Microfluidic Invasion Chemotaxis Platform for 3D Neurovascular Co-Culture
Abstract
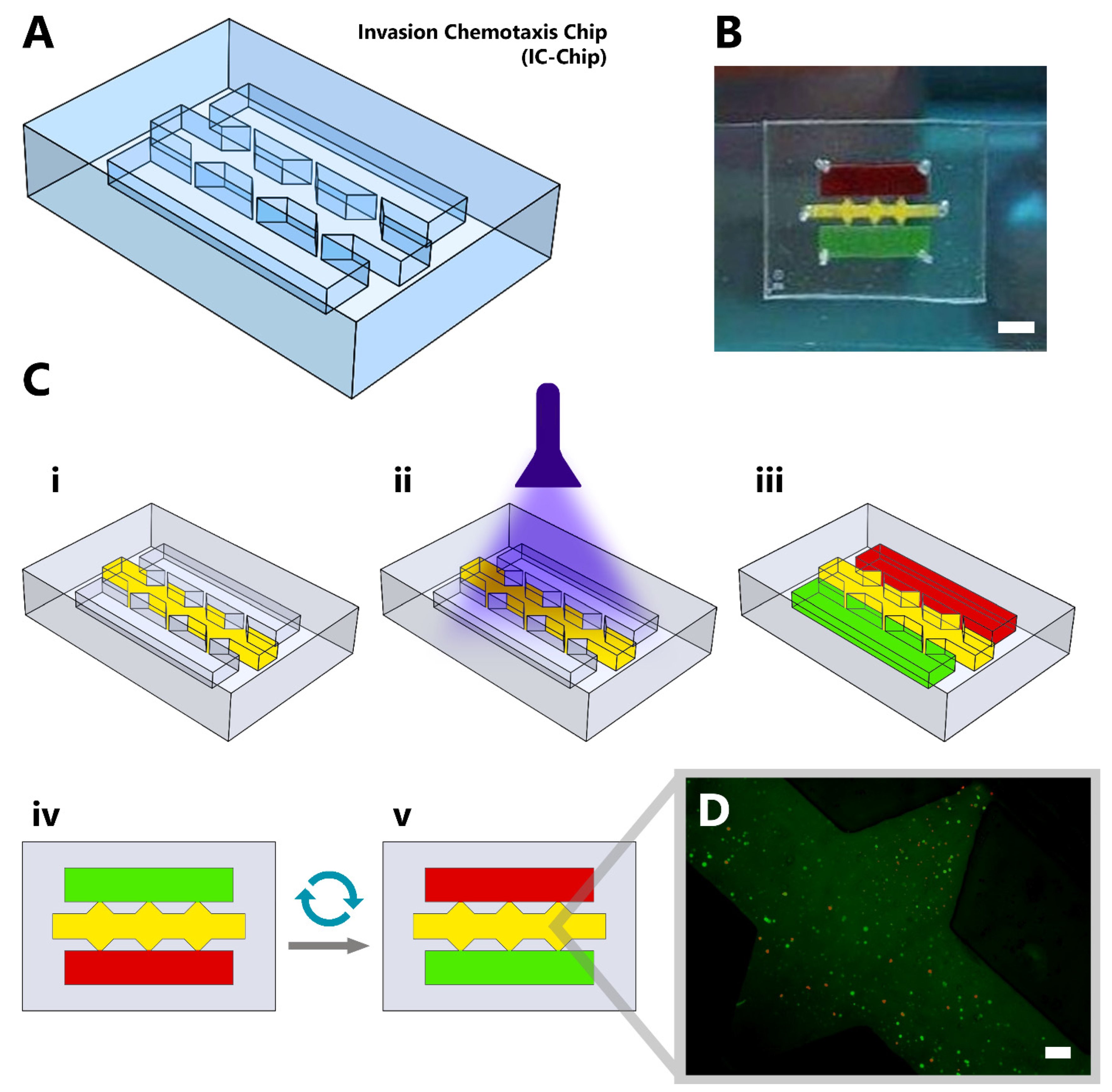
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. GelMA Synthesis
2.2.2. Surface Coating of Coverslips
2.2.3. Field Emission Scanning Electron Microscopy Imaging of GelMA
2.2.4. Preparation of the Pre-Polymer Solution
2.2.5. Preparation of Cells
2.2.6. UV Curing of GelMA and Cell Seeding Inside Microfluidic Chip
2.2.7. Preparation of GelMA for Cell Viability Analysis
2.2.8. Cell Viability Assay
2.2.9. Atomic Force Microscopy Analysis of GelMA
2.2.10. Viability Imaging of Encapsulated Cells Inside the Microfluidic Chip
2.2.11. Swelling Test
2.2.12. Finite-Difference Simulation
2.2.13. Functional Immunofluorescent Staining
3. Results
3.1. Field Emission Scanning Electron Microscopy Imaging of GelMA
3.2. Atomic Force Microscopy Analysis of GelMA
3.3. Fourier Transform Infrared Spectroscopy Analysis of GelMA
3.4. Swelling Test of the GelMA Inside the Microfluidic Chip
3.5. Simulation Results
3.6. Cell Viability Test
3.7. Cell Viability Imaging of GelMA Inside the Microfluidic Chip
3.8. Functional Immunostaining of Cells
4. Discussion
4.1. Hydrogel Fabrication Inside the Microfluidic Chip Using UV Photopolymerization
4.2. Microfluidic Applications for Co-Culture and Migration Studies
5. Conclusions
6. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttenplan, A.P.M.; Birgani, Z.T.; Giselbrecht, S.; Truckenmüller, R.K.; Habibović, P. Chips for Biomaterials and Biomaterials for Chips: Recent Advances at the Interface between Microfabrication and Biomaterials Research. Adv. Healthc. Mater. 2021, 10, 2100371. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Tagle, D.A. Tissue chips—Innovative tools for drug development and disease modeling. Lab Chip 2017, 17, 3026–3036. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, C. Organoids and Microphysiological Systems: New Tools for Ophthalmic Drug Discovery. Front. Pharmacol. 2020, 11, 407. [Google Scholar] [CrossRef] [Green Version]
- Kleinstreuer, N.; Holmes, A. Harnessing the power of microphysiological systems for COVID-19 research. Drug Discov. Today 2021, 26, 2496–2501. [Google Scholar] [CrossRef]
- Signore, M.A.; De Pascali, C.; Giampetruzzi, L.; Siciliano, P.A.; Francioso, L. Gut-on-Chip microphysiological systems: Latest advances in the integration of sensing strategies and adoption of mature detection mechanisms. Sens. Bio-Sens. Res. 2021, 33, 100443. [Google Scholar] [CrossRef]
- Sarabi, M.R.; Yetisen, A.K.; Tasoglu, S. Magnetic levitation for space exploration. Trends Biotechnol. 2022; in press. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Sarabi, M.R.; Birtek, M.T.; Mustafaoglu, N.; Zhang, Y.S.; Tasoglu, S. 3D bioprinted organ-on-chips. Aggregate 2022, e197. [Google Scholar] [CrossRef]
- Yigci, D.; Sarabi, M.R.; Ustun, M.; Atceken, N.; Sokullu, E.; Onder, T.B.; Tasoglu, S. 3D Bioprinted Glioma Models. Prog. Biomed. Eng. 2022. [Google Scholar] [CrossRef]
