Impacts of Mask Wearing and Leakages on Cyclic Respiratory Flows and Facial Thermoregulation
Abstract
:1. Introduction
- (1)
- Develop an integrated ambient–mask–face–airway computational model with varying gaps for respiratory flows and heat transfer under tidal breathing.
- (2)
- Validate the computational model against corresponding experimental measurements, including the mask–face surface temperatures using infrared imaging, the air temperature using thermal probes, and leakage flow velocities using an anemometer.
- (3)
- Quantify the temperature variations and thermal regulations under the facemask under varying leakage flows in comparison to the case with no mask.
- (4)
- Examine the airflow characteristics underlying the spatiotemporal variation in face and airflow temperatures.
2. Materials and Methods
2.1. Mask–Face–Airway Model with Gaps
2.2. Facemask Physical Properties
2.3. Boundary Conditions for the Airway and Face
2.4. Numerical Methods
2.5. Experimental Methods
3. Results
3.1. Model Validation
3.2. Airflow and Temperature Variations with/without Mask Wearing
3.3. Vortex Dynamics and Thermoregulation
3.4. Gap Size Effects on Facial Theomoregulation
3.4.1. Airflow and Facial Temperatures
3.4.2. Temperature Variations at the Philtrum
3.4.3. Temperature Change Rate at the Philtrum
3.4.4. Temperature Variations at the Chin
3.4.5. Temperature Variations at the Right/Left Cheeks and Nose Bridge
3.4.6. Temperature Change Rate at the Chin, Right/Left Cheeks, and Nose Bridge
4. Discussion
4.1. Thermal Regulation Mechanisms with Mask Wearing
4.2. Unique Patterns of Temperature Variations vs. Gap Size/Location and Sampling Points
4.3. Implications for Development of Fit Test Tool for Disposable Masks
4.4. Implications for Mask Wearing as a Public Health Measure in Viral Control
5. Conclusions
- Wearing a surgical mask with a perfect fit caused temperatures up to 4.3 °C higher at the philtrum than no mask in ambient conditions of 25 °C under normal breathing.
- A minor gap of 0.51 cm2 at the nose top led to 5.6% leakage and a 28% decrease in warming effect, while a gap of 4.27 cm2 led to 41.7% leakage and a 62% decrease in warming effect, relative to a perfect fit.
- The cooling effect occurred predominantly during inhalation and was more significant in the gap proximity.
- For a given sampling point, the temporal temperature profiles (T and Dt/dt) were distinctive among incrementally increasing gap sizes.
- Combining the temperature variations (T, dT/dt, and warming/cooling) at multiple sample points yielded a unique pattern for one gap size/location. It would be promising to reversely determine the gap size/location using temperature measurements at multiple points across the mask.
