Versatile Polysaccharides for Application to Semi-Solid and Fluid Foods: The Pectin Case
Abstract
:1. Introduction
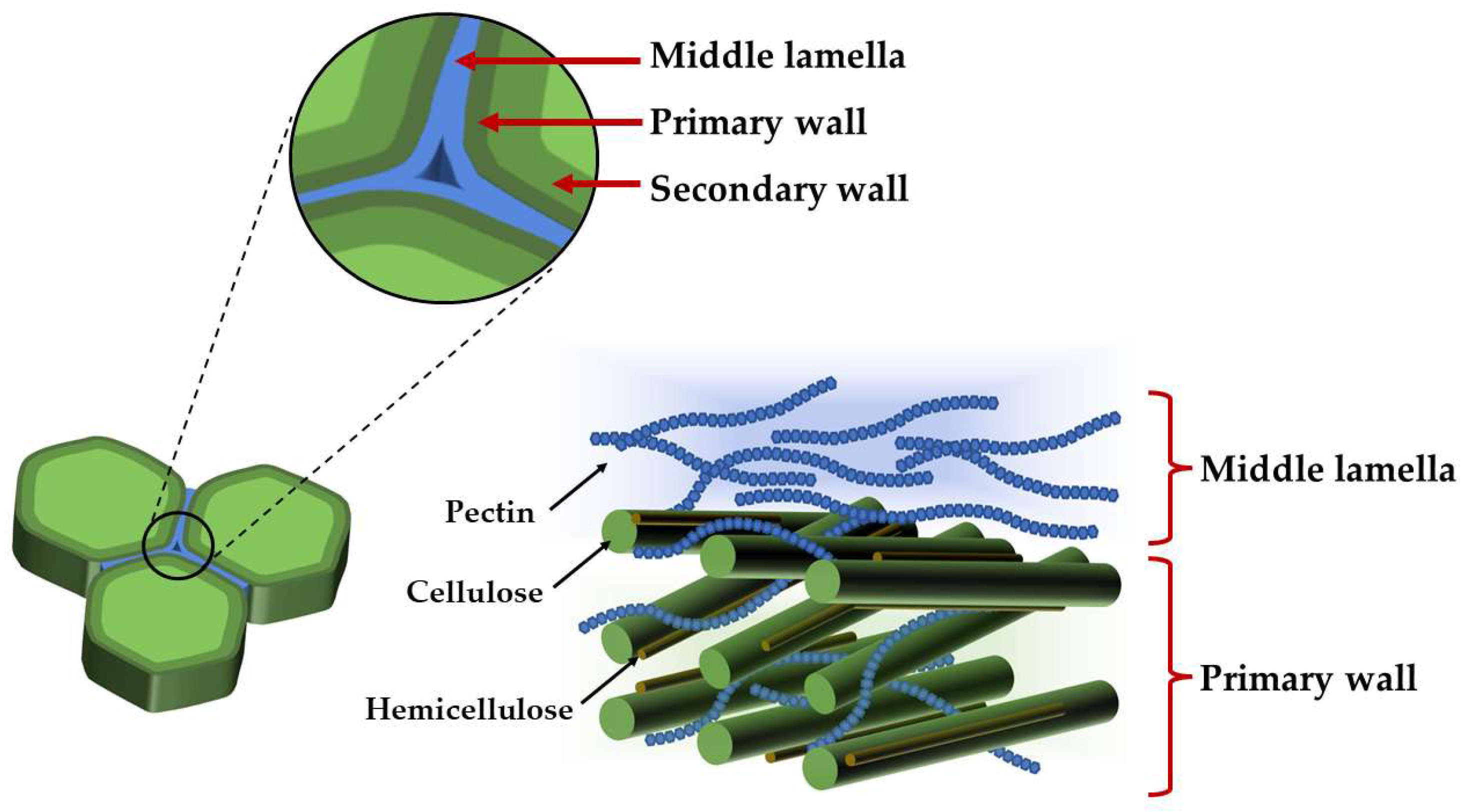
2. Chemical Structure of Pectin—A Heterogeneous Polysaccharide
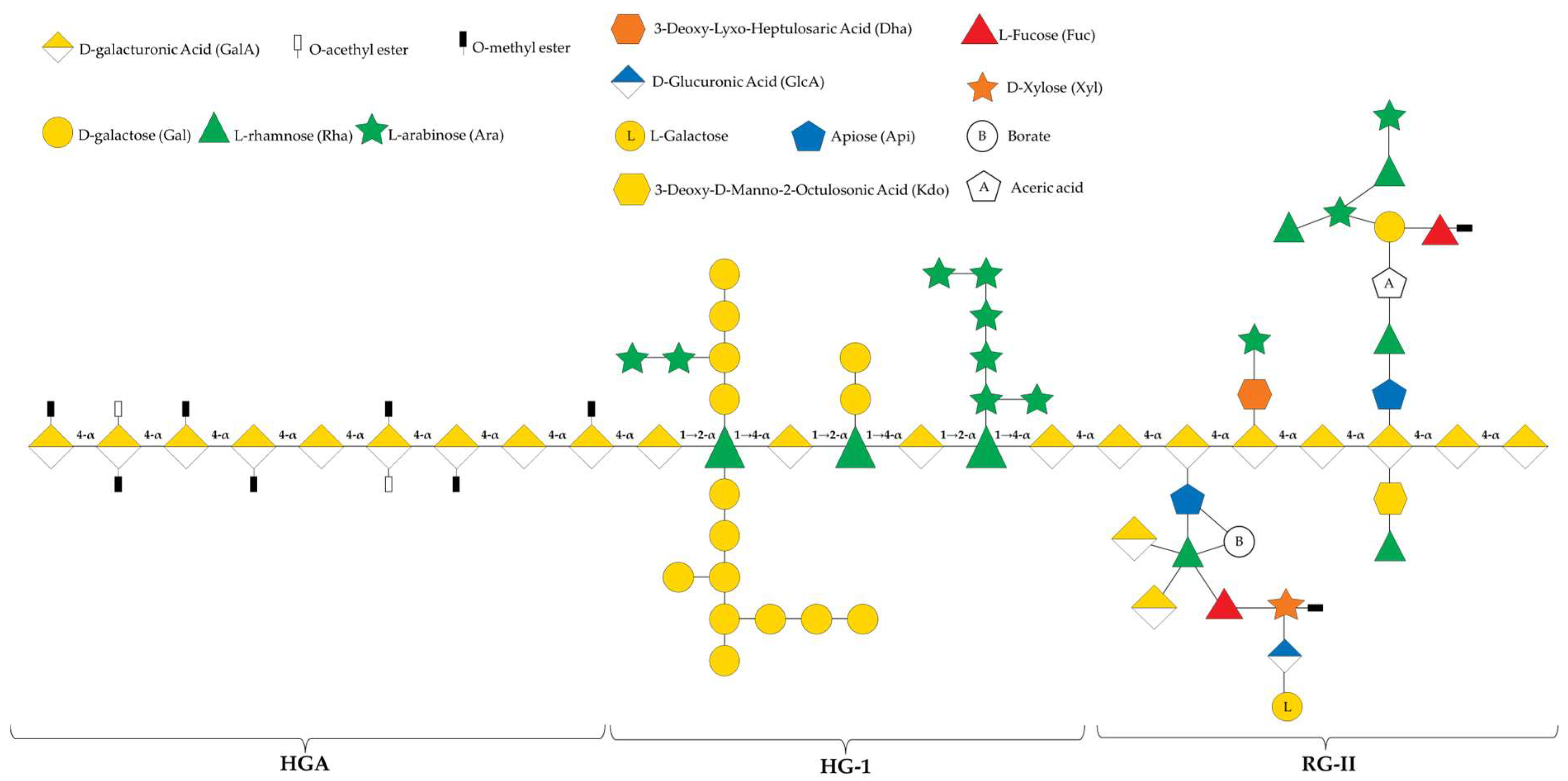
2.1. Homogalacturonan
2.2. Rhamnogalacturonan-I
2.3. Rhamnogalacturonan-II
3. Pectin and Its Potential Sources
| Type of by-Products | Extraction Methods | Optimized Parameters | Yield (%) * | References |
|---|---|---|---|---|
| Artichoke by-products | UAE + EE (Celluclast) | 20 KHz, 30% amplitude, pulse (2 s on/1 s off), pH: 5.0, 200 rpm, 6 h, 50 °C, S:L * 1:15 | 13.9 | [79] |
| Banana peels | UAE | 40 KHz, 185 W, pH: 3.68, 200 rpm, 17.12 min, 33.12 °C, S:L 1:12 | 2.62 | [80] |
| Berry fruits (raspberry) | CE | 58 °C, 60 min, orbital shaking, 250 rpm, pH: 3, citric acid, S:L 1:4 | 8.8 | [81] |
| Berry fruits (raspberry) | EE (Celluclast) | 40 °C, 90 min, orbital shaking, 200 rpm, pH: 5, S:L 1:20 | 9.9 | [81] |
| Dragon fruit peels | UAE | 37 KHz, 330 W, pH: 2, 25 min, 65 °C, S:L 1:20 | 6.27 | [82] |
| Eggplant peels | UAE | 50 W, pH: 2.25, 30 min | 27.60 | [83] |
| Jabuticaba peels | UAE + Heating 40 °C + Microwave reactor | 20 KHz, 500 W, 15 min + 40 °C + 150 W, 3 min, pH: 1.8, S:L 1:29 | 17.79 | [84] |
| Pistachio hulls | CE | 90 °C, 30 min, magnetic stirrer, 200 rpm, pH: 0.5, S:L 1:50 | 32.3 | [85] |
| Citrus limetta peels | UAE | 20 KHz, 500 W, 37% amplitude, pulse (15 s on/15 s off) pH: 1.9, 24 min, 40 °C, S:L 1:30 | 28.73 | [86] |
| Grapefruit and or/tangerine wastes | UAE | 20 KHz, 130 W, 90% amplitude, pulse (5 min on/2 min off) pH: 2.5, 30 min (grapefruit), 15 min (tangerine), 80 °C, S:L 1:30 | 26.05 and or/13.46 | [87] |
| Papaya pulp (fourth day after harvest) | CE | 30 min, magnetic stirrer, 80% boiling ethanol, S:L 1:40 | 35.45 | [78] |
| Apple pomace | CE | 90 °C, 120 min, pH: 1.5, citric acid, S:L 1:10 | 38.91 | [88] |
| Apple pomace | MAE | 420 W, pH: 1.5, 120 s, S:L 1:15 | 45.15 | [88] |
| Orange peels | CE | 500 rpm, 1 h, 65 °C, citric acid | 19.65 | [89] |
| Custard apple peels | UAE | 20 KHz, 70% amplitude, pH: 2.3, 18.04 min, 63.22 °C, S:L 1:24 | 8.93 | [90] |
| Cocoa pod husks | MAE | 400 W, pH: 1.16, 15 min, S:L 1:25 | 9.64 | [91] |
| Watermelon rinds | CE | 95 °C, 90 min, pH: 1.36, magnetic stirring, S:L 1:20 | 13.4 | [92] |
| Mango peels | MAE | 606 W, pH: 1.83, 5.15 min, S:L 1:20 | 18.94 | [93] |
| Pequi mesocarp | CE | 80 °C, 160 min, magnetic stirring, 1500 rpm, citric acid, S:L 1:31 | 26.6 | [94] |
| Pineapple peels | CE | 95 °C, 60 min, water bath, pH: 2.2–2.4, citric acid, S:L 1:40 | 1.02 | [95] |
| Pineapple peels | ME * | 420 W, 85–90 °C, pH: 2.2–2.4, citric acid, 60 min, S:L 1:40 | 2.12 | [95] |
| Persimmon peels | CE | 90 °C, 120 min, water bath, 500 rpm, pH: 2, citric acid, S:L 1:20 | NA | [96] |
| Durian rinds | CE | 93.3 °C, 185 min, shaking water bath, 90 rpm, S:L 1:50 | 12.12 | [97] |
| Cinnamomum cassia barks | MAE | 600 W, pH: 2, 3 min, S:L 1:40 | 13.48 | [98] |
4. Pectin as a Thickening and Gelling Agent and Its Application to Semi-Solid and Fluid Foods
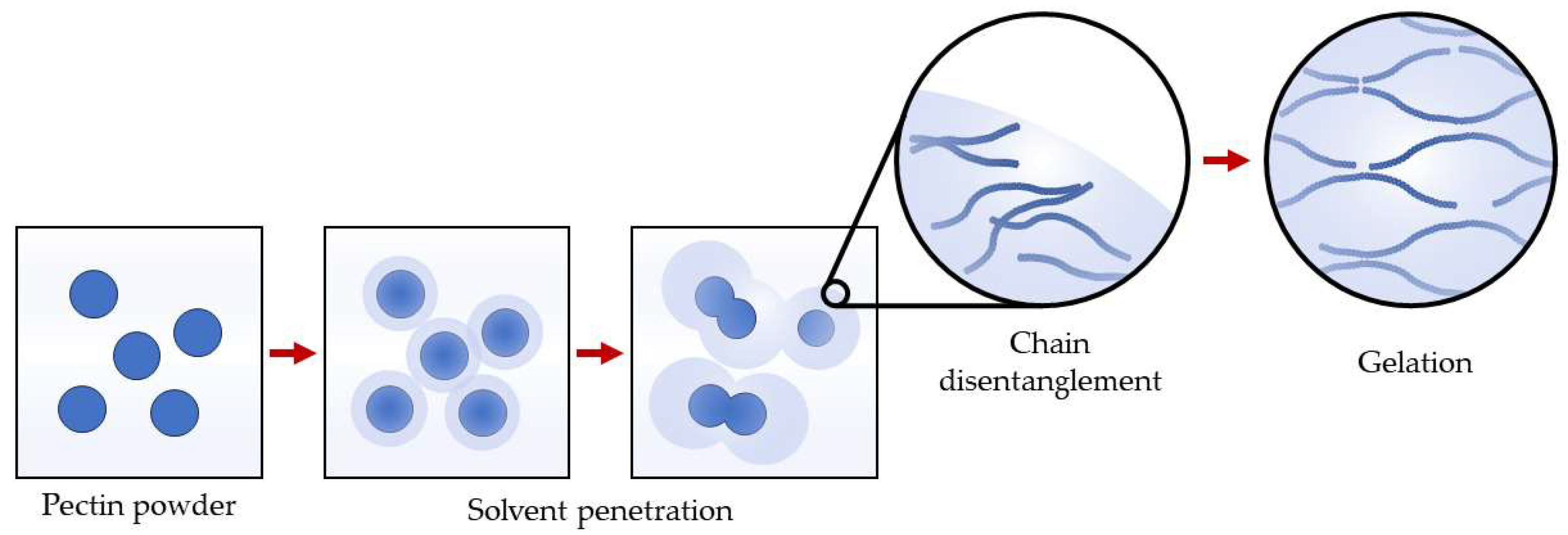
Water Inclusion Ability of Pectin