- Shi, T.; Cheung, M. Urine-derived induced pluripotent/neural stem cells for modeling neurological diseases. Cell Biosci. 2021, 11, 85. [Google Scholar] [CrossRef]
- Hernandez, H. Engineering Human iPSC-Derived Skeletal Muscle to Model Pompe Disease: Towards Novel Gene and Regenerative Therapies; Erasmus University Rotterdam: Rotterdam, The Netherlands, 2021. [Google Scholar]
- Santoso, J.W.; McCain, M.L. Neuromuscular disease modeling on a chip. Dis. Model. Mech. 2020, 13, dmm044867. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Ting, H.-C.; Liu, C.-A.; Su, H.-L.; Chiou, T.-W.; Lin, S.-Z.; Harn, H.-J.; Ho, T.-J. Induced pluripotent stem cell (iPSC)-based neurodegenerative disease models for phenotype recapitulation and drug screening. Molecules 2020, 25, 2000. [Google Scholar]
- Lynch, E.; Peek, E.; Reilly, M.; FitzGibbons, C.; Robertson, S.; Suzuki, M. Current Progress in the Creation, Characterization, and Application of Human Stem Cell-derived in Vitro Neuromuscular Junction Models. Stem Cell Rev. Rep. 2021, 18, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Fabre, K.M.; Livingston, C.; Tagle, D.A. Organs-on-chips (microphysiological systems): Tools to expedite efficacy and toxicity testing in human tissue. Exp. Biol. Med. 2014, 239, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.M.; Hoffman, T.; Luu, B.Q.; Ashammakhi, N.; Li, S. Application of lung microphysiological systems to COVID-19 modeling and drug discovery: A review. Bio-Design Manuf. 2021, 4, 757–775. [Google Scholar] [CrossRef]
- Doherty, E.L.; Aw, W.Y.; Hickey, A.J.; Polacheck, W.J. Microfluidic and Organ-on-a-Chip Approaches to Investigate Cellular and Microenvironmental Contributions to Cardiovascular Function and Pathology. Front. Bioeng. Biotechnol. 2021, 9, 624435. [Google Scholar] [CrossRef]
- Lesher-Perez, S.C.; Frampton, J.P.; Takayama, S. Microfluidic systems: A new toolbox for pluripotent stem cells. Biotechnol. J. 2012, 8, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Haring, A.P.; Sontheimer, H.; Johnson, B.N. Microphysiological Human Brain and Neural Systems-on-a-Chip: Potential Alternatives to Small Animal Models and Emerging Platforms for Drug Discovery and Personalized Medicine. Stem Cell Rev. Rep. 2017, 13, 381–406. [Google Scholar] [CrossRef]
- Holmes, A.M.; Solari, R.; Holgate, S.T. Animal models of asthma: Value, limitations and opportunities for alternative approaches. Drug Discov. Today 2011, 16, 659–670. [Google Scholar] [CrossRef]
- Singh, Y.P.; Moses, J.C.; Bhardwaj, N.; Mandal, B.B. Overcoming the Dependence on Animal Models for Osteoarthritis Therapeutics—The Promises and Prospects of In Vitro Models. Adv. Healthc. Mater. 2021, 10, 2100961. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.; Yenilmez, B.; Tasoglu, S. Towards Single-Step Biofabrication of Organs on a Chip via 3D Printing. Trends Biotechnol. 