Abstract
Laminar flow aircraft may potentially save fuel and reduce the emission of pollutants and greenhouse gases. However, laminar flow aircraft face challenges caused by contaminations on the wings, such as insect impact residue. To study insect residue on an aircraft airfoil, a new setup was developed that used rotary wings and shot an insect toward the leading edge. This setup kept insects intact before impact while airflow was maintained throughout the experiment. Additionally, the setup enabled the long-term observation of the impact residue while the test speed was adjusted. Two experiments were carried out to investigate inconsistencies from past studies about insect rupture velocity and the effect of airflow on residue. Drosophila Hydei was the insect used, and aluminum was used as the baseline substrate, which was also coated with polyurethane, acrylic, and two superhydrophobic coatings. Instead of a threshold velocity for the minimum rupture velocity of the insect, a range from initial insect rupture to the velocity at which insects ruptured in all instances was determined (i.e., 17–30 m/s). Furthermore, the presence of a coating (polyurethane) on the airfoil did not affect the minimum rupture velocity. It was observed that airflow, which has been previously mentioned as a mitigation method, did not change the residue amount after coagulation for all coatings.
1. Introduction
The aviation sector plays a crucial role in enabling worldwide interconnectedness, but it faces the complex task of reducing its environmental footprint. Pre-pandemic, in 2019, about 38.9 million flights were undertaken by the airline industry, which consumed about 445 million cubic meters of fuel and emitted about 905 million tons of CO2 [1,2,3]. The concept of laminar flow aircraft is becoming ever more important due to its potential to reduce CO2 pollution and improve flight efficiency. Reduced fuel usage by laminar flow aircraft could help to reduce the global climate temperature increase by approximately 0.0001 °C yearly (the average increase per decade is 0.18 °C) [4,5].
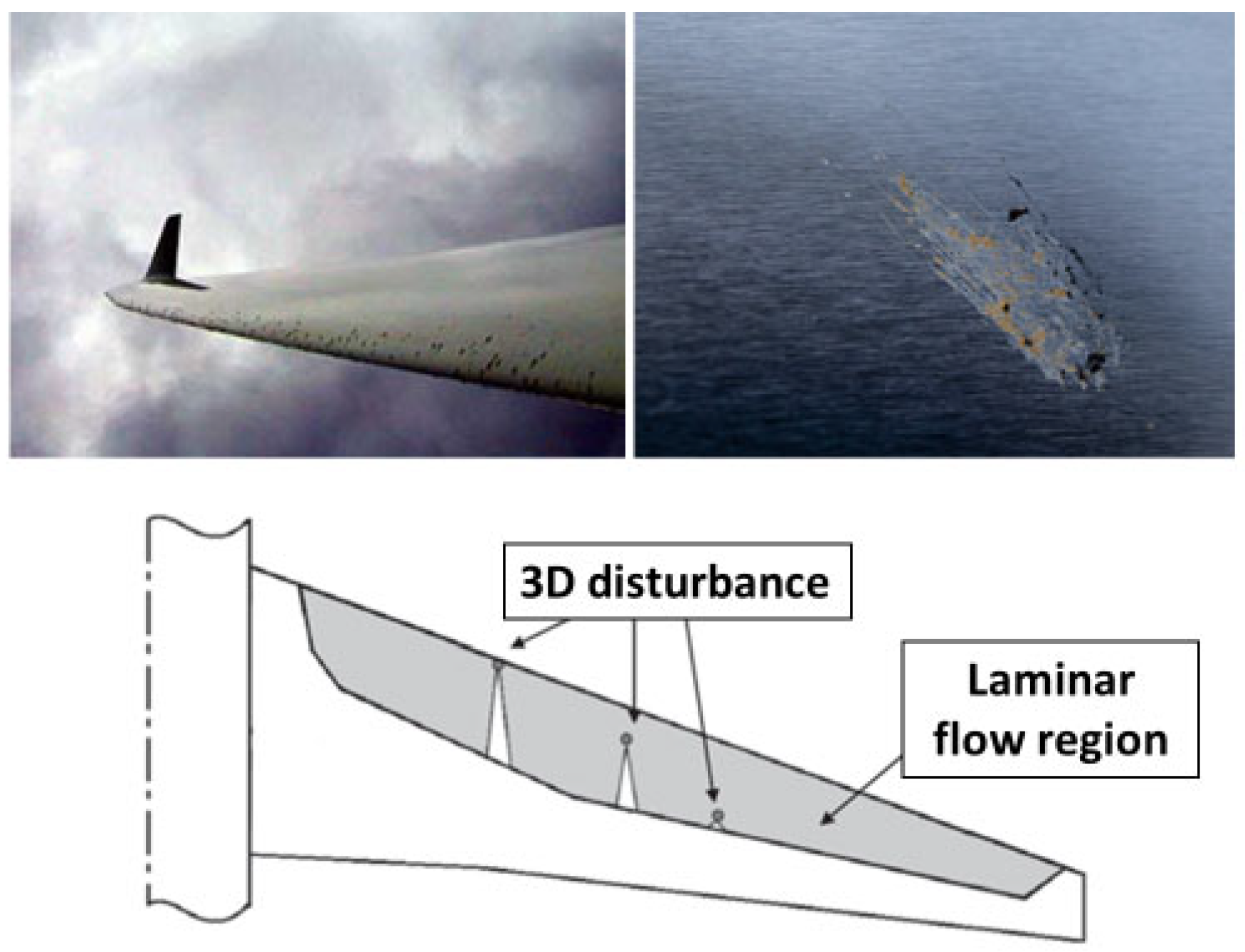
A laminar flow aircraft is characterized by having a laminar flow over most of the airfoil chord. Progress in the development of laminar flow control (LFC) aircraft has partly been impeded due to the susceptibility of the airfoil to contamination. Contaminants are mainly composed of sand and dust particles, ice, and ruptured insect residue; these cause the early transition from laminar to turbulent flow. There has been considerable research into the effects of icing on the aerodynamics of the wing (e.g., [6]) and ways to mitigate icing (e.g., using coatings [7]). In comparison, less attention has been paid to contamination due to insect residue, even though minute insect residue has the potential to disrupt the laminar flow of an aircraft (see bottom-row image in Figure 1) and compromise its overall efficiency due to the promotion of turbulent flow [8].