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knorr, D.; Watzke, H. Food Processing at a Crossroad. Front. Nutr. 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Petrus, R.R.; Sobral, P.J.D.; Tadini, C.C.; Goncalves, C.B. The NOVA classification system: A critical perspective in food science. Trends Food Sci. Technol. 2021, 116, 603–608. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.; Windhab, E.J. Rheology of food materials. Curr. Opin. Colloid Interface Sci. 2011, 16, 36–40. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid and Semisolid Foods: Principles and Aplications; Springer: New York, NY, USA, 2007. [Google Scholar]
- Stanley, N.L.; Taylor, L.J. Rheological basis of oral characteristics of fluid and semisolid foods—A review. Acta Psychol. 1993, 84, 79–92. [Google Scholar] [CrossRef]
- Murray, B.S.; Ettelaie, R.; Sarkar, A.; Mackie, A.R.; Dickinson, E. The perfect hydrocolloid stabilizer: Imagination versus reality. Food Hydrocoll. 2021, 117, 106696. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.Q.; Li, X.X.; Sun, L.J.; Guo, Y.R. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Introduction to food hydrocolloids. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–26. [Google Scholar]
- Garti, N.; Reichman, D.; Hendrickx, H.; Dickinson, E.; Jackson, L.K.; Bergenstahl, B. Hydrocolloids as food emulsifiers and stabilizers. Food Struct. 1993, 12, 411–426. [Google Scholar]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Tang, Q.L.; Huang, G.L. Improving method, properties and application of polysaccharide as emulsifier. Food Chem. 2022, 376, 131937. [Google Scholar] [CrossRef]
- Zhao, R.X.; Qi, J.R.; Liu, Q.R.; Zeng, W.Q.; Yang, X.Q. Fractionation and characterization of soluble soybean polysaccharide esterified of octenyl succinic anhydride and its effect as a stabilizer in acidified milk drinks. Food Hydrocoll. 2018, 85, 215–221. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E.; Mazoyer, J.; Langendorff, V. Emulsifying properties of depolymerized citrus pectin: Role of the protein fraction. In Gums and Stabilisers for the Food Industry 11; The Royal Society of Chemistry: Cambridge, UK, 2002; pp. 311–317. [Google Scholar]
- Einhorn-Stoll, U. Pectin-water interactions in foods—From powder to gel. Food Hydrocoll. 2018, 78, 109–119. [Google Scholar] [CrossRef]
- Kravtchenko, T.P.; Renoir, J.; Parker, A.; Brigand, G. A novel method for determining the dissolution kinetics of hydrocolloid powders. Food Hydrocoll. 1999, 13, 219–225. [Google Scholar] [CrossRef]
- Kurita, O.; Miyake, Y.; Yamazaki, E. Chemical modification of citrus pectin to improve its dissolution into water. Carbohydr. Polym. 2012, 87, 1720–1727. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Benthin, A.; Zimathies, A.; Gorke, O.; Drusch, S. Pectin-water interactions: Comparison of different analytical methods and influence of storage. Food Hydrocoll. 2015, 43, 577–583. [Google Scholar] [CrossRef]
- Cuq, B.; Rondet, E.; Abecassis, J. Food powders engineering, between knowhow and science: Constraints, stakes and opportunities. Powder Technol. 2011, 208, 244–251. [Google Scholar] [CrossRef]
- Shah, U.V.; Karde, V.; Ghoroi, C.; Heng, J.Y.Y. Influence of particle properties on powder bulk behaviour and processability. Int. J. Pharm. 2017, 518, 138–154. [Google Scholar] [CrossRef]
- Dacanal, G.C.; Menegalli, F.C. Selection of operational parameters for the production of instant soy protein isolate by pulsed fluid bed agglomeration. Powder Technol. 2010, 203, 565–573. [Google Scholar] [CrossRef]
- Hirata, T.A.M.; Dacanal, G.C.; Menegalli, F.C. Effect of operational conditions on the properties of pectin powder agglomerated in pulsed fluid bed. Powder Technol. 2013, 245, 174–181. [Google Scholar] [CrossRef]
