Nebulization Criteria and Quantification
Abstract
1. Introduction
1.1. Medical Nebulizers
1.2. Nebulizer Characterization
2. Literature Review on Nebulization
2.1. Methods
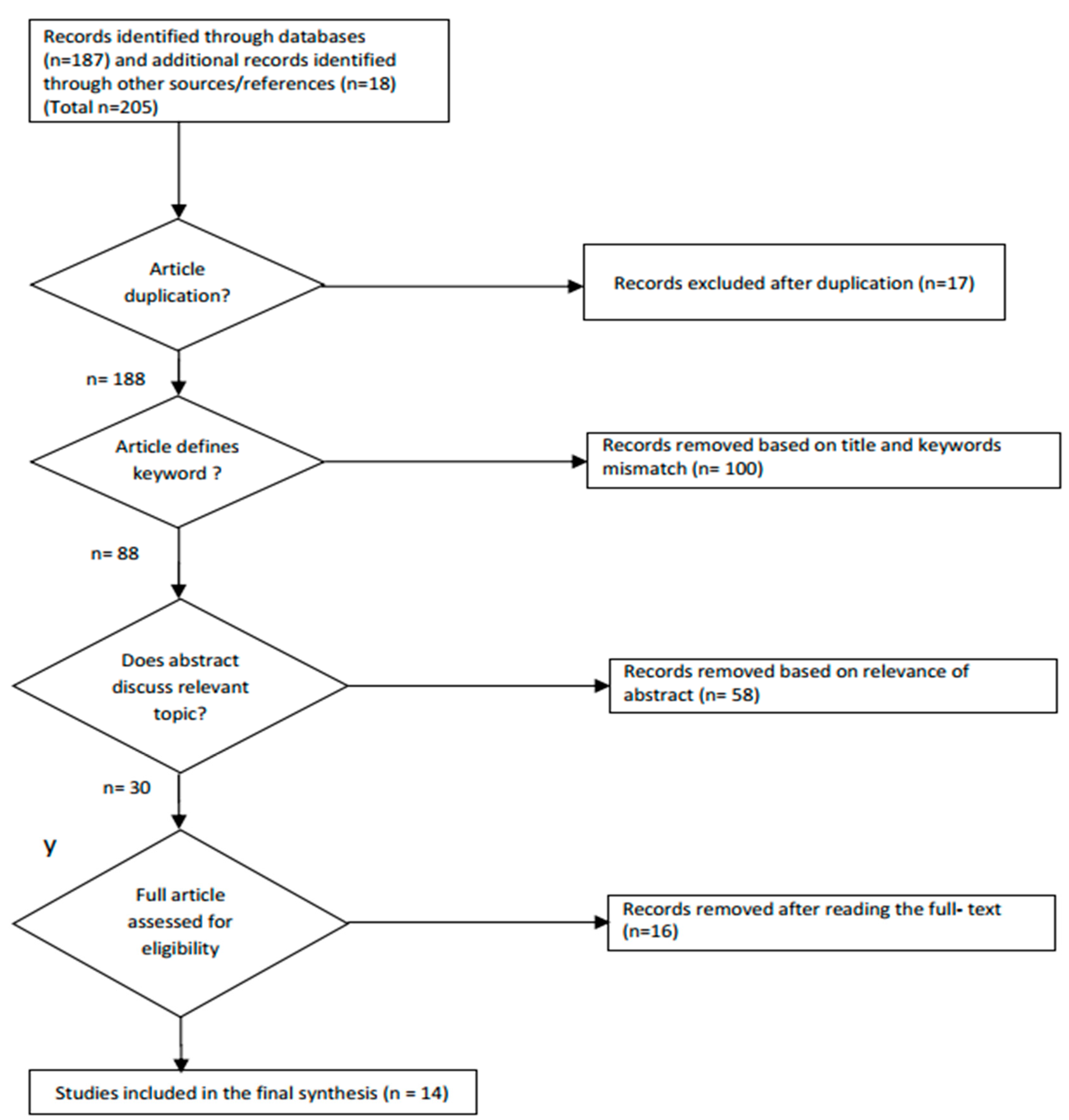
2.1.1. Search Strategy and Eligibility Criteria
2.1.2. Data Extraction and Study Quality Evaluation
2.2. Influencing Factors
2.3. Droplet Size and Velocity Measurement
- Mechanical method: In this method, first, droplet samples are directly collected on a solid surface or in a cell containing special fluid. Next, their size is measured through photography or microscopy. There are different types of mechanical methods including droplet capture, cascade impaction and sedimentation techniques. In the droplet capture method, samples of the droplet are collected on a surface where different kinds of surface coatings such as white Vaseline and magnesium oxide are applied. Then size of the droplets is determined using a microscope by measuring the mechanical deformation that occurred on the surface coatings by the droplet impact. Although this method is simple, size determination and counting is difficult due to the overlap of droplets on the slides. Besides, the collected sample of droplets might not represent the whole droplet [80]. In case of cascade impactors, the droplet travels with airflow at high velocity and then is impacted with slides coated with a carbon and magnesium oxide mixture, which retains the large droplets, whereas the small droplets will follow the airflow around the slide. As the velocity of the small droplets increases, they will hit the slide, and then after are gathered in a collector. The size of the droplet is then determined by analyzing the cumulative droplet sizes in each slide. This method is convenient for droplet sizes between 1.5 and 50 µm, whereas droplets larger than 50 µm impose problems on the first slide [84]. In the case of the sedimentation method, the spray of droplets is injected into a horizontally moving uniform airflow, which passes through a settling chamber. Then the droplets split up and fall down at varying distances into the settling chamber because of their different terminal velocity and size.