- This was a physiology-based simulation study, and future human studies are recommended to confirm the results.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [PubMed]
- Okazaki, S.; Yamanami, H.; Nakagawa, F.; Takuwa, N.; Kawabata Duncan, K.J. Mask wearing increases eye involvement during smiling: A facial EMG study. Sci. Rep. 2021, 11, 20370. [Google Scholar] [CrossRef] [PubMed]
- Rinck, M.; Primbs, M.A.; Verpaalen, I.A.M.; Bijlstra, G. Face masks impair facial emotion recognition and induce specific emotion confusions. Cogn. Res. Princ. Implic. 2022, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Langbehn, A.T.; Yermol, D.A.; Zhao, F.; Thorstenson, C.A.; Niedenthal, P.M. Wearing N95, surgical, and cloth face masks compromises the perception of emotion. Affect. Sci. 2022, 3, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Han, J.; Yeon, Y.M.; Kang, N.Y.; Kim, E. Effect of face mask on skin characteristics changes during the COVID-19 pandemic. Skin Res. Technol. 2021, 27, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhu, Y.; Zhou, X.; Guo, Z.; Mao, Y.; Jiang, H.; Fang, Z.; Zheng, Z.; Chen, X. Investigation of the effects of face masks on thermal comfort in Guangzhou, China. Build. Environ. 2022, 214, 108932. [Google Scholar] [CrossRef]
- Venugopal, V.; Lennqvist, R.; Latha, P.K.; Rekha, S.; Suraya, A.; Jakobsson, K.; Kjellstrom, T. Challenges in conducting epidemiological field studies evaluating associations between heat stress and renal health among workers in informal sectors: Experiences from India. Environ. Res. 2021, 200, 111343. [Google Scholar] [CrossRef]
- Roberge, R.J.; Kim, J.-H.; Coca, A. Protective facemask impact on human thermoregulation: An overview. Ann. Occup. Hyg. 2012, 56, 102–112. [Google Scholar]
- Cates, V.C.; Marullo, A.L.; Isakovich, R.; Bird, J.D.; Keess, J.L.; Ricord, K.C.; Leslie, K.M.; Janssens, A.A.; Bhardwaj, R.; Day, T.A. Comparison of mild physiological effects of surgical masks and N95 respirators over 60 min at rest. J. Appl. Physiol. 2023, 135, 227–237. [Google Scholar] [CrossRef]
- Pan, X.; Li, X.; Kong, P.; Wang, L.; Deng, R.; Wen, B.; Xiao, L.; Song, H.; Sun, Y.; Zhou, H.; et al. Assessment of use and fit of face masks among individuals in public during the COVID-19 pandemic in China. JAMA Netw. Open 2021, 4, e212574. [Google Scholar] [CrossRef]
- Su, W.-C.; Lee, J.; Xi, J.; Zhang, K. Investigation of mask efficiency for loose-fitting masks against ultrafine particles and effect on airway deposition efficiency. Aerosol Air Qual. Res. 2022, 22, 210228. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Wang, J. A critical review on the role of leakages in the facemask protection against SARS-CoV-2 infection with consideration of vaccination and virus variants. Indoor Air 2022, 32, e13127. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Barari, K.; Si, X.A.; Abdollahzadeh Jamalabadi, M.Y.; Park, J.H.; Rein, M. Inspiratory leakage flow fraction for surgical masks with varying gaps and filter materials. Phys. Fluids 2022, 34, 041908. [Google Scholar] [CrossRef]
