Computational Fluid Dynamics Modeling of Sweep Gas Flow Rate-Dependent Carbon Dioxide Removal in Oxygenators
Abstract
1. Introduction
- Develop a theoretical model that incorporates the blood pressure drop and gas transfer influenced by sweep gas flow rates;
- Implement the theoretical model into a CFD framework and validate it by comparing model predictions with experimental data;
- Apply the validated CFD model to investigate carbon dioxide removal in oxygenators under varying sweep gas flow rates.
2. Materials and Methods
2.1. Theory Formulation
2.1.1. Transfer of Oxygen
2.1.2. Carbon Dioxide Removal
2.1.3. Effects of Sweep Gas Rate
2.1.4. Blood Flow and Pressure Drop
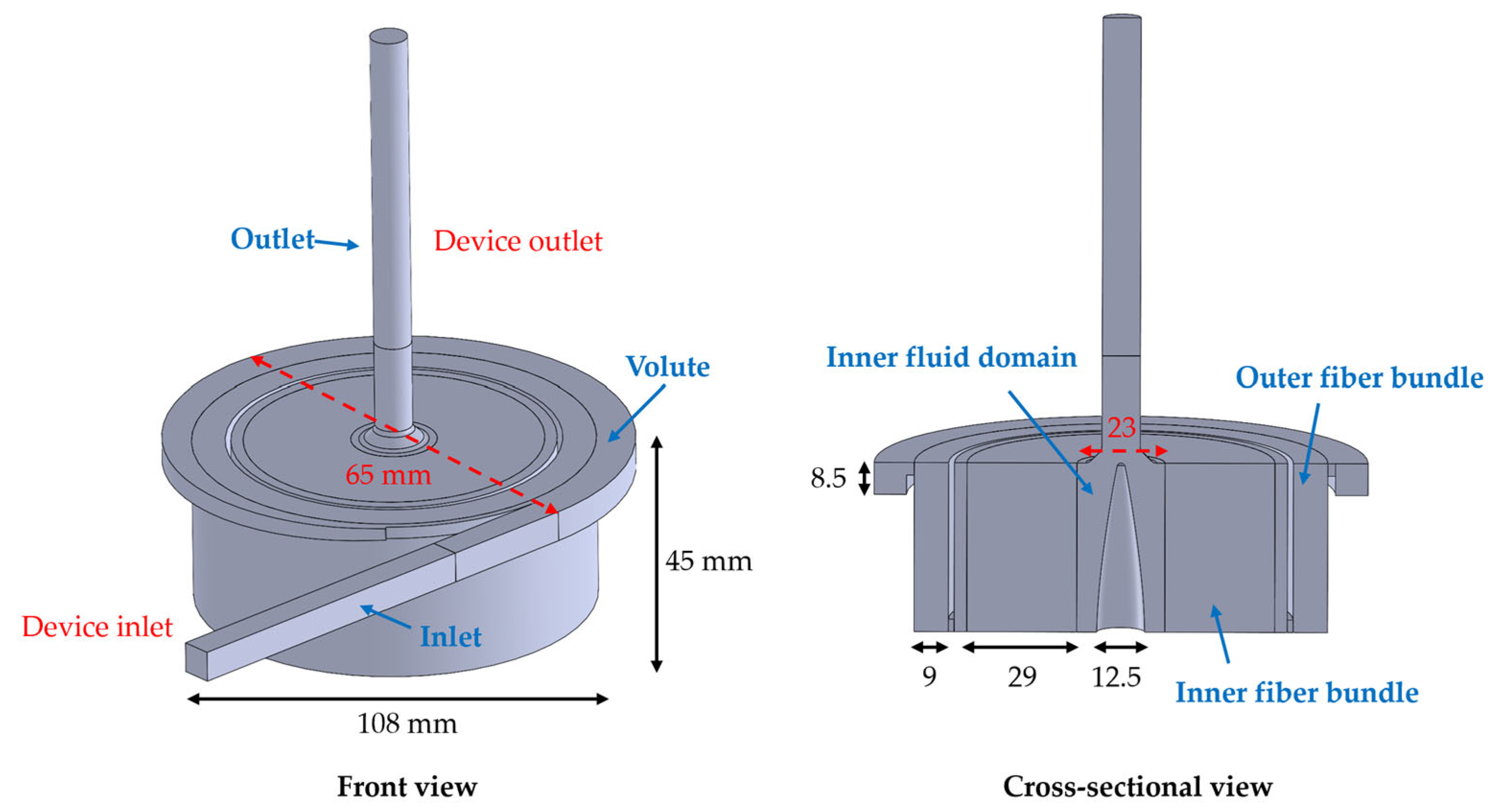
2.2. Simulation Setup
2.2.1. Model Implementation
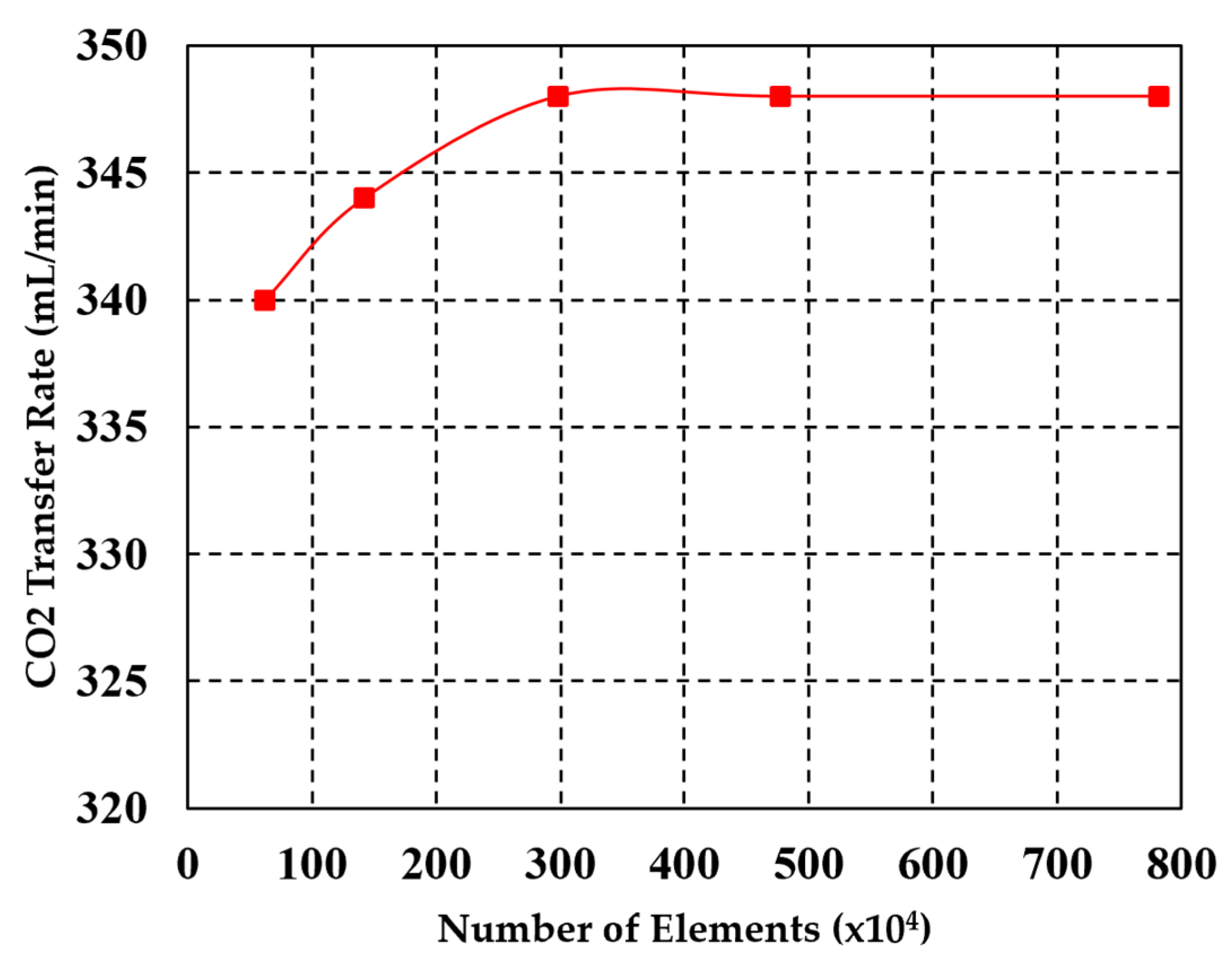
2.2.2. Mesh Sensitivity Study
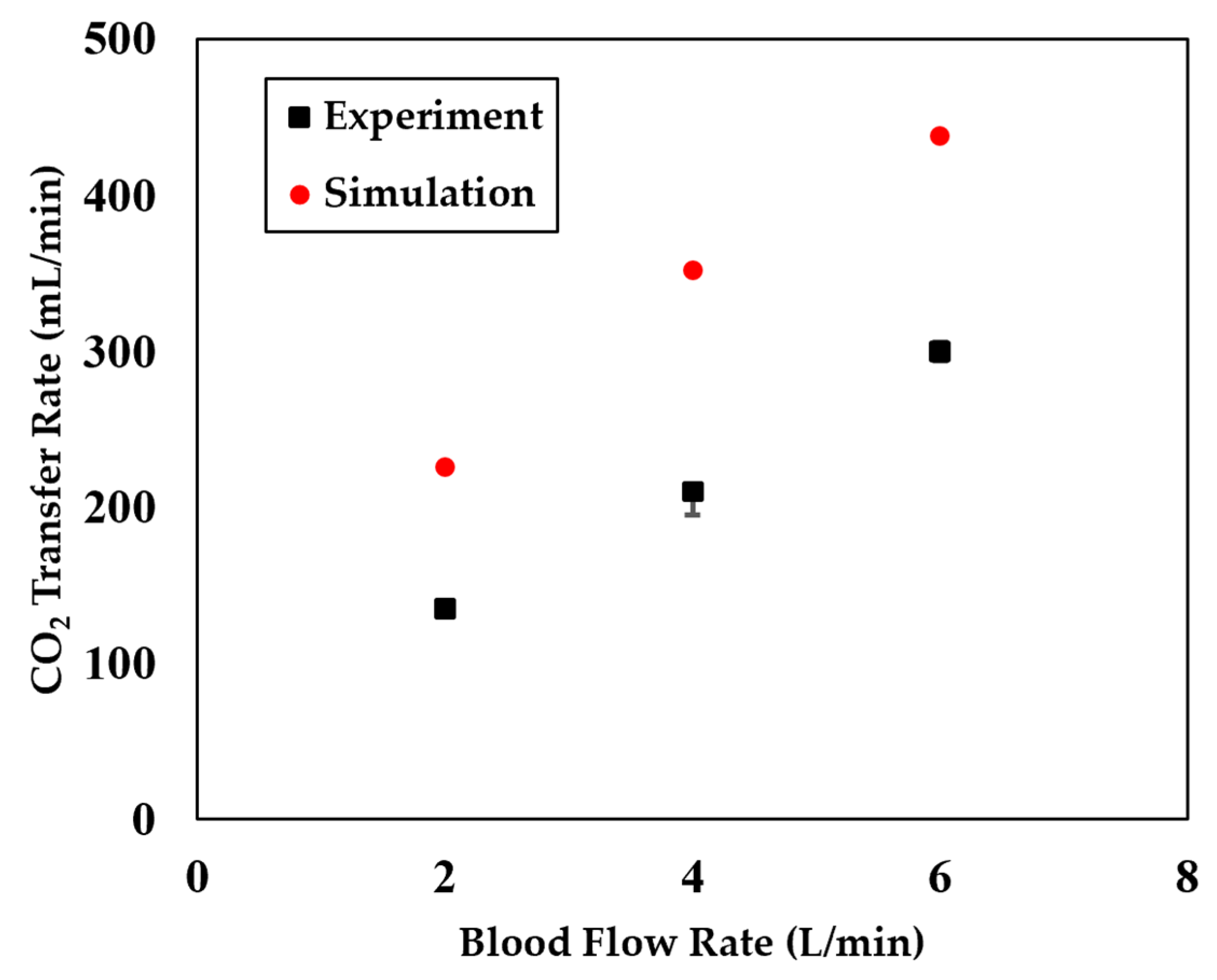
2.2.3. Model Validation
3. Analysis of Simulation Results
3.1. CO2 Patial Pressure Under Different Sweep Gas Rate
3.2. CO2 Concentration Under Different Sweep Gas Rate
3.3. Fiber Lumen CO2 Patial Pressure Under Different Sweep Gas Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Subramaniyan, V.; Fuloria, S.; Sekar, M.; Shanmugavelu, S.; Vijeepallam, K.; Kumari, U.; Narain, K.; Meenakshi, D.U.; Maziz, M.N.H.; Fuloria, N.K. Introduction to Lung Disease. In Targeting Epigenetics in Inflammatory Lung Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–16. [Google Scholar]
- Han, D.; Zhang, J.; Shah, A.; Griffith, B.P.; Wu, Z.J. A New Multifunction Oxygenator with a Dual-Chamber Gas Exchanger to Reduce Dependence on Oxygen Supply. ASAIO J. 2025. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, T.; Zhang, J.; Griffith, B.P.; Wu, Z.J. Model-Based Design and Optimization of Blood Oxygenators. J. Med. Devices, Trans. ASME 2020, 14, 041001. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Griffith, B.; Zhang, J.; He, G. Blood Gas Exchanger for Multifunctional Respiratory Support. PCT/US2024/012927, 25 February 2024. [Google Scholar]