- Electrical: This method depends on the detection and analysis of electronic pulses produced by drops for the construction of drop size distributions. The electrical techniques include the Wicks–Dukler approach, the charged wire probe, and the hot wire anemometer [84]. In the Wicks–Dukler method, the droplet contact frequency between two sharp needles with potential difference is recorded. The distance between the needles is adjustable, and electric contact among them occurs if the droplets are in contact with them simultaneously. In order to monitor the closure frequency, a counter is utilized. The distance between the needles is varied, and closure frequency is measured again and then droplet size distribution is determined. In case of the charged wire method, the droplet is made to hit electrically charged wire, which results in the draw of charge from the wire. The amount of charge drawn from the wire depends on the size of the droplet. In the case of a hot wire anemometer, a drop is captured in a resistively heated wire, which results in the local cooling of the heated wire as the droplet evaporates. Due to this cooling, the wire resistance will fall down. Therefore, in order to restore the resistance to the originally preset value, a voltage pulse is produced, and the analysis of this voltage pulse leads to the droplet size determination [84].
- Optical: includes both imaging (photography, cinematography, holography) and nonimaging (single drop counters, ensemble multi drop counters) techniques.
2.3.1. Laser Diffraction Analyzer
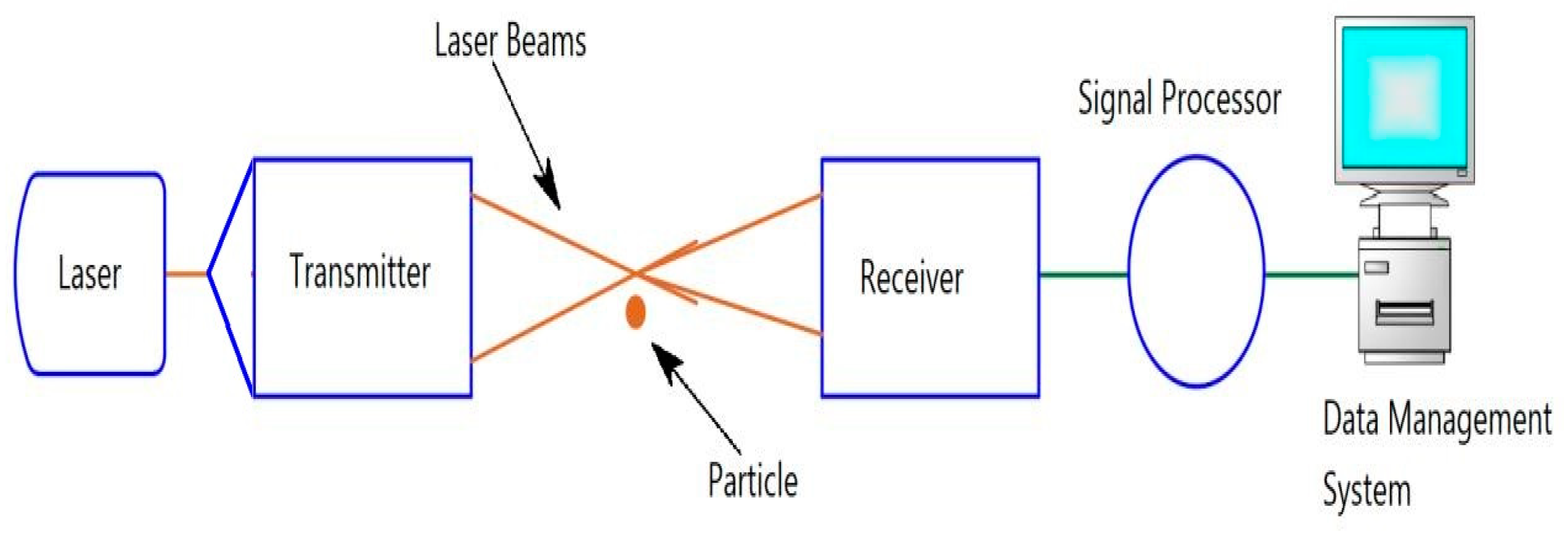
2.3.2. Laser Doppler Velocimetry
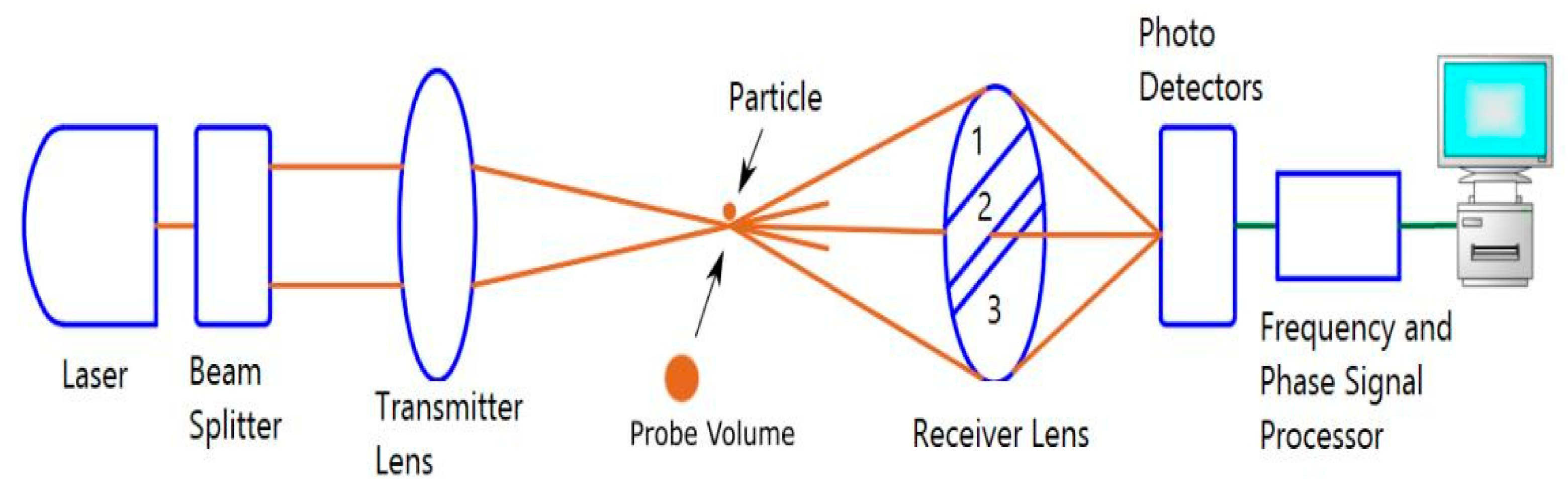
2.3.3. Phase Doppler Anemometer
3. Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Merkus, H.G.; Meesters, G.M.H. (Eds.) Production, Handling and Characterization of Particulate Materials; Springer International Publishing: Cham, Switzerland, 2016; Volume 25. [Google Scholar]
- Bayvel, L.P.; Orzechowski, Z. Liquid Atomization; Taylor & Francis: Washington, DC, USA, 1993. [Google Scholar]
- Khavkin, Y. The Theory and Practice of Swirl Atomizers; Taylor & Francis: New York, NY, USA, 2004. [Google Scholar]
- Lefebvre, A.H. Atomization and Sprays; Hemisphere Pub. Corp.: New York, NY, USA, 1989. [Google Scholar]
- ANSYS FLUENT 12.0 Theory Guide—15.9.2 The Pressure-Swirl Atomizer Model. Available online: http://www.afs.enea.it/project/neptunius/docs/fluent/html/th/node269.htm (accessed on 18 August 2019).
- Ang, A.S.M.; Berndt, C.C. A review of testing methods for thermal spray coatings. Int. Mater. Rev. 2014, 59, 179–223. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, J.; Feng, M.; Yang, B.; Cai, X. Study on imaging method for measuring droplet size in large sprays. Particuology 2015, 22, 100–106. [Google Scholar] [CrossRef]
- Broeders, M.E.A.C.; Sanchis, J.; Levy, M.L.; Crompton, G.K.; Dekhuijzen, P.N.R.; ADMIT Working Group. The ADMIT series—Issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim. Care Respir. J. 2009, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Smola, M.; Vandamme, T.; Sokolowski, A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int. J. Nanomed. 2008, 3, 1–19. [Google Scholar]