2016, 34, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Ustun, M.; Dabbagh, S.R.; Ilci, I.; Bagci-Onder, T.; Tasoglu, S. Glioma-on-a-Chip Models. Micromachines 2021, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mosavati, B.; Oleinikov, A.V.; Du, E. Biosensors for Detection of Human Placental Pathologies: A Review of Emerging Technologies and Current Trends. Transl. Res. 2019, 213, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Mosavati, B.; Oleinikov, A.V.; Du, E. Development of an Organ-on-a-Chip-Device for Study of Placental Pathologies. Int. J. Mol. Sci. 2020, 21, 8755. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.P.B.; Domingues, R.M.A.; Shevchuk, M.; Gomes, M.E.; Peppas, N.A.; Reis, R.L. Biomaterials for Sequestration of Growth Factors and Modulation of Cell Behavior. Adv. Funct. Mater. 2020, 30, 1909011. [Google Scholar] [CrossRef]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact. Mater. 2021, 9, 198–220. [Google Scholar] [CrossRef]
- Geraili, A.; Jafari, P.; Hassani, M.S.; Araghi, B.H.; Mohammadi, M.H.; Ghafari, A.M.; Tamrin, S.H.; Modarres, H.P.; Kolahchi, A.R.; Ahadian, S.; et al. Controlling Differentiation of Stem Cells for Developing Personalized Organ-on-Chip Platforms. Adv. Healthc. Mater. 2017, 7, 1700426. [Google Scholar] [CrossRef] [Green Version]
- Sarabi, M.; Ahmadpour, A.; Yetisen, A.; Tasoglu, S. Finger-Actuated Microneedle Array for Sampling Body Fluids. Appl. Sci. 2021, 11, 5329. [Google Scholar] [CrossRef]
- Herbert, R.; Lim, H.; Park, S.; Kim, J.; Yeo, W. Recent Advances in Printing Technologies of Nanomaterials for Implantable Wireless Systems in Health Monitoring and Diagnosis. Adv. Healthc. Mater. 2021, 10, e2100158. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Wu, Z.; Wang, J.; Wang, Z.L. A review on emerging biodegradable polymers for environmentally benign transient electronic skins. J. Mater. Sci. 2021, 56, 16765–16789. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hernandez, A.L.; Unluturk, B.D.; Quintero, S.A.; de Barros, N.R.; Apu, E.H.; Shams, A.B.; Ostrovidov, S.; Li, J.; Contag, C.; et al. Biodegradable Implantable Sensors: Materials Design, Fabrication, and Applications. Adv. Funct. Mater. 2021, 31, 2104149. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable Materials for Sustainable Health Monitoring Devices. ACS Appl. Bio Mater. 2020, 4, 163–194. [Google Scholar] [CrossRef]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-Based Scaffolds in Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Yenilmez, B.; Temirel, M.; Knowlton, S.; Lepowsky, E.; Tasoglu, S. Development and characterization of a low-cost 3D bioprinter. Bioprinting 2019, 13, e00044. [Google Scholar] [CrossRef]
- Knowlton, S.; Cho, Y.; Li, X.J.; Khademhosseini, A.; Tasoglu, S. Utilizing stem cells for three-dimensional neural tissue engineering. Biomater. Sci. 2016, 4, 768–784. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Chiong, J.A.; Tran, H.; Lin, Y.; Zheng, Y.; Bao, Z. Integrating Emerging Polymer Chemistries for the Advancement of Recyclable, Biodegradable, and Biocompatible Electronics. Adv. Sci. 2021, 8, 2101233. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad, F.; Akhtar, B. Biodegradable nanoparticles as drug delivery devices. J. Drug Deliv. Sci. Technol. 2021, 64, 102638. [Google Scholar] [CrossRef]
