Detailed Assessment of Nasal Inter-Chamber Anatomical Variations and Its Effect on Flow Apportionment and Inhalation Exposure Patterns
Abstract
:1. Introduction
2. Method
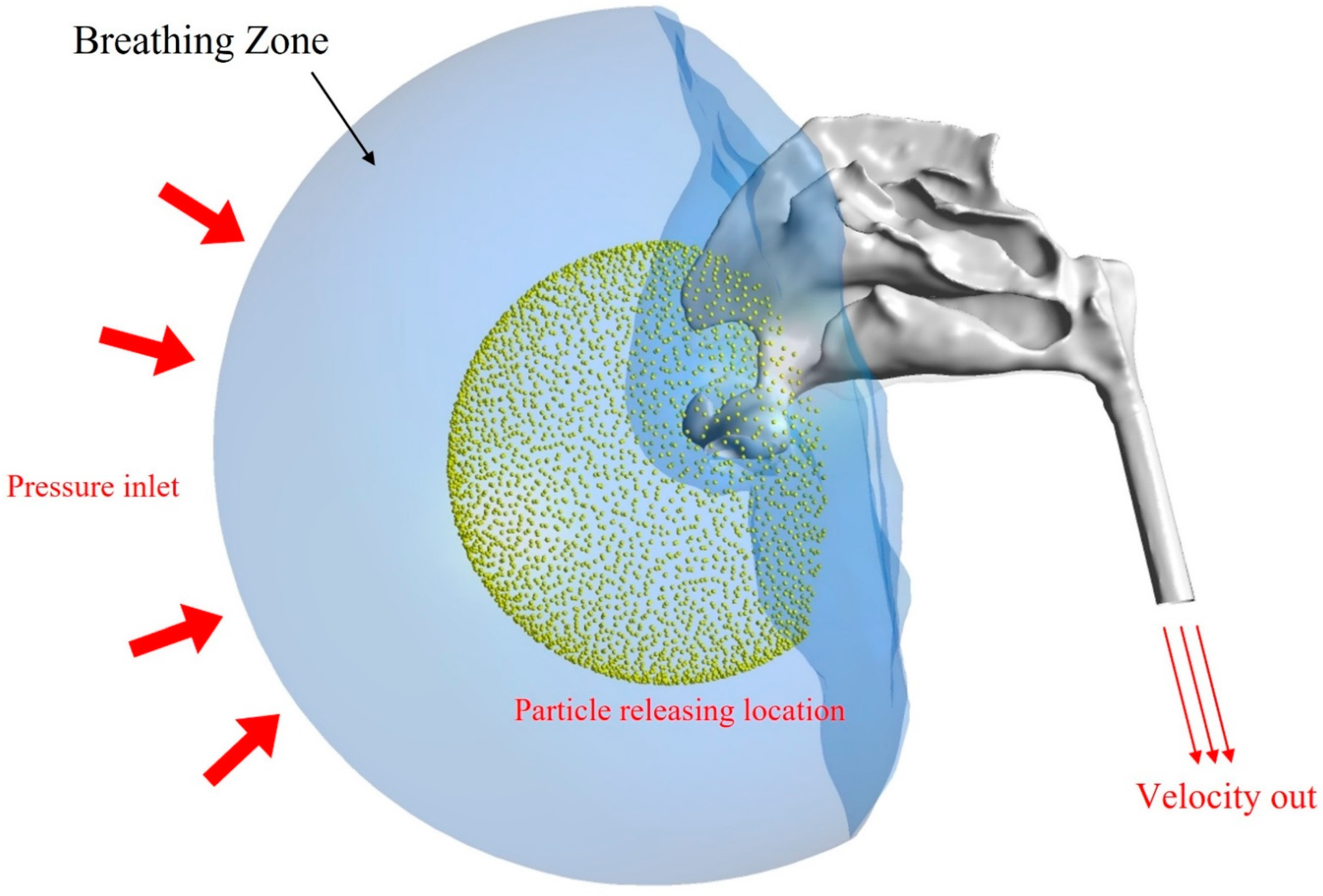
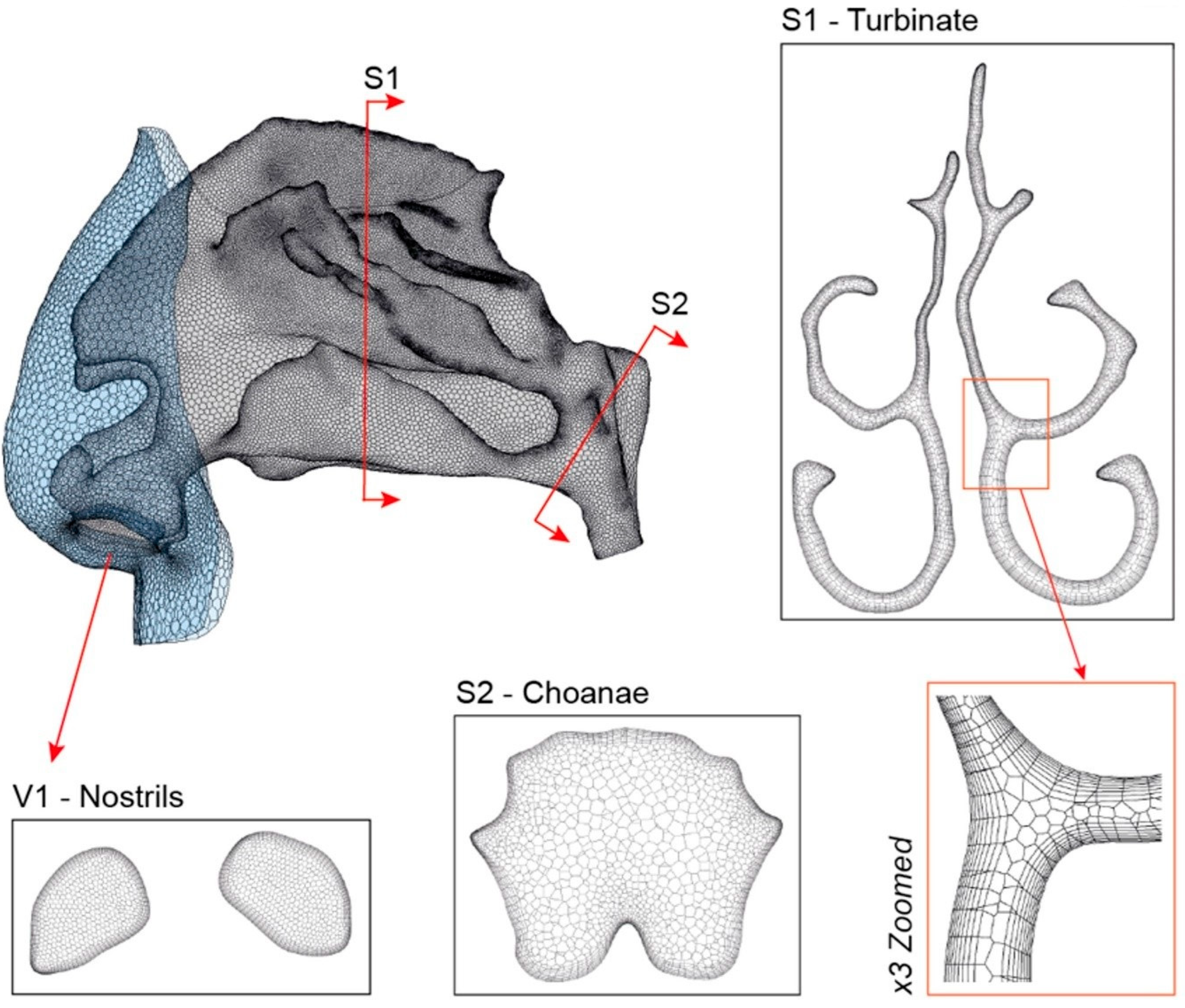
2.1. Airway Model and Computational Mesh
2.2. Particle Simulation
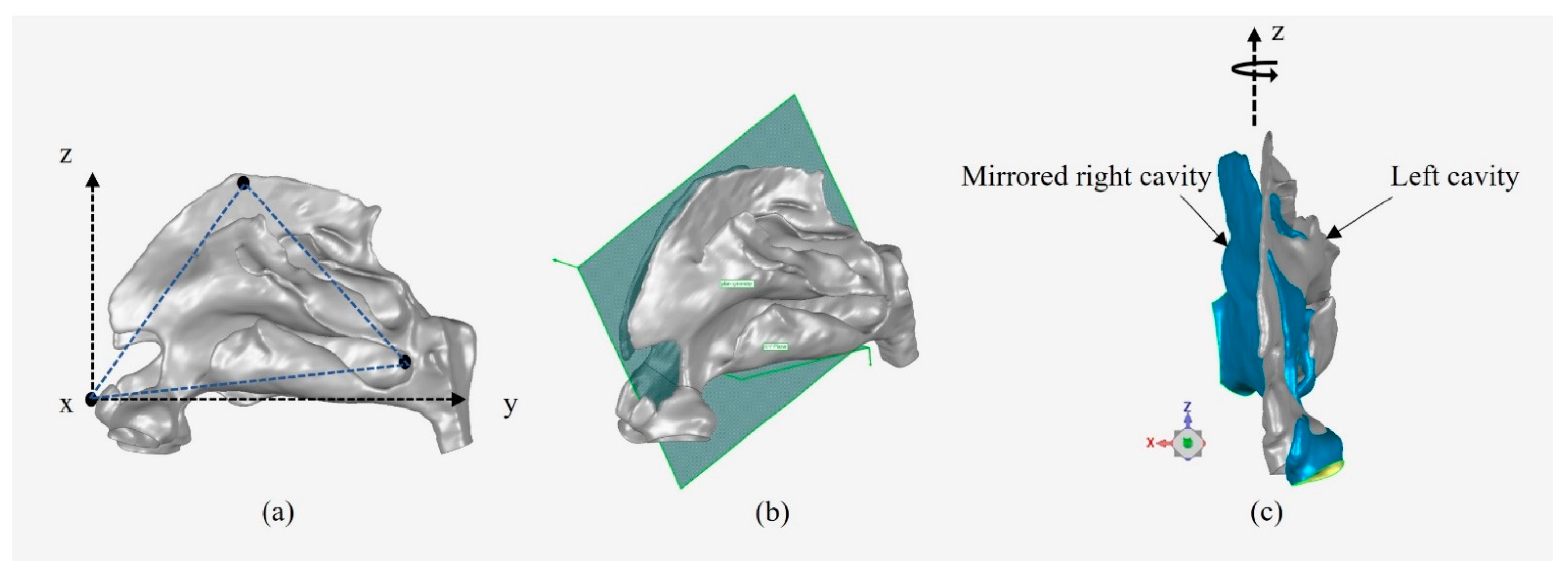
2.3. Alignment Method for Inter-Chamber Anatomical Comparison
3. Results and Discussion
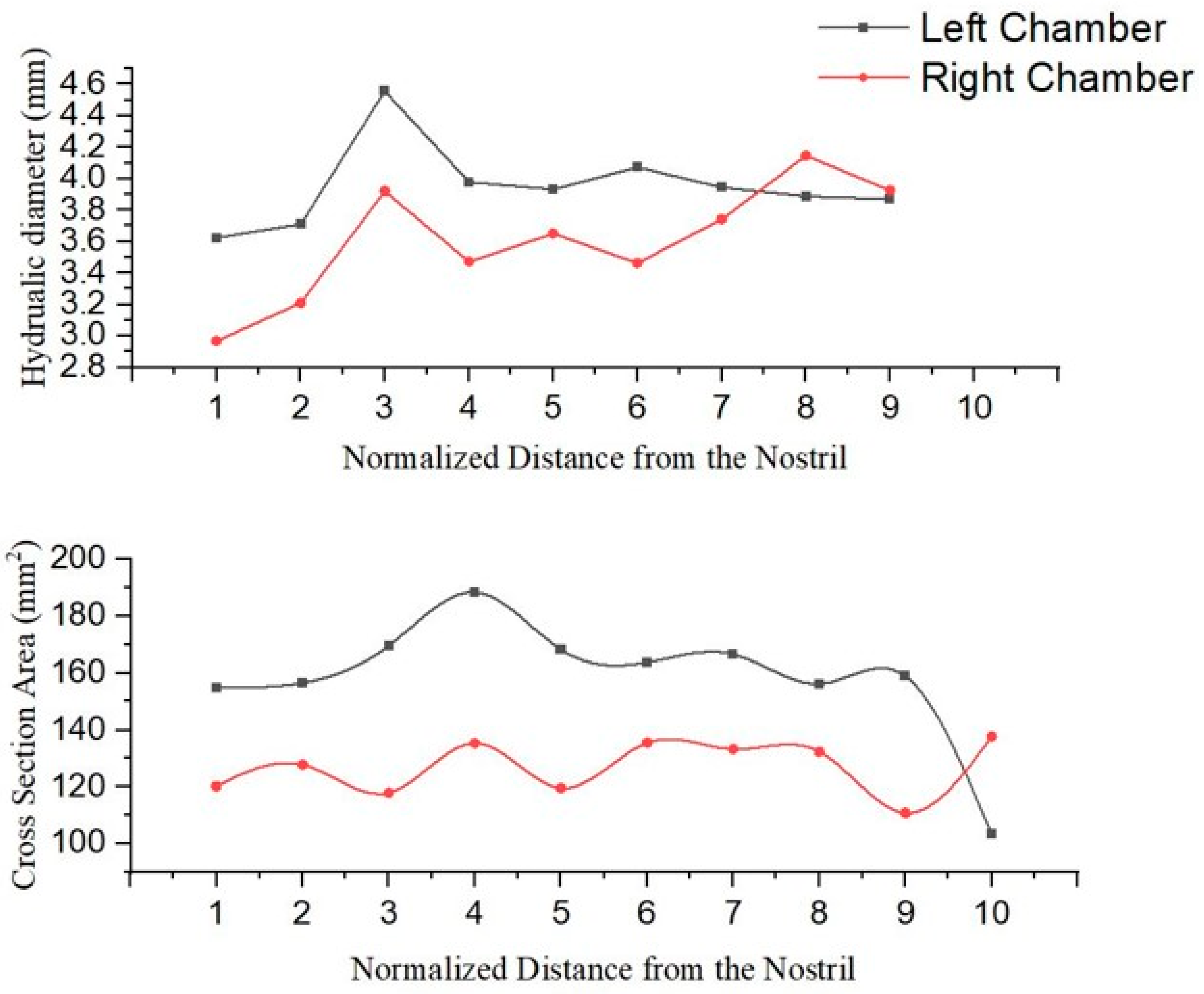
3.1. Anatomical Comparison
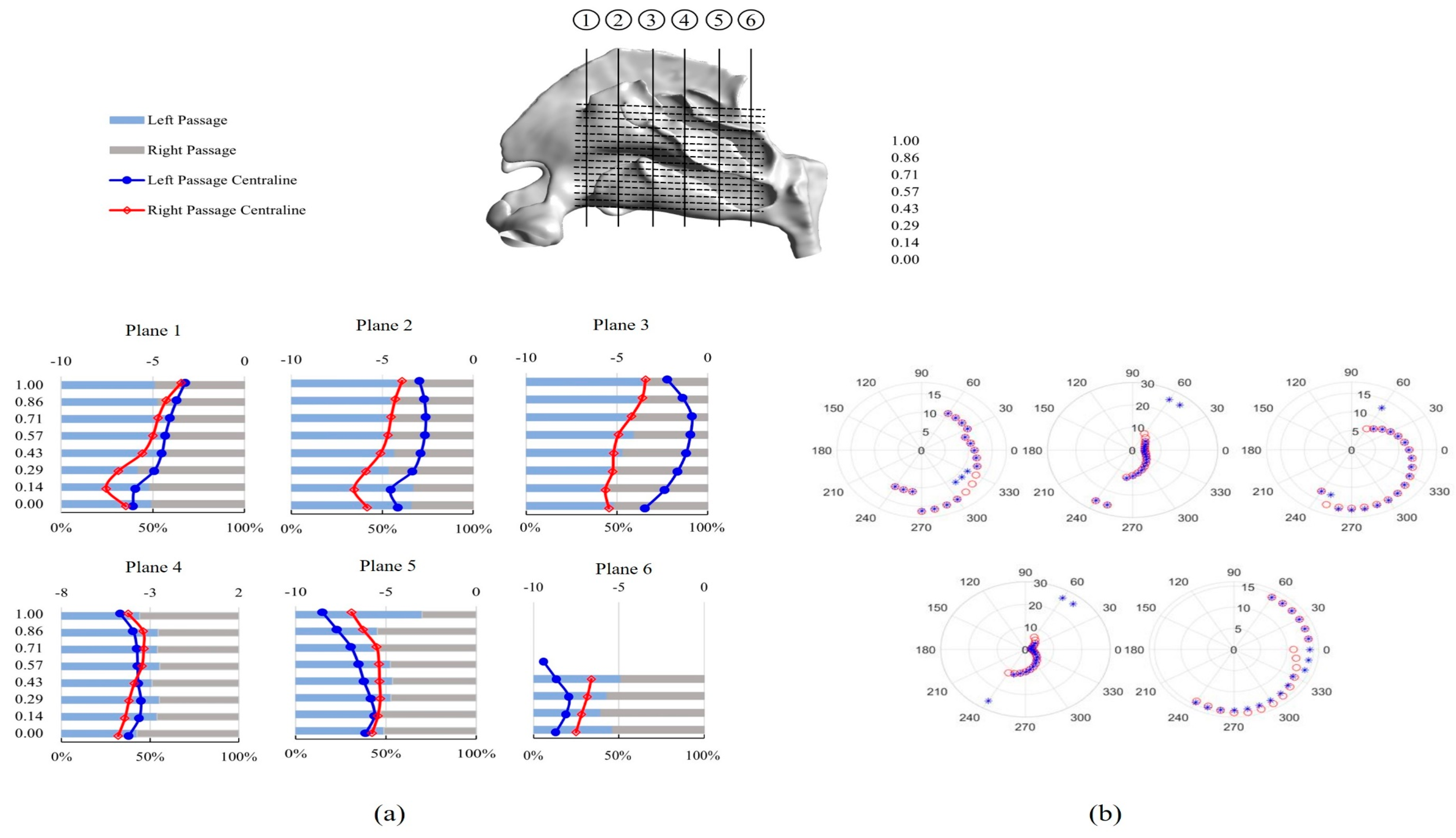
3.2. Inter-Chamber Variability Analysis
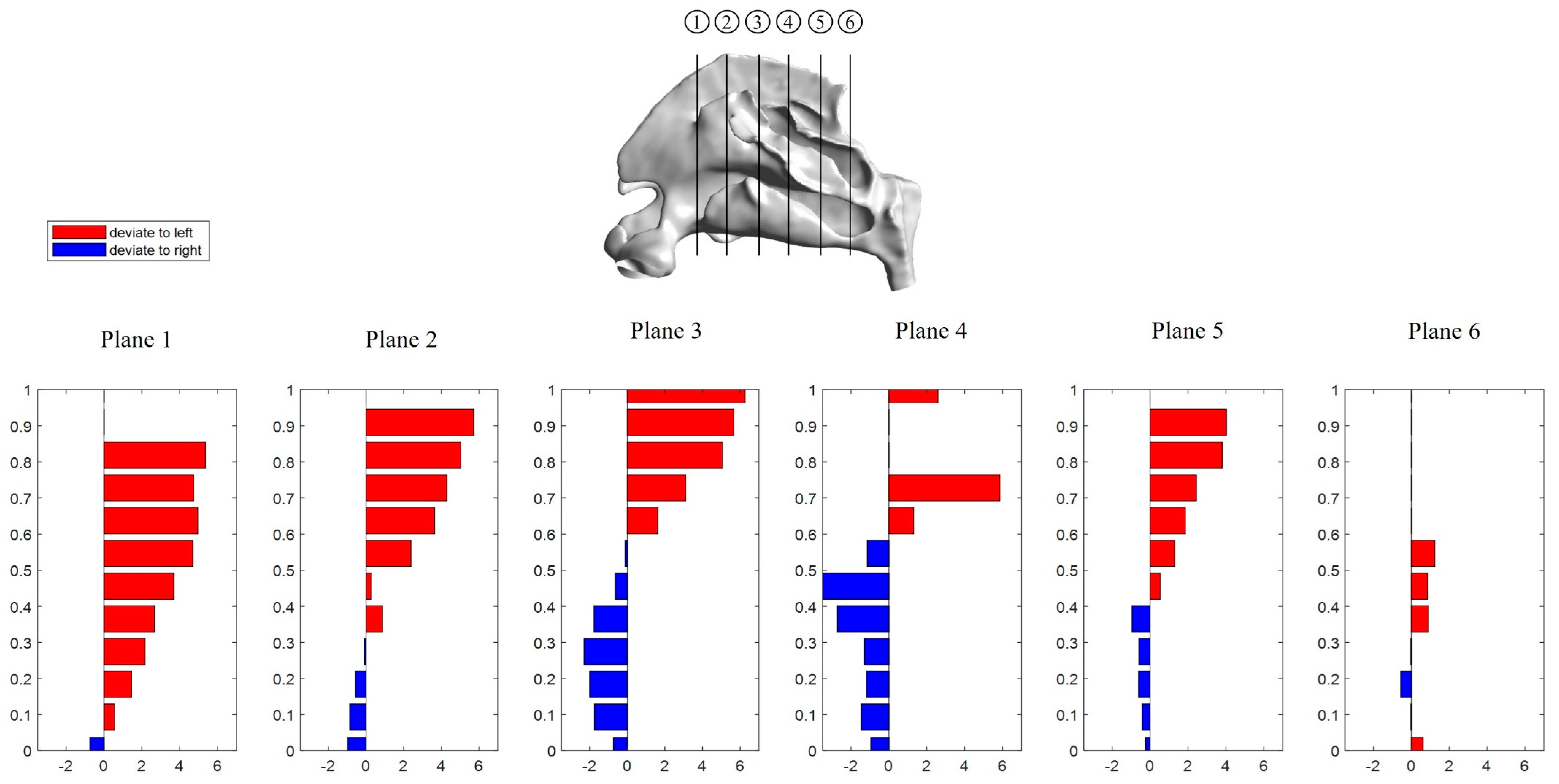
3.2.1. Septum Deviation
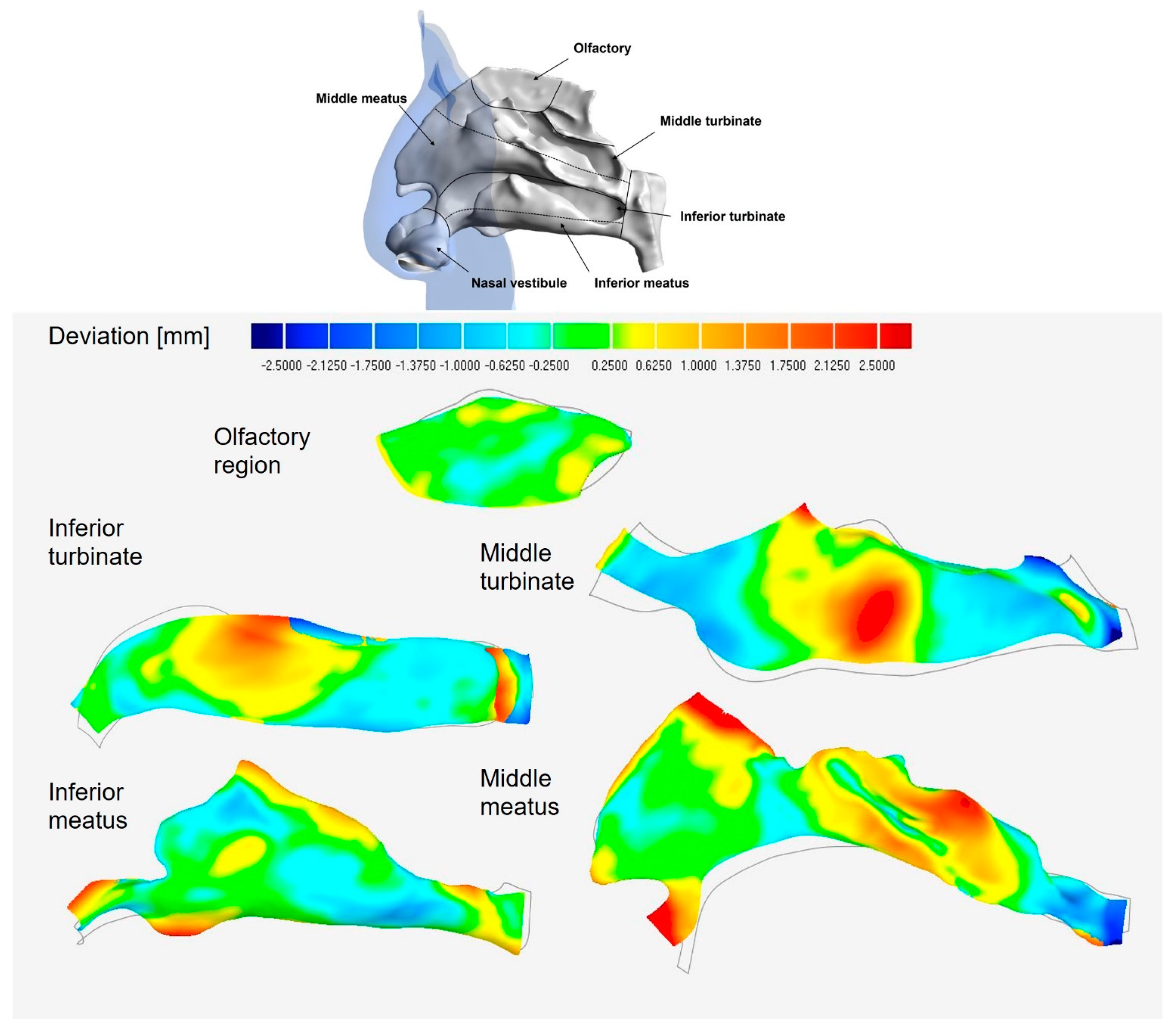
3.2.2. Lateral Wall Regional Deviation Analysis
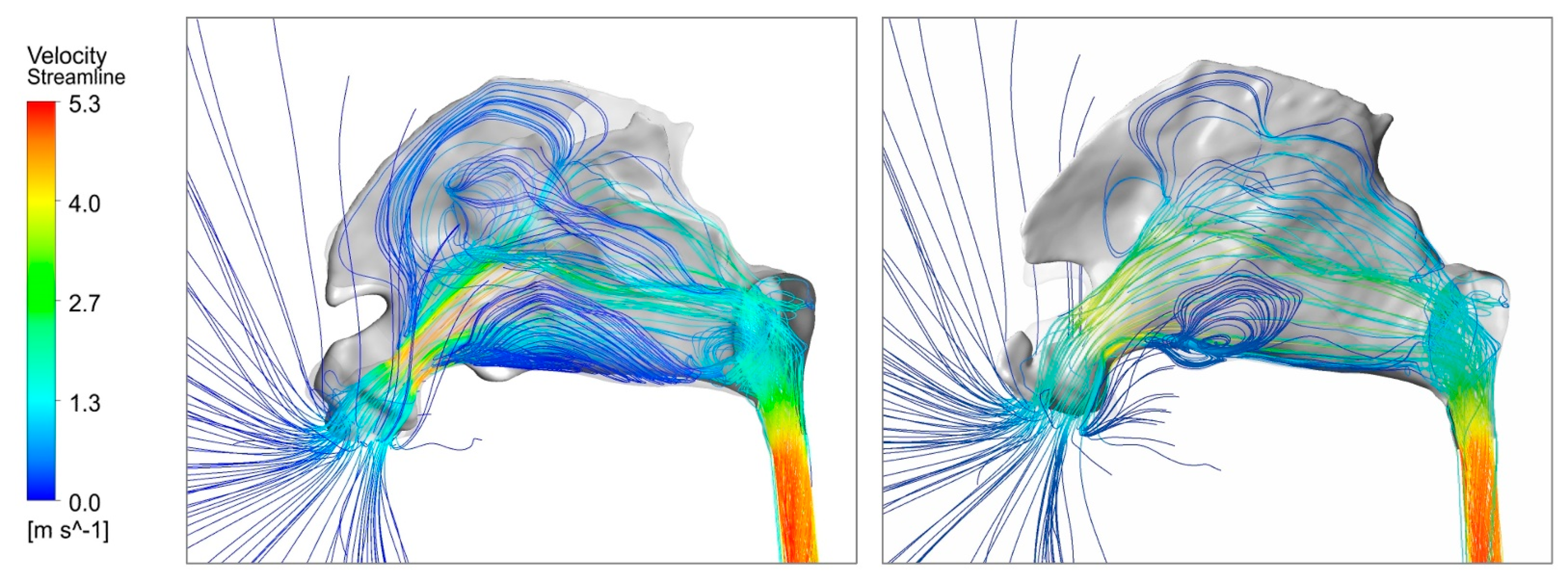
3.3. Airflow Dynamics
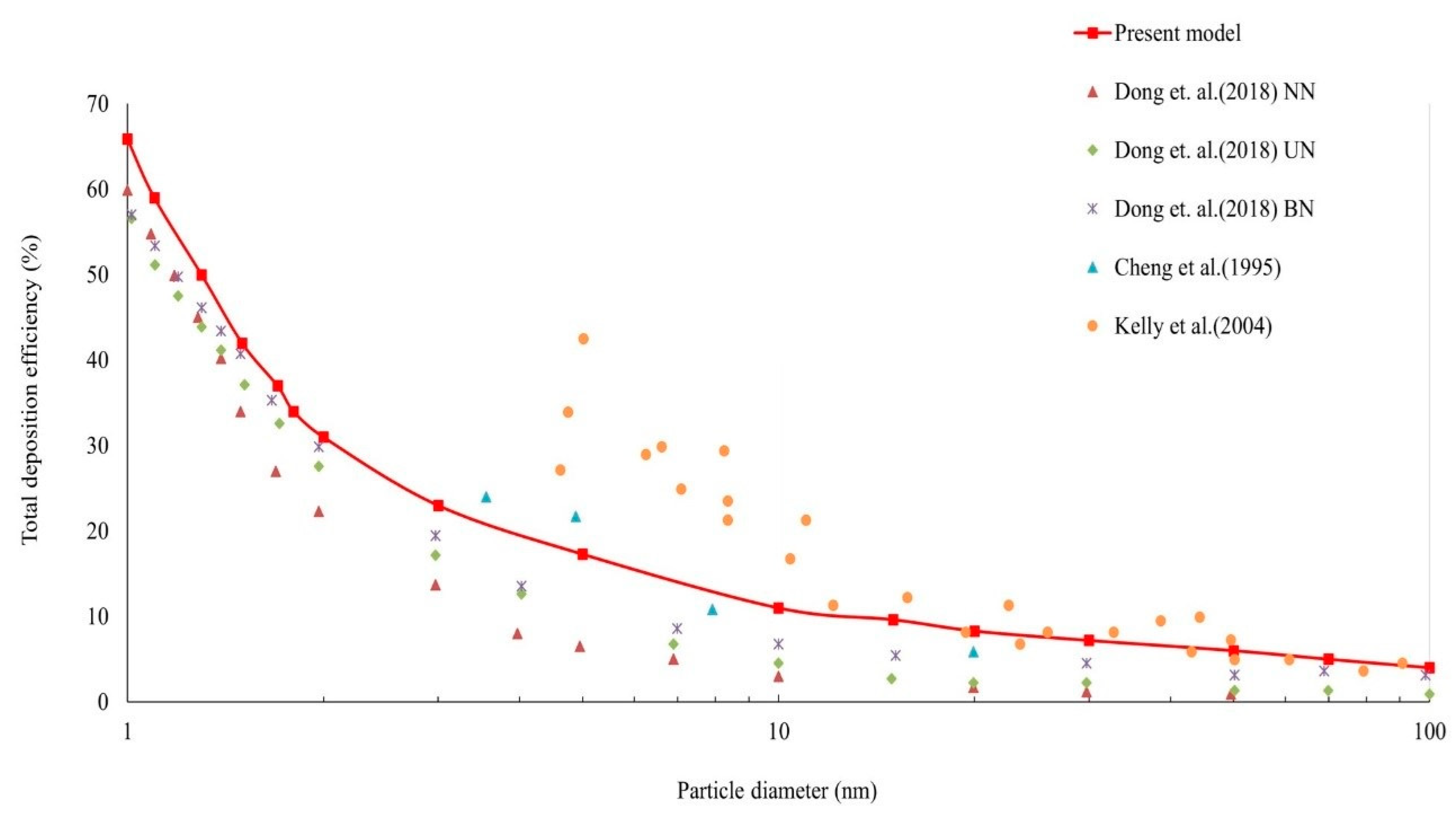
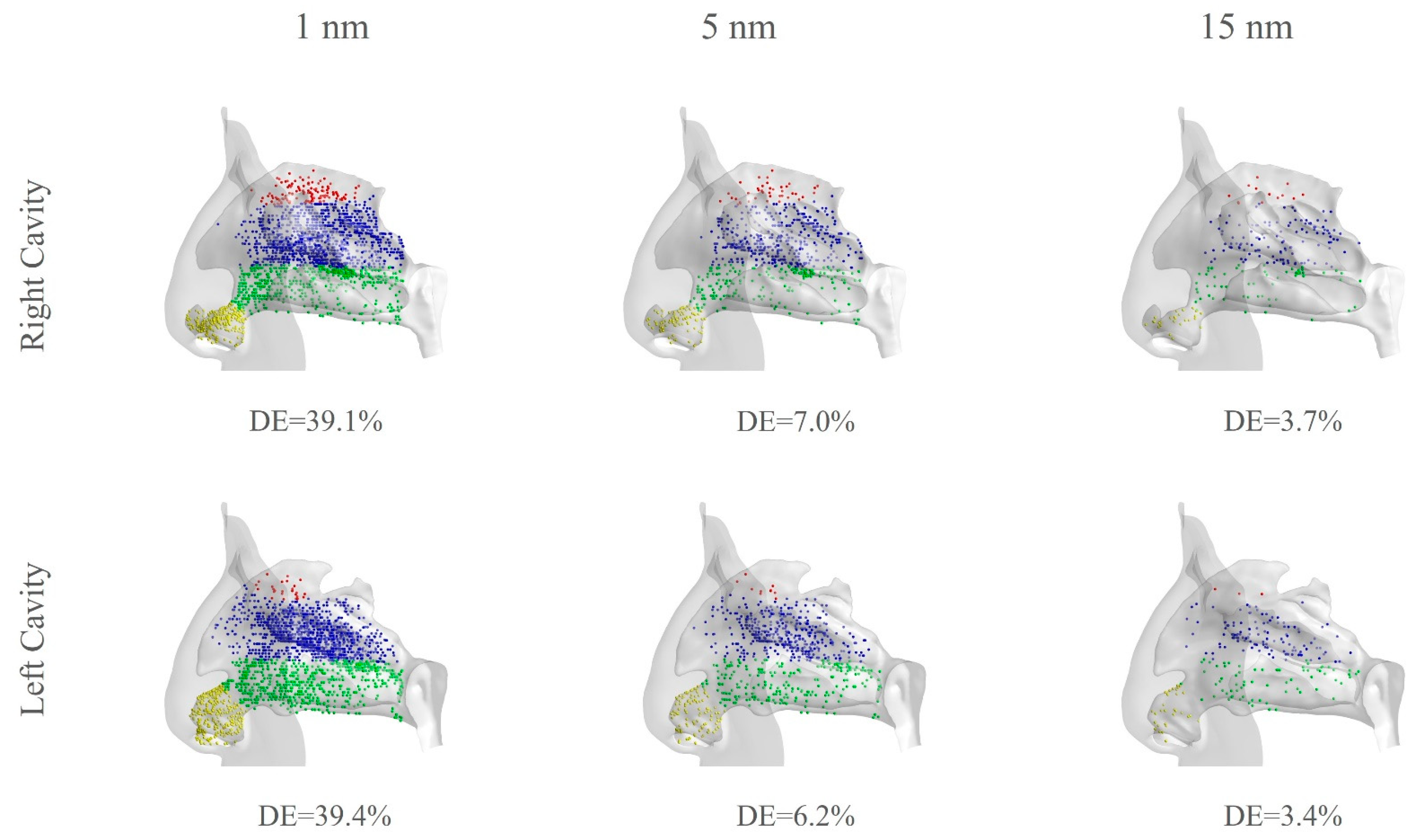
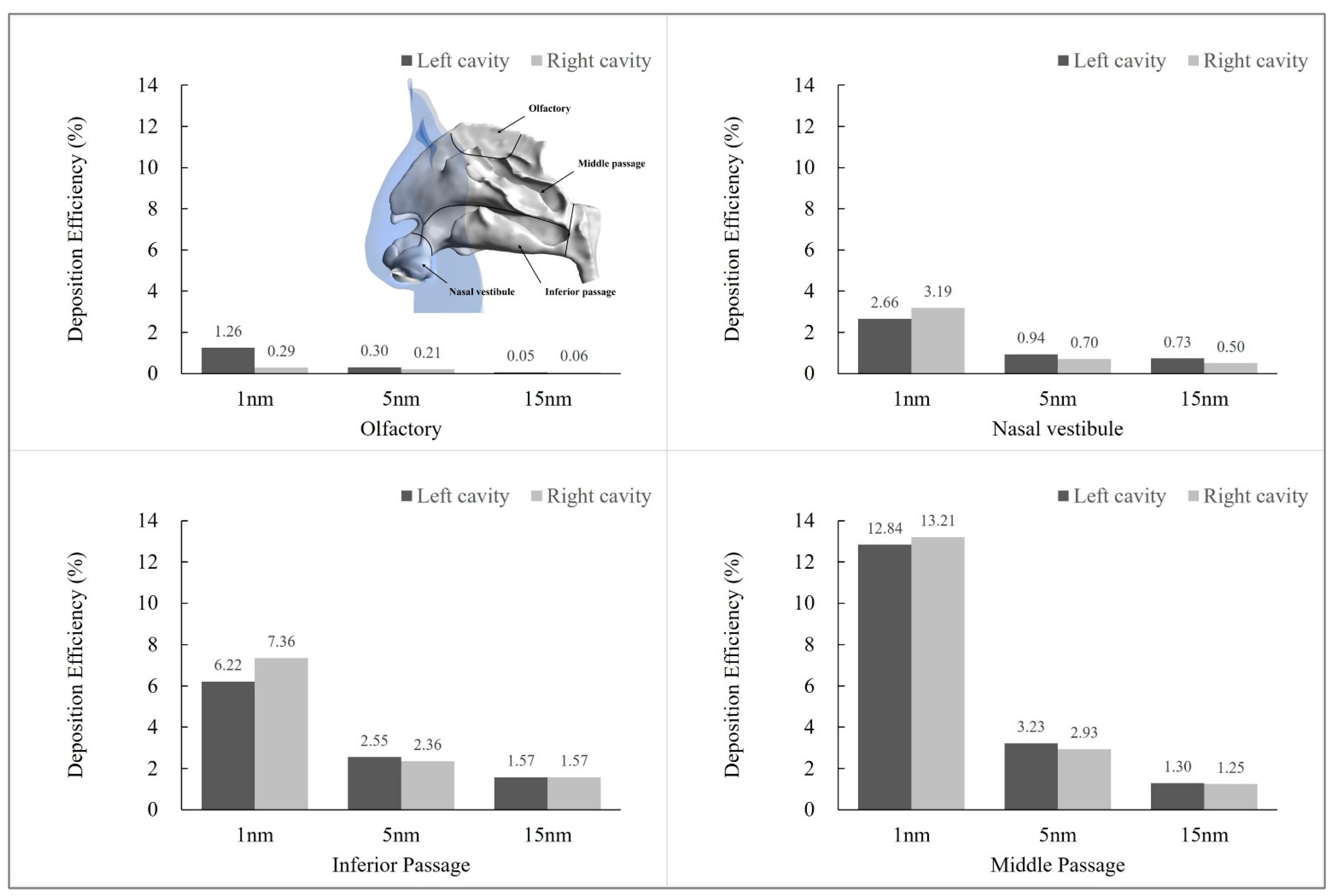