- Abduljabbar, M.; Kalthoum, D.E.; Bakarman, M.; Wahby Salem, I.; Alsulaimani, Z.; Alharbi, W.; Shawish, S.; Alsobhi, R. The correlation between wearing face masks and skin damage in adults during the COVID-19 pandemic: A cross-sectional study in eddah, Saudi Arabia. Cureus 2022, 14, e31521. [Google Scholar] [CrossRef] [PubMed]
- Gül, Ü. COVID-19 and dermatology. Turk. J. Med. Sci. 2020, 50, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Zhu, S.; Huang, Y.; Li, L.; Tao, J.; Lei, T.; Song, J.; Liu, D.; Chen, L.; Shi, Y.; et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: A survey in Wuhan and its surrounding regions. Br. J. Dermatol. 2020, 183, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.E.; Bin Dakhil, A.A.; Albrkheel, A.A.; Almutlq, M.M.; Alolayan, O.K.; Alqahtani, M.A.; Alsubaie, F.S.; Alessa, D.S.; Alzamil, F.M. Face mask-induced skin changes: A new common phenomenon during the coronavirus disease 2019 pandemic. Dermatol. Rep. 2023, 15, 9526. [Google Scholar] [CrossRef]
- Teo, W.L. The “Maskne” microbiome–pathophysiology and therapeutics. Int. J. Dermatol. 2021, 60, 799–809. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Gonçalves, T.; Peixoto, D.; Pires, P.C.; Velsankar, K.; Jha, N.K.; Chavda, V.P.; Mohammad, I.S.; Cefali, L.C.; Mazzola, P.G.; et al. Rosacea topical treatment and care: From traditional to new drug delivery systems. Mol. Pharm. 2023, 20, 3804–3828. [Google Scholar] [CrossRef]
- Rathi, S.K.; Dsouza, J.M. Maskne: A new acne variant in COVID-19 era. Indian J. Dermatol. 2022, 67, 552–555. [Google Scholar]
- Thatiparthi, A.; Liu, J.; Martin, A.; Wu, J.J. Adverse Effects of COVID-19 and Face Masks: A Systematic Review. J. Clin. Aesthet. Dermatol. 2021, 14, S39–S45. [Google Scholar] [PubMed]
- Nahm, W.J.; Nagler, A.R.; Milam, E.C. Association of perioral dermatitis with facial mask usage during the COVID-19 pandemic: A retrospective study. JAAD Int. 2023, 10, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Kisielinski, K.; Giboni, P.; Prescher, A.; Klosterhalfen, B.; Graessel, D.; Funken, S.; Kempski, O.; Hirsch, O. Is a mask that covers the mouth and nose free from undesirable side effects in everyday use and free of potential hazards? Int. J. Environ. Res. Public Health 2021, 18, 4344. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Militký, J.; Venkataraman, M.; Steyn, H.; Vermaas, J. The effect of mask style and fabric selection on the comfort properties of fabric masks. Materials 2022, 15, 2559. [Google Scholar] [CrossRef]