- Virchow, J.C.; Crompton, G.K.; Dal Negro, R.; Pedersen, S.; Magnan, A.; Seidenberg, J.; Barnes, P.J. Importance of inhaler devices in the management of airway disease. Respir. Med. 2008, 102, 10–19. [Google Scholar] [CrossRef]
- Vincken, W.; Dekhuijzen, P.R.; Barnes, P.; ADMIT Group. The ADMIT series—Issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim. Care Respir. J. 2010, 19, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Chrystyn, H.; Price, D. Not all asthma inhalers are the same: Factors to consider when prescribing an inhaler. Prim. Care Respir. J. 2009, 18, 243–249. [Google Scholar] [CrossRef]
- Geller, D.E. The science of aerosol delivery in cystic fibrosis. Pediatr. Pulmonol. 2008, 43, S5–S17. [Google Scholar] [CrossRef]
- Longest, P.W.; Hindle, M. Evaluation of the Respimat Soft Mist Inhaler using a concurrent CFD and in vitro approach. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 99–112. [Google Scholar] [CrossRef]
- Newman, S.P.; Wilding, I.R.; Hirst, P.H. Human lung deposition data: The bridge between in vitro and clinical evaluations for inhaled drug products? Int. J. Pharm. 2000, 208, 49–60. [Google Scholar] [CrossRef]
- Finlay, W.H. Estimating the type of hygroscopic behavior exhibited by aqueous droplets. J. Aerosol Med. 1998, 11, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Longest, P.W.; Hindle, M.; Choudhuri, S.D. Effects of generation time on spray aerosol transport and deposition in models of the mouth-throat geometry. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Fletcher, D.; Chan, H.-K.; Raper, J. A Comparative Study of Two Marketed Pulmonary Drug Delivery Devices Using Computational Fluid Dynamics; Horwood International Publishing: Bethesda, MD, USA, 2004; pp. 821–824. [Google Scholar]
- Tibbatts, J.; Mendes, P.J.; Vlllax, P. Understanding the power requirements for efficient dispersion in powder inhalers: Comparing CFD predictions and experimental measurements. Respir. Drug Deliv. 2010, 1, 323–330. [Google Scholar]
- Nichols, S.; Wynn, E. New approaches to optimizing dispersion in dry powder inhalers-dispersion force mapping and adhesion measurements. Respir. Drug Deliv. 2008, 1, 175–184. [Google Scholar]
- Tashkin, D.P. A review of nebulized drug delivery in COPD. Int. J. Chron. Obstruct Pulmon. Dis. 2016, 11, 2585–2596. [Google Scholar] [CrossRef]
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2015, 12, 889–900. [Google Scholar] [CrossRef]
- Turner, M.O.; Gafni, A.; Swan, D.; FitzGerald, J.M. A review and economic evaluation of bronchodilator delivery methods in hospitalized patients. Arch. Intern. Med. 1996, 156, 2113–2118. [Google Scholar] [CrossRef]
- Cates, C.J.; Welsh, E.J.; Rowe, B.H. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: The Role of Inhalant Delivery Devices and Drug Formulations in Therapeutic Effectiveness of Aerosolized Medications. Available online: https://pubmed.ncbi.nlm.nih.gov/14616419/ (accessed on 5 May 2020).