- McCann, M.C.; Roberts, K. Plant cell wall architecture: The role of pectins. Pectins Pectinases 1996, 14, 91–107. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Dey, P.M.; Brinson, K. Plant cell-walls. Adv. Carbohydr. Chem. Biochem. 1984, 42, 265–382. [Google Scholar] [CrossRef]
- Wang, W.J.; Chen, W.J.; Zou, M.M.; Lv, R.L.; Wang, D.L.; Hou, F.R.; Feng, H.; Ma, X.B.; Zhong, J.J.; Ding, T.; et al. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Brownleader, M.D.; Jackson, P.; Mobasheri, A.; Pantelides, A.T.; Sumar, S.; Trevan, M.; Dey, P.M. Molecular aspects of cell wall modifications during fruit ripening. Crit. Rev. Food Sci. Nutr. 1999, 39, 149–164. [Google Scholar] [CrossRef]
- Willats, W.G.T.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Liu, J.N.; Bi, J.F.; McClements, D.J.; Liu, X.; Yi, J.Y.; Lyu, J.; Zhou, M.; Verkerk, R.; Dekker, M.; Wu, X.Y.; et al. Impacts of thermal and non-thermal processing on structure and functionality of pectin in fruit-and vegetable-based products: A review. Carbohydr. Polym. 2020, 250, 116890. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.M.; Li, T.; Liang, R.H.; Luo, S.J. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. An Introduction to Pectins: Structure and Properties; ACS Publications: Washington, DC, USA, 1986. [Google Scholar]
- Durand, D.; Bertrand, C.; Clark, A.H.; Lips, A. Calcium-induced gelation of low methoxy pectin solutions—Thermodynamic and rheological considerations. Int. J. Biol. Macromol. 1990, 12, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Almagro, N.; Garrido-Galand, S.; Taladrid, D.; Moreno-Arribas, M.V.; Villamiel, M.; Montilla, A. Use of natural low-methoxyl pectin from sunflower by-products for the formulation of low-sucrose strawberry jams. J. Sci. Food Agric. 2022, 102, 5957–5964. [Google Scholar] [CrossRef]
- Albersheim, P.; Darvill, A.G.; Oneill, M.A.; Schols, H.A.; Voragen, A.G.J. An hypothesis: The same six polysaccharides are components of the primary cell walls of all higher plants. Pectins Pectinases 1996, 14, 47–55. [Google Scholar] [CrossRef]
- Lau, J.M.; McNeil, M.; Darvill, A.G.; Albersheim, P. Structure of the backbone of rhamnogalacturonan-I, a pectic polysaccharide in the primary-cell walls of plants. Carbohydr. Res. 1985, 137, 111–125. [Google Scholar] [CrossRef]
- Chen, S.G.; Zheng, J.Q.; Zhang, L.M.; Cheng, H.; Orfila, C.; Ye, X.Q.; Chen, J.L. Synergistic gelling mechanism of RG-I rich citrus pectic polysaccharide at different esterification degree in calcium-induced gelation. Food Chem. 2021, 350, 129177. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Guo, Y.; Zhang, Z.H.; Jia, L.R.; Gao, H.; Xing, Z.H.; Duan, F.X. Okra polysaccharides reduced the gelling-required sucrose content in its synergistic gel with high-methoxyl pectin by microphase separation effect. Food Hydrocoll. 2019, 95, 506–516. [Google Scholar] [CrossRef]
- Wan, L.; Wang, H.Y.; Zhu, Y.; Pan, S.Y.; Cai, R.; Liu, F.X. Comparative study on gelling properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, atmospheric enzymatic, and alkaline de-esterification. Carbohydr. Polym. 2019, 226, 115285. [Google Scholar] [CrossRef]
- Wu, D.M.; Zheng, J.Q.; Mao, G.Z.; Hu, W.W.; Ye, X.Q.; Linhardt, R.J.; Chen, S.G. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. Nutr. 2020, 60, 2938–2960. [Google Scholar] [CrossRef]
- O’NEILL, M.; Albersheim, P.; Darvill, A. The pectic polysaccharides of primary cell walls. In Methods in Plant Biochemistry; Elsevier: Amsterdam, The Netherlands, 1990; Volume 2, pp. 415–441. [Google Scholar]