- Lachaux, J.; Hwang, G.; Arouche, N.; Naserian, S.; Harouri, A.; Lotito, V.; Casari, C.; Lok, T.; Menager, J.B.; Issard, J.; et al. A compact integrated microfluidic oxygenator with high gas exchange efficiency and compatibility for long-lasting endothelialization. Lab Chip 2021, 21, 4791–4804. [Google Scholar] [CrossRef] [PubMed]
- Franke, B.; Goubergrits, L. Beveled shaped axial oxygenator with improved hemodynamics. medRxiv 2024. ahead of printing. [Google Scholar] [CrossRef]
- Han, D.; Zhang, J.; He, G.; Griffith, B.P.; Wu, Z.J. Computational fluid dynamics-based design and in vitro characterization of a novel pediatric pump-lung. Artif. Organs 2023, 48, 130–140. [Google Scholar] [CrossRef]
- Han, D.; Leibowitz, J.L.; Han, L.; Wang, S.; He, G.; Griffith, B.P.; Wu, Z.J. Computational fluid dynamics analysis and experimental hemolytic performance of three clinical centrifugal blood pumps: Revolution, Rotaflow and CentriMag. Med. Nov. Technol. Devices 2022, 15, 100153. [Google Scholar] [CrossRef]
- He, G.; Han, L.; Zhang, J.; Shah, A.; Kaczorowski, D.J.; Griffith, B.P.; Wu, Z. Numerical study of the effect of LVAD inflow cannula positioning on thrombosis risk. Comput. Methods Biomech. Biomed. Engin. 2022, 25, 852–860. [Google Scholar] [CrossRef]
- He, G.; Zhang, J.; Shah, A.; Berk, Z.B.; Han, L.; Dong, H.; Griffith, B.P.; Wu, Z.J. Flow characteristics and hemolytic performance of the new Breethe centrifugal blood pump in comparison with the CentriMag and Rotaflow pumps. Int. J. Artif. Organs 2021, 44, 829–837. [Google Scholar] [CrossRef]