- Nebuliser systems for drug delivery in cystic fibrosis—Daniels, T—2013 | Cochrane Library. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007639.pub2/full (accessed on 6 May 2020).
- Tuinman, P.R.; Dixon, B.; Levi, M.; Juffermans, N.P.; Schultz, M.J. Nebulized anticoagulants for acute lung injury—A systematic review of preclinical and clinical investigations. Crit. Care 2012, 16, R70. [Google Scholar] [CrossRef]
- Rouby, J.J.; Bouhemad, B.; Monsel, A.; Brisson, H.; Arbelot, C.; Lu, Q. Aerosolized antibiotics for ventilator-associated pneumonia: Lessons from experimental studies. Anesthesiology 2012, 117, 1364–1380. [Google Scholar] [CrossRef]
- Boyden, J.Y.; Connor, S.R.; Otolorin, L.; Nathan, S.D.; Fine, P.G.; Davis, M.S.; Muir, J.C. Nebulized medications for the treatment of dyspnea: A literature review. J. Aerosol Med. Pulm. Deliv. Pulm. Drug Deliv. 2015, 28, 1–19. [Google Scholar] [CrossRef]
- Finlay, W.H. Pharmaceutical Aerosol Sprays for Drug Delivery to the Lungs. In Handbook of Atomization and Sprays: Theory and Applications; Ashgriz, N., Ed.; Springer US: Boston, MA, USA, 2011; pp. 899–907. [Google Scholar]
- Ari, A. Jet, Ultrasonic, and Mesh Nebulizers: An Evaluation of Nebulizers for Better Clinical Outcomes. Euras J. Pulm. 2014, 16, 1–7. [Google Scholar] [CrossRef]
- Yeo, L.; Friend, J.; Mcintosh, M.; Meeusen, E.; Morton, D. Ultrasonic Nebulization Platforms for Pulmonary Drug Delivery. Expert Opin. Drug Deliv. 2010, 7, 663–679. [Google Scholar] [CrossRef]
- Ari, A.; Restrepo, R.D.; American Association for Respiratory Care. Aerosol delivery device selection for spontaneously breathing patients: 2012. Respir. Care 2012, 57, 613–626. [Google Scholar] [CrossRef]
- Ari, A.; Fink, J.B. Guidelines for aerosol devices in infants, children and adults: Which to choose, why and how to achieve effective aerosol therapy. Expert Rev. Respir. Med. 2011, 5, 561–572. [Google Scholar] [CrossRef]
- Watts, A.B.; McConville, J.T.; Williams, R.O. Current therapies and technological advances in aqueous aerosol drug delivery. Pharm. Drug Dev. Ind. Pharm. 2008, 34, 913–922. [Google Scholar] [CrossRef]
- Taylor, K.M.G.; McCallion, O.N.M. Ultrasonic nebulisers for pulmonary drug delivery. Int. J. Pharm. 1997, 153, 93–104. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Yazaki, T.; Arai, M.; Asai, K.; Kanke, M. The nebulization of budesonide suspensions by a newly designed mesh nebulizer. Respir. Drug Deliv. 2002, 8, 487–489. [Google Scholar]
- Elhissi, A.M.A.; Karnam, K.K.; Danesh-Azari, M.-R.; Gill, H.S.; Taylor, K.M.G. Formulations generated from ethanol-based proliposomes for delivery via medical nebulizers. J. Pharm. Pharmacol. 2006, 58, 887–894. [Google Scholar] [CrossRef]
- Elhissi, A.M.A.; Taylor, K.M.G. Delivery of liposomes generated from proliposomes using air-jet, ultrasonic, and vibrating-mesh nebulisers. J. Drug Deliv. Sci. Technol. 2005, 15, 261–265. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K.; Katinger, H. Nebulization of liposomal rh-Cu/Zn-SOD with a novel vibrating membrane nebulizer. J. Liposome Res. 2006, 16, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.; Gardenhire, D.; Ari, A.; Hess, D. A Guide to Aerosol Delivery Devices for Respiratory Therapists, 3rd ed.; American Association for Respiratory Care: Irving, TX, USA, 2013. [Google Scholar]
- Waldrep, J.C.; Dhand, R. Advanced nebulizer designs employing vibrating mesh/aperture plate technologies for aerosol generation. Curr. Drug Deliv. 2008, 5, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Dhand, R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir. Care 2002, 47, 1406–1416. [Google Scholar]
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011, 377, 1032–1045. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Hickey, R.D.T. Medical Devices for the Delivery of Therapeutic Aerosols to the Lungs. December 2006. Available online: https://www.rti.org/publication/medical-devices-delivery-therapeutic-aerosols-lungs (accessed on 30 August 2019).