- John, N. Bio-Implants Derived from Biocompatible and Biodegradable Biopolymeric Materials. In Advanced Studies in Experimental and Clinical Medicine; Apple Academic Press: Palm Bay, FL, USA, 2021; pp. 53–81. [Google Scholar] [CrossRef]
- Pyarasani, R.D.; Jayaramudu, T.; John, A. Polyaniline-based conducting hydrogels. J. Mater. Sci. 2018, 54, 974–996. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugan, S.; Parcha, S.R. Fabrication techniques involved in developing the composite scaffolds PCL/HA nanoparticles for bone tissue engineering applications. J. Mater. Sci. Mater. Med. 2021, 32, 93. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, M.R.; Bediz, B.; Falo, L.D.; Korkmaz, E.; Tasoglu, S. 3D printing of microneedle arrays: Challenges towards clinical translation. J. 3D Print. Med. 2021, 5, 65–70. [Google Scholar] [CrossRef]
- Tyagi, N.; Gambhir, K.; Kumar, S.; Gangenahalli, G.; Verma, Y.K. Interplay of reactive oxygen species (ROS) and tissue engineering: A review on clinical aspects of ROS-responsive biomaterials. J. Mater. Sci. 2021, 56, 16790–16823. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-stimuli-responsive hydrogels and their medical applications. New J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Goldhahn, C.; Cabane, E.; Chanana, M. Sustainability in wood materials science: An opinion about current material development techniques and the end of lifetime perspectives. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2021, 379, 20200339. [Google Scholar] [CrossRef]
- Sampson, K.L.; Deore, B.; Go, A.; Nayak, M.A.; Orth, A.; Gallerneault, M.; Malenfant, P.R.L.; Paquet, C. Multimaterial Vat Polymerization Additive Manufacturing. ACS Appl. Polym. Mater. 2021, 3, 4304–4324. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, E.; Wang, Y. Advances in the applications of polymer biomaterials for in vitro follicle culture. Biomed. Pharmacother. 2021, 140, 111422. [Google Scholar] [CrossRef]
- Green, R.; Baek, S.; Poole-Warren, L.; Martens, P.J. Conducting polymer-hydrogels for medical electrode applications. Sci. Technol. Adv. Mater. 2010, 11, 014107. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; Dohmen, C.; Philipp, A.; Kiener, D.; Maiwald, G.; Scheu, C.; Ogris, M.; Wagner, E. Synthesis and biological evaluation of a bioresponsive and endosomolytic siRNA−Polymer conjugate. Mol. Pharm. 2009, 6, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mubarak, N.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A Critical Review on the Synthesis of Natural Sodium Alginate Based Composite Materials: An Innovative Biological Polymer for Biomedical Delivery Applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Xu, W.; Jambhulkar, S.; Zhu, Y.; Ravichandran, D.; Kakarla, M.; Vernon, B.; Lott, D.G.; Cornella, J.L.; Shefi, O.; Miquelard-Garnier, G.; et al. 3D printing for polymer/particle-based processing: A review. Compos. Part B Eng. 2021, 223, 109102. [Google Scholar] [CrossRef]