- Han, D.; Zhang, J.; He, G.; Griffith, B.P.; Wu, Z.J. A prestressed intracellular biomechanical model for the platelet to capture the disc-to-sphere morphological change from resting to activated state. Int. J. Comput. Methods 2022, 19, 2250021. [Google Scholar] [CrossRef]
- Hormes, M.; Borchardt, R.; Mager, I.; Schmitz-Rode, T.; Behr, M.; Steinseifer, U. A validated CFD model to predict O2 and CO2 transfer within hollow fiber membrane oxygenators. Int. J. Artif. Organs 2011, 34, 317–325. [Google Scholar] [CrossRef]
- Asiltürk, A.Y.; Atalık, K. Computational Pulsatile Flow and Efficiency Analysis of Biocompatible Microfluidic Artificial Lungs for Different Fiber Configurations. J. Biomech. Eng. 2024, 146, 081002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nolan, T.D.C.; Zhang, T.; Griffith, B.P.; Wu, Z.J. Characterization of membrane blood oxygenation devices using computational fluid dynamics. J. Memb. Sci. 2007, 288, 268–279. [Google Scholar] [CrossRef]
- Barbian, K.P.; Hirschwald, L.T.; Linkhorst, J.; Neidlin, M.; Steinseifer, U.; Wessling, M.; Wiegmann, B.; Jansen, S.V. Flow and mass transfer prediction in anisotropic TPMS-structures as extracorporeal oxygenator membranes using reduced order modeling. J. Memb. Sci. 2024, 690, 122160. [Google Scholar] [CrossRef]
- Focke, J.M.; Bonke, P.L.; Gendron, N.; Call, T.; Steinseifer, U.; Arens, J.; Neidlin, M. The influence of membrane fiber arrangement on gas exchange in blood oxygenators: A combined numerical and experimental analysis. J. Memb. Sci. 2024, 710, 123147. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Fu, X.; Xi, Y.; Sun, A.; Chen, Z.; Fan, Y. A comprehensive study of oxygenator gas transfer efficiency and thrombosis risk. Phys. Fluids 2024, 36, 081916. [Google Scholar] [CrossRef]