- Kleinstreuer, C.; Zhang, Z. Airflow and Particle Transport in the Human Respiratory System. Ann. Rev. Fluid Mech. 2010, 42, 301–334. [Google Scholar] [CrossRef]
- Carvalho, T.C.; McConville, J.T. The Function and Performance of Aqueous Aerosol Devices for Inhalation Therapy. Available online: https://pubmed.ncbi.nlm.nih.gov/27061412/ (accessed on 5 May 2020).
- Arulmuthu, E.R.; Williams, D.J.; Baldascini, H.; Versteeg, H.K.; Hoare, M. Studies on aerosol delivery of plasmid DNA using a mesh nebulizer. Biotechnol. Bioeng. 2007, 98, 939–955. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Holmes, T.D.; Gao, J.; Guilmette, R.A.; Li, S.; Surakitbanharn, Y.; Rowlings, C. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. J. Aerosol Med. 2001, 14, 267–280. [Google Scholar] [CrossRef]
- Yang, J.Z.; Young, A.L.; Chiang, P.-C.; Thurston, A.; Pretzer, D.K. Fluticasone and budesonide nanosuspensions for pulmonary delivery: Preparation, characterization, and pharmacokinetic studies. J. Pharm. Sci. 2008, 97, 4869–4878. [Google Scholar] [CrossRef] [PubMed]
- Chemmalasseri, E.A. Numerical Modelling of Droplet Formation in Mesh Nebulizers. Master’s Thesis, Delft University of Technology, Delft, The Nederland, 2012. [Google Scholar]
- Haddrell, A.E.; Lewis, D.; Church, T.; Vehring, R.; Murnane, D.; Reid, J.P. Pulmonary aerosol delivery and the importance of growth dynamics. Ther. Deliv. 2017, 8, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Ashgriz, N. Numerical Techniques for Simulating the Atomization Process; Springer: Boston, MA, USA, 2011; pp. 339–357. [Google Scholar]
- Wong, W.; Fletcher, D.; Traini, D.; Chan, H.-K.; Young, P. The use of computational approaches in inhaler development. Adv. Drug Deliv. Rev. 2011, 64, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Ruzycki, C.A.; Javaheri, E.; Finlay, W.H. The use of computational fluid dynamics in inhaler design. Expert Opin. Drug Deliv. 2013, 10, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Salary, R.R.; Lombardi, J.P.; Samie Tootooni, M.; Donovan, R.; Rao, P.K.; Borgesen, P.; Poliks, M.D. Computational Fluid Dynamics Modeling and Online Monitoring of Aerosol Jet Printing Process. J. Manuf. Sci. Eng. 2017, 139. [Google Scholar] [CrossRef]
- Weber, A.; Morlin, G.; Cohen, M.; Williams-Warren, J.; Ramsey, B.; Smith, A. Effect of nebulizer type and antibiotic concentration on device performance. Pediatr. Pulmonol. 1997, 23, 249–260. [Google Scholar] [CrossRef]
- Ochowiak, M.; Doligalski, M.; Broniarz-Press, L.; Matuszak, M.; Gosciniak, A. Characterization of sprays for thermo-stabilized pneumatic nebulizer. Available online: https://pubmed.ncbi.nlm.nih.gov/26825254/ (accessed on 5 May 2020).
- Broniarz-Press, L.; Ochowiak, M.; Matuszak, M.; Wlodarczak, S. The Effect of Shear and Extensional Viscosity on Atomization in Medical Inhaler. Available online: https://pubmed.ncbi.nlm.nih.gov/24746416/ (accessed on 5 May 2020).
- Broniarz-Press, L.; Sosnowski, T.R.; Matuszak, M.; Ochowiak, M.; Jablczynska, K. The Effect of Shear and Extensional Viscosities on Atomization of Newtonian and Non-Newtonian Fluids in Ultrasonic Inhaler. Available online: https://europepmc.org/article/med/25735665 (accessed on 5 May 2020).
- Jeng, Y.R.; Su, C.C.; Feng, G.H.; Peng, Y.Y. An investigation into a piezoelectrically actuated nebulizer with μEDM-made micronozzle array. Exp. Therm. Fluid Sci. 2007, 31, 1147–1156. [Google Scholar] [CrossRef]
- Su, G.; Longest, P.W.; Pidaparti, R.M. A novel micropump droplet generator for aerosol drug delivery: Design simulations. Biomicrofluidics 2010, 4, 044108. [Google Scholar] [CrossRef]
- Amirav, I.; Oron, A.; Tal, G.; Cesar, K.; Ballin, A.; Houri, S.; Naugolny, L.; Mandelberg, A. Aerosol delivery in respiratory syncytial virus bronchiolitis: Hood or face mask? J. Pediatr. 2005, 147, 627–631. [Google Scholar] [CrossRef]
- Amirav, I.; Balanov, I.; Gorenberg, M.; Groshar, D.; Luder, A.S. Nebuliser hood compared to mask in wheezy infants: Aerosol therapy without tears! Arch. Dis. Child. 2003, 88, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.F.; Finlay, W.H. Liquid atomizing: Nebulizing and other methods of producing aerosols. J. Aerosol Med. 2006, 19, 28–35. [Google Scholar] [CrossRef]
- Finlay, W.H.; Martin, A.R. Modeling of aerosol deposition with interface devices. J. Aerosol Med. 2007, 20 (Suppl. 1), S19–S26; [Google Scholar] [CrossRef] [PubMed]
- Longest, P.W.; Spence, B.M.; Holbrook, L.; Mossi, T.; K. Son, M.Y.J.; Hindle, M. Production of inhalable submicrometer aerosols from conventional mesh nebulizers for improved respiratory drug delivery. Available online: https://pubmed.ncbi.nlm.nih.gov/22707794/ (accessed on 5 May 2020).
