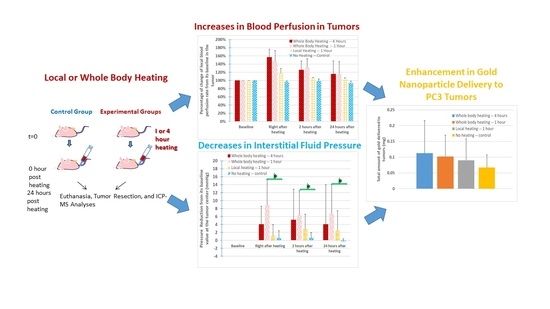
Nanoparticle Delivery in Prostate Tumors Implanted in Mice Facilitated by Either Local or Whole-Body Heating
Abstract
1. Introduction
2. Materials and Methods
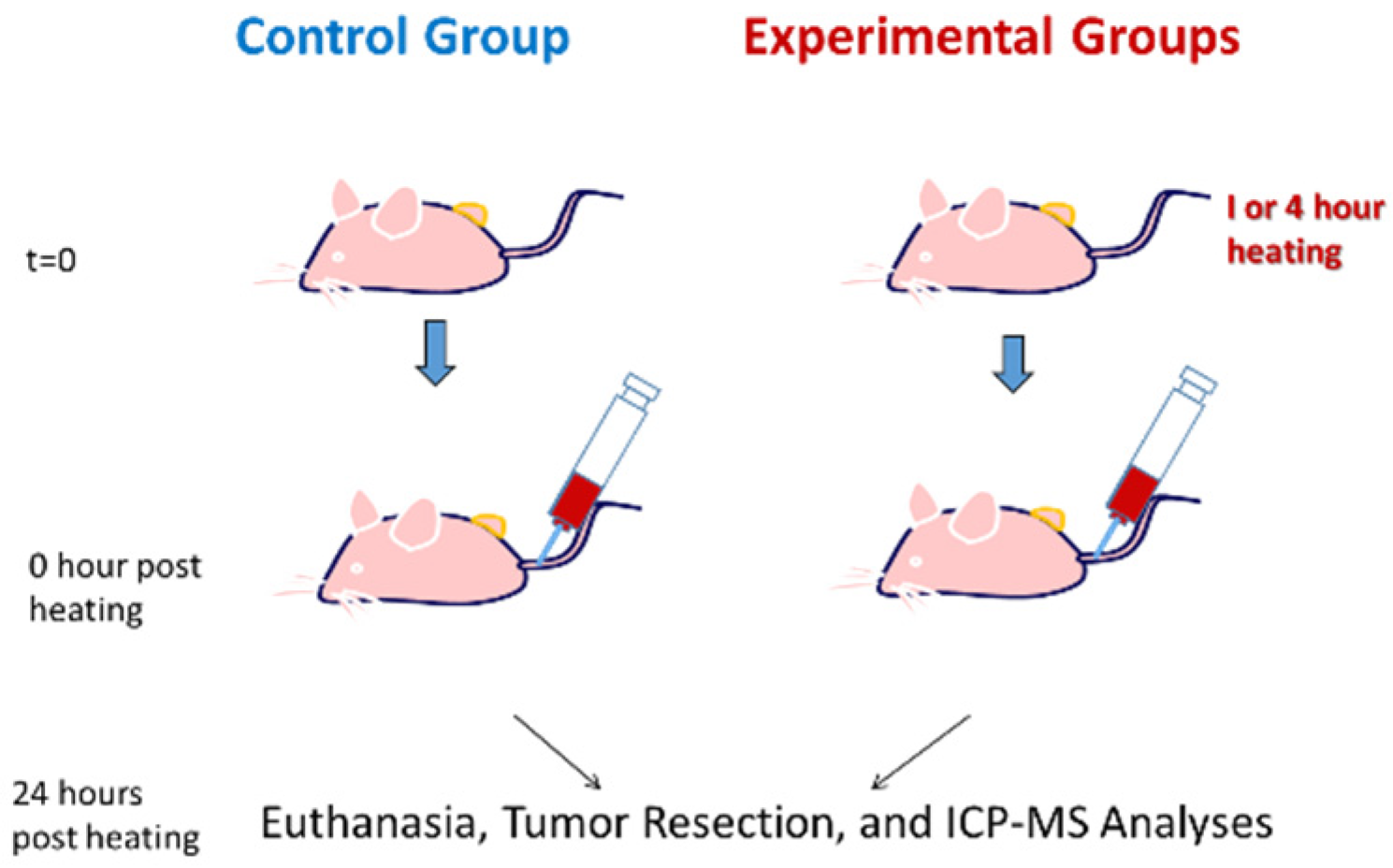
2.1. Animals and Tumor Model
2.2. Experimental Measurements of Temperatures, IFPs, and Blood Perfusion Rates
2.3. Experimental Protocols
2.4. Statistical Analyses
3. Results
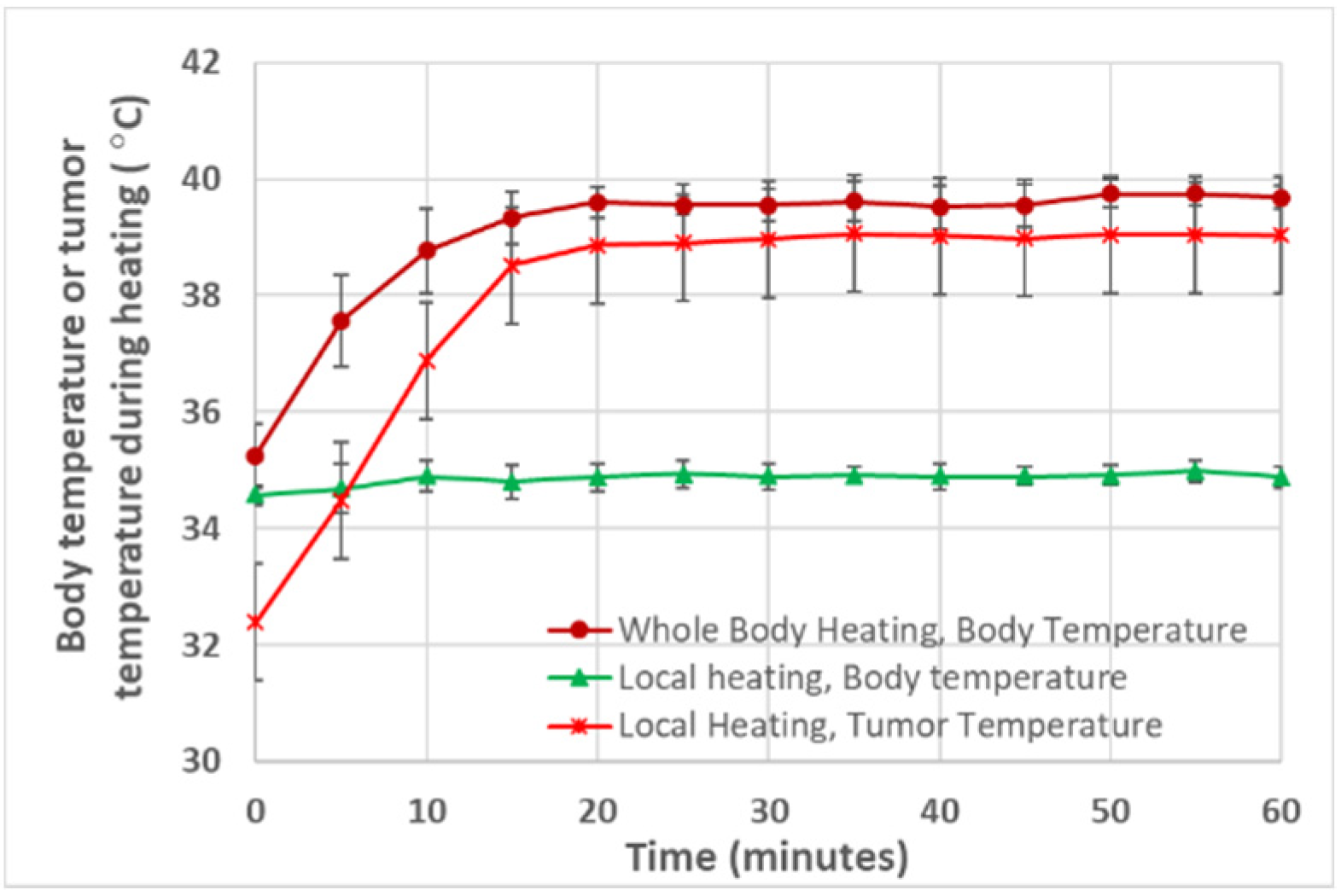
3.1. Tumor and Body Temperatures
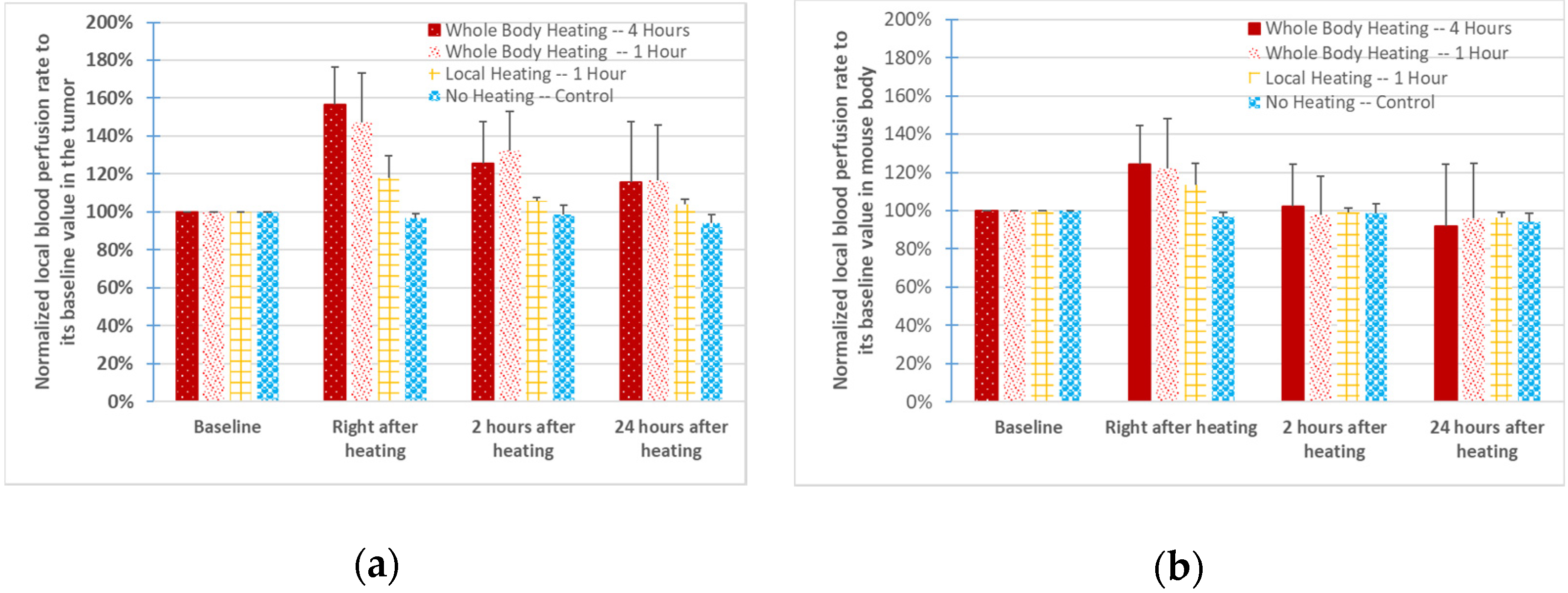
3.2. Blood Perfusion Rates in the Tumor and Mouse Body
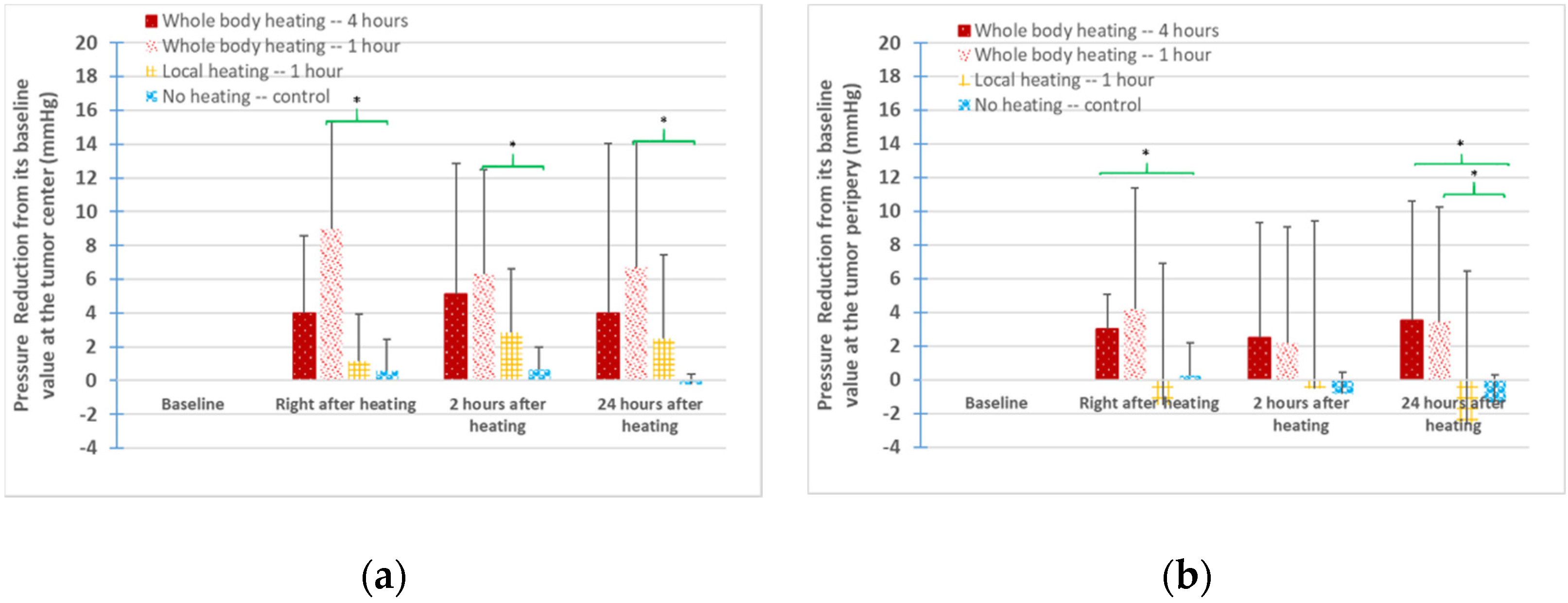
3.3. IFPs in the Tumors
3.4. ICP–MS Quantification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherukuri, P.; Glazer, E.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Basha, M. Nanotechnology as a Promising Strategy for Anticancer Drug Delivery. Curr. Drug Deliv. 2018, 15, 497–509. [Google Scholar] [CrossRef]
- Dockery, L.; Daniel, M.-C. Dendronized Systems for the Delivery of Chemotherapeutics. Mol. Cell. Basis Metastasis Road Ther. 2018, 139, 85–120. [Google Scholar]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective mac-romolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, F.; Mishra, R.K.; Khan, R. Precision Cancer Nanotherapy: Evolving Role of Multifunctional Nanoparticles for Cancer Active Targeting. J. Med. Chem. 2019, 62, 10475–10496. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sawa, T.; Fang, J.; Tanaka, S.; Miyamoto, Y.; Akaike, T.; Maeda, H. Pegylated zinc protoporphyrin: A wa-ter-soluble heme oxygenase inhibitor with tumor-targeting capacity. Bioconjug. Chem. 2002, 13, 1031–1038. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, M.; Ning, X.; Zhou, C.; Yang, S.; Zheng, J. PEGylation and Zwitterionization: Pros and Cons in the Renal Clearance and Tumor Targeting of Near-IR-Emitting Gold Nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 12572–12576. [Google Scholar] [CrossRef]
- van Vlerken, L.E.; Duan, Z.; Little, S.R.; Seiden, M.V.; Amiji, M.M. Biodistribution and Pharmacokinetic Analysis of Paclitaxel and Ceramide Administered in Multifunctional Polymer-Blend Nanoparticles in Drug Resistant Breast Cancer Model. Mol. Pharm. 2008, 5, 516–526. [Google Scholar] [CrossRef][Green Version]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; A Barrand, M. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 1–32. [Google Scholar] [CrossRef]
- A Nagy, J.; Chang, S.-H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef]
- Bockhorn, M.; Jain, R.K.; Munn, L.L. Active versus passive mechanisms in metastasis: Do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007, 8, 444–448. [Google Scholar] [CrossRef]
- Song, C.W. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984, 44, 4721s–4730s. [Google Scholar]
- Hauck, M.L.; Dewhirst, M.W.; Bigner, D.D.; Zalutsky, M.R. Local hyperthermia improves uptake of a chimeric monoclonal antibody in a subcutaneous xenograft model. Clin. Cancer Res. 1997, 3, 63–70. [Google Scholar]
- Lammers, T.; Peschke, P.; Kühnlein, R.; Subr, V.; Ulbrich, K.; Debus, J.; Huber, P.; Hennink, W.; Storm, G. Effect of radiother-apy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery system. J. Controll. Release 2007, 117, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Leunig, M.; Goetz, A.E.; Dellian, M.; Zetterer, G.; Gamarra, F.; Jain, R.K.; Messmer, K. Interstitial fluid pressure in solid tu-mors following hyperthermia: Possible correlation with therapeutic response. Cancer Res. 1992, 52, 487–490. [Google Scholar]