- Patova, O.A.; Golovchenko, V.V.; Ovodov, Y.S. Pectic polysaccharides: Structure and properties. Russ. Chem. Bull. 2014, 63, 1901–1924. [Google Scholar] [CrossRef]
- Stevenson, T.T.; Darvill, A.G.; Albersheim, P. 3-Deoxy-D-lyxo-2-heptulosaric acid, a component of the plant cell-wall polysaccharide rhamnogalacturonan-II. Carbohydr. Res. 1988, 179, 269–288. [Google Scholar] [CrossRef]
- Ovodov, Y.S. Current views on pectin substances. Russ. J. Bioorganic Chem. 2009, 35, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Neelamegham, S.; Aoki-Kinoshita, K.; Bolton, E.; Frank, M.; Lisacek, F.; Lutteke, T.; O’Boyle, N.; Packer, N.H.; Stanley, P.; Toukach, P.; et al. Updates to the Symbol Nomenclature for Glycans guidelines. Glycobiology 2019, 29, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Thibault, J.F.; Renard, C.; Axelos, M.A.V.; Roger, P.; Crepeau, M.J. Studies of the length of homogalacturonic regions in pectins by acid-hydrolysis. Carbohydr. Res. 1993, 238, 271–286. [Google Scholar] [CrossRef]
- MacDougall, A.J.; Needs, P.W.; Rigby, N.M.; Ring, S.G. Calcium gelation of pectic polysaccharides isolated from unripe tomato fruit. Carbohydr. Res. 1996, 293, 235–249. [Google Scholar] [CrossRef]
- Pauly, M.; Scheller, H.V. O-Acetylation of plant cell wall polysaccharides: Identification and partial characterization of a rhamnogalacturonan O-acetyl-transferase from potato suspension-cultured cells. Planta 2000, 210, 659–667. [Google Scholar] [CrossRef]
- Rombouts, F.M.; Thibault, J.F. Feruloylated pectic substances from sugar-beet pulp. Carbohydr. Res. 1986, 154, 177–187. [Google Scholar] [CrossRef]
- Kikuchi, A.; Edashige, Y.; Ishii, T.; Satoh, S. A xylogalacturonan whose level is dependent on the size of cell clusters is present in the pectin from cultured carrot cells. Planta 1996, 200, 369–372. [Google Scholar] [CrossRef]
- Yu, L.Y.; Mort, A.J. Partial characterization of xylogalacturonans from cell walls of ripe watermelon fruit: Inhibition of endopolygalacturonase activity by xylosylation. Pectins Pectinases 1996, 14, 79–88. [Google Scholar] [CrossRef]
- Schols, H.A.; Bakx, E.J.; Schipper, D.; Voragen, A.G.J. A xylogalacturonan subunit present in the modified hairy regions of apple pectin. Carbohydr. Res. 1995, 279, 265–279. [Google Scholar] [CrossRef]
- Longland, J.M.; Fry, S.C.; Trewavas, A.J. Developmental control of apiogalacturonan biosynthesis and udp-apiose production in a duckweed. Plant Physiol. 1989, 90, 972–976. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.; Darvill, A.G.; Albersheim, P. Structure of plant cell walls: X. Rhamnogalacturonan I, a structurally complex pectic polysaccharide in the walls of suspension-cultured sycamore cells. Plant Physiol. 1980, 66, 1128–1134. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary-cell walls in flowering plants—Consistency of molecular-structure with the physical-properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Guillon, F.; Thibault, J.F. Structural investigation of the neutral sugar side-chains of sugar-beet pectins. 1. methylation analysis and mild acid-hydrolysis of the hairy fragments of sugar-beet pectins. Carbohydr. Res. 1989, 190, 85–96. [Google Scholar] [CrossRef]
- Schols, H.A.; Voragen, A.G.J. Complex pectins: Structure elucidation using enzymes. Pectins Pectinases 1996, 14, 3–19. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Warrenfeltz, D.; Kates, K.; Pellerin, P.; Doco, T.; Darvill, A.G.; Albersheim, P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester: In vitro conditions for the formation and hydrolysis of the dimer. J. Biol. Chem. 1996, 271, 22923–22930. [Google Scholar] [CrossRef]