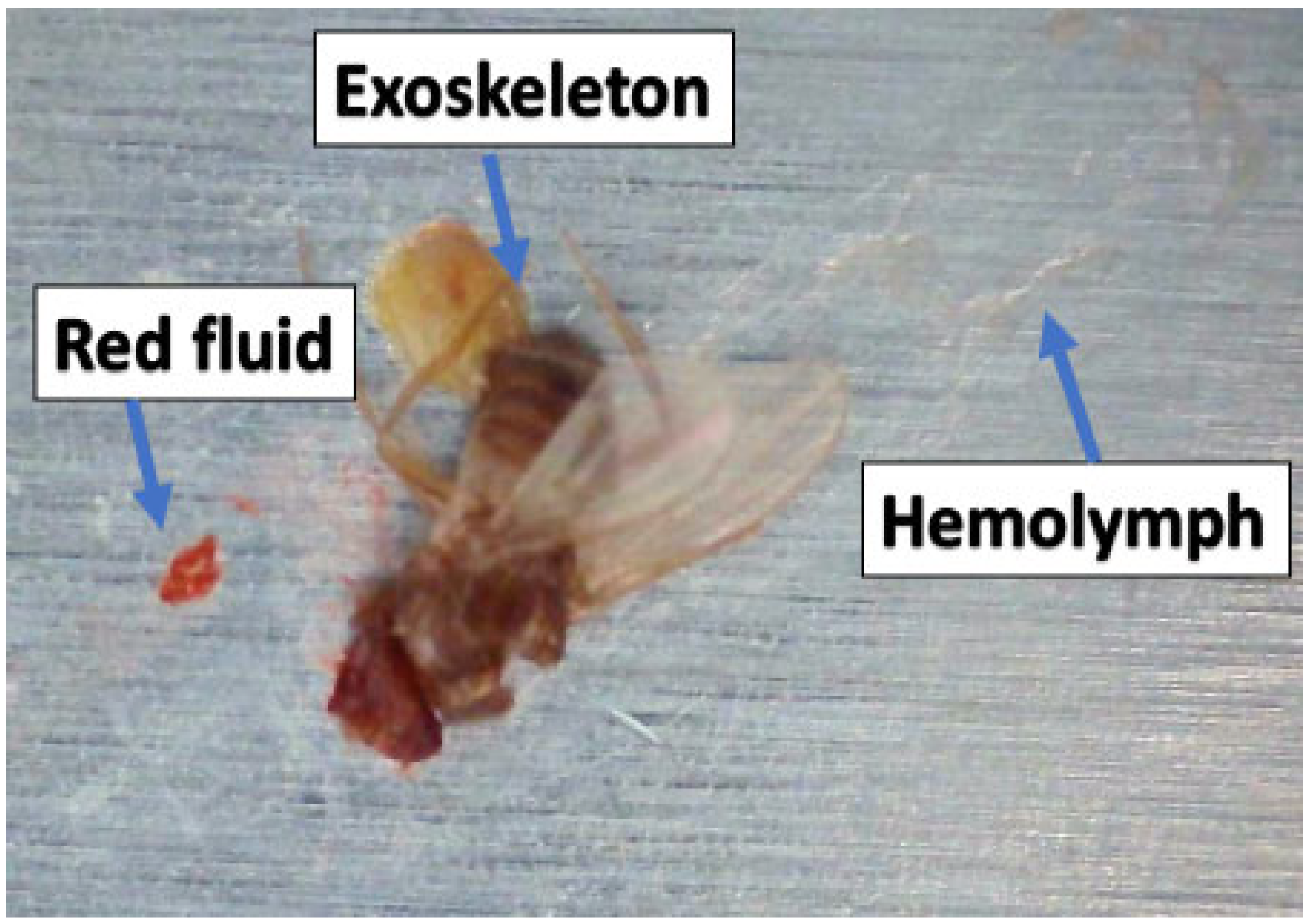
If the impact velocity is sufficiently high, an insect ruptures upon impact with the surface, and various parts of the insect (including fluids) are ejected; this ejection is similar to the splashing seen in drop-impact studies (e.g., [9]). The top-row images in Figure 1 show residue formed as a result of impact; the height and the area of this residue determine how the boundary layer flow is disrupted. For the majority of commercial airliners, a residue height of between 100 and 150 μm is deemed sufficient to trigger flow transitions from laminar to turbulent [10]. Previous studies have focused on characterizing the size and shape of insect residue [11,12,13]. Generally, there are three main components of residue: the exoskeleton, which contains the outer parts of the insect; the hemolymph, which is mostly related to abdominal fluid; and red coagulated fluid, which belongs to the head/eyes (Figure 2). Insect hemolymph coagulates in a short time (10 s or less) and acts as a glue that adheres the hard parts of the insect (legs, wings, parts of broken exoskeleton, etc.) to the airfoil. These hard parts are mainly responsible for the transition from laminar to turbulent flow.
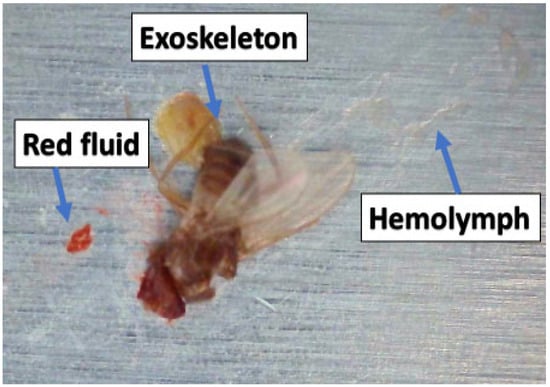
Figure 2.
Fruit fly residue components.