- Yousefi, M.; Pourmehran, O.; Gorji-Bandpy, M.; Inthavong, K.; Yeo, L.; Tu, J. CFD simulation of aerosol delivery to a human lung via surface acoustic wave nebulization. Biomech. Model Mechanobiol. 2017, 16, 2035–2050. [Google Scholar] [CrossRef]
- Santati, S.; Thongsri, J.; Sarntima, P. Modified Small-Volume Jet Nebulizer Based on CFD Simulation and Its Clinical Outcomes in Small Asthmatic Children. J. Healthc. Eng. 2019. Available online: https://www.hindawi.com/journals/jhe/2019/2524583/ (accessed on 10 December 2019).
- Shen, S.-C.; Wang, Y.-J.; Chen, Y.-Y. Design and fabrication of medical micro-nebulizer. Sens. Actuators A Phys. 2008, 144, 135–143. [Google Scholar] [CrossRef]
- Pirozynski, M.; Sosnowski, T.R. Inhalation devices: From Basic Science to Practical Use, Innovative vs. Generic Products. Available online: https://pubmed.ncbi.nlm.nih.gov/27267298/ (accessed on 5 May 2020).
- Shakked, T.; Katoshevski, D.; Broday, D.M.; Amirav, I. Numerical simulation of air flow and medical-aerosol distribution in an innovative nebulizer hood. J. Aerosol Med. 2005, 18, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Xi, J.; Si, X.; Berlinski, A.; Su, W.C. Hood nebulization: Effects of head direction and breathing mode on particle inhalability and deposition in a 7-month-old infant model. J. Aerosol Med. Pulm. Deliv. Pulm. Drug Deliv. 2014, 27, 209–218. [Google Scholar] [CrossRef]
- Delvadia, R.R.; Longest, P.W.; Hindle, M.; Byron, P.R. In vitro tests for aerosol deposition. III: Effect of inhaler insertion angle on aerosol deposition. J. Aerosol Med. Pulm. Deliv. Pulm. Drug Deliv. 2013, 26, 145–156. [Google Scholar] [CrossRef]
- Tong, X.; Dong, J.; Shang, Y.; Inthavong, K.; Tu, J. Effects of nasal drug delivery device and its orientation on sprayed particle deposition in a realistic human nasal cavity. Comput. Biol. Med. 2016, 77, 40–48. [Google Scholar] [CrossRef]
- Radhakrishnan, H.; Kassinos, S. CFD modeling of turbulent flow and particle deposition in human lungs. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis/St. Paul, MN, USA, 2–6 September 2009; pp. 2867–2870. [Google Scholar] [CrossRef]
- Frank, D.O.; Kimbell, J.S.; Pawar, S.; Rhee, J.S. Effects of anatomy and particle size on nasal sprays and nebulizers. Otolaryngol. Head Neck Surg. 2012, 146, 313–319. [Google Scholar] [CrossRef]
- Optical Imaging of Sprays—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/036012859190011B (accessed on 12 August 2019).