- Tang, T.Q.; Hsu, S.Y.; Dahiya, A.; Soh, C.H.; Lin, K.C. Numerical modeling of pulsatile blood flow through a mini-oxygenator in artificial lungs. Comput. Methods Programs Biomed. 2021, 208, 106241. [Google Scholar] [CrossRef]
- Karabulut, H.; Toraman, F.; Tarcan, S.; Demirhisa, Ö.; Alhan, C. Adjustment of sweep gas flow during cardiopulmonary bypass. Perfusion 2002, 17, 353–356. [Google Scholar] [CrossRef]
- Turri, F.; Yanagihara, J.I. Computer-assisted numerical analysis for oxygen and carbon dioxide mass transfer in blood oxygenators. Artif. Organs 2011, 35, 579–592. [Google Scholar] [CrossRef]
- Manap, H.H.; Abdul Wahab, A.K.; Zuki, F.M. Mathematical Modelling of Carbon Dioxide Exchange in Hollow Fiber Membrane Oxygenator. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 210, p. 012003. [Google Scholar] [CrossRef]
- Vaslef, S.N.; Mockros, L.F.; Anderson, R.W.; Leonard, R.J. Use of a mathematical model to predict oxygen transfer rates in hollow fiber membrane oxygenators. ASAIO J. 1994, 40, 990–996. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of the molecules of hemoglobin on its dissociation curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar]
- Hewitt, T.J.; Hattler, B.G.; Federspiel, W.J. A mathematical model of gas exchange in an intravenous membrane oxygenator. Ann. Biomed. Eng. 1998, 26, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Svitek, R.G.; Federspiel, W.J. A mathematical model to predict CO2 removal in hollow fiber membrane oxygenators. Ann. Biomed. Eng. 2008, 36, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Barrett, N.A.; Hart, N.; Camporota, L. In-vitro performance of a low flow extracorporeal carbon dioxide removal circuit. Perfusion 2020, 35, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Scaravilli, V.; Zanella, A.; Sangalli, F.; Patroniti, N. Basic aspects of physiology during ECMO support. In ECMO-Extracorporeal Life Support Adults; Springer: Berlin/Heidelberg, Germany, 2014; pp. 19–36. [Google Scholar]
