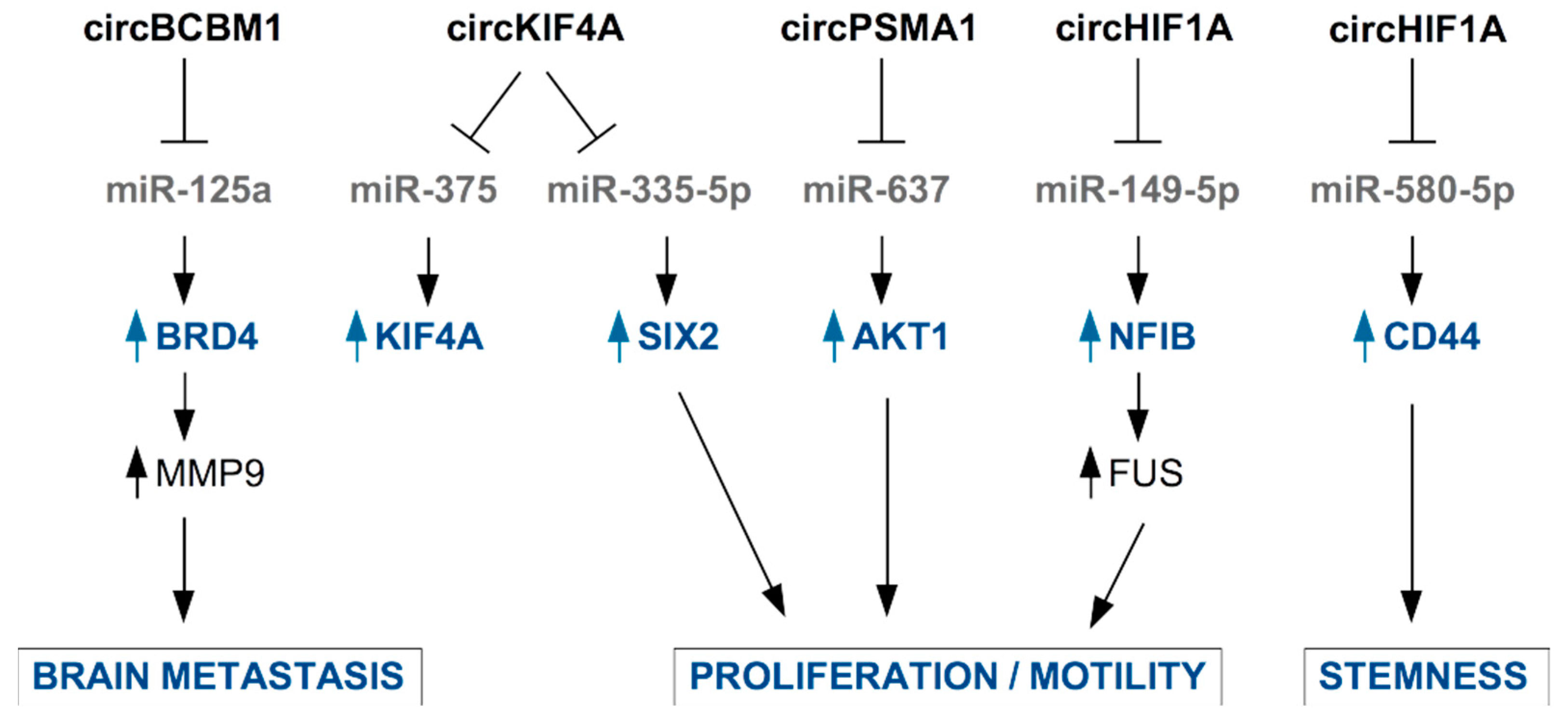
- Jia, Q.X.; Ye, L.L.; Xu, S.W.; Xiao, H.; Xu, S.D.; Shi, Z.Y.; Li, J.S.; Chen, Z.Q. Circular RNA 0007255 regulates the progression of breast cancer through miR-335-5p/SIX2 axis. Thorac. Cancer 2020, 11, 619–630. [Google Scholar] [CrossRef]
- Tang, H.L.; Huang, X.J.; Wang, J.; Yang, L.; Kong, Y.A.; Gao, G.F.; Zhang, L.J.; Chen, Z.S.; Xie, X.M. circKIF4A acts as a prognostic factor and mediator to regulate the progression of triple-negative breast cancer. Mol. Cancer 2019, 18, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, B.; Zhang, A.Q.; Li, M.L.; Pan, L.; Tang, W.Q.; An, M.; Liu, W.; Zhang, J.Q. Circular RNA profile of breast cancer brain metastasis: Identification of potential biomarkers and therapeutic targets. Epigenomics 2018, 10, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Liu, W.; Zhu, C.; Li, P.; Wang, L.; Pan, L.; Li, K.; Cai, P.Y.; Meng, M.; Wang, Y.T.; et al. Circular RNA circBCBM1 promotes breast cancer brain metastasis by modulating miR-125a/BRD4 axis. Int. J. Biol. Sci. 2021, 17, 3104–3117. [Google Scholar] [CrossRef]
- Chen, T.; Wang, X.L.; Li, C.; Zhang, H.W.; Liu, Y.; Han, D.W.; Li, Y.M.; Li, Z.; Luo, D.; Zhang, N.; et al. CircHIF1A regulated by FUS accelerates triple-negative breast cancer progression by modulating NFIB expression and translocation. Oncogene 2021, 40, 2756–2771. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.X.; Du, J.X.; Min, Z.H.; Ma, L.; Zhang, W.; Zhu, W.; Liu, Y.L. Carcinoma-associated fibroblasts derived exosomes modulate breast cancer cell stemness through exonic circHIF1A by miR-580-5p in hypoxic stress. Cell Death Discov. 2021, 7, 141. [Google Scholar] [CrossRef]
- Yang, S.J.; Wang, D.D.; Zhong, S.L.; Chen, W.Q.; Wang, F.L.; Zhang, J.; Xu, W.X.; Xu, D.; Zhang, Q.; Li, J.; et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/beta-catenin (cyclin D1) axis. Cell Death Dis. 2021, 12, 420. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Su, P.; Liang, Y.; Li, Z.; Zhang, H.; Song, X.; Han, D.; Wang, X.; Liu, Y.; et al. circ-EIF6 encodes EIF6-224aa to promote TNBC progression via stabilizing MYH9 and activating the Wnt/beta-catenin pathway. Mol. Ther. 2021, 30, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zheng, S.; Lin, Y.; Ke, L.M. Circular RNA circ-ERBB2 Elevates the Warburg Effect and Facilitates Triple-Negative Breast Cancer Growth by the MicroRNA 136-5p/Pyruvate Dehydrogenase Kinase 4 Axis. Mol. Cell Biol. 2021, 41, e00609-20. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Xu, W.; Xia, Z.H.; Liu, W.Y.; Pan, G.F.; Ding, J.B.; Li, J.D.; Wang, J.F.; Xie, X.F.; Jiang, D.W. Hsa_circ_0000199 facilitates chemo-tolerance of triple-negative breast cancer by interfering with miR-206/613-led PI3K/Akt/mTOR signaling. Aging 2021, 13, 4522–4551. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.H.; Jiang, L.; Lu, L.L.; Dai, T.T.; Li, A.S.; Chen, Y.; Zhang, L.F. CircWAC induces chemotherapeutic resistance in triple-negative breast cancer by targeting miR-142, upregulating WWP1 and activating the PI3K/AKT pathway. Mol. Cancer 2021, 20, 43. [Google Scholar] [CrossRef]
- Xing, Z.Y.; Wang, R.J.; Wang, X.; Liu, J.Q.; Zhang, M.L.; Feng, K.X.; Wang, X. CircRNA circ-PDCD11 promotes triple-negative breast cancer progression via enhancing aerobic glycolysis. Cell Death Discov. 2021, 7, 218. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Agostino, S.; Vahabi, M.; Turco, C.; Fontemaggi, G. Secreted Non-Coding RNAs: Functional Impact on the Tumor Microenvironment and Clinical Relevance in Triple-Negative Breast Cancer. Non-Coding RNA 2022, 8, 5. https://doi.org/10.3390/ncrna8010005
Di Agostino S, Vahabi M, Turco C, Fontemaggi G. Secreted Non-Coding RNAs: Functional Impact on the Tumor Microenvironment and Clinical Relevance in Triple-Negative Breast Cancer. Non-Coding RNA. 2022; 8(1):5. https://doi.org/10.3390/ncrna8010005
Chicago/Turabian StyleDi Agostino, Silvia, Mahrou Vahabi, Chiara Turco, and Giulia Fontemaggi. 2022. "Secreted Non-Coding RNAs: Functional Impact on the Tumor Microenvironment and Clinical Relevance in Triple-Negative Breast Cancer" Non-Coding RNA 8, no. 1: 5. https://doi.org/10.3390/ncrna8010005
APA StyleDi Agostino, S., Vahabi, M., Turco, C., & Fontemaggi, G. (2022). Secreted Non-Coding RNAs: Functional Impact on the Tumor Microenvironment and Clinical Relevance in Triple-Negative Breast Cancer. Non-Coding RNA, 8(1), 5. https://doi.org/10.3390/ncrna8010005