- May, C.D. Industrial pectins—Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Funami, T.; Zhang, G.Y.; Hiroe, M.; Noda, S.; Nakauma, M.; Asai, I.; Cowman, M.K.; Al-Assaf, S.; Phillips, G.O. Effects of the proteinaceous moiety on the emulsifying properties of sugar beet pectin. Food Hydrocoll. 2007, 21, 1319–1329. [Google Scholar] [CrossRef]
- Williams, P.A.; Sayers, C.; Viebke, C.; Senan, C.; Mazoyer, J.; Boulenguer, P. Elucidation of the emulsification properties of sugar beet pectin. J. Agric. Food Chem. 2005, 53, 3592–3597. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Wang, T.; Tao, Y.H.; Lai, C.H.; Huang, C.X.; Ling, Z.; Yong, Q. Influence of extraction methods on navel orange peel pectin: Structural characteristics, antioxidant activity and cytoprotective capacity. Int. J. Food Sci. Technol. 2023, 58, 1382–1393. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, J.X.; Chen, L.; Jin, S.; An, C.; Yang, B.; Schols, H.A.; de Vos, P.; Bai, W.B.; Tian, L.M. Effects of thermal treatments on the extraction and in vitro fermentation patterns of pectins from pomelo (Citrus grandis). Food Hydrocoll. 2023, 141, 108755. [Google Scholar] [CrossRef]
- Liu, N.; Yang, W.N.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.P.; Huang, L.Q.; Gao, W.Y. Comparison of characterization and antioxidant activity of different citrus peel pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef]
- Das, I.; Arora, A. One stage hydrothermal treatment: A green strategy for simultaneous extraction of food hydrocolloid and co-products from sweet lime (Citrus limetta) peels. Food Hydrocoll. 2023, 134, 107947. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical properties of pectin from Malus domestica ‘Falticeni’ apple pomace as affected by non-conventional extraction techniques. Food Hydrocoll. 2020, 100, 105383. [Google Scholar] [CrossRef]
- Zhou, J.B.; Liu, D.; Xia, W.H.; Guo, Y.R.; Luo, Y.C.; Xue, J. Physicochemical and functional properties of RG-I enriched pectin extracted from thinned-young apples. Int. J. Biol. Macromol. 2023, 236, 123953. [Google Scholar] [CrossRef]
- Morales-Contreras, B.E.; Wicker, L.; Rosas-Flores, W.; Contreras-Esquivel, J.C.; Gallegos-Infante, J.A.; Reyes-Jaquez, D.; Morales-Castro, J. Apple pomace from variety “Blanca de Asturias” as sustainable source of pectin: Composition, rheological, and thermal properties. Lwt-Food Sci. Technol. 2020, 117, 108641. [Google Scholar] [CrossRef]
- del Amo-Mateos, E.; Lopez-Linares, J.C.; Garcia-Cubero, T.; Lucas, S.; Coca, M. Green biorefinery for sugar beet pulp valorisation: Microwave hydrothermal processing for pectooligosaccharides recovery and biobutanol production. Ind. Crops Prod. 2022, 184, 115060. [Google Scholar] [CrossRef]
- Bindereif, B.; Eichhofer, H.; Bunzel, M.; Karbstein, H.P.; Wefers, D.; van Der Schaaf, U.S. Arabinan side-chains strongly affect the emulsifying properties of acid-extracted sugar beet pectins. Food Hydrocoll. 2021, 121, 106968. [Google Scholar] [CrossRef]
- Reichembach, L.H.; Petkowicz, C.L.D. Pectins from alternative sources and uses beyond sweets and jellies: An overview. Food Hydrocoll. 2021, 118, 106824. [Google Scholar] [CrossRef]
- FAO. 2022. Available online: https://www.un.org/en/observances/end-food-waste-day (accessed on 23 May 2023).