- Mumma, J.; Liu, F.; Ng, N.L.; Morgan, J.; Lane, M.; Gannon, P. Designing better cloth masks: The effect of fabric and attachment-style on discomfort. J. Occup. Environ. Hyg. 2023, 20, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kwong, L.H.; Wilson, R.; Kumar, S.; Crider, Y.S.; Reyes Sanchez, Y.; Rempel, D.; Pillarisetti, A. Review of the breathability and filtration efficiency of common household materials for face masks. ACS Nano 2021, 15, 5904–5924. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.K.; Leung, L.; Lau, P.; Tong, J. The effect of wearing a face mask on body temperature. Hong Kong J. Emerg. Med. 2005, 12, 23–27. [Google Scholar] [CrossRef]
- Pequeña, A.R.; Prasetyo, Y.T. The Effect of mask & face shield on the general discomfort of the workers in the food industry: A structural equation modeling approach. J. Phys. Conf. Ser. 2021, 1996, 012012. [Google Scholar]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Facial skin temperature and discomfort when wearing protective face masks: Thermal infrared imaging evaluation and hands moving the mask. Int. J. Environ. Res. Public Health 2020, 17, 4624. [Google Scholar] [CrossRef]
- Salati, H.; Khamooshi, M.; Vahaji, S.; Christo, F.C.; Fletcher, D.F.; Inthavong, K. N95 respirator mask breathing leads to excessive carbon dioxide inhalation and reduced heat transfer in a human nasal cavity. Phys. Fluids 2021, 33, 081913. [Google Scholar] [CrossRef]
- Inthavong, K.; Fletcher, D.F.; Khamooshi, M.; Vahaji, S.; Salati, H. Wet surface wall model for latent heat exchange during evaporation. Int. J. Numer. Methods Biomed. Eng. 2022, 38, e3581. [Google Scholar] [CrossRef] [PubMed]
- Lembo, M.; Vedetta, C.; Moscato, U.; Del Gaudio, M. Thermal discomfort in healthcare workers during the COVID-19 pandemic. Med. Lav. 2021, 112, 123–129. [Google Scholar] [PubMed]
- Zhu, Y.; Mao, Y.; Li, Y.; Tang, T.; Jiang, H.; Qiao, S.; Lin, S.; Zheng, Z.; Fang, Z.; Chen, X. Field investigation of the heat stress in outdoor of healthcare workers wearing personal protective equipment in South China. Front. Public Health 2023, 11, 1166056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qiao, S.; Wu, W.; Li, Y.; Jian, H.; Lin, S.; Tang, T.; Zheng, Z.; Mao, Y.; Chen, X.; et al. Thermal discomfort caused by personal protective equipment in healthcare workers during the delta COVID-19 pandemic in Guangzhou, China: A case study. Case Stud. Therm. Eng. 2022, 34, 101971. [Google Scholar] [CrossRef]
- Bonell, A.; Nadjm, B.; Samateh, T.; Badjie, J.; Perry-Thomas, R.; Forrest, K.; Prentice, A.M.; Maxwell, N.S. Impact of personal cooling on performance, comfort and heat strain of healthcare workers in PPE, a study from west Africa. Front. Public Health 2021, 9, 712481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tokura, H.; Guo, Y.P.; Wong, A.S.W.; Wong, T.; Chung, J.; Newton, E. Effects of wearing N95 and surgical facemasks on