- Stepleton, S.; Dunne, M.; Milosevic, M.; Tran, C.W.; Gold, M.J.; Vedadi, A.; Mckee, T.D.; Ohashi, P.S.; Allen, C.; Jaffray, D.A. Radiation and heat improve the delivery and efficacy of nanotherapeutics by modulating intratumoral fluid dynamic. ACS Nano 2018, 12, 7583–7600. [Google Scholar] [CrossRef]
- Hauck, M.L.; Coffin, D.O.; Dodge, R.K.; Dewhirst, M.W.; Mitchell, J.B.; Zalutsky, M.R. A local hyperthermia treatment which enhances antibody uptake in a glioma xenograft model does not affect tumor interstitial fluid pressure. Int. J. Hyperth. 1997, 13, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Capitano, M.L.; Spernyak, J.A.; Schueckler, J.T.; Thomas, S.; Singh, A.K.; Evans, S.S.; Hylander, B.L.; Repasky, E.A. Mild Elevation of Body Temperature Reduces Tumor Interstitial Fluid Pressure and Hypoxia and Enhances Efficacy of Radiotherapy in Murine Tumor Models. Cancer Res. 2011, 71, 3872–3880. [Google Scholar] [CrossRef]
- Winslow, T.B.; Eranki, A.; Ullas, S.; Singh, A.K.; Repasky, E.A.; Sen, A. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: Analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int. J. Hyperth. 2015, 31, 693–701. [Google Scholar] [CrossRef]
- Gu, Q.; Liu, S.; Ray, A.S.; Florinas, S.; Christie, R.J.; Daniel, M.-C.; Bieberich, C.; Ma, R.; Zhu, L. Mild Whole-Body Hyperthermia-Induced Interstitial Fluid Pressure Reduction and Enhanced Nanoparticle Delivery to PC3 Tumors: In Vivo Studies and Micro-Computed Tomography Analyses. J. Therm. Sci. Eng. Appl. 