3.4. Particle Exposure Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, G.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Interindividual Variability in Nasal Filtration as a Function of Nasal Cavity Geometry. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Inthavong, K.; Tu, J.; Wang, S. Numerical simulations for detailed airflow dynamics in a human nasal cavity. Respir. Physiol. Neurobiol. 2008, 161, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Katelaris, C.H.; Kemp, A.S. Allergic rhinitis—practical management strategies. Aust. Fam. Physician 2008, 37, 214–220. [Google Scholar] [PubMed]
- Mladina, R.; Čujić, E.; Šubarić, M.; Vuković, K. Nasal septal deformities in ear, nose, and throat patients. An international study. Am. J. Otolaryngol.-Head Neck Med. Surg. 2008, 29, 75–82. [Google Scholar]
- Moghaddam, M.G.; Garcia, G.J.M.; Frank-Ito, D.O.; Kimbell, J.S.; Rhee, J.S. Virtual septoplasty: A method to predict surgical outcomes for patients with nasal airway obstruction. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Laine, T.; Warren, D.W. Effects of age, gender, and body size on nasal cross-sectional area in children. Eur. J. Orthod. 1991, 13, 311–316. [Google Scholar] [CrossRef]
- Huang, Z.L.; Wang, D.Y.; Zhang, P.C.; Dong, F.; Yeoh, K.H. Evaluation of nasal cavity by acoustic rhinometry in Chinese, Malay and Indian ethnic groups. Acta Oto-Laryngol. 2001, 121, 844–848. [Google Scholar]
- Chen, X.B.; Lee, H.P.; Chong, V.F.H.; Wang, D.Y. Numerical Simulation of the Effects of Inferior Turbinate Surgery on Nasal Airway Heating Capacity. Am. J. Rhinol. Allergy 2010, 24, e118–e122. [Google Scholar] [CrossRef]
- Ma, J.; Dong, J.; Shang, Y.; Inthavong, K.; Tu, J.; Frank-Ito, D.O. Air conditioning analysis among human nasal passages with anterior anatomical variations. Med. Eng. Phys. 2018, 57, 19–28. [Google Scholar] [CrossRef]
- Dong, J.; Ma, J.; Shang, Y.; Inthavong, K.; Qiu, D.; Tu, J.; Frank-Ito, D. Detailed nanoparticle exposure analysis among human nasal cavities with distinct vestibule phenotypes. J. Aerosol Sci. 2018, 121, 54–65. [Google Scholar] [CrossRef]