- Pedrosa, L.D.; Lopes, R.G.; Fabi, J.P. The acid and neutral fractions of pectins isolated from ripe and overripe papayas differentially affect galectin-3 inhibition and colon cancer cell growth. Int. J. Biol. Macromol. 2020, 164, 2681–2690. [Google Scholar] [CrossRef]
- Sabater, C.; Sabater, V.; Olano, A.; Montilla, A.; Corzo, N. Ultrasound-assisted extraction of pectin from artichoke by-products. An artificial neural network approach to pectin characterisation. Food Hydrocoll. 2020, 98, 105238. [Google Scholar] [CrossRef]
- Phaiphan, A. Ultrasound assisted extraction of pectin from banana peel waste as a potential source for pectin production. Acta Sci. Pol. Technol. Aliment. 2022, 21, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Almagro, N.; Ruiz-Torralba, A.; Mendez-Albinana, P.; Guerra-Hernandez, E.; Garcia-Villanova, B.; Moreno, R.; Villamiel, M.; Montilla, A. Berry fruits as source of pectin: Conventional and non-conventional extraction techniques. Int. J. Biol. Macromol. 2021, 186, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.L.; Tang, S.F.; Ali, A.; Chow, Y.H. Optimisation of pectin production from dragon fruit peels waste: Drying, extraction and characterisation studies. Sn Appl. Sci. 2020, 2, 621. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. Lwt-Food Sci. Technol. 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Resende, L.M.; Franca, A.S. Jabuticaba (Plinia sp.) Peel as a Source of Pectin: Characterization and Effect of Different Extraction Methods. Foods 2023, 12, 117. [Google Scholar] [CrossRef]
- Baris, S.; Elik, A.; Gogus, F.; Yanik, D.K. Pistachio hull as an alternative pectin source: Its extraction and use in oil in water emulsion system. Prep. Biochem. Biotechnol. 2023, 53, 433–442. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Ultrasound-assisted extraction of pectin from Citrus limetta peels: Optimization, characterization, and its comparison with commercial pectin. Food Biosci. 2023, 51, 102231. [Google Scholar] [CrossRef]
- Polanco-Lugo, E.; Martinez-Castillo, J.I.; Cuevas-Bernardino, J.C.; Gonzalez-Flores, T.; Valdez-Ojeda, R.; Pacheco, N.; Ayora-Talavera, T. Citrus pectin obtained by ultrasound-assisted extraction: Physicochemical, structural, rheological and functional properties. Cyta-J. Food 2019, 17, 463–471. [Google Scholar] [CrossRef]
- Dranca, F.; Talon, E.; Vargas, M.; Oroian, M. Microwave vs. conventional extraction of pectin from Malus domestica ’Falticeni’ pomace and its potential use in hydrocolloid-based films. Food Hydrocoll. 2021, 121, 107026. [Google Scholar] [CrossRef]
- Hamai-Amara, H.; Abdoun-Ouallouche, K.; Nacer-Khodja, A.; Abdelhafid, K.; Benmouloud, A.; Djefal-Kerrar, A. Optimization of the extraction of orange peel pectin and evaluation of its antiproliferative activity towards HEp2 cancer cells. Euro-Mediterr. J. Environ. Integr. 2020, 5, 43. [Google Scholar] [CrossRef]
- Shivamathi, C.S.; Moorthy, I.G.; Kumar, R.V.; Soosai, M.R.; Maran, J.P.; Kumar, R.S.; Varalakshmi, P. Optimization of ultrasound assisted extraction of pectin from custard apple peel: Potential and new source. Carbohydr. Polym. 2019, 225, 115240. [Google Scholar] [CrossRef] [PubMed]
- Pangestu, R.; Amanah, S.; Juanssilfero, A.B.; Yopi; Perwitasari, U. Response surface methodology for microwave-assisted extraction of pectin from cocoa pod husk (Theobroma cacao) mediated by oxalic acid. J. Food Meas. Charact. 2020, 14, 2126–2133. [Google Scholar] [CrossRef]
- Mendez, D.A.; Fabra, M.J.; Gomez-Mascaraque, L.; Lopez-Rubio, A.; Martinez-Abad, A. Modelling the Extraction of Pectin towards the Valorisation of Watermelon Rind Waste. Foods 2021, 10, 738. [Google Scholar] [CrossRef]
- Singaram, A.J.V.; Ganesan, N.D. Modeling the influence of extraction parameters on the yield and chemical characteristics of microwave extracted mango (Mangifera indica L.) peel pectin by response surface methodology. Prep. Biochem. Biotechnol. 2022, 52, 711–723. [Google Scholar] [CrossRef]
- Siqueira, R.A.; Veras, J.M.L.; de Sousa, T.L.; de Farias, P.M.; de Oliveira, J.G.; Bertolo, M.R.V.; Egea, M.B.; Placido, G.R. Pequi mesocarp: A new source of pectin to produce biodegradable film for application as food packaging. Food Sci. Technol. 2022, 42, e71421. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornuit, R. Preparation and characterization of pectin fraction from pineapple peel as a natural plasticizer and material for biopolymer film. Food Bioprod. Process. 2019, 118, 198–206. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.H.; Li, F.; Li, D.P.; Huang, Q.R. Pectin extracted from persimmon peel: A physicochemical characterization and emulsifying properties evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Jong, S.H.; Abdullah, N.; Muhammad, N. Optimization of low-methoxyl pectin extraction from durian rinds and its physicochemical characterization. Carbohydr. Polym. Technol. Appl. 2023, 5, 100263. [Google Scholar] [CrossRef]
- Al-Ajalein, A.A.S.; Shafie, M.H.; Yap, P.G.; Kassim, M.A.; Naharudin, I.; Wong, T.W.; Gan, C.Y. Microwave-assisted extraction of polysaccharide from Cinnamomum cassia with anti-hyperpigmentation properties: Optimization and characterization studies. Int. J. Biol. Macromol. 2023, 226, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a phenomenological definition of the term ‘gel’. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Rossmurphy, S.B. Structure-property relationships in food biopolymer gels and solutions. J. Rheol. 1995, 39, 1451–1463. [Google Scholar] [CrossRef]