heart rate, thermal stress and subjective sensations. Int. Arch. Occup. Environ. Health 2005, 78, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, B.; Fernandes, S. Exercise with facemask; Are we handling a devil’s sword? A physiological hypothesis. Med. Hypotheses 2020, 144, 110002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Poon, E.T.; Wan, K.; Dai, Z.; Wong, S.H. Effects of wearing a mask during exercise on physiological and psychological outcomes in healthy individuals: A systematic review and meta-analysis. Sports Med. 2023, 53, 125–150. [Google Scholar] [CrossRef]
- Person, E.; Lemercier, C.; Royer, A.; Reychler, G. Effect of a surgical mask on six minute walking distance. Rev. Mal. Respir. 2018, 35, 264–268. [Google Scholar] [CrossRef]
- Jesus, J.P.; Gomes, M.; Dias-Gonçalves, A.; Correia, J.M.; Pezarat-Correia, P.; Mendonca, G.V. Effects of surgical masks on the responses to constant work-rate cycling performed at different intensity domains. Clin. Physiol. Funct. Imaging 2022, 42, 43–52. [Google Scholar] [CrossRef]
- Pimenta, T.; Tavares, H.; Ramos, J.; Oliveira, M.; Reis, D.; Amorim, H.; Rocha, A. Facemasks during aerobic exercise: Implications for cardiac rehabilitation programs during the COVID-19 pandemic. Rev. Port. Cardiol. 2021, 40, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lee, C.W. Physiological effects of N95 respirators on rescuers during cardiopulmonary resuscitation. Heliyon 2023, 9, e18970. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, B.; Seibt, R.; Gabriel, J.; Bär, M.; Dilek, Ü.; Brandt, A.; Martus, P.; Rieger, M.A. Influence of face masks on physiological and subjective response during 130 min of simulated light and medium physical manual work—An explorative study. Healthcare 2023, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Thi Ngoc Le, H.; Xuan Nguyen, K.; Duy Nguyen, T.; Quang La, H.; Thanh Nguyen, X.; Tien Le, D.; Quoc Pham, H.; Xuan Nguyen, K.; Hoang Nguyen, H.; Van Pham, V.; et al. Effects of three-hour wearing personal protective equipment on heart rate variability in healthcare workers for the treatment of COVID-19 patients. Int. J. Gen. Med. 2023, 16, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, V.; Marek, E.-M.; Sucker, K.; Jettkant, B.; Kendzia, B.; Strauß, B.; Ulbrich, M.; Deckert, A.; Berresheim, H.; Eisenhawer, C.; et al. Influence of face masks on the subjective impairment at different physical workloads. Sci. Rep. 2023, 13, 8133. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, G.; He, Y.; Zhang, Z.; Ding, Y. Effects of wearing masks on human health and comfort during the COVID-19 pandemic. IOP Conf. Ser. Earth Environ. Sci. 2020, 531, 012034. [Google Scholar] [CrossRef]