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Florinas, S.; Liu, M.; Fleming, R.; Van Vlerken-Ysla, L.; Ayriss, J.; Gilbreth, R.; DiMasi, N.; Gao, C.; Wu, H.; Xu, Z.-Q.; et al. A Nanoparticle Platform to Evaluate Bioconjugation and Receptor-Mediated Cell Uptake Using Cross-Linked Polyion Complex Micelles Bearing Antibody Fragments. Biomacromolecules 2016, 17, 1818–1833. [Google Scholar] [CrossRef]
- Chen, S.; Florinas, S.; Teitgen, A.; Xu, Z.-Q.; Gao, C.; Wu, H.; Kataoka, K.; Cabral, H.; Christie, R.J. Controlled Fab installation onto polymeric micelle nanoparticles for tuned bioactivity. Sci. Technol. Adv. Mater. 2017, 18, 666–680. [Google Scholar] [CrossRef]
- Pan, H.; Grow, M.E.; Wilson, O.M.; Daniel, M.-C. A New PPI dendron as potential convenient building-block in con-struction of multifunctional systems for biomedical applications. Tetrahedron 2013, 69, 2799–2806. [Google Scholar] [CrossRef]
- Noireaux, J.; Grall, R.; Hullo, M.; Chevillard, S.; Oster, C.; Brun, E.; Sicard-Roselli, C.; Loeschner, K.; Fisicaro, P. Gold nano-particle uptake in tumor cells: Quantification and size distribution by SP-ICPMS. Separations 2019, 6, 3. [Google Scholar] [CrossRef]
- Allabashi, R.; Stach, W.; De La Escosura-Muñiz, A.; Liste-Calleja, L.; Merkoçi, A.; Escosura-Muñiz, A. ICP-MS: A powerful technique for quantitative determination of gold nanoparticles without previous dissolving. J. Nanopart. Res. 2009, 11, 2003–2011. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Martin, J.; Chauhan, V.; Jain, S.R.; Diop-Frimpong, B.; Bardeesy, N.; Smith, B.L.; Ferrone, C.R.; Hornicek, F.J.; Boucher, Y.; et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 15101–15108. [Google Scholar] [CrossRef]