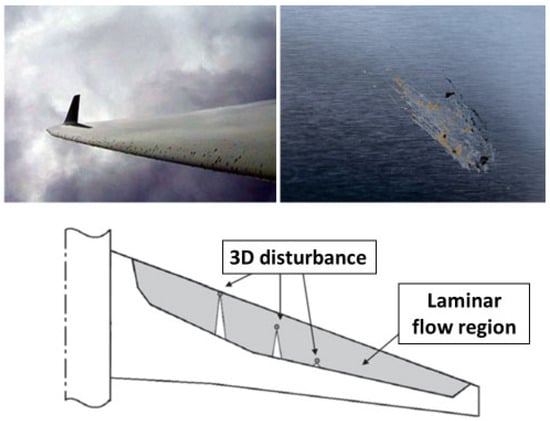
Figure 1.
Insect residue on an airfoil and laminar flow transition caused by insect residue (top-right image credited to David C. Bowman from NASA Langley; top-left image sourced from [14] with permission; and bottom image sourced from [8] with permission from Elsevier).
Figure 1.
Insect residue on an airfoil and laminar flow transition caused by insect residue (top-right image credited to David C. Bowman from NASA Langley; top-left image sourced from [14] with permission; and bottom image sourced from [8] with permission from Elsevier).
The amount of insect residue remaining on the surface or airfoil is a function of the insect type and characteristics of the impact (e.g., the speed and angle of impact), and other variables, such as the presence of airflow and surface/airfoil coating. Another parameter of interest is the minimum impact velocity at which an insect ruptures.
To maintain laminar flow on an LFC aircraft, airfoils must either be cleaned or, using other means, the contamination height must be kept below 100–400 μm, depending on the application [8,13,15,16,17,18]. Different methods for mitigating insect residue have been suggested and investigated, which generally fall into three main categories: washing systems, the use of coatings, and sweeping away the residue via airflow (a more detailed list can be found in [19]).
The washing systems investigated [19,20] are functional, but they generally seem impractical [21] due to the requirement of having to carry additional fluid in the wings of an aircraft and needing auxiliary systems and frequent maintenance. (This applies not only to aircraft but also to other systems impacted by insect residue, e.g., wind turbines.) Similar to the methods used to combat icing contamination, coatings have received considerable attention as a passive means to mitigate the effects of insect residue, especially in recent years [8,10,21,22,23,24,25,26,27,28]. In the latest studies on insect contamination reduction, coatings were determined to be a promising method to reduce the insect residue footprint after rupture, with hydrophobic coatings being more effective in general. Coatings are considered to either reduce the adhesion of the residue to the surface or to limit the spread of the ruptured insect during the impact process (generally limiting the contact of the insect with the surface). Reduced adhesion means that the presence of an airflow may cause detachment of the residue from the surface. Reduced spreading of insect fluids after rupture can reduce the chances of exoskeleton fragments being attached to coagulating fluids. We believe that this should be the underlying mechanism seen in the literature (e.g., [21]) for a reduction in exoskeleton debris on coated surfaces compared with uncoated surfaces. Finally, because hemolymph coagulates in seconds, there may be sufficient time for insect fluid to be swept away from the surface and leave no or a small amount of residue; for high air velocities and low adhesion surfaces, there is potential for the residue to be eroded or removed from the surface. Similar observations have been reported for contamination due to icing for airfoils [29], but this matter has not been explored in full detail for insect residue, as discussed below.
An airflow effect was simulated in some previous studies. For example, in [30], airflow was reported to remove up to half of the insects remaining on an airfoil. However, Peterson and Fisher [15] reported that during a flight test at 92 m/s, insect residues were not eroded. A reason for this contradiction might be that most of the insect residue is eroded immediately after impact and the remaining residue is in a stable condition [15]. Also, right after impact, before hemolymph coagulation, airflow can smear the residue [8,22]. A study [25] comparing the results of two setups, one with the presence of airflow and the other without airflow, indicated that the presence of airflow causes a higher residue area and lower residue height. Taken together, it appears that the presence of airflow changes the residue height and area immediately after impact. However, contradictory results for the effect of airflow on residue after a period of time need further investigation to clarify whether or not airflow continuously removes insect residue.
The relative impact velocity of the insect and surface/airfoil determines the rupture energy of the insect’s body. Test results with Drosophila [25,31] show that at low velocities, the insect either does not rupture or partially ruptures; at high velocities, the insect ruptures completely. Therefore, there should be a transition velocity range for insects from partial to full rupture. For instance, the speed at which the exoskeleton hits the airfoil and ruptures is not enough for the insect to stick to the airfoil because there is no hemolymph for adherence. Therefore, considering the natural variation in a population of insects, it is logical to think that insect rupture velocity should have a range (i.e., a velocity) from which a few insects rupture and stick to a velocity at which all insects completely rupture and stick. Kok et al. [25] showed that lower body parts like the abdomen of fruit flies break at a lower speed (24 m/s) than the exoskeleton (30 m/s; it should be noted that a statistical analysis of the data was not provided). Other studies [13,20,23,31,32,33,34] reported a threshold number instead of a range for the rupture velocity of the same insect (Drosophila Melanogaster), e.g., Coleman [20,31] and Wohl [23] reported about 10 and 14 m/s, respectively; Krishnan [13] reported the rupture velocity to be 21 m/s. Taken together, further investigation is needed to study rupture velocity because of these seemingly inconsistent results for the same species.
The present study formulates the following research inquiry: What is the impact of airflow on insect residue? Does there exist a velocity transition that can explain the reported inconsistent values for insect rupture of the same species? Given the importance of surface coatings in mitigating insect residue, we conducted tests with various surface coatings in this work.
2. Methodology
2.1. Experimental Apparatus
To enable this study, a novel experimental setup was developed. In contrast to setups from past studies, this setup keeps insects intact before impact while airflow is maintained during the experiment. Also, the setup allows for the long-term observation of the impact residue and the modification of speed during the test to mimic the take-off procedure of an aircraft. The newly developed setup is more compact compared with traditional wind tunnels commonly used in past studies. The details of the experimental setup can be found in [35].
A schematic of the conceptual design of insect impact on the airfoils is shown in Figure 3. Insects are the particles used in this study. A particle dropper presents one particle at a time to the particle launcher. The particle launcher directs the particle toward the moving airfoils, aiming for the airfoil’s leading edge. The airfoils are mounted on a rotary system.