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Papkovsky, D.B.; Dmitriev, R.I. Biological detection by optical oxygen sensing. Chem. Soc. Rev. 2013, 42, 8700–8732. [Google Scholar] [CrossRef]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef] [Green Version]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic Electronics: An El Dorado in the Quest of New Photocatalysts for Polymerization Reactions. Acc. Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef]
- Kaya, K.; Jockusch, S.; Yagci, Y. Mussel-Inspired Coatings by Photoinduced Electron-Transfer Reactions: Photopolymerization of Dopamine under UV, Visible, and Daylight under Oxygen-Free Conditions. Macromolecules 2021, 54, 5991–5999. [Google Scholar] [CrossRef]
- Ghaderinezhad, F.; Ceylan Koydemir, H.; Tseng, D.; Karinca, D.; Liang, K.; Ozcan, A.; Tasoglu, S. Sensing of electrolytes in urine using a miniaturized paper-based device. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Li, L.; Tasoglu, S. Assessing reusability of microfluidic devices: Urinary protein uptake by PDMS-based channels after long-term cyclic use. Talanta 2019, 192, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, S. Toilet-based continuous health monitoring using urine. Nat. Rev. Urol. 2022, 19, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Temirel, M.; Yenilmez, B.; Tasoglu, S. Long-term cyclic use of a sample collector for toilet-based urine analysis. Sci. Rep. 2021, 11, 2170. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Rabbi, F.; Doğan, Z.; Yetisen, A.K.; Tasoglu, S. Machine learning-enabled multiplexed microfluidic sensors. Biomicrofluidics 2020, 14, 061506. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Aldana, A.A.; Malatto, L.; Rehman, M.A.U.; Boccaccini, A.R.; Abraham, G.A. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomaterials 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, H. The origin and present status of Fick’s diffusion law. J. Chem. Educ. 1964, 41, 397. [Google Scholar] [CrossRef]
- Miller, C.C. The Stokes-Einstein law for diffusion in solution. Proc. R. Soc. A Math. Phys. Eng. Sci. 1924, 106, 724–749. [Google Scholar]
- Millington, R.J.; Quirk, J.P. Permeability of porous solids. Trans. Faraday Soc. 1961, 57, 1200–1207. [Google Scholar] [CrossRef]
- Leggio, L.; Vivarelli, S.; L’Episcopo, F.; Tirolo, C.; Caniglia, S.; Testa, N.; Marchetti, B.; Iraci, N. microRNAs in Parkinson’s Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasoglu, S.; Yu, C.; Gungordu, H.I.; Guven, S.; Vural, T.; Demirci, U. Guided and magnetic self-assembly of tunable magnetoceptive gels. Nat. Commun. 2014, 5, 4702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasoglu, S.; Diller, E.; Guven, S.; Sitti, M.; Demirci, U. Untethered micro-robotic coding of three-dimensional material composition. Nat. Commun. 2014, 5, 3124. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.; Yenilmez, B.; Anand, S.; Tasoglu, S. Photocrosslinking-based bioprinting: Examining crosslinking schemes. Bioprinting 2017, 5, 10–18. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Sani, E.S.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; Franca, C.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Shahani, S.; Sun, D.D.; Sharma, B.; Elisseeff, J.H.; Leong, K.W. Biodegradable and photocrosslinkable polyphosphoester hydrogel. Biomaterials 2006, 27, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Rezapour Sarabi, M.; Alseed, M.M.; Karagoz, A.A.; Tasoglu, S. Machine Learning-Enabled Prediction of 3D-Printed Microneedle Features. Biosensors 2022, 12, 491. [Google Scholar] [CrossRef]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef]
- Hwangbo, H.; Lee, H.; Jin, E.; Lee, J.; Jo, Y.; Ryu, D.; Kim, G. Bio-printing of aligned GelMa-based cell-laden structure for muscle tissue regeneration. Bioact. Mater. 2022, 8, 57–70. [Google Scholar] [CrossRef] [PubMed]




| Abbreviation/Symbol | Explanation |
|---|---|
| HUVEC | Human umbilical vein endothelial cell line |
| SH-SY5Y | Human neuroblastoma cell line |
| Diffusive flux | |
| The fluid diffusion coefficient | |
| The diffusion coefficient inside the porous matrix | |
| Concentration | |
| The Boltzmann constant | |
| Temperature | |
| Dynamic viscosity | |
| Radius of the diffusing particle | |
| Porosity of the matrix |
| Side Channels | Middle Channel | |
|---|---|---|
| Height | 150 μm | 150 μm |
| Width | 3250 μm | 1125 μm (the narrow parts); 3375 μm (the wide parts) |
| Injection Volume | 20 μL | 20 μL |
| Diameter of Inlets | 1000 μm | 1000 μm |
| UV Exposure Duration | 50 s |
| Microchip Distance from the UV Source | 60 mm |
| Irradiance Level | 6.25 W cm−2 |
| GelMA Concentration | 10% |
| Photoinitiator Concentration | 1% |
| Cell Concentration | 5 × 105 mL−1 |
| Area | 100.8 pm2 |
| Sa | 7.3136 nm |
| Sq | 9.9188 nm |
| Sy | 116.32 nm |
| Sp | 74.854 nm |
| Sv | −41.464 nm |
| Sm | −5.7364 fm |
| Mean | Std Deviation | N | |
|---|---|---|---|
| IC-Chip | 82.500 | 5.632 | 12 |
| Petri Dish | 86.416 | 11.766 | 12 |
| KERRYPNX | IC-Chip | Petri Dish | |
|---|---|---|---|
| IC-Chip | Pearson Correlation | 1 | 0.704 |
| Sig. (2-tailed) | - | 0.011 | |
| N | 12 | 12 | |
| Petri Dish | Pearson Correlation | 0.704 | 1 |
| Sig. (2-tailed) | 0.011 | - | |
| N | 12 | 12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokullu, E.; Cücük, Z.L.; Sarabi, M.R.; Birtek, M.T.; Bagheri, H.S.; Tasoglu, S. Microfluidic Invasion Chemotaxis Platform for 3D Neurovascular Co-Culture. Fluids 2022, 7, 238. https://doi.org/10.3390/fluids7070238
Sokullu E, Cücük ZL, Sarabi MR, Birtek MT, Bagheri HS, Tasoglu S. Microfluidic Invasion Chemotaxis Platform for 3D Neurovascular Co-Culture. Fluids. 2022; 7(7):238. https://doi.org/10.3390/fluids7070238
Chicago/Turabian StyleSokullu, Emel, Zeynel Levent Cücük, Misagh Rezapour Sarabi, Mehmet Tugrul Birtek, Hesam Saghaei Bagheri, and Savas Tasoglu. 2022. "Microfluidic Invasion Chemotaxis Platform for 3D Neurovascular Co-Culture" Fluids 7, no. 7: 238. https://doi.org/10.3390/fluids7070238
APA StyleSokullu, E., Cücük, Z. L., Sarabi, M. R., Birtek, M. T., Bagheri, H. S., & Tasoglu, S. (2022). Microfluidic Invasion Chemotaxis Platform for 3D Neurovascular Co-Culture. Fluids, 7(7), 238. https://doi.org/10.3390/fluids7070238