- Andrã, R.; Vuyk, H.D.; Ahmed, A.B.; Graamans, K.; Trenitã, G.N. Correlation between subjective and objective evaluation of the nasal airway. A systematic review of the highest level of evidence. Clin. Otolaryngol. 2009, 34, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Johnson, M.R.; Matida, E.A.; Kherani, S.; Marsan, J. Creation of a standardized geometry of the human nasal cavity. J. Appl. Physiol. 2009, 106, 784–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.F.; Abdullah, M.Z.; Ahmad, K.A.; Shuaib, I.L. Analytical Comparisons of Standardized Nasal Cavity. J. Med. Imaging Health Inform. 2014, 4, 14–20. [Google Scholar] [CrossRef]
- Keustermans, W.; Huysmans, T.; Schmelzer, B.; Sijbers, J.; Dirckx, J.J. The effect of nasal shape on the thermal conditioning of inhaled air: Using clinical tomographic data to build a large-scale statistical shape model. Comput. Biol. Med. 2020, 117, 103600. [Google Scholar] [CrossRef] [PubMed]
- Brüning, J.; Hildebrandt, T.; Heppt, W.; Schmidt, N.; Lamecker, H.; Szengel, A.; Amiridze, N.; Ramm, H.; Bindernagel, M.; Zachow, S.; et al. Characterization of the Airflow within an Average Geometry of the Healthy Human Nasal Cavity. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inthavong, K.; Tu, J.; Ahmadi, G. Computational Modelling of Gas-Particle Flows with Different Particle Morphology in the Human Nasal Cavity. J. Comput. Multiph. Flows 2009, 1, 57–82. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Dong, J.; Inthavong, K.; Tu, J. Comparative numerical modeling of inhaled micron-sized particle deposition in human and rat nasal cavities. Inhal. Toxicol. 2015, 27, 694–705. [Google Scholar] [CrossRef]
- Inthavong, K.; Ge, Q.J.; Li, X.; Tu, J.Y. Source and trajectories of inhaled particles from a surrounding environment and its deposition in the respiratory airway. Inhal. Toxicol. 2013, 25, 280–291. [Google Scholar] [CrossRef]
- Inthavong, K.; Ge, Q.J.; Li, X.D.; Tu, J.Y. Detailed predictions of particle aspiration affected by respiratory inhalation and airflow. Atmos. Environ. 2012, 62, 107–117. [Google Scholar] [CrossRef]