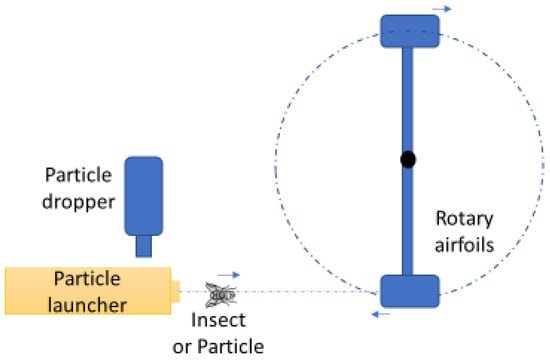
Figure 3.
Top-view schematic of the conceptual design of the insect impact apparatus.
2.2. Sample Preparation
2.2.1. Coatings
Aluminum 1100 was chosen as the baseline substrate because of its common application in the aircraft industry. For ease of folding over an airfoil template, a thin sheet of aluminum foil (0.03 mm) was used. The formed aluminum sheet was held in place by taping its end to the airfoil template. Some aluminum foils were coated in order to study the effect of different coatings. After each experiment, the aluminum foil was unfolded, and the impact region was cut out and attached to a microscope slide for analysis.
Prior to the application of the coatings, the aluminum foil sheet underwent a cleaning process using ethanol and acetone. This was performed to ensure that the surface was free from contaminants and to promote strong adhesion for the subsequent coatings. It should be noted that the coated surfaces were used immediately after preparation to ascertain that they were not re-contaminated. As such, it was not necessary to clean the coated surfaces before use. Each surface was used once for each test. Polyurethane (PU)- and acrylic-based coatings were chosen because these are often used as coatings for aircraft. The chosen PU was a quick-drying water-based matte polyurethane from BEHR that needed at least two applications. The chosen acrylic coating was a water-based premium diamond wood finish from Varathane. The coatings were applied using a dip-coating technique. These specific coatings were chosen partly in order to study the effectiveness of common coatings in reducing insect residue in industry.
According to previous studies [10,36], the most effective coatings for the mitigation of insect residue are superhydrophobic coatings. As such, a pair of commercially available superhydrophobic coatings (i.e., UltraEverDry and NeverWet) were also used. Superhydrophobic coatings were applied according to the manufacturer’s instructions.
2.2.2. Coating Characterization
The roughness of each coating was determined using a BRUKER ContourGT-K (Bruker, Tuscan, AZ, USA) non-contact surface measuring profilometer and Vision64 software (Version 2). For each coating, the arithmetic mean surface roughness (Ra) was determined for three separate areas of the sample, and the average values were reported.
The contact angle of each coating was determined using a KRUSS drop-shape analyzer. Before measuring, aluminum samples were cleaned with acetone and ethanol, and the coated samples were used immediately after preparation. Using the sessile drop method and the Young–Laplace fitting method, the contact angles for deionized water on three separate points of each sample were recorded, and the average values were reported. All measurements were done at room temperature.
2.2.3. Insects
The Drosophila or, in common terms, the fruit fly, is the most commonly impacted insect in flight due to their ubiquity; their size is 2 to 3 mm. Two common types of Drosophilas are Melanogaster and Hydei. Hydei is about 3 mm, which is 1 mm larger than Melanogaster. Past experiments were mostly performed using Melanogaster; however, experimenting with both is valid and comparable [10]. Because Drosophila Hydei has more hemolymph and body fluids, it is more likely to adhere to the airfoil after rupture. As a result, wingless Drosophila Hydei was selected as the impacting insect in this study.
Prior to the experiments, the insects were anesthetized using CO2 to ensure they were in a dormant state and did not experience distress during impact. Then, they were loaded into the particle launcher. The methods used in this study were rigorously evaluated to verify compliance with ethical research guidelines for exploiting insects. A formal request was sent to the university’s ethical committee to inquire about the ethical implications of using insects in a state of dormancy for impact tests. The committee issued a response stating that obtaining ethical clearance was not required for this project. However, every attempt was made to reduce the utilization of insects and guarantee that the experimental methodology was optimized and effective in order to necessitate the smallest possible sample number to ensure validity.
A Satorius TE214S scale (Satorius, Ann Arbor, MI, USA) with a precision of 0.1 mg was used to weigh eleven Drosophila Hydei, and the average weight was calculated. Three were measured for length using ImageJ (1.53u), and the average length was calculated.
2.3. Experimental Procedures
2.3.1. Impact Tests
To understand the insect rupture phenomenon, it is necessary to investigate the speed at which rupture starts. An experiment was designed to investigate the rupture velocity and whether or not the coating type affects rupture velocity. Insects impacted the airfoil at intervals of 5 m/s from low to high speeds. Three insect impact samples were taken for each speed. When the number of ruptured insects started to increase, the interval between velocity measurements decreased from 5 m/s to 2.5 m/s. Aluminum- and PU-coated aluminum samples/airfoils were examined. Previous investigations reported the rupture velocity for aluminum; as such, aluminum was utilized to compare the newly developed apparatus’s results with results from past studies. Furthermore, it is possible that the coating characteristics affect rupture velocity. For instance, due to roughness, insects rupture at lower velocities when rougher coatings are used or when there are differences in compliance. Therefore, PU was chosen as a coating to investigate what happens to rupture velocity when a coating is used.
As mentioned in Section 2.1, the newly developed setup allows for the long-term observation of residue. The effect of airflow at 60 m/s on the residue of all coatings was investigated by monitoring the residue closely at the time of impact and after 10 min to observe the effect of continuous airflow on removal. The area of residue was the monitored parameter.
2.3.2. Data Processing
To evaluate the residue mitigation ability of coatings, the height and area of the insect residue need to be measured. The area of insect residue was selected because it was used in past studies. To measure the area of residue, top-view images of the samples were taken with a smartphone at a distance of 10 cm from the sample. The microscope slide’s edge was used as the reference length. ImageJ was used to select the edges of the residue in order to manually measure the area of the samples (see Figure 4). The measured area was used to evaluate the coatings.

Figure 4.
Using ImageJ (the contour of the residue is marked by dots) to measure a residue’s area.