- Singh, M.; Ma, R.; Zhu, L. Theoretical evaluation of enhanced gold nanoparticle delivery to PC3 tumors due to increased hydraulic conductivity or recovered lymphatic function after mild whole body hyperthermia. Med. Biol. Eng. Comput. 2021, 59, 301–313. [Google Scholar] [CrossRef]
- Mariana, V.F.; De Fátima, G.G.M.; Maria, P.D.G.J. The effect of mechanical lymph drainage accompanied with heat on lymphedema. J. Res. Med. Sci. 2011, 16, 1448–1451. [Google Scholar]
- Leu, A.J.; A Berk, D.; Lymboussaki, A.; Alitalo, K.; Jain, R.K. Absence of functional lymphatics within a murine sarcoma: A molecular and functional evaluation. Cancer Res. 2000, 60, 4324–4327. [Google Scholar]
- Baxter, L.T.; Jain, R.K. Vascular permeability and interstitial diffusion in superfused tissues: A two-dimensional model. Microvasc. Res. 1988, 36, 108–115. [Google Scholar] [CrossRef]
- Baxter, L.T.; Jain, R.K. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc. Res. 1989, 37, 77–104. [Google Scholar] [CrossRef]
- Kareh, A.W.; Secomb, T. Effect of increasing vascular hydraulic conductivity on delivery of macromolecular drugs to tumor cells. Int. J. Radiat. Oncol. 1995, 32, 1419–1423. [Google Scholar] [CrossRef]
- Su, D.; Ma, R.; Salloum, M.; Zhu, L. Multi-scale study of nanoparticle transport and deposition in tissues during an injection process. Med. Biol. Eng. Comput. 2010, 48, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, S.; Milosevic, M.; Allen, C.; Zheng, J.; Dunne, M.; Yeung, I.; Jaffray, D.A. A Mathematical Model of the Enhanced Permeability and Retention Effect for Liposome Transport in Solid Tumors. PLoS ONE 2013, 8, e81157. [Google Scholar] [CrossRef] [PubMed]