- Hopkins, S.R.; Dominelli, P.B.; Davis, C.K.; Guenette, J.A.; Luks, A.M.; Molgat-Seon, Y.; Sá, R.C.; Sheel, A.W.; Swenson, E.R.; Stickland, M.K. Face masks and the cardiorespiratory response to physical activity in health and disease. Ann. Am. Thorac. Soc. 2021, 18, 399–407. [Google Scholar] [CrossRef]
- Wang, J.; Xi, J.; Han, P.; Wongwiset, N.; Pontius, J.; Dong, H. Computational analysis of a flapping uvula on aerodynamics and pharyngeal wall collapsibility in sleep apnea. J. Biomech. 2019, 94, 88–98. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Z.; Nevorski, D.; White, T.; Zhou, Y. Nasal and olfactory deposition with normal and bidirectional intranasal delivery techniques: In vitro tests and numerical simulations. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 118–131. [Google Scholar] [CrossRef]
- Xi, J.; Si, X.A.; Nagarajan, R. Effects of mask-wearing on the inhalability and deposition of airborne SARS-CoV-2 aerosols in human upper airway. Phys. Fluids 2020, 32, 123312. [Google Scholar] [CrossRef]
- Xi, J.; Kim, J.; Si, X.; Corley, R.A.; Zhou, Y. Modeling of inertial depositions in scaled models of rat and human nasal airways: Towards in vitro regional dosimetry in small animals. J. Aerosol Sci. 2016, 99, 78–93. [Google Scholar] [CrossRef]
- Xi, J.; Longest, P.W. Numerical predictions of submicrometer aerosol deposition in the nasal cavity using a novel drift flux approach. Int. J. Heat Mass Transf. 2008, 51, 5562–5577. [Google Scholar] [CrossRef]
- Xi, J.; Longest, P. Effects of oral airway geometry characteristics on the diffusional deposition of inhaled nanoparticles. J. Biomech. Eng. 2008, 130, 011008. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.N.J. Side-leakage of face mask. Eur. Phys. J. E Soft Matter. 2021, 44, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.; Solano, T.; Shoele, K. Bridge the gap: Correlate face mask leakage and facial features with 3D morphable face models. J. Expo. Sci. Environ. Epidemiol. 2021, 32, 735–743. [Google Scholar] [CrossRef]
- Lee, K.P.; Yip, J.; Kan, C.W.; Chiou, J.C.; Yung, K.F. Reusable face masks as alternative for disposable medical masks: Factors that affect their wear-comfort. Int. J. Environ. Res. Public Health 2020, 17, 6623. [Google Scholar] [CrossRef] [PubMed]
- Oner, E.; Seçkin, A.; Egeli, D.; Seçkin, M. Investigation of the thermal comfort properties of masks used during the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2022, 19, 11275. [Google Scholar] [CrossRef]
- Noto, T.; Zhou, G.; Schuele, S.; Templer, J.; Zelano, C. Automated analysis of breathing waveforms using BreathMetrics: A respiratory signal processing toolbox. Chem. Senses 2018, 43, 583–597. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Cai, L.; Song, A.Y.; Wu, P.; Hsu, P.-C.; Peng, Y.; Chen, J.; Liu, C.; Catrysse, P.B.; Liu, Y.; Yang, A.; et al. Warming up human body by nanoporous metallized polyethylene textile. Nat. Commun. 2017, 8, 496. [Google Scholar] [CrossRef]