The airfoil’s speed was computed by multiplying the angular velocity of the arm (see Figure 3) by the distance between the collision point and the center of rotation (33 ± 1 cm).
A high-speed camera was used to capture the movement of insects. The insect flew at a speed of 2.3 ± 0.2 m/s, with and without the presence of airflow (calculated using the insect’s distance traveled and the camera’s imaging time stamp). The impact of airflow on insect speed was determined to be a 0.2 m/s reduction in its velocity at maximum speed (using the camera’s imaging time stamp); 0.2 m/s was considered insignificant compared with the insect’s speed and the accuracy of its measurement. As a result, it was assumed that the insects moved at a constant speed of 2.3 m/s.
Given that both the airfoil and the insect traveled toward each other, the following formula was used to determine relative velocity:
where ω is the angular velocity and 0.33 m is the radius of rotation.
V = 2.3 + ω × 0.33 (m/s)
3. Results and Discussion
The average weight of Drosophila Hydei was 1.6 0.1 mg. The average length of Drosophila Hydei was 3.05 0.11 mm. The contact angle and roughness of each coating are given in Table 1.

Table 1.
Contact angle and roughness of the coatings.
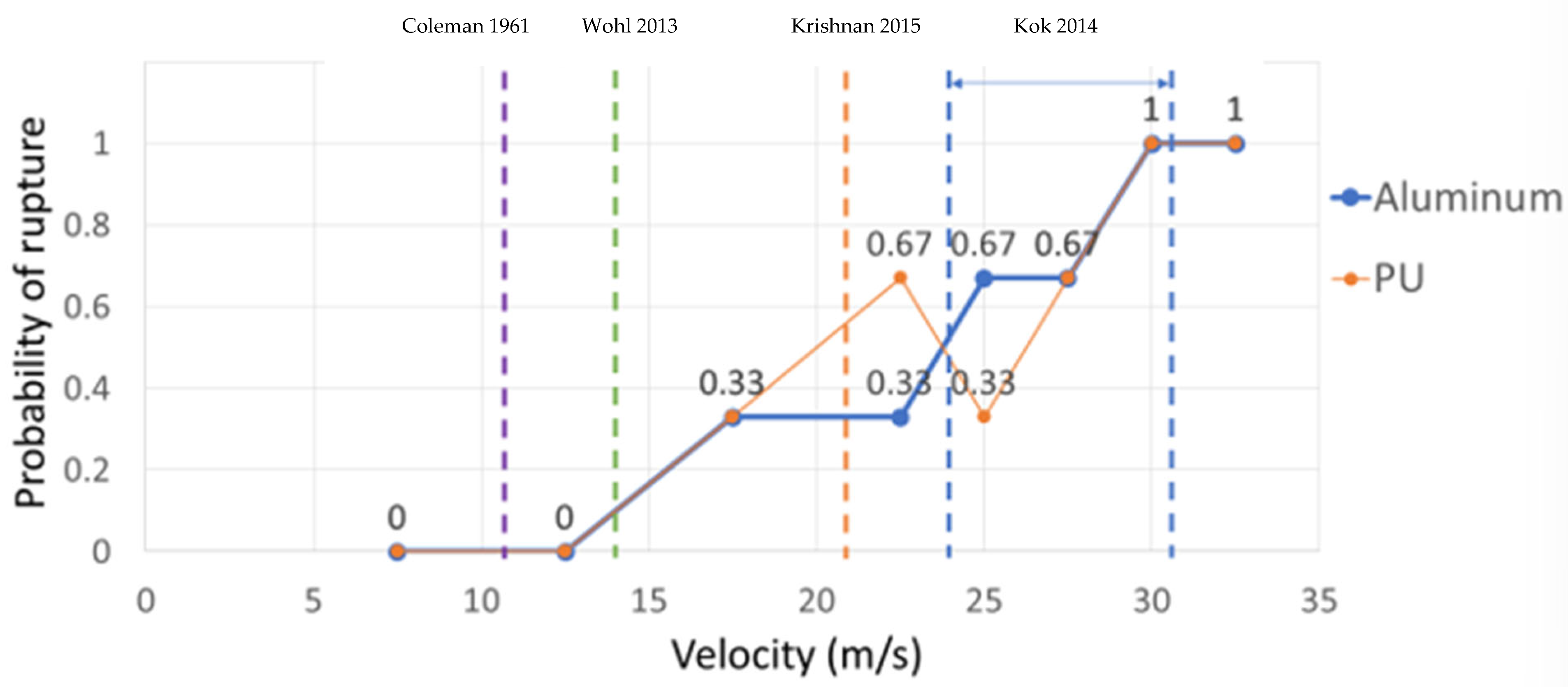
Rupture probability was defined as the number of successful ruptures over the total number of trials. As shown in Figure 5, rupture was seen occasionally at 17.5 m/s; at 30 m/s, rupture always occurred. By comparing the results of aluminum and PU, it can be concluded that rupture velocity is not a function of the coating characteristics but is only related to impact velocity; PU was selected from other coatings because it is not superhydrophobic and traces of rupture could be observed (using acrylic would produce the same results because it is also not superhydrophobic). Past studies have shown that for superhydrophobic coatings, rupture is possible, but the insect does not adhere to the airfoils due to the low wettability of superhydrophobic surfaces. Therefore, superhydrophobic coatings were not used in the rupture velocity experiments. The results from this study show that rupture velocity is a phenomenon that is better defined as a range of velocities rather than reported as a single threshold, given the probabilistic nature of the event. Unless one is interested in cases where a fruit fly ruptures 100% of the time, then the higher bound of rupture velocity (i.e., 30 m/s) should be used. In the studies shown in Figure 5 (i.e., [11,22,23,25]), the target impacting area was either an airfoil or a tilted plate. Both types of studies (airfoil and tilted plate) examined a range of impact angles, including the normal impact angle (as in the current study) in which all the impact force was applied to the insect’s body. Because the ratio of insect to airfoil or tilted plate size is negligible, and all mentioned studies examined the normal impact angle, comparing the results from these studies is acceptable. Studies [11,20,23] do not mention that the reported velocity was the threshold at which the insects started to rupture or that they ruptured every time. However, based on Figure 5, they probably reported the lower and middle bounds of impact velocity at which some insects started to rupture. In [37], impact velocity was reported for both the soft parts of insects (24 m/s) and the hard parts of insects (30 m/s). However, the study does not mention if the reported number was the threshold velocity at which insects started to rupture or if they ruptured every time. Based on Figure 5, both numbers probably represent the higher bound of impact velocity for the soft and hard parts of the insect.