- Lefebvre, A.H.; McDonell, V.G. Atomization and Sprays; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Finlay, W.; Stapleton, K. Undersizing of droplets from a vented nebulizer caused by aerosol heating during transit through an Anderson impactor. J. Aerosol Sci. 1999, 30, S0021–S8502. [Google Scholar] [CrossRef]
- Dennis, J.; Berg, E.; Sandell, D.; Ali, A.; Lamb, P.; Tservistas, M.; Karlsson, M.; Mitchell, J. Cooling the NGI—an approach to size a nebulised aerosol more accurately. Pharmeur Sci. Notes 2008, 2008, 27–30. [Google Scholar]
- Kwong, W.T.; Ho, S.L.; Coates, A.L. Comparison of nebulized particle size distribution with Malvern laser diffraction analyzer versus Andersen cascade impactor and low-flow Marple personal cascade impactor. J. Aerosol Med. 2000, 13, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R. A review of drop size measurement—The application of techniques to dense fuel sprays. Prog. Energy Combust. Sci. 1977, 3, 225–234. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Nagel, M.W. Cascade impactors for the size characterization of aerosols from medical inhalers: Their uses and limitations. J. Aerosol Med. 2003, 16, 341–377. [Google Scholar] [CrossRef] [PubMed]
- Gurses, B.K.; Smaldone, G.C. Effect of tubing deposition, breathing pattern, and temperature on aerosol mass distribution measured by cascade impactor. J. Aerosol Med. 2003, 16, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef]
- McCallion, O.N.M.; Taylor, K.M.G.; Thomas, M.; Taylor, A.J. The Influence of Surface Tension on Aerosols Produced By Medical Nebulisers International. J. Pharm. 1996, 129, 123–136. [Google Scholar]
- Mitchell, J.P.; Nagel, M.W.; Nichols, S.; Nerbrink, O. Laser diffractometry as a technique for the rapid assessment of aerosol particle size from inhalers. J. Aerosol Med. 2006, 19, 409–433. [Google Scholar] [CrossRef]
- Pilcer, G.; Vanderbist, F.; Amighi, K. Correlations between cascade impactor analysis and laser diffraction techniques for the determination of the particle size of aerosolised powder formulations. Int. J. Pharm. 2008, 358, 75–81. [Google Scholar] [CrossRef]
- Lelong, N.; Junqua-Moullet, A.; Diot, P.; Vecellio, L. Comparison of laser diffraction measurements by mastersizer X and spraytec to characterize droplet size distribution of medical liquid Aerosols. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 94–102. [Google Scholar] [CrossRef]
- Kippax, P.W. Appraisal of the Laser Diffraction Particle-Sizing Technique. Pharm. Technol. 2005, 3, 88–89. [Google Scholar]
- Etzler, F.M.; Deanne, R. Particle size analysis: A comparison of various methods ii. Part. Syst. Charact. 1997, 14, 278–282. [Google Scholar] [CrossRef]
- FDA (CDRH). Reviewer Guidance for Nebulizers, Metered Dose Inhalers, Spacers and Actuators. U.S. Food and Drug Administration. 1993. Available online: http://www.fda.gov/regulatory-information/search-fda-guidance-documents/reviewer-guidance-nebulizers-metered-dose-inhalers-spacers-and-actuators (accessed on 25 June 2019).
- FDA (CDRH). Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action; Center for Drug Evaluation and Research: Rockville, MD, USA, 2003; p. 37.
- ISO13320-1. Particle Size Analysis—Laser Diffraction Methods. Part 1, General Principles; BSI: London, UK, 1999. [Google Scholar]
- Jones, S.A.; Martin, G.P.; Brown, M.B. High-pressure aerosol suspensions—A novel laser diffraction particle sizing system for hydrofluoroalkane pressurised metered dose inhalers. Int. J. Pharm. 2005, 302, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.C.; Liu, B.Y.H. Aerodynamic particle size measurement by laser-doppler velocimetry. J. Aerosol Sci. 1980, 11, 139–150. [Google Scholar] [CrossRef]
- Doughty, D.V.; Vibbert, C.; Kewalramani, A.; Bollinger, M.E.; Dalby, R.N. Automated actuation of nasal spray products: Determination and comparison of adult and pediatric settings. Pharm. Drug Dev. Ind. Pharm. 2011, 37, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kippax, P.; Krarup, H.; Suman, J.D. Application for droplet sizing: Manual versus automated actuation of nasal sprays. Pharm. Technol. 2004, 28, 30–40. [Google Scholar]
- Liu, X.F.; Doub, W.H.; Guo, C.N. Evaluation of droplet velocity and size from nasal spray devices using phase Doppler anemometry (PDA). Int. J. Pharm. 2010, 388, 82–87. [Google Scholar] [CrossRef]
- Albrecht, H.-E.; Borys, M.; Damaschke, N.; Tropea, C. Laser Doppler and Phase Doppler Measurement Techniques; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; pp. 9–26. [Google Scholar]
- Azevedo, C.; Costa, F.; Andrade, J.C. Effects of nozzle exit geometry on spray characteristics of a blurry injector. At. Sprays 2013, 23. [Google Scholar] [CrossRef]

| Particle Characteristics | Nebulizer System | Patient Condition |
|---|---|---|
| Diameter Shape Tap density Bulk density Charge Hydroscopy Surface tension Viscosity Concentration effect Mass median diameter | Residual volume Continuous delivery Baffle design Flow rate Pressure Frequency | Airway obstruction Respiratory rate Reduced vital capacity Nebulization time Occluded airways Residence time Nasal breathing Nozzle insertion angle |
| Authors | Title of Study | Technique | Major Focus | Method Used | Journal Impact Factor | No. of Citations |
|---|---|---|---|---|---|---|
| Worth Longest et al. [68] | Production of Inhalable Submicrometer Aerosols from Conventional Mesh Nebulizers for Improved Respiratory Drug Delivery | Mesh nebulizer | Investigate how submicron meter and nanometer particles are efficient during drug delivery and explore their formation from mesh nebulizers | The low Reynolds number (LRN) K–ω approach was used for modeling the laminar flow and Lagrangian method was used for particle tracking | 2.866 | 69 |