- Meermann, B.; Nischwitz, V. ICP-MS for the analysis at the nanoscale—A tutorial review. J. Anal. At. Spectrom. 2018, 33, 1432–1468. [Google Scholar] [CrossRef]
- Laborda, F.; Bolea, E.; Cepriá, G.; Gómez, M.T.; Jiménez, M.S.; Pérez-Arantegui, J.; Castillo, J.R. Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples. Anal. Chim. Acta 2016, 904, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, K.L.; Gordon, W.H.; Ash, K.O. Inductively coupled plasma mass spectrometry for trace element analysis in the clin-ical laboratory. Ann. Clin. Lab. Sci. 1995, 25, 264–271. [Google Scholar]
- Liu, J.; Murphy, K.E.; Winchester, M.R.; Hackley, V.A. Overcoming challenges in single particle inductively coupled plasma mass spectrometry measurement of silver nanoparticles. Anal. Bioanal. Chem. 2017, 409, 6027–6039. [Google Scholar] [CrossRef]
- LeBrun, A.; Ma, R.; Zhu, L. MicroCT Image based simulation to design heating protocols in magnetic nanoparticle hyperther-mia for cancer treatment. J. Therm. Biol. 2016, 62, 129–137. [Google Scholar] [CrossRef]
- Singh, M.; Gu, Q.; Ma, R.; Zhu, L. Heating protocol design affected by nanoparticles re-distribution and thermal damage model in magnetic nanoparticle hyperthermia for cancer treatment. ASME J. Heat Transf. 2020, 142, 072501. [Google Scholar] [CrossRef]
- Manuchehrabadi, N.; Zhu, L. Development of a computational simulation paradigm to design a protocol for treating prostate tumor using transurethral laser photothermal therapy. Int. J. Hyperth. 2014, 30, 349–361. [Google Scholar] [CrossRef]



| Time Instances | 1 h Whole-Body | 1 h Local | Control | |
|---|---|---|---|---|
| 4 h whole body | 0 h | 0.32 | 0.03 | 0.004 |