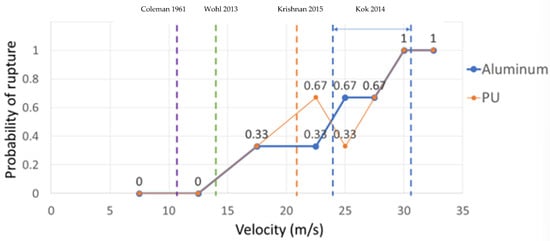
Figure 5.
Rupture velocity for Drosophila impacting aluminum- and polyurethane (PU)-coated surfaces. The vertical dashed lines are reported velocities for insect rupture from other studies; from left to right, the reported velocities are from references [11], [2], [23], and [37], respectively.
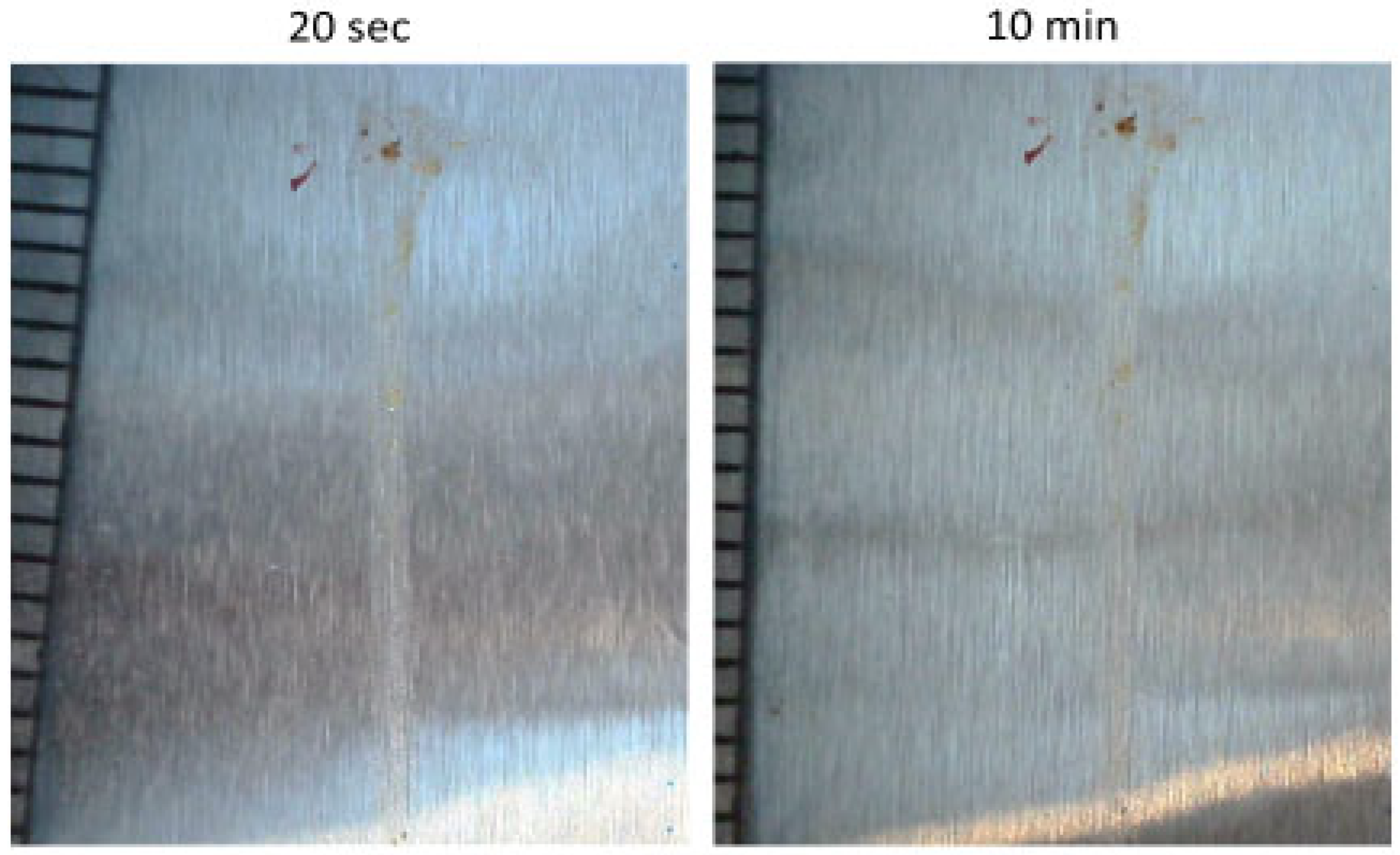
Pictures of residues from 60 m/s insect impact on acrylic coating are shown in Figure 6. The pictures were taken a short time after impact (less than 20 s because turning off the system takes a few seconds) and after 10 min exposure to airflow. Based on Table 2, there was not a significant change in the residue area after exposure to airflow. Because removing residues prior to coagulation is simpler, and every residue that is intended to be removed by airflow is completely removed prior to coagulation, this could account for all the residues being in steady conditions with airflow after coagulation (less than 10 s). In conclusion, the effect of airflow after insect impact on insect residue is negligible after coagulation. This conclusion aligns with the results of [15]. However, in [30], it was reported that airflow can remove half of the residue. This study, similar to earlier studies, used aluminum airfoil and compared residue size before and after a long period of being affected by airflow at the cruising velocity of an aircraft. However, in [30], first, an aircraft was impacted by insects, and then, after measurement of residue sizes, it flew. Therefore, because airflow was absent during coagulation (10 s after impact), nothing was eroded. After the flight, some parts were eroded due to airflow. Because airflow exists during impact, in reality, the results from [15] are likely more representative.