- Li, C.; Guan, G.; Reif, R.; Huang, Z.; Wang, R.K. Determining elastic properties of skin by measuring surface waves from an impulse mechanical stimulus using phase-sensitive optical coherence tomography. J. R. Soc. Interface 2012, 9, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, D.C. Formulation of the k-w Turbulence model revisited. AIAA J. 2008, 46, 2823–2838. [Google Scholar] [CrossRef]
- Szanyi, M.L.; Hemmingsen, C.S.; Yan, W.; Walther, J.H.; Glimberg, S.L. Near-wellbore modeling of a horizontal well with Computational Fluid Dynamics. J. Pet. Sci. Eng. 2018, 160, 119–128. [Google Scholar] [CrossRef]
- Xi, J.; Longest, P.W. Transport and deposition of micro-aerosols in realistic and simplified models of the oral airway. Ann. Biomed. Eng. 2007, 35, 560–581. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Talaat, M.; Xi, J. SARS-CoV-2 virus-laden droplets coughed from deep lungs: Numerical quantification in a single-path whole respiratory tract geometry. Phys. Fluids 2021, 33, 023306. [Google Scholar]
- Xi, J.; Zhang, Z.; Si, X. Improving intranasal delivery of neurological nanomedicine to the olfactory region using magnetophoretic guidance of microsphere carriers. Int. J. Nanomed. 2015, 10, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Staymates, M. Flow visualization of an N95 respirator with and without an exhalation valve using schlieren imaging and light scattering. Phys. Fluids 2020, 32, 111703. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Liebner, T.J.; Craven, B.A.; Settles, G.S. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J. R. Soc. Interface 2009, 6 (Suppl. S6), S727–S736. [Google Scholar] [CrossRef]
- Taylor, S.; Asmundson, G.J.G. Negative attitudes about facemasks during the COVID-19 pandemic: The dual importance of perceived ineffectiveness and psychological reactance. PLoS ONE 2021, 16, e0246317. [Google Scholar] [CrossRef]
- Brooks, J.T.; Beezhold, D.H.; Noti, J.D.; Coyle, J.P.; Derk, R.C.; Blachere, F.M.; Lindsley, W.G. Maximizing fit for cloth and medical procedure masks to improve performance and reduce SARS-CoV-2 transmission and exposure, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 254–257. [Google Scholar] [CrossRef]
- Abaluck, J.; Kwong, L.H.; Styczynski, A.; Haque, A.; Kabir, M.A.; Bates-Jefferys, E.; Crawford, E.; Benjamin-Chung, J.; Raihan, S.; Rahman, S.; et al. Impact of community masking on COVID-19: A cluster-randomized trial in Bangladesh. Science 2022, 375, eabi9069. [Google Scholar] [CrossRef] [PubMed]