| Arulmuthu et al. [49] | Studies on Aerosol Delivery of Plasmid DNA using a Mesh Nebulizer | MicroAIR NE-U22 mesh nebulizer | Aerosolization of Plasmid DNA without getting deformed by the stress–strain | Assuming laminar flow, the solution controls were SIMPLE pressure–velocity coupling and second order pressure with third-order monotone upstream-centered schemes for conservation laws (MUSCL) momentum discretization. The transient flow simulations were carried out using Fluent 6.2 computational fluid dynamics (CFD_ commercial code. | 4.26 | 45 |
| Shen et al. [71] | Design and Fabrication of Medical Micro-Nebulizer | Micropiezo electrically-actuated nebulizer | The optimal operating conditions for micropiezo, electrically actuated nebulizers. The optimal was estimated for the best droplet size and velocity output | CFD-RC commercial code was used for the CFD simulation while volume of fluid (VOF) module was applied to calculate the ejection process and grid deformation module simulated the vibration micro-nozzle plate | 2.739 | 42 |
| Frank et al. [78] | Effects of Anatomy and Particle Size on Nasal Sprays and Nebulizers | Not specified | Effect of a nasal deformity to the penetration of drug through a nebulizer | ANSYS Fluent 12.1.4 software bases on the finite volume method and discrete phase model to calculate particle trajectories | 2.175 | 37 |
| Su et al. [63] | A Novel Micropump Droplet Generator for Aerosol Drug Delivery | Micropump droplet generator (MDG) | Design feasibility of a valveless micropump droplet generator and effects of nozzle geometry and frequency in determining the effectiveness of the therapy were investigated | CFD simulation based on solver fluent 12 based finite volume method and the transient solution was implemented by the implicit marching technique solving the Navier–Stokes equations. The SIMPLE algorithm was used for solving the pressure–velocity coupling | 2.531 | 19 |
| Yousefi et al. [69] | CFD Simulation of Aerosol Delivery to a Human Lung via Surface Acoustic Nebulization | Nebulizer driven by a surface acoustic wave | The transport and deposition of drugs into in-silico lung model by a surface acoustic wave (SAW) nebulizer | A Eulerian approach was used to solve the Navier–Stokes equations (using the K−ω low Reynolds number (LRN)model) that govern fluid flow and a Lagrangian and discrete phase model were used for particle tracking | 2.829 | 19 |
| Kim et al. [74] | Hood Nebulization: Effects of Head Direction and Breathing Mode on Particle Inhale Ability and Deposition in a 7-Month-old Infant Model | Mesh nebulizer | The inhalability and deposition of drugs on an infant under different head positions and breathing conditions | For the CFD simulation, ANSYS Fluent 6.3 commercial code was used while the low Reynolds number k−ω approach and Lagrangian were used to track the particles | 2.866 | 18 |
| Radhakrishnan et al. [77] | CFD Modeling of Turbulent Flow and Particle Deposition in Human Lungs | Not specified | Effects of turbulence on the deposition and dispersion of drugs due to the geometry of the upper airways | Navier–Stokes equation and mass continuity equation to study the flow, finite volume method based solver using the LES (Large eddy simulation) model with Smagorinsky sub-grid scale were used to study turbulent airflow and particle deposition | 0.76 | 16 |
| Tong et al. [76] | Effects of Nasal Drug Delivery Device and its Orientation on Sprayed Particle Deposition in a Realistic Human Nasal Cavity | Nasal drug delivery device | Analyses of influencing factors such as patients breathing mode, nozzle, drug droplet size, and releasing direction | The full Navier–Stokes equations solved on ANSYS-fluent v14.5 software and SIMPLE method for pressure–velocity coupling | 2.286 | 14 |
| Jeng et al. [62] | An Investigation into Piezoelectrically Actuated Nebulizer with the μEDM-made Micronozzle Array | Piezoelectrically actuated nebulizer | Traditional ultrasonic nebulizers under varying operating conditions such as frequency and properties of drugs such as viscosity and surface tension | The SIMPLEC (SIMPLE-Consistent) algorithm was used for the velocity and pressure fields and VOF (volume of fluid) and PLIC (piecewise linear interface calculation) for factors of volume fractions of liquids | 3.493 | 11 |
| Shakked et al. [73] | Numerical Simulation of air Flow and Medical-Aerosol Distribution in an Innovative Nebulizer Hood | Nebulizer with hood | Investigate numerically the airflow induced drug dispersion inside the hood and drug droplet dispersion with respect to three breathing phases: inspiration, expiration, and apnea | FLUENT 6.1 CFD software package describing the airflow and the trajectories of drug droplets to solve Navier–Stokes equation and GAMBIT package to generate geometry and mesh | 2.866 | 9 |
| Santati et al. [70] | Modified Small-Volume Jet Nebulizer Based on CFD Simulation and its Clinical Outcomes in Small Asthmatic Children | Small volume jet nebulizer (SVJN) | Redesign of SVJN by adding corrugated tube in order to slow down the drug velocity so that they are suitable for small children | ANSYS Fluent 17.1 CFD program was used to solve conservation equations. The shear stress transport k−ω turbulance model was used where particle path lines were calculated using discrete phase model | 1.295 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hailu, N.; Postema, M.; Krejcar, O.; Assefa, D. Nebulization Criteria and Quantification. Fluids 2020, 5, 91. https://doi.org/10.3390/fluids5020091
Hailu N, Postema M, Krejcar O, Assefa D. Nebulization Criteria and Quantification. Fluids. 2020; 5(2):91. https://doi.org/10.3390/fluids5020091
Chicago/Turabian StyleHailu, Nardos, Michiel Postema, Ondrej Krejcar, and Dawit Assefa. 2020. "Nebulization Criteria and Quantification" Fluids 5, no. 2: 91. https://doi.org/10.3390/fluids5020091
APA StyleHailu, N., Postema, M., Krejcar, O., & Assefa, D. (2020). Nebulization Criteria and Quantification. Fluids, 5(2), 91. https://doi.org/10.3390/fluids5020091