- Li, J.M.; Nie, S.P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. Mysore 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid gel particles: Formation, characterization, and application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef]
- Oakenfull, D.; Scott, A. Hydrophobic interaction in the gelation of high methoxyl pectins. J. Food Sci. 1984, 49, 1093–1098. [Google Scholar] [CrossRef]
- Kastner, H.; Einhorn-Stoll, U.; Senge, B. Structure formation in sugar containing pectin gels–Influence of Ca2+ on the gelation of low-methoxylated pectin at acidic pH. Food Hydrocoll. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- Evageliou, V.; Richardson, R.K.; Morris, E.R. Effect of pH, sugar type and thermal annealing on high-methoxy pectin gels. Carbohydr. Polym. 2000, 42, 245–259. [Google Scholar] [CrossRef]
- Fennema, O.R. Food Chemistry; CRC Press: Boca Raton, FL, USA, 1996; Volume 76. [Google Scholar]
- Ako, K. Influence of elasticity on the syneresis properties of κ-carrageenan gels. Carbohydr. Polym. 2015, 115, 408–414. [Google Scholar] [CrossRef]
- Lin, Y.P.; An, F.P.; He, H.; Geng, F.; Song, H.B.; Huang, Q. Structural and rheological characterization of pectin from passion fruit (Passiflora edulis f. flavicarpa) peel extracted by high-speed shearing. Food Hydrocoll. 2021, 114, 106555. [Google Scholar] [CrossRef]
- Feng, L.Y.; Zhou, Y.; Ashaolu, T.J.; Ye, F.Y.; Zhao, G.H. Physicochemical and rheological characterization of pectin-rich fraction from blueberry (Vaccinium ashei) wine pomace. Int. J. Biol. Macromol. 2019, 128, 629–637. [Google Scholar] [CrossRef]
- Sarmadi, B.; Nikmaram, P.; Mortazavian, A.M.; Kiani, H.; Mousavi, M.; Khanniri, E.; Mohammadi, R.; da Cruz, A.G. High-Methoxyl Apple Pectin Improves Rheological Properties and Storage Stability of the Flavored Probiotic Yogurt Drinks, Compared to Pomegranate Pectin. Appl. Food Biotechnol. 2022, 9, 91–102. [Google Scholar] [CrossRef]
- Wang, X.Y.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Wilbanks, D.J.; Yazdi, S.R.; Lucey, J.A. Effects of varying casein and pectin concentrations on the rheology of high-protein cultured milk beverages stored at ambient temperature. J. Dairy Sci. 2022, 105, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Zidi, D.; Gharsallaoui, A.; Dupas-Farrugia, C.; Attia, H.; Ayadi, M.A. Physicochemical and rheological changes of acidified camel milk added with commercial low methoxyl-pectin. Int. J. Biol. Macromol. 2019, 128, 347–353. [Google Scholar] [CrossRef]
- Khubber, S.; Chaturvedi, K.; Thakur, N.; Sharma, N.; Yadav, S.K. Low-methoxyl pectin stabilizes low-fat set yoghurt and improves their physicochemical properties, rheology, microstructure and sensory liking. Food Hydrocoll. 2021, 111, 106240. [Google Scholar] [CrossRef]
- Estaji, M.; Mohammadi-Moghaddam, T.; Gholizade-Eshan, L.; Firoozzare, A.; Hooshmand-Dalir, M.A. Physicochemical characteristics, sensory attributes, and antioxidant activity of marmalade prepared from black plum peel. Int. J. Food Prop. 2020, 23, 1979–1992. [Google Scholar] [CrossRef]
- Ghiraldi, M.; Franco, B.G.; Moraes, I.C.F.; Pinho, S.C. Emulsion-Filled Pectin Gels for Vehiculation of Vitamins D3 and B12: From Structuring to the Development of Enriched Vegan Gummy Candies. Acs Food Sci. Technol. 2021, 1, 1945–1952. [Google Scholar] [CrossRef]
- Huang, J.H.R.; Wu, C.Y.; Chan, H.M.; Ciou, J.Y. Printing Parameters of Sugar/Pectin Jelly Candy and Application by Using a Decision Tree in a Hot-Extrusion 3D Printing System. Sustainability 2022, 14, 11618. [Google Scholar] [CrossRef]
- Sun, W.X.; Yang, W.H.; Zheng, Y.X.; Zhang, H.L.; Fang, H.T.; Liu, D.H.; Kong, X.L.; Chen, S.G.; Ye, X.Q.; Tian, J.H. Effect of Potato Pulp Pectic Polysaccharide on the Stability of Acidified Milk Drinks. Molecules 2020, 25, 5632. [Google Scholar] [CrossRef] [PubMed]
| Source of Pectin | Type of by-Products | Extraction Methods | Mw (KDa) | References |
|---|---|---|---|---|
| Navel orange (Citrus sinensis) | Orange peels | CE * (acid; HCl) | 212.9 | [67] |
| CE (alkali; NaOH) | 153.0 | [67] | ||
| Hydrothermal | 109.2 | [67] | ||
| Pomelo (Citrus grandis) | Pomelo peels | Hydrothermal | 134.0 | [68] |
| CE (acid; citric acid) | 188.5 | [68] | ||
| CE (EDTA-2Na) | 220.4 | [68] | ||
| SWE * (hydrothermal) | 56.3 | [68] | ||
| SWE (acid; citric acid) | 88.4 | [68] | ||
| SWE (EDTA-2Na) | 137.1 | [68] | ||
| Lukan | Citrus peels | CE (acid; acetic acid) | 130 | [69] |
| Ponkan | CE (acid; acetic acid) | 173 | [69] | |
| Shatangju | CE (acid; acetic acid) | 40 | [69] | |
| Wogan | CE (acid; acetic acid) | 163 | [69] | |
| Citrus Limetta | Citrus peels | Hydrothermal | 330.9 | [70] |
| CE (acid; HCl) | 296.2 | [70] | ||
| Malus domestica “Fălticeni” apples | Apple pomace | CE (acid; citric acid) | 263 | [71] |
| MAE * | 264 | [71] | ||
| UAE * | 386 | [71] | ||
| EE * (cellulase or/ Celluclast®1.5 L) | (118/117) | [71] | ||
| UAEH * | 260 | [71] | ||
| EAU * | 117 | [71] | ||
| Fuji | Apple pomace | CE (acid; citric acid) | 1541 | [72] |
| Jingshiji | CE (acid; citric acid) | 1787 | [72] | |
| Ruixue | CE (acid; citric acid) | 1626 | [72] | |
| Blanca de Asturias | Apple pomace | CE (AP *:HCl; 1:15; 20 min) | 865 | [73] |
| CE (AP:HCl; 1:20; 20 min) | 850 | [73] | ||
| CE (AP:HCl; 1:25; 20 min) | 836 | [73] | ||
| Beet | Sugar beet pulp | MAE * (precipitate A) | 488 | [74] |
| MAE (precipitate B1) | 474 | [74] | ||
| MAE (precipitate B2) | 523 | [74] | ||
| Beet | Sugar beet pulp | CE (acid; nitric acid; 1 h) | 913 | [75] |
| CE (acid; nitric acid; 2 h) | 824 | [75] | ||
| CE (acid; nitric acid; 4 h) | 695 | [75] | ||