- Fill, B.; Gartner, M.; Johnson, G.; Horner, M.; Ma, J. Computational fluid flow and mass transfer of a functionally integrated pediatric pump-oxygenator configuration. ASAIO J. 2008, 54, 214–219. [Google Scholar] [CrossRef]
- Fu, X.; Su, Z.; Wang, Y.; Sun, A.; Wang, L.; Deng, X.; Chen, Z.; Fan, Y. Comparison of hemodynamic features and thrombosis risk of membrane 497 oxygenators with different structures: A numerical study. Comput. Biol. Med. 2023, 159, 106907. [Google Scholar] [CrossRef]
- Ertan Taskin, M.; Zhang, T.; Fraser, K.H.; Griffith, B.P.; Wu, Z.J. Design Optimization of a Wearable Artificial Pump-Lung Device With Computational Modeling. J. Med. Devices Trans. ASME 2012, 6, 031009. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askew, K.; Rizzo, J.; Fan, L.; He, G. Computational Fluid Dynamics Modeling of Sweep Gas Flow Rate-Dependent Carbon Dioxide Removal in Oxygenators. Fluids 2025, 10, 158. https://doi.org/10.3390/fluids10060158
Askew K, Rizzo J, Fan L, He G. Computational Fluid Dynamics Modeling of Sweep Gas Flow Rate-Dependent Carbon Dioxide Removal in Oxygenators. Fluids. 2025; 10(6):158. https://doi.org/10.3390/fluids10060158
Chicago/Turabian StyleAskew, Keira, Julia Rizzo, Lei Fan, and Ge He. 2025. "Computational Fluid Dynamics Modeling of Sweep Gas Flow Rate-Dependent Carbon Dioxide Removal in Oxygenators" Fluids 10, no. 6: 158. https://doi.org/10.3390/fluids10060158
APA StyleAskew, K., Rizzo, J., Fan, L., & He, G. (2025). Computational Fluid Dynamics Modeling of Sweep Gas Flow Rate-Dependent Carbon Dioxide Removal in Oxygenators. Fluids, 10(6), 158. https://doi.org/10.3390/fluids10060158