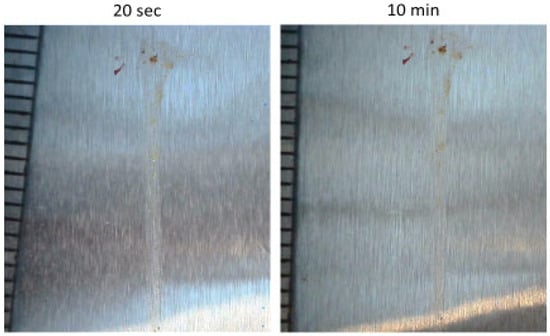
Figure 6.
Insect residue on acrylic after exposure to airflow for 20 s and 10 min.

Table 2.
Area measurements after exposure to airflow for 20 s and 10 min.
Based on Table 2, superhydrophobic coatings (NeverWet and UltraEverDry) show good residue mitigating ability compared with the other coatings.
4. Conclusions
This study shows the usefulness of a novel setup developed to study insect impact and its residue on airfoils. The findings from the experiments reveal that fruit flies start to rupture at a velocity of 17.5 m/s; insect rupture progresses such that all impacted fruit flies rupture at 30 m/s. These results support the notion that representing rupture velocity as a range, rather than a single threshold, is more appropriate due to the probabilistic characteristics of the event. Furthermore, the examination of surface coatings emphasized that the rupture velocity is inherently linked to the velocity of impact, regardless of the specific coating used.
This investigation into the impact of airflow on insect residue, particularly in light of contrasting results from prior research, yields the conclusion that airflow does not modify insect residue after coagulation. In addition, it was observed that superhydrophobic coatings are effective in reducing the surface area covered by residue.
Author Contributions
The roles of the authors are identified by their initials as follows: conceptualization, A.A.; data curation, M.G.; formal analysis, M.G. and A.A.; funding acquisition, A.A.; investigation, M.G. and A.A.; methodology, M.G. and A.A.; project administration, A.A.; supervision, A.A.; validation, M.G.; visualization, M.G.; roles in Writing, M.G. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant.
Data Availability Statement
Data for this study can be made available upon request from the corresponding author.
Acknowledgments
The authors acknowledge the technical editorial support of Maeve O’Connell.
Conflicts of Interest
The authors declare no conflict of interest of any type in the preparation and reporting of this work.
References
- CO2 Emissions of Airlines Worldwide 2004–2022|Statista. Available online: https://www.statista.com/statistics/1186820/co2-emissions-commercial-aviation-worldwide/ (accessed on 26 June 2022).
- Commercial Airlines: Worldwide Fuel Consumption 2005–2022|Statista. Available online: https://www.statista.com/statistics/655057/fuel-consumption-of-airlines-worldwide/ (accessed on 26 June 2022).
- Airline Industry Worldwide–Number of Flights 2004–2022|Statista. Available online: https://www.statista.com/statistics/564769/airline-industry-number-of-flights/ (accessed on 26 June 2022).
- A New Study Puts Temperature Increases Caused by CO2 Emissions on the Map. Available online: https://phys.org/news/2016-01-temperature-co2-emissions.html (accessed on 18 July 2022).
- Climate Change: Global Temperature|NOAA Climate.gov. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature (accessed on 18 July 2022).
- Bragg, M.; Broeren, A.; Blumenthal, L. Iced-Airfoil and Wing Aerodynamics; SAE Technical Paper 2003-01-2098; SAE International: Warrendale, PA, USA, 2003. [Google Scholar] [CrossRef]
- Antonini, C.; Innocenti, M.; Horn, T.; Marengo, M.; Amirfazli, A. Understanding the Effect of Superhydrophobic Coatings on Energy Reduction in Anti-Icing Systems. Cold Reg. Sci. Technol. 2011, 67, 58–67. [Google Scholar] [CrossRef]
- Kok, M.; Smith, J.G., Jr.; Wohl, C.J.; Siochi, E.J.; Young, T.M. Critical considerations in the mitigation of insect residue contamination on aircraft surfaces–A review. Prog. Aerosp. Sci. 2015, 75, 1–14. [Google Scholar] [CrossRef]
- Burzynski, D.A.; Roisman, I.V.; Bansmer, S.E. On the splashing of high-speed drops impacting a dry surface. J Fluid Mech. 2020, 892, A2. [Google Scholar] [CrossRef]
- Kok, M.; Mertens, T.; Raps, D.; Young, T.M. Influence of surface characteristics on insect residue adhesion to aircraft leading edge surfaces. Prog. Org. Coat. 2013, 76, 1567–1575. [Google Scholar] [CrossRef]
- Krishnan, K.G.H.; Milionis, A.; Starr, M.; Loth, E. Fruit fly impact outcomes and residue components on an aerodynamic surface. In Proceedings of the 53rd AIAA Aerospace Sciences Meeting, Kissimmee, FL, USA, 5–9 January 2015; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2015; pp. 181–192. [Google Scholar] [CrossRef]
- Krishnan, K.G.H.; Robison, R.; Tetteh, F.; Loth, E.; Farrell, T.; Crouch, J.D.; Berry, D. Characterization of insect residue on an aerodynamic leading edge. In Proceedings of the 8th AIAA Atmospheric and Space Environments Conference, Washington, DC, USA, 13–17 June 2016; p. 3445. [Google Scholar] [CrossRef]
- Krishnan, K.G.; Milionis, A.; Tetteh, F.; Loth, E. Fruit fly impact on an aerodynamic surface: Types of outcomes and residue components. Aerosp. Sci. Technol. 2017, 69, 181–192. [Google Scholar] [CrossRef]
- Withease Ceramic Coating. Available online: https://weceramiccoating.com/aircraft (accessed on 16 July 2022).