| CE (acid; nitric acid; 16 h) | 286 | [75] |
| Source/Family of Pectin | Food Application | Rheological Measurements (Rheometer or Viscometer) | Pectin Concentration (g/100 mL or g/100 g) | Mains Results of Rheological Properties |
|---|---|---|---|---|
| Apple peels/HM * [112] | Flavored probiotic Yogurt drinks | Geometry: cone and plate (4°, 40 mm in diameter). Oscillatory measurements: frequency range of (0.1 to 10) Hz. Linear viscoelastic behavior region found (10% strain) | 0.1, 0.2, 0.3, 0.4, 0.5 | Shear-thinning flow behavior, gel-liked network at low frequencies. A total of 0.5 g/100 mL of pectin showed the highest G′ and G″ and stability during storage |
| Apple pomace (AP) powder/NA [113] | Set type yogurt | Geometry: concentric cylinder (diameter of cup and bob: 28.92 and 26.66 mm, respectively). Oscillatory measurements: frequency of 1 Hz and 0.5% strain. Fermentation of skim milk fortified with AP was performed in situ in the rheometer | 0.1, 0.5, 1.0 | Yogurts containing 1.0 g/100 g of apple pomace and cooled to 4 °C showed a higher G′ than that the control, causing a firmer and more consistent gel without disrupting gelation |
| Citrus peels/HM [114] | High-protein cultured milk beverages | Geometry: vane (ST24–4V-30). Oscillatory measurements: frequency range of 0.1 Hz and strain of 0.5% | 0.15, 0.50, 0.85, 1.00 | All samples formed gels. It was indicated that in aged samples a high concentration of pectin slowed the formation of elastic bonds |
| Citrus peels/LMA * [115] | Acidified camel milk | Geometry: cone and plate (3.5 cm diameter; 2° angle). Oscillatory measurements: frequency range of (00.1 to 20) Hz. Linear viscoelastic behavior region found (0.1% strain) | 0.5, 1.0, 1.5, 2.0 | Higher pectin concentration led to a strong gel with higher G′ values. This result could be attributed to the formation of complexes, and the mechanical spectra prove the hypothesis that pectin forms strands with caseins micelles |
| Citrus peels/LM * [116] | Low-fat set yogurt | Geometry: concentric cylinder (32.40 mm bob diameter, 12 mm length). Oscillatory measurements: frequency range of (00.1 to 16) Hz. Linear viscoelastic behavior region found (0.1% strain) | 0.05, 0.10,0.20, 0,40, 0.60, 0.80, 1.00 | Low-fat yogurt revealed pseudoplastic shear-thinning behavior. When increasing the concentrations of LMP, both G′ and G′′ increased. The increasing elastic modulus evinced that the LMP addition might have enhanced the strength of bonds in protein structure |
| Citrus peels/HM [117] | Marmalade prepared from black plum peel | Geometry: bob and cup (bob length: 60 mm; bob diameter: 14 mm; gap width: 1 mm). Flow curves measurements: shear rate (20 to 300/s over a timelapse of 400 s). Apparent viscosity: shear rate of 40/s | 0.3, 0.4, 0.5, and 0.6 | The increase in pectin led to a rise in apparent viscosity, firmness, and consistency |
| Citrus peels/PLMA * [118] | Vegan gummy candies | Geometry: cone and plate geometry (2°, 60 mm diameter). Oscillatory measurements: frequency range of (0.01 to 10) Hz. Linear viscoelastic behavior region found (8 Pa) | 4 | All samples tested presented gel behavior as G′, G″. Nonfilled gels presented a lower G′ than emulsion-filled gels (EFG), indicating that the former had a weaker three-dimensional polymer network structure |
| NA—/HM [119] | Jelly candy (3D printing) | Geometry: parallel plate. Oscillatory measurements: frequency range of (0.01 to 0.16) Hz. Linear viscoelastic behavior region found (5% strain) | 10, 12, 14 and 16 | A total of 16 (g/100 g) of pectin had the height of G′ and G″, and all pectin jelly candy showed the characteristic of pseudoplastic shear-thinning |
| Pomegranate peels/HM [112] | Flavored probiotic yogurt | Geometry: cone and plate (4°, 40 mm in diameter). Oscillatory measurements: frequency range of (0.1 to 10) Hz. Linear viscoelastic behavior region found (10% strain) | 0.1, 0.2, 0.3, 0.4, 0.5 | All samples showed Newtonian flow behavior and liquid-like behavior over the frequency range |
| Potato pulp/LM [120] | Acidified milk drinks | Geometry: NA. Oscillatory measurements: frequency range of (0.1 to 10) Hz. Linear viscoelastic behavior region found (1% strain) | 0.1, 0.2, 0.3, 0.4, 0.5 | The storage modulus (G′) was higher than the loss modulus (G″) for all the samples, indicating a gel-like structure |
| Sunflower by-products /LM [37] | Low-sucrose strawberry jams | Geometry: parallel plate. Oscillatory measurements: frequency range of (0.1 to 10) Hz. Controlled stress of 5 Pa | 1.0 | G′ was higher than G″ over the whole frequency range for almost every formulation. A sample presented a sol–gel transition at higher frequencies. This fact could be related to the use of glucose and fructose syrup instead of sucrose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toniazzo, T.; Fabi, J.P. Versatile Polysaccharides for Application to Semi-Solid and Fluid Foods: The Pectin Case. Fluids 2023, 8, 243. https://doi.org/10.3390/fluids8090243
Toniazzo T, Fabi JP. Versatile Polysaccharides for Application to Semi-Solid and Fluid Foods: The Pectin Case. Fluids. 2023; 8(9):243. https://doi.org/10.3390/fluids8090243
Chicago/Turabian StyleToniazzo, Taíse, and João Paulo Fabi. 2023. "Versatile Polysaccharides for Application to Semi-Solid and Fluid Foods: The Pectin Case" Fluids 8, no. 9: 243. https://doi.org/10.3390/fluids8090243
APA StyleToniazzo, T., & Fabi, J. P. (2023). Versatile Polysaccharides for Application to Semi-Solid and Fluid Foods: The Pectin Case. Fluids, 8(9), 243. https://doi.org/10.3390/fluids8090243