- Van der Westhuizen, H.M.; Kotze, K.; Tonkin-Crine, S.; Gobat, N.; Greenhalgh, T. Face coverings for covid-19: From medical intervention to social practice. BMJ 2020, 370, m3021. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, M.; Lim, W.W.; Yeung, A.; Adam, D.C.; Ali, S.T.; Lau, E.H.Y.; Wu, P.; Yuen, K.-Y.; Leung, G.M.; Cowling, B.J. COVID-19 transmission in Hong Kong despite universal masking. J. Infect. 2021, 83, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Bundgaard, J.S.; Raaschou-Pedersen, D.E.T.; von Buchwald, C.; Todsen, T.; Norsk, J.B.; Pries-Heje, M.M.; Vissing, C.R.; Nielsen, P.B.; Winsløw, U.C.; et al. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in Danish mask wearers: A randomized controlled trial. Ann. Intern. Med. 2021, 174, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, H.; Cao, L.; Lai, Z.; Cheng, Z.; Chen, Q.; Liu, T.; Liu, X.; Wen, Y.; Xu, C.; et al. Genetic analysis of a SARS-CoV-2 Omicron variant from a Chinese traveller returning from overseas. Emerg. Microbes. Infect. 2022, 11, 306–309. [Google Scholar] [CrossRef]
- Schmidt, N.B.; Richey, J.A.; Cromer, K.R.; Buckner, J.D. Discomfort intolerance: Evaluation of a potential risk factor for anxiety psychopathology. Behav. Ther. 2007, 38, 247–255. [Google Scholar] [CrossRef]
- Xu, Q.; Mao, Z.; Wei, D.; Fan, K.; Liu, P.; Wang, J.; Wang, X.; Lou, X.; Lin, H.; Wang, C.; et al. Association between mask wearing and anxiety symptoms during the outbreak of COVID 19: A large survey among 386,432 junior and senior high school students in China. J. Psychosom. Res. 2022, 153, 110709. [Google Scholar] [CrossRef]
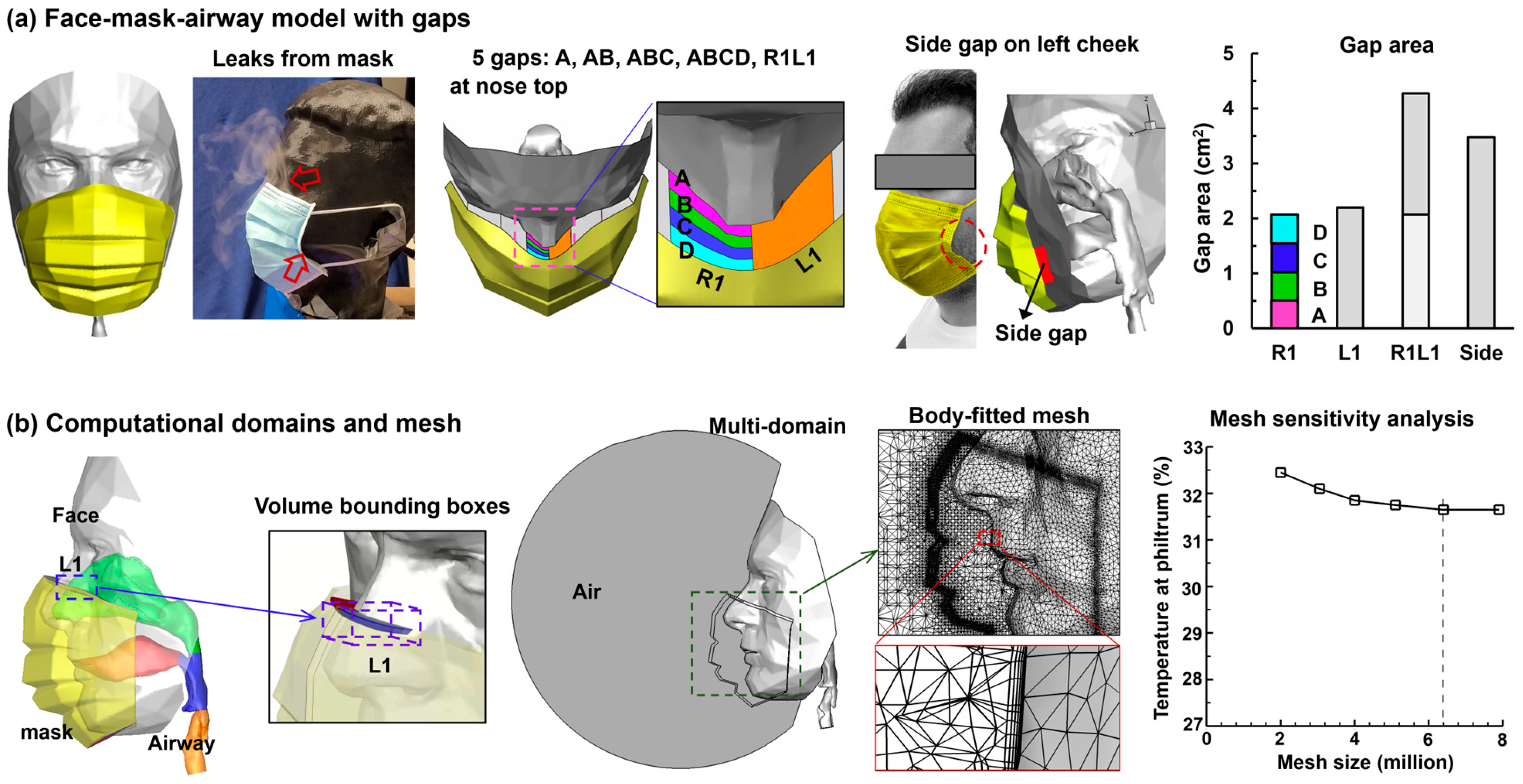
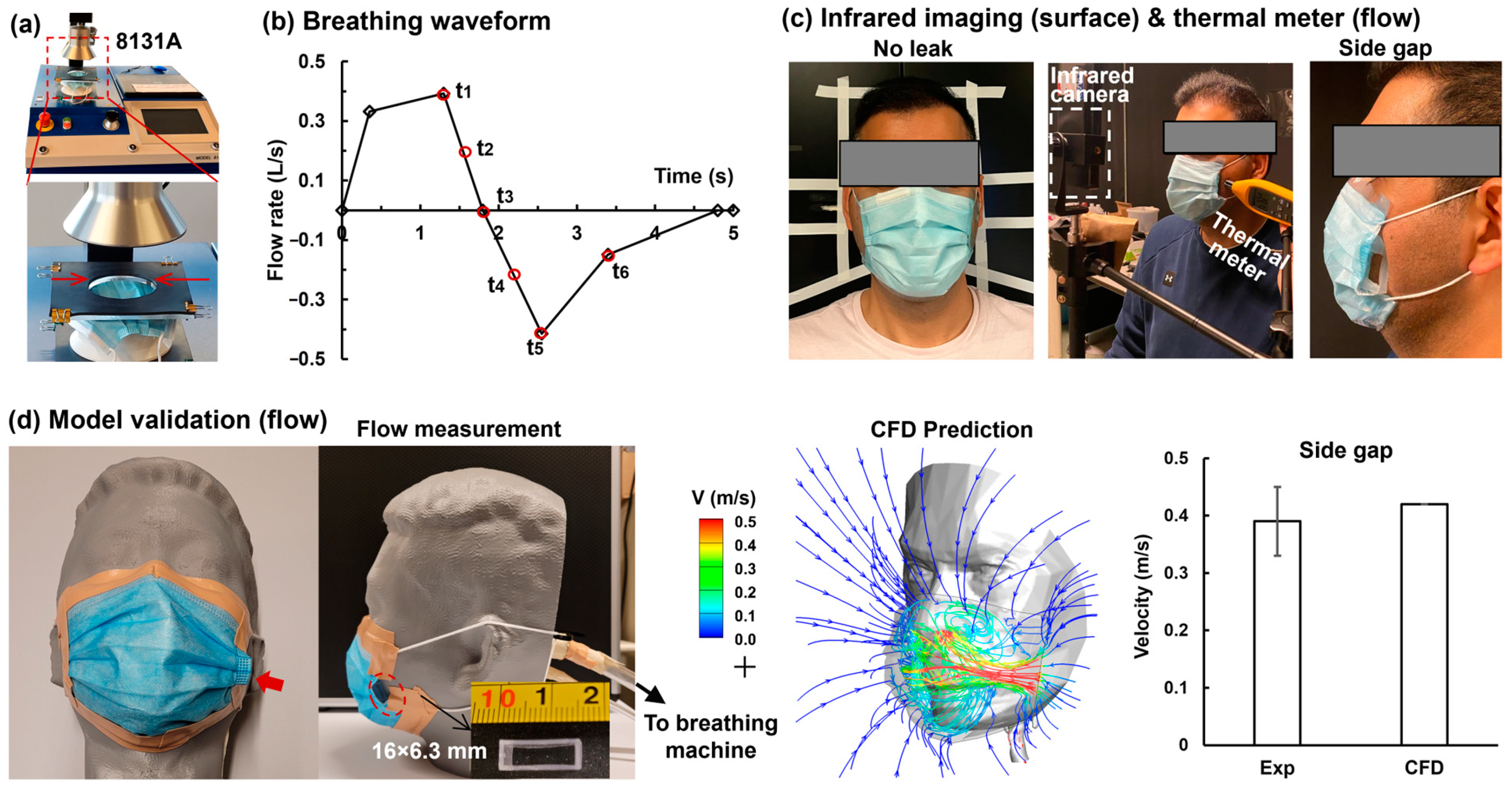


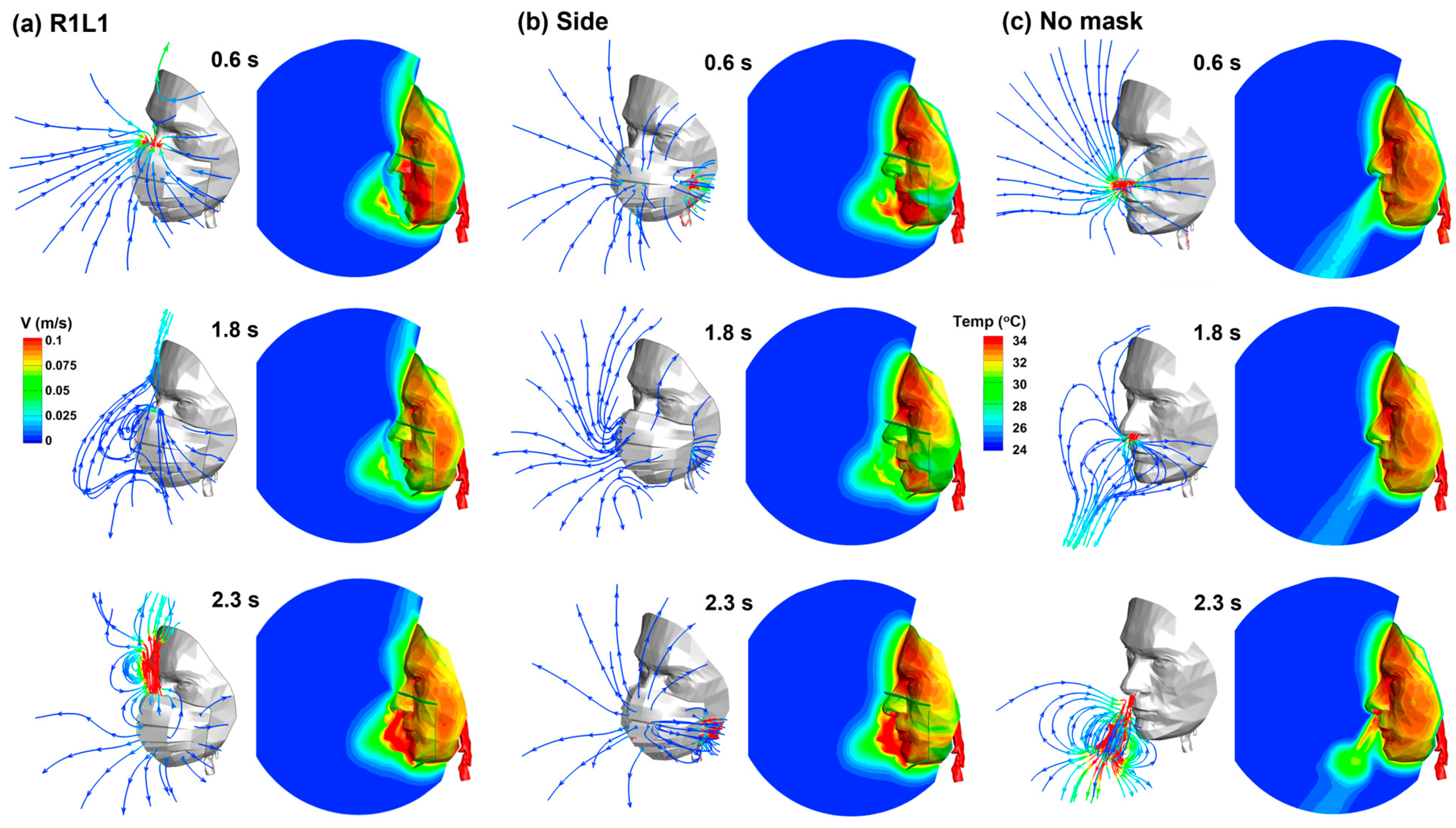

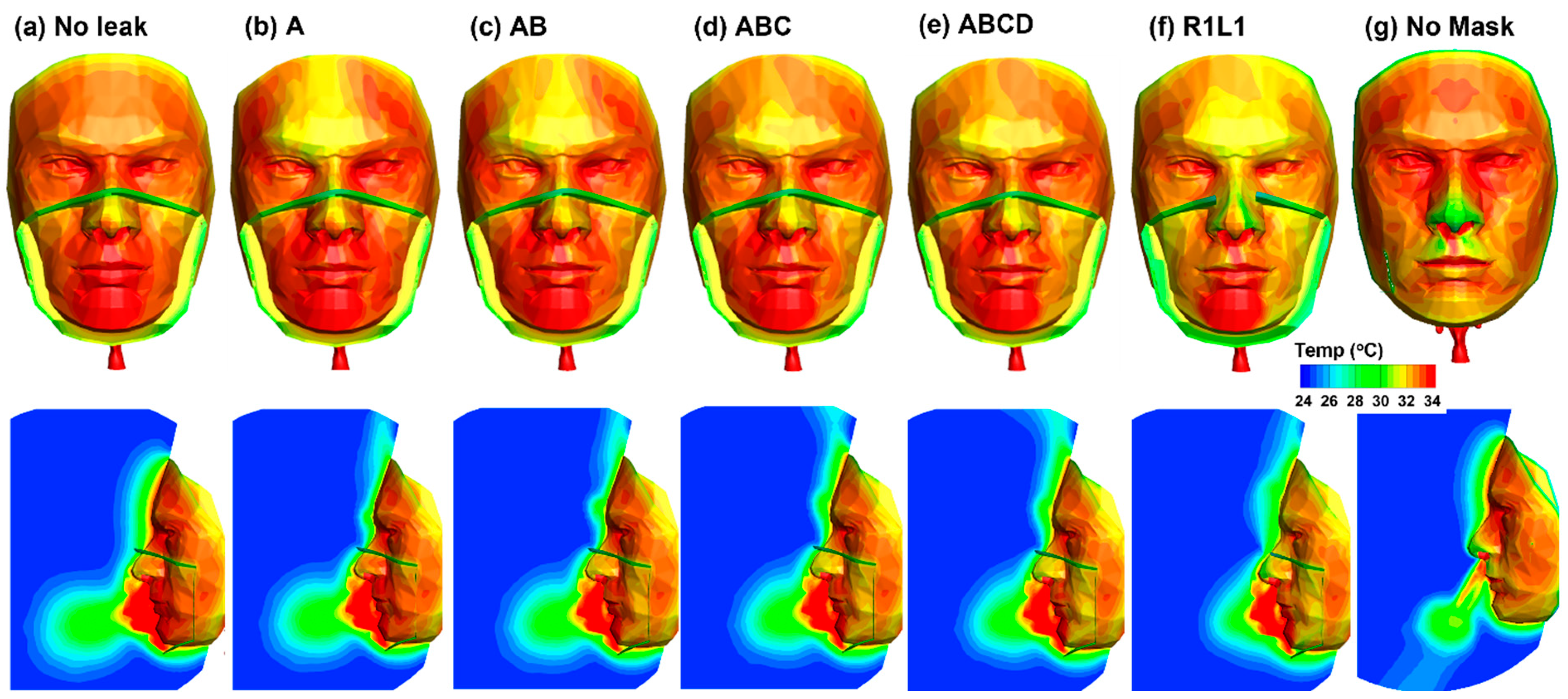



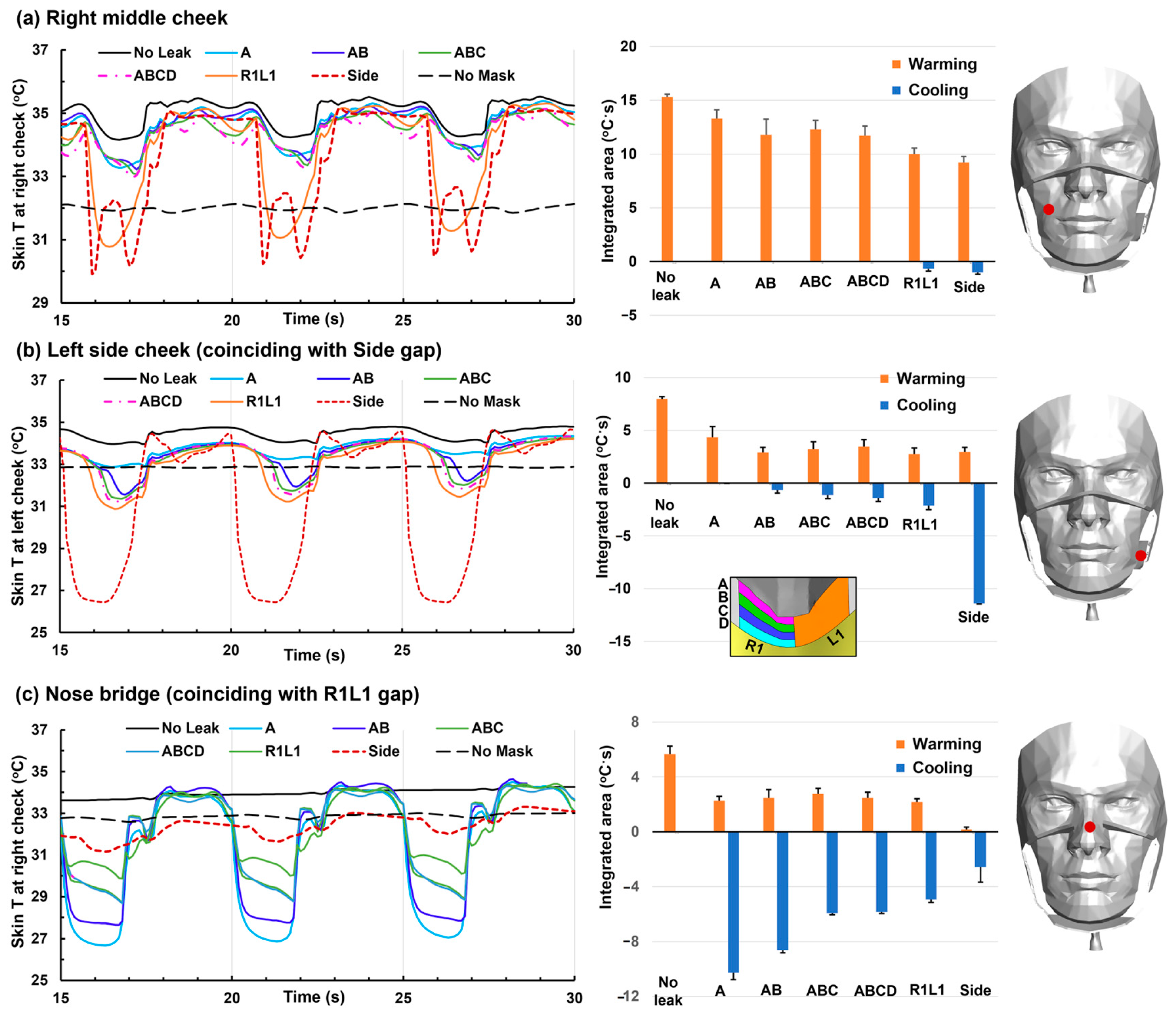
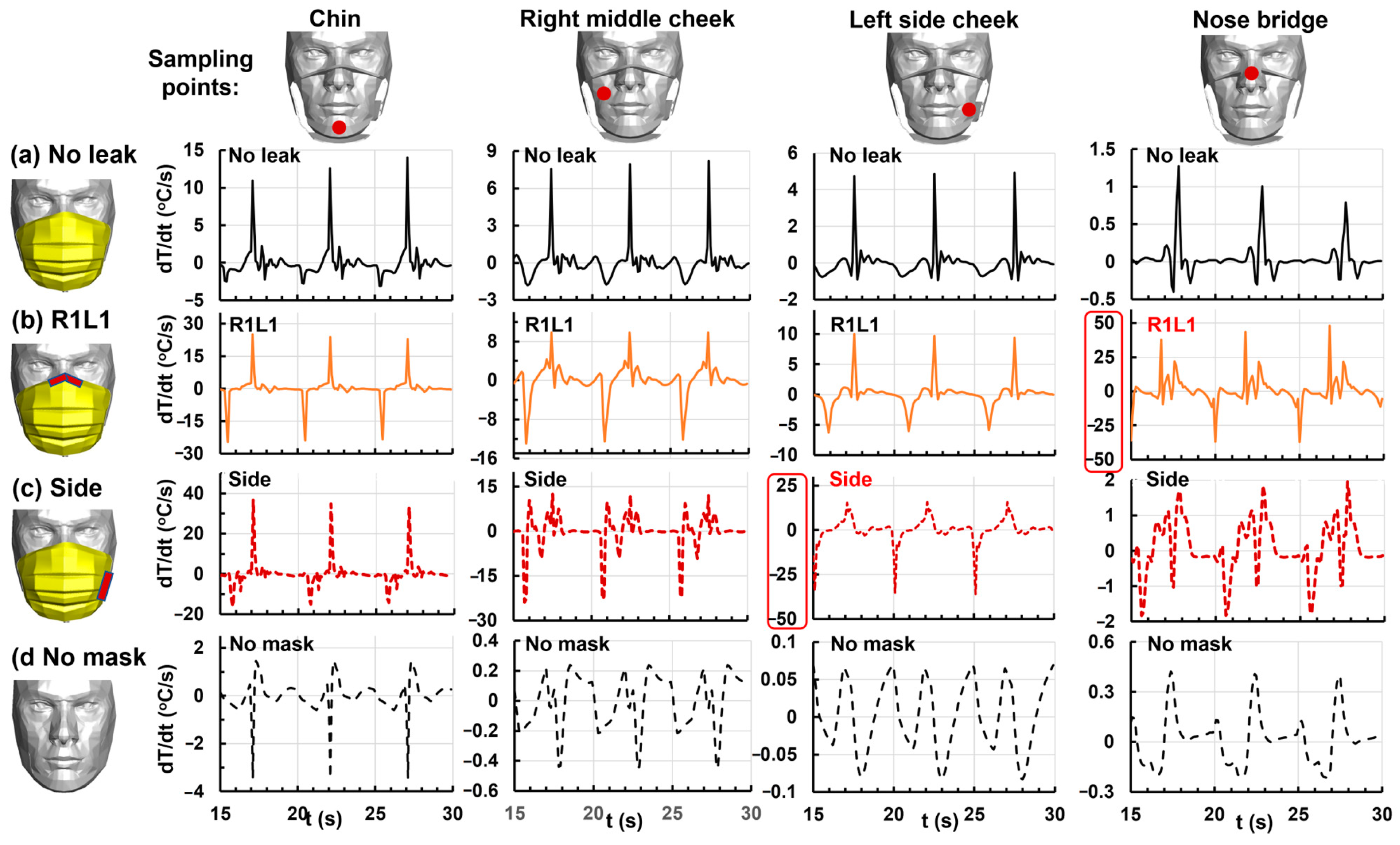
| Gap | No Leak | A | AB | ABC | ABCD | R1L1 | Side |
|---|---|---|---|---|---|---|---|
| A (cm2) | 0 | 0.51 | 1.02 | 1.55 | 2.07 | 4.27 | 3.48 |
| Gap leak (%) | 0 | 5.6 | 11.1 | 16.2 | 21.5 | 41.7 | 42.8 |
| Mask front (%) | 82.6 | 77.9 | 73.2 | 68.9 | 64.3 | 48.7 | 48.1 |
| Interface (%) | 17.4 | 16.5 | 15.7 | 14.9 | 14.2 | 9.6 | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barari, K.; Si, X.; Xi, J. Impacts of Mask Wearing and Leakages on Cyclic Respiratory Flows and Facial Thermoregulation. Fluids 2024, 9, 9. https://doi.org/10.3390/fluids9010009
Barari K, Si X, Xi J. Impacts of Mask Wearing and Leakages on Cyclic Respiratory Flows and Facial Thermoregulation. Fluids. 2024; 9(1):9. https://doi.org/10.3390/fluids9010009
Chicago/Turabian StyleBarari, Kian, Xiuhua Si, and Jinxiang Xi. 2024. "Impacts of Mask Wearing and Leakages on Cyclic Respiratory Flows and Facial Thermoregulation" Fluids 9, no. 1: 9. https://doi.org/10.3390/fluids9010009
APA StyleBarari, K., Si, X., & Xi, J. (2024). Impacts of Mask Wearing and Leakages on Cyclic Respiratory Flows and Facial Thermoregulation. Fluids, 9(1), 9. https://doi.org/10.3390/fluids9010009