- Peterson, J.B., Jr.; Fisher, D.F. Flight investigation of insect contamination and its alleviation. In Proceedings of the CTOL Transport Technology Conference, Hampton, VA, USA, 28 February–3 March 1978; pp. 357–373. [Google Scholar]
- Maddalon, D.V.; Braslow, A.L. Simulated-Airline-Service Flight Tests of Laminar-Flow Control with Perforated-Surface Suction System; NASA/TP 2966; NASA: Hampton, VA, USA, 1990. [Google Scholar]
- Marsden, D.; Toogood, R. Wind tunnel tests of a slotted flapped wing section. Technol. Soar. 1978, 12, 4–9. [Google Scholar]
- Elsenaar, A.; Haasnoot, H.N. A survey on Schiphol airport of the contamination of wing leading edges of three different aircraft types under operating conditions. In Proceeding of the First European Forum on Laminar Flow Technology, Hamburg, Germany, 16–18 March 1992; pp. 256–261. [Google Scholar]
- O’Donoghue, D.; Young, T.M.; Pembroke, J.T.; O’Dwyer, T.F. An investigation of surfactant and enzyme formulations for the alleviation of insect contamination on hybrid laminar flow control surfaces. Aerosp. Sci. Technol. 2002, 6, 19–29. [Google Scholar] [CrossRef]
- Coleman, W.S. Roughness due to insects. In Boundary Layer and Flow Control; Lachmann, G.V., Ed.; Pergamon Press: Oxford, UK, 1961; Volume II, pp. 682–747. [Google Scholar] [CrossRef]
- Bayer, I.S.; Krishnan, K.G.; Robison, R.; Loth, E.; Berry, D.H.; Farrell, T.E.; Crouch, J.D. Thermal Alternating Polymer Nanocomposite (TAPNC) Coating Designed to Prevent Aerodynamic Insect Fouling. Sci. Rep. 2016, 6, 38459. [Google Scholar] [CrossRef] [PubMed]
- Wohl, C.J.; Smith, J.G., Jr.; Penner, R.K.; Lorenzi, T.M.; Lovell, C.S.; Siochi, E.J. Evaluation of commercially available materials to mitigate insect residue adhesion on wing leading edge surfaces. Prog. Org. Coat. 2013, 76, 42–50. [Google Scholar] [CrossRef]
- Wohl, C.J.; Smith, J.; Connell, J.; Siochi, E.; Penner, R.; Gardner, J. Engineered surfaces for mitigation of insect residue adhesion. In Proceedings of the 51st AIAA Aerospace Sciences Meeting, Grapevine, TX, USA, 7–10 January 2013; p. 413. [Google Scholar] [CrossRef]
- Kok, M.; Young, T.M. The evaluation of hierarchical structured superhydrophobic coatings for the alleviation of insect residue to aircraft laminar flow surfaces. Appl. Surf. Sci. 2014, 314, 1053–1062. [Google Scholar] [CrossRef]
- Kok, M.; Tobin, E.F.; Zikmund, P.; Raps, D.; Young, T.M. Laboratory investigation into anti-contamination coatings for mitigating insect contamination with application to laminar flow technologies. In Contamination Mitigating Polymeric Coatings for Extreme Environments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 291–313. [Google Scholar] [CrossRef]
- Wohl, C.J.; Shanahan, M.H.; Bosh, A.J.; Smith, J.G., Jr.; Penner, R.K.; Connell, J.W.; Siochi, E.J. Contamination-mitigating epoxy coatings for aircraft leading edges. In Proceedings of the Annual Meeting of the Adhesion Society, San Antonio, TX, USA, 21–25 February 2016. [Google Scholar]
- Wohl, C.J.; Doss, J.R.; Shanahan, M.H.; Smith, J.G., Jr.; Penner, R.K.; Connell, J.W.; Siochi, E.J. Influence of surface properties and impact conditions on adhesion of insect residues. In Proceedings of the 38th Annual Meeting of the Adhesion Society, Savannah, GA, USA, 20–25 February 2015; pp. 20–25. [Google Scholar]
- Smith, J.; Lorenzi, T.; Wohl, C.; Penner, R.; Siochi, E. Influence of surface energy on insect residue adhesion. In Proceedings of the 35th Annual Meeting of the Adhesion Society, New Orleans, LA, USA, 26 February 2012; pp. 26–29. [Google Scholar]
- Mangini, D.; Antonini, C.; Marengo, M.; Amirfazli, A. Runback ice formation mechanism on hydrophilic and superhydrophobic surfaces. Cold Reg. Sci. Technol. 2015, 109, 53–60. [Google Scholar] [CrossRef]
- Lachmann, I.G.C. Aspects of Insect Contamination in Relation to Laminar Flow Aircraft; Technical Report C.P. No., 484; Ministry of Aviation Aeronautical Research Council, A.R.C: Atlantic, NJ, USA, 1960. [Google Scholar]
- Coleman, W.S. The characteristics of roughness from insects as observed for two-dimensional, incompressible flow past airfoils. J. Aerosp. Sci. 1959, 26, 264–280. [Google Scholar] [CrossRef]
- Maresh, J.; Bragg, M. The role of airfoil geometry in minimizing the effect of insect contamination of laminar flow sections. In Proceedings of the 2nd Applied Aerodynamics Conference, Seattle, WA, USA, 21–23 August 1984; p. 2170. [Google Scholar] [CrossRef]
- Croom, C.C.; Holmes, B.J. Flight Evaluation of an Insect Contamination Protection System for Laminar Flow Wings; SAE Transactions: Warrendale, PA, USA, 1985; pp. 486–494. [Google Scholar]
- Joslin, R.D. Overview of Laminar Flow Control; NASA/TP 208705; NASA: Hampton, VA, USA, 1998. [Google Scholar]
- Ghasemzadeh, M. Design of and Experimentation with an Insect Impact Simulator Setup. Master’s Thesis, York University, Toronto, ON, Canada, 2022. [Google Scholar]
- Kok, M.; Raps, D.; Young, T.M. Effects of surface roughness and energy on insect residue adhesion to aircraft leading edge surfaces. In Proceedings of the 36th Annual Meeting of the Adhesion Society, Daytona Beach, FL, USA, 9–10 February 2013; pp. 3–6. [Google Scholar]
- Kok, M.; Tobin, E.F.; Zikmund, P.; Raps, D.; Young, T.M. Laboratory testing of insect contamination with application to laminar flow technologies, Part I: Variables affecting insect impact dynamics. Aerosp. Sci. Technol. 2014, 39, 605–613. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).