- Zubair, M.; Riazuddin, V.N.; Abdullah, M.Z.; Ismail, R.; Shuaib, I.L.; Hamid, S.A.; Ahmad, K.A. Airflow inside the nasal cavity: Visualization using computational fluid dynamics. Asian Biomed. 2010, 4, 657–661. [Google Scholar] [CrossRef] [Green Version]
- Zubair, M.; Riazuddin, V.N.; Abdullah, M.Z.; Rushdan, I.; Shuaib, I.L.; Ahmad, K.A. Computational Fluid Dynamics Study of Pull and Plug Flow Boundary Condition on Nasal Airflow. Biomed. Eng. Appl. Basis Commun. 2013, 25, 1350044. [Google Scholar] [CrossRef]
- Segal, R.A.; Kepler, G.M.; Kimbell, J.S. Effects of Differences in Nasal Anatomy on Airflow Distribution: A Comparison of Four Individuals at Rest. Ann. Biomed. Eng. 2008, 36, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Asgharian, B.; Kimbell, J.S.; Wong, B.A. Particle Deposition in Human Nasal Airway Replicas Manufactured by Different Methods. Part II: Ultrafine Particles. Aerosol Sci. Technol. 2004, 38, 1072–1079. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Cheng, Y.-S.; Yeh, H.-C.; Swift, D.L. Deposition of Ultrafine Aerosols in the Head Airways During Natural Breathing and During Simulated Breath Holding Using Replicate Human Upper Airway Casts. Aerosol Sci. Technol. 1995, 23, 465–474. [Google Scholar] [CrossRef]
- Dong, J.; Sun, Q.; Shang, Y.; Zhang, Y.; Tian, L.; Tu, J. Numerical comparison of inspiratory airflow patterns in human nasal cavities with distinct age differences. Int. J. Numer. Methods Biomed. Eng. 2021, e3565. [Google Scholar] [CrossRef]


| Left Cavity | Right Cavity | Total Cavity | |
|---|---|---|---|
| Surface area [cm2] | 100.45 | 92.48 | 192.93 |
| Volume [cm3] | 10.44 | 9.36 | 19.80 |
| Surface to volume ratio | 9.62 | 9.88 | 9.74 |
| Middle Turbinate | Inferior Turbinate | Middle Meatus | Infer Meatus | Olfactory | |
|---|---|---|---|---|---|
| Max. deviation positive | 3.16 | 3.08 | 5.31 | 2.76 | 0.61 |
| Max. deviation negative | −3.56 | −2.82 | −2.76 | −1.34 | −0.58 |