| 2 h | 0.33 | 0.08 | 0.045 | |
| 24 h | 0.48 | 0.18 | 0.42 | |
| 1 h whole body | 0 h | / | 0.02 | 0.0003 |
| 2 h | / | 0.014 | 0.007 | |
| 24 h | / | 0.14 | 0.40 | |
| 1 h local | 0 h | / | / | 0.0002 |
| 2 h | / | / | 0.22 | |
| 24 h | / | / | 0.31 |
| Time Instances | 1 h Whole-Body | 1 h Local | Control | |
|---|---|---|---|---|
| 4 h whole body | 0 h | 0.42 | 0.16 | 0.002 |
| 2 h | 0.31 | 0.35 | 0.32 | |
| 24 h | 0.39 | 0.34 | 0.42 | |
| 1 h whole body | 0 h | / | 0.20 | 0.02 |
| 2 h | / | 0.48 | 0.42 | |
| 24 h | / | 0.48 | 0.43 | |
| 1 h local | 0 h | / | / | 0.01 |
| 2 h | / | / | 0.39 | |
| 24 h | / | / | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Q.; Dockery, L.; Daniel, M.-C.; Bieberich, C.J.; Ma, R.; Zhu, L. Nanoparticle Delivery in Prostate Tumors Implanted in Mice Facilitated by Either Local or Whole-Body Heating. Fluids 2021, 6, 272. https://doi.org/10.3390/fluids6080272
Gu Q, Dockery L, Daniel M-C, Bieberich CJ, Ma R, Zhu L. Nanoparticle Delivery in Prostate Tumors Implanted in Mice Facilitated by Either Local or Whole-Body Heating. Fluids. 2021; 6(8):272. https://doi.org/10.3390/fluids6080272
Chicago/Turabian StyleGu, Qimei, Lance Dockery, Marie-Christine Daniel, Charles J. Bieberich, Ronghui Ma, and Liang Zhu. 2021. "Nanoparticle Delivery in Prostate Tumors Implanted in Mice Facilitated by Either Local or Whole-Body Heating" Fluids 6, no. 8: 272. https://doi.org/10.3390/fluids6080272
APA StyleGu, Q., Dockery, L., Daniel, M.-C., Bieberich, C. J., Ma, R., & Zhu, L. (2021). Nanoparticle Delivery in Prostate Tumors Implanted in Mice Facilitated by Either Local or Whole-Body Heating. Fluids, 6(8), 272. https://doi.org/10.3390/fluids6080272