| Average deviation | 0.98 | 0.94 | 0.89 | 0.52 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Tian, L.; Tu, J.; Dong, J. Detailed Assessment of Nasal Inter-Chamber Anatomical Variations and Its Effect on Flow Apportionment and Inhalation Exposure Patterns. Fluids 2022, 7, 89. https://doi.org/10.3390/fluids7030089
Sun Q, Tian L, Tu J, Dong J. Detailed Assessment of Nasal Inter-Chamber Anatomical Variations and Its Effect on Flow Apportionment and Inhalation Exposure Patterns. Fluids. 2022; 7(3):89. https://doi.org/10.3390/fluids7030089
Chicago/Turabian StyleSun, Qinyuan, Lin Tian, Jiyuan Tu, and Jingliang Dong. 2022. "Detailed Assessment of Nasal Inter-Chamber Anatomical Variations and Its Effect on Flow Apportionment and Inhalation Exposure Patterns" Fluids 7, no. 3: 89. https://doi.org/10.3390/fluids7030089
APA StyleSun, Q., Tian, L., Tu, J., & Dong, J. (2022). Detailed Assessment of Nasal Inter-Chamber Anatomical Variations and Its Effect on Flow Apportionment and Inhalation Exposure Patterns. Fluids, 7(3), 89. https://doi.org/10.3390/fluids